Abstract
F-ATPases synthesize ATP from ADP and phosphate coupled with an electrochemical proton gradient in bacterial or mitochondrial membranes and can hydrolyse ATP to form the gradient. F-ATPases consist of a catalytic F1 and proton channel F0 formed from the α3β3γδϵ and ab2c10 subunit complexes, respectively. The rotation of γϵc10 couples catalyses and proton transport. Consistent with the threefold symmetry of the α3β3 catalytic hexamer, 120° stepped revolution has been observed, each step being divided into two substeps. The ATP-dependent revolution exhibited stochastic fluctuation and was driven by conformation transmission of the β subunit (phosphate-binding P-loop/α-helix B/loop/β-sheet4). Recent results regarding mechanically driven ATP synthesis finally proved the role of rotation in energy coupling.
Keywords: F-ATPase, ATP synthase, rotational catalysis, F1, F0
1. Introduction
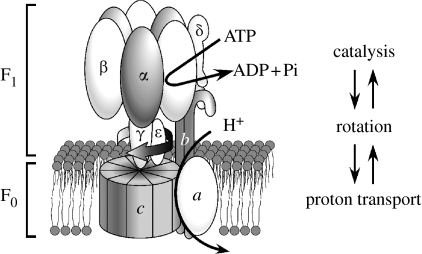
Cellular ATP is synthesized by a proton pumping F-ATPase (ATP synthase) coupled with an electrochemical proton gradient generated by the respiratory chain. The entire mechanism (oxidative- or photophosphorylation) is carried out in the membranes of mitochondria, bacteria or chloroplasts. F-ATPase, named after coupling factors of phosphorylation (Pedersen & Carafoli 1987), is a membrane enzyme formed from a catalytic sector, F1, and a proton pathway, F0 (figure 1). The basic structure of F1 is formed from the α, β, γ, δ and ϵ subunits with a stoichiometry of 3 : 3 : 1 : 1 : 1, and that of F0 from the a, b and c subunits (1 : 2 : 10–14).
Figure 1.
Schematic illustration of F-ATPases (F1 and F0 sectors). ATP synthesis/hydrolysis is coupled with proton transport through mechanical rotation. The number of c subunit monomers varies between 10 and 14 depending on the source. The model is for E. coli or yeast F-ATPase with a ring formed from 10 monomers.
Taking advantage of its stability, the Bacillus F-ATPase formed from the TF1 and TF0 sectors (T for thermophilic) was purified and reconstituted (Sone et al. 1975; Yoshida et al. 1975). The reconstituted F-ATPase could generate an electrochemical proton gradient upon ATP hydrolysis, and, in the reverse direction, could synthesize ATP coupled with the gradient (Sone et al. 1977). These results biochemically defined F-ATPase as a chemiosmotic enzyme (Mitchell 1979).
Functional F1 was obtained from Escherichia coli (Futai et al. 1974) and reconstituted from the five isolated subunits (Dunn & Futai 1980). The subunits of F0 and F1 were determined from the transducing λ phage carrying the entire genes for F-ATPases (Foster et al. 1980). F-ATPase purified from an overproducing strain could be functionally reconstituted in liposomes (Moriyama et al. 1991). Escherichia coli F-ATPase is the first enzyme whose genes and subunit sequences were determined (Kanazawa & Futai 1982; Futai & Kanazawa 1983; Walker et al. 1984) and has been studied with genetic manipulation.
X-ray structure of bovine F1 (Abrahams et al. 1994) clearly supported the binding change mechanism for ATP synthesis, including mechanical rotation of subunits (Boyer 1997). It became possible to correlate mutational results to the higher ordered structure.
In this article, discussion is focused on rotational catalysis and energy coupling by F-ATPases. We apologize to those whose works are not cited due to space limitation. For the areas not discussed in detail, readers could refer to the reviews (Futai et al. 1989, 2003; Stock et al. 2000; Senior et al. 2002; Fillingame et al. 2003). Other reviews are cited elsewhere where it is appropriate.
2. Catalysis, transport and energy coupling by F-ATPases
(a) Catalysis by F-ATPases
F-ATPases couple proton transport in F0 and chemistry in F1 through mechanical rotation. As expected from the presence of the three catalytic β subunits in an α3β3 hexamer, F-ATPases follow the binding change mechanism (Boyer 1997): briefly, ADP and Pi (phosphate) bind to a loose site that changes the conformation of the site to a tight one for ATP synthesis, while ATP is released from another site. The mechanism proposes that the reaction ‘ADP +Pi↔ATP +H2O’ at the tight site is reversible with no energy change, and that three sites are involved in the ATP synthesis sequentially through rotation of the α3β3 hexamer as to the γ subunit.
ATP hydrolysis in the F1 can be measured under single site (unisite catalysis) or steady-state (multisite catalysis) conditions: the steady-state ATPase rate is 105–106 fold faster than that of the ATP hydrolysis at a single site assayed with an ATP : F1 ratio of less than 1 : 3 (Cross et al. 1982). Further kinetic studies indicated that the equilibrium constant at the catalytic site is near unity, supporting that ATP is synthesized with no energy change (Grubmeyer et al. 1982). Three asymmetric catalytic sites were suggested by kinetic studies on wild-type and mutant enzymes, nucleotide binding being detected with an intrinsic tryptophan probe, affinity labelling with ATP analogues or chemical modification with inhibitors (Boyer 1997; Senior et al. 2002; Futai et al. 2003). Consistent with the asymmetric mechanism, the X-ray structure showed three different catalytic sites in βE, βDP and βTP corresponding to empty, ADP- and ATP-bound β subunits, respectively (Abrahams et al. 1994).
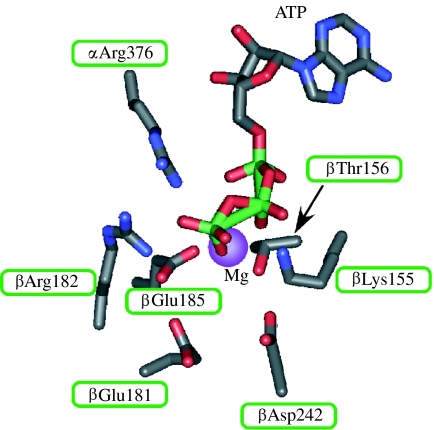
Catalytic residues were identified in the E. coli F1 (Ida et al. 1991; Omote et al. 1992; Senior & Al-Shawi 1992; Senior et al. 1993; Park et al. 1994; Löbau et al. 1997; figure 2). βLys155 of the β subunit is required for the binding of the γ phosphate moiety of ATP, as shown by enzymes such as the ones with βLys155 changed to Ala, Ser, Thr, Gln or Glu, and the affinity labelling of ATP analogues. The hydroxyl moiety of βThr156 is possibly essential for Mg2+ binding. βGlu181 is a critical catalytic residue forming a hydrogen bond with a water molecule near the ATP γ phosphate.
Figure 2.
Catalytic site of F-ATPases. The catalytic site of E. coli F-ATPase is shown together with bound ATP. The amino acid positions are according to the bovine F1 (Abrahams et al. 1994).
Analysis of intrinsic tryptophan probe (βTyr331Trp) indicated that the βLys155, βGlu181 and βAsp242 are catalytic residues: βLys155 interacts with MgATP, βGlu181 is a major catalytic residue and βAsp242 interacts with magnesium (Löbau et al. 1997). βGlu185 (Omote et al. 1995) and α Arg376 (Le et al. 2000) of the β and α subunits, respectively, are required for catalytic cooperativity. These residues could be located near ATP in the crystal structure of F1 (figure 2; Abrahams et al. 1994) and the transition state of the catalytic site (Braig et al. 2000).
(b) F0 sector and proton pathway
Subunit a has five transmembrane helices and the conserved essential aArg210 is in the fourth helix (Wada et al. 1999), close to the amino-terminal helix of the c subunit: a model of the interaction of the two helices was discussed (Fillingame et al. 2000). The b subunit is a long helical protein whose amino-terminal is embedded in the membrane, and its carboxyl terminus interacts with α and δ at the top of F1 (Fillingame et al. 2000). The structure of the c subunit, two transmembrane α-helices connected by a polar loop, has been solved by NMR (Girvin et al. 1998). Low-resolution electron (Birkenhäger et al. 1990) and atomic force microscopy (AFM; Singh et al. 1996; Takeyasu et al. 1996) suggested a ring structure formed from multiple c subunits. Refined AFM studies indicated that chloroplast and bacterial (Ilyobacter tartaricus) rings comprise 14 (Seelert et al. 2000) and 11 (Stahlberg et al. 2001) monomers, respectively. The X-ray structure of yeast F1 with a 10 monomer ring was solved (Stock et al. 1999). The X-ray diffraction has recently revealed an 11 monomer ring of I. tartaricus F-ATPase (Meier et al. 2005) and a 10 monomer ring of Enterococcus hirae V-ATPase (Murata et al. 2005). Cross-linking studies on the E. coli F-ATPase are consistent with 10 monomers, with amino and carboxyl helical domains located inside and outside, respectively (Fillingame et al. 2000).
Functional c rings were suggested by expressing covalently fused E. coli or Bacillus genes (Jiang et al. 2001; Mitome et al. 2004). The 10 oligomer formed from the fused genes was active. A similar experiment was carried out by nature: a gene encoding 13 homologous domains of the c subunits covalently connected was found in archae Methanopyrus kandleri (Lolkema & Boekema 2003). These results established a c subunit ring structure, the number of monomers differing with the species.
cAsp61, at the middle of the second transmembrane helix of the E. coli c subunit, is responsible for proton transport, and the results of mutational studies are consistent with rotary movement of the c10 ring (Fillingame & Dmitriev 2002). The stoichiometry (1 : 10–14) of the a and c subunits indicates that one aArg210 sequentially interacts with multiple carboxyl moieties for continuous proton translocation.
(c) Role of the γ subunit in energy coupling
An essential role of the γ subunit in catalysis and assembly was suggested by early experiments including reconstitution of the α3β3γ complex with ATPase activity (Dunn & Futai 1980) and analysis of defective termination mutants (Iwamoto et al. 1990). A role of chloroplast γ in regulation was indicated by the presence of a unique domain for reversible formation of a disulfide bond (Miki et al. 1988). Although the sequences of γ are weakly homologous among species, the amino- and carboxyl-terminal regions are significantly conserved (Nakamoto et al. 1992).
Of the mutants suggesting an active role of the γ subunit, γMet23→Lys and γMet23→Arg substitutions (Shin et al. 1992) should be briefly mentioned. They are impaired in ATP synthesis, and can only form a low electrochemical proton gradient dependent on ATP hydrolysis. The γMet23 residue is located close to the DELSEED loop (βAsp380–βAsp386) of β. Thermodynamic studies suggested that the mutant Lys23 could form an ionized hydrogen bond with βGlu381 of one of the three β subunits (Al-Shawi et al. 1997). These results suggest that the interaction between the region including γMet23 and DELSEED is involved in energy coupling.
The defect of the Lys23 mutant was suppressed by a second mutation in the carboxyl-terminal region of the γ subunit (Nakamoto et al. 1993, 1995). Most of the second mutations were mapped at positions far from position 23, but near the region where γ could interact with β. These results suggested that the α-helices of γ located at the centre of the α3β3 hexamer undergo large conformational changes during catalysis, as expected from the different orientation of γ as to the three β (βE, βDP and βTP; Abrahams et al. 1994) subunits.
3. Rotational catalysis of F-ATPase
(a) γ subunit rotation in the F1 sector
The binding change mechanism predicted that the catalytic sites in the three β subunits sequentially participate in ATP synthesis or hydrolysis through conformation transmission via rotation of the γ located at the central space of the α3β3. Observing rotation became possible using available X-ray structures (Abrahams 1994), since an appropriate probe could be introduced at a defined position of immobilized F1 or F0F1. Rotation of γ was suggested by cross-linking between γCys87 and introduced βCys380 in the DELSEED loop (Asp380–Asp386) of E. coli F1 (Duncan et al. 1995), and analysis of polarized absorption recovery after photobleaching of a probe linked to the carboxyl terminus of chloroplast F1 (Sabbert et al. 1996).
The rotation of γ was unambiguously video recorded by Noji et al. (1997). They observed ATP-dependent rotation of an actin filament connected to γ of the immobilized the Bacillus α3β3γ complex. The anticlockwise revolution speed became slower with an increase in filament length, generating frictional torque of approximately 40 pN·nm. The rotation of the ϵ subunit was shown subsequently (Kato-Yamada et al. 1998). Using an actin probe, we confirmed E. coli γ subunit rotation sensitive to F1 ATPase inhibitor azide, and generated torque of approximately 40 pN·nm (Omote et al. 1999). This finding prompted us to study the rotation of the F-ATPase holoenzyme and its mechanism by analysing previously isolated mutants.
(b) Subunit rotation in the F-ATPase holoenzyme
Revolution of γ should be transmitted to the F0 during ATP-dependent proton translocation in F-ATPase. Assuming that γ rotates with the c ring, purified F-ATPase was immobilized through the α subunit. An actin filament connected to the c ring rotated upon ATP hydrolysis and generated similar torque to that observed for the γ rotation (Sambongi et al. 1999). A similar experiment was carried out using a different method to connect the actin probe to the c ring (Pänke et al. 2000). Experimental systems for observing rotation were critically discussed (Sambongi et al. 2000; Wada et al. 2000). These results suggest that γ, ϵ and the c ring form a rotor, consistent with the finding that cross-linking between γ and c or ϵ and c did not affect ATPase activity (Schulenberg et al. 1999). Obviously, the cross-linking between rotor and stator such as β and γ or β and ϵ, respectively, resulted in the loss of the activity (Aggeler et al. 1999).
Furthermore, an actin filament connected to the a or α subunit rotated upon ATP hydrolysis in F-ATPase immobilized through the c ring (Tanabe et al. 2001). Similar results were obtained with membrane-bound F-ATPase, which was not subjected to solubilization with a detergent (Nishio et al. 2002). The rotation of gold beads attached to the Bacillus F0F1 c ring was shown recently, the rates being approximately 300 revolutions per second (rps) at 37°C (Ueno et al. 2005). These results established that the γϵc10 and α3β3δab2 complexes are an interchangeable rotor and a stator, respectively.
(c) Rotational synthesis of ATP
Chemiosmotic theory had been supported by ATP synthesis driven by an electrochemical proton gradient applied artificially (Mitchell 1979). Subunit rotation during ATP synthesis was shown recently with an F-ATPase in liposomes by fluorescence resonance energy transfer analysis (Diez et al. 2004). Thus, the electrochemical gradient should rotate the γϵc10 in F-ATPase, followed by ATP synthesis at the β subunit catalytic site.
ATP synthesis driven by the artificial γ revolution was shown (Itoh et al. 2004). Rotation of a bead in the magnetic field to the clockwise direction (viewed from the membrane) resulted in ATP synthesis. Rondelez et al. (2005) also observed ATP synthesis when γ was rotated artificially. Further studies will address the mechanistic questions including whether or not the rotation speed is proportional to the torque applied, or revolution is initiated upon applying torque higher than a certain value.
4. Stepping stochastic rotation
(a) Stepping rotation
As predicted by the sequential catalysis at the three sites, three 120° steps of Bacillus F1 were observed using an actin filament, when the ATP concentration was lowered (Yasuda et al. 1998). The stepped rotation was confirmed using beads with low viscous drag (Yasuda et al. 2001) or a fluorophore (Adachi et al. 2000). Furthermore, the stepping was observed with ATP concentrations of approximately 30-fold higher than the Km values (Yasuda et al. 2001; Nakanishi-Matsui et al. 2007).
Further analysis using smaller probes has shown that each step is divided into 90 and 30° substeps (Yasuda et al. 2001), later revised to 80 and 40°, respectively (Shimabukuro et al. 2003). They were attributed to ATP binding and phosphate release, respectively (Adachi et al. 2007). Thus, the rotation pauses before the 80° step, when the ATP concentration was lowered. The 120° steps observed with a higher ATP concentration most probably reflected ATP hydrolysis/product release (Nakanishi-Matsui et al. 2007).
(b) Stochastic rotation
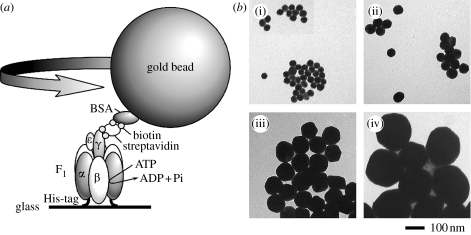
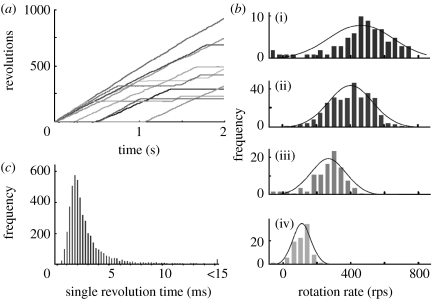
The maximal speed of an actin filament connected to the γ subunit was approximately 10 rps (Sabbert et al. 1996; Noji et al. 1997; Omote et al. 1999), i.e. slower than the rate expected from the turnover number of the steady-state ATPase. The ATPase rate calculated from the filament rotation was approximately 30 s−1, assuming that three ATP molecules were hydrolysed for one revolution (Nakanishi-Matsui et al. 2006). A gold bead attached to γ rotated faster (figure 3), possibly at a rate close to that of γ without a probe, since ones of 40 and 60 nm diameter rotated essentially at the same speed. The average rate during 250 ms observation was approximately 380 rps, i.e. approximately 10 times faster than the value expected from the ATPase. These results suggested that approximately 10% of the F1 molecules were rotating at the milliseconds time resolution. This interpretation became more convincing on observing longer time courses of randomly selected beads (Nakanishi-Matsui et al. 2007): individual beads paused randomly and rotated again (figure 4a). Histograms of the rotation speed indicated that the rotation exhibited stochastic fluctuation (figure 4b) essentially independent of the probe sizes (40–200 nm diameter; Nakanishi-Matsui & Futai 2006).
Figure 3.
Rotation of gold beads attached to the γ subunit of F1. (a) Experimental set-up for observing gold bead rotation. F1 was immobilized on a glass surface, a gold bead was attached and ATP-dependent rotation was followed. (b) Electron microscopy of beads used ((i) 40 nm, (ii) 60 nm, (iii) 100 nm, and (iv) 200 nm).
Figure 4.
Stochastic fluctuation of the γ subunit rotation. (a) Time courses for the gold beads. Rotation of randomly selected beads (60 nm diameter) was followed. (b) Histograms of rotation rates. The rotation rates of beads were estimated every 10 ms ((i) 40 nm, (ii) 60 nm, (iii) 100 nm, and (iv) 200 nm). (c) Single revolution time of gold beads. The time course of 60 nm beads was followed, and single revolution time (time required for the 360° revolution) is shown as histogram. Histograms obtained for 10 randomly selected beads are shown (see Ref. Nakanishi-Matsui et al. 2007 for coloured version).
When the beads were followed for a longer time, we observed that they unexpectedly paused sometimes for longer than 0.1 s, possibly due to MgADP inhibition (Nakanishi-Matsui et al. 2007). Longer pause of single beads should certainly lower the steady-state ATPase, which corresponds to the average rate of randomly selected beads.
The γ rotated in a stepwise manner, indicating that overall speed depends on the pausing dwells (approx. ms) and the time required for revolution between the steps (0→120°, 120→240°, 240→360° per 0°). The time required for the 120° revolution was mostly ≦0.25 ms, whereas pausing dwells were longer and variable. Thus, we used a single revolution time, i.e. the time for the 360° revolution, to analyse bead rotation with variable rates (figure 4c). This parameter could express all revolutions of a bead, even those including long pauses in a single figure. The histograms of the single revolution time for each bead and those of multiple beads combined were similar.
The fluctuation of the rotation rate was independent of the probe size (40–200 nm; figure 3b) when normalized (Nakanishi-Matsui & Futai 2006). Rotation of a single fluorophore attached to the γ subunit also exhibited variable dwelling times (Adachi et al. 2000). We have observed similar fluctuation of gold beads rotation in F0F1 (in preparation). These results indicate that the fluctuation is an intrinsic property of F-ATPase.
The effect of the ϵ subunit, an inhibitor of F1 ATPase, on the rotation was clearly observed when a gold bead was used as the probe (Nakanishi-Matsui et al. 2006). Histograms of the rates in the presence of excess ATP showed two populations, one peak at less than 40 rps and another at 200–240 rps. Most of the former peak was due to increased pausings, and the second peak was slower than that without ϵ. These results indicated that ϵ is inhibitory by increasing the pausing duration.
5. Towards understanding the rotation mechanism
(a) Mutation studies of rotation
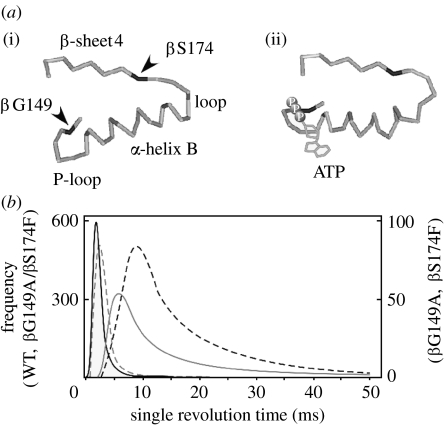
The mechanisms of catalysis and energy coupling of F-ATPase have been studied by analysing the mutants (Futai et al. 1989, 2003). Substitution of βSer174 had interesting effects on ATPase activity: the larger the side-chain volume of the residue introduced, the lower the ATPase activity became (Omote et al. 1994), and F1 with Phe or Leu exhibited approximately 10% wild-type activity. The introduced residues may affect the conformation of the β-sheet4 and the α-helix B domain (figure 5a), because introduced Phe may interact with the βIle166 or βIle163 residue in α-helix B (Iko et al. 2001).
Figure 5.
β subunit domain essential for F1 rotation. (a) β Subunit domain between β-sheet4 and P-loop. A β subunit domain is shown for an (i) empty (βE) β subunit or (ii) ATP bound (βTP). The positions of Ser174, Gly149 and ATP are shown according to the bovine F1 (Abrahams et al. 1994). (b) Effects of the βPhe174 mutation and a second mutation (βGly149Ala) on rotation. Fitted curves were obtained from the histogram of the single revolution time of the wild-type and mutant F1 (wild-type, black solid curve; Phe174, grey solid curve; Phe174/Ala149, grey dashed curve; and Ala149, black dashed curve).
The defect caused by βPhe174 was suppressed by a second mutation replacing βGly149 in the phosphate-binding P-loop (Iwamoto et al. 1993), indicating that the interaction between β-sheet4 and P-loop is important for ATPase activity. However, torque generated by an actin filament connected to βPhe174 or βLeu174 did not correspond to the ATPase activity, suggesting that actin is not suitable for studying mutant F1 (Iko et al. 2001).
Gold beads were attached to the mutant γ subunit (Nakanishi-Matsui et al. 2007), expecting that they reflect rotation as the speed of the γ subunit. Similarly, in the case of the wild-type, the single revolution time of a 60 nm gold bead showed stochastic fluctuation. Both βPhe174 and βLeu174 exhibited longer single revolution time than the wild-type (figure 5b), whereas those with a second mutation (βGly149Ala) were essentially similar to the wild-type. The βGly149 residue is in the P-loop where catalytic residues such as βLys155 and βThr156 are present (figure 2). These results suggest that the conformational transmission between the P-loop and β-sheet4 should be an initial change for driving rotation.
We were interested in the γLys23 mutation, which causes a defect in energy coupling between ATPase and proton translocation (Shin et al. 1992). Using an actin filament, we observed that the F1 generated essentially the same torque regardless of the mutation (Omote et al. 1999), suggesting that the mutant γMet23Lys is defective in transforming mechanical work into proton transport. However, the mutant's rotation should be studied using probe with lower viscous drag. Furthermore, it is of interest to examine the effect of replacing cAsp61 or aArg210 on rotation, which impairs proton transport (Hosokawa et al. 2005).
(b) For understanding the rotation mechanism
It has been accepted that F-ATPase couples proton transport and ATP synthesis/hydrolysis through subunit rotation. An important concept is stochastic fluctuation of F-ATPase rotation. The H+/ATP ratio for each step may also be stochastic. As discussed above, F1 has three catalytic sites, whereas proton transporting aspartate or glutamate in F0 has number 10, 11, 13 or 14 depending on the source. Assuming that all carboxyl moieties are used and three ATP molecules are synthesized or hydrolysed in one 360° revolution, the H+/ATP ratio should be 3.3, 3.7, 4.3 and 4.6 for the different species. To accommodate the non-integer H+/ATP ratio, the number of protons transported in each 120° step may be variable: for example, three protons in two 120° steps and four protons in one 120° step may be transported in E. coli F-ATPase having a c ring of 10 monomers (figure 1).
An enzyme is generally defined with kinetic parameters such as Km and Vmax obtained from steady-state kinetics. However, each enzyme molecule should exhibit variable rates, as shown for the rotation of a bead connected to F1. It should be noted that kinetic parameters determined on bulk phase measurement for ATPase are the averages for individual molecules.
Acknowledgments
We wish to thank our co-workers, whose names appear in the references. Also we appreciate Dr A. Yamamoto (Nagahama Institute of Bioscience and Technology) for the electron microscopy of gold beads. We are grateful for the support of CREST, Japan Science and Technology Agency, and grants-in-aid from the Ministry of Education, Science and Culture of Japan. We also thank Daiichi Pharmaceutical Company and Eisai Pharmaceutical Company for their support.
Footnotes
One contribution of 17 to a Theme Issue ‘Japan: its tradition and hot topics in biological sciences’.
References
- Abrahams J.P, Leslie A.G.W, Lutter R, Walker J. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. doi:10.1038/370621a0 [DOI] [PubMed] [Google Scholar]
- Adachi K, Yasuda R, Noji H, Itoh H, Harada Y, Yoshida M, Kinoshita K., Jr Stepping rotation of F1-ATPase visualized through angle-resolved single fluorophore imaging. Proc. Natl Acad. Sci. USA. 2000;97:7243–7247. doi: 10.1073/pnas.120174297. doi:10.1073/pnas.120174297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K, Oiwa K, Nishizawa T, Furuike S, Noji H, Itoh H, Yoshida M, Kinoshita K., Jr Coupling of rotation and catalysis in F1-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. doi: 10.1016/j.cell.2007.05.020. doi:10.1016/j.cell.2007.05.020 [DOI] [PubMed] [Google Scholar]
- Aggeler R, Haughton M.A, Capaldi R.A. Disulfide bond formation between the COOH-terminal domain of the β subunits and γ and ϵ subunits of the Escherichia coli F1-ATPase. Structural implications and functional consequences. J. Biol. Chem. 1999;270:9185–9191. doi: 10.1074/jbc.270.16.9185. [DOI] [PubMed] [Google Scholar]
- Al-Shawi M.K, Ketcham C.J, Nakamoto R.K. Energy coupling, turnover, and stability of the F0F1 ATP synthase are dependent on the energy of interaction between γ and β subunits. J. Biol. Chem. 1997;272:2300–2306. doi: 10.1074/jbc.272.4.2300. doi:10.1074/jbc.272.4.2300 [DOI] [PubMed] [Google Scholar]
- Birkenhäger R, Hoppert M, Deckers-Hebestreit G, Mayer F, Altendorf K. The F0 complex of the Escherichia coli ATP synthase. Investigation by electron spectroscopic imaging and immunoelectron microscopy. Eur. J. Biochem. 1990;230:58–67. doi:10.1111/j.1432-1033.1995.0058i.x [PubMed] [Google Scholar]
- Boyer P. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. doi:10.1146/annurev.biochem.66.1.717 [DOI] [PubMed] [Google Scholar]
- Braig K, Menz R.I, Leslie A.G, Walker J.E. Structure of bovine mitochondrial F1-ATPase inhibited by Mg2+ADP and aluminium fluoride. Structure. 2000;8:567–573. doi: 10.1016/s0969-2126(00)00145-3. doi:10.1016/S0969-2126(00)00145-3 [DOI] [PubMed] [Google Scholar]
- Cross R.L, Grubmeyer C, Penefsky H.S. Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate enhancement resulting from cooperative interactions between multiple catalytic sites. J. Biol. Chem. 1982;257:12 101–12 105. [PubMed] [Google Scholar]
- Diez M, et al. Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nature Struct. Mol. Biol. 2004;11:135–141. doi: 10.1038/nsmb718. doi:10.1038/nsmb718 [DOI] [PubMed] [Google Scholar]
- Duncan T.M, Bulygin V.V, Zhou Y, Hutcheon M.L, Cross R.L. Rotation of subunits during catalysis of Escherichia coli F1 ATPase. Proc. Natl Acd. Sci. USA. 1995;92:10 964–10 968. doi: 10.1073/pnas.92.24.10964. doi:10.1073/pnas.92.24.10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S.D, Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J. Biol. Chem. 1980;255:113–118. [PubMed] [Google Scholar]
- Fillingame R.H, Dmitriev U.Y. Structural model of the transmembrane F0 rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ. Biochim. Biophys. Acta. 2002;1565:232–245. doi: 10.1016/s0005-2736(02)00572-2. doi:10.1016/S0005-2736(02)00572-2 [DOI] [PubMed] [Google Scholar]
- Fillingame R.H, Jiang W, Dmitriev O.Y. Coupling H+ transport to rotary catalysis in F-type ATP synthase: structure and organization of the transmembrane rotary motor. J. Exp. Biol. 2000;203:9–17. doi: 10.1242/jeb.203.1.9. [DOI] [PubMed] [Google Scholar]
- Fillingame R.H, Angevine C.M, Dimitriev O.Y. Mechanism of coupling proton movements to c-ring rotation in ATP synthesis. FEBS Lett. 2003;555:29–34. doi: 10.1016/s0014-5793(03)01101-3. doi:10.1016/S0014-5793(03)01101-3 [DOI] [PubMed] [Google Scholar]
- Foster D.L, Mosher M.E, Futai M, Fillingame R.H. Subunits of the H+ ATPase of Escherichia coli. Overproduction of an eight-subunit F0F1 ATPase following induction of a λ-transducing phage carrying the unc operon. J. Biol. Chem. 1980;255:12 037–12 041. [PubMed] [Google Scholar]
- Futai M, Kanazawa H. Structure and function of proton translocating ATPase (F0F1): biochemical and molecular biological approaches. Microbial. Rev. 1983;47:285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M, Sternweis P.C, Heppel L.A. Purification and properties of reconstitutively active and inactive adenosine-triphosphatase from Escherichia coli. Proc. Natl Acad. Sci. USA. 1974;71:2725–2729. doi: 10.1073/pnas.71.7.2725. doi:10.1073/pnas.71.7.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M, Noumi T, Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu. Rev. Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. doi:10.1146/annurev.bi.58.070189.000551 [DOI] [PubMed] [Google Scholar]
- Futai M, Sun-Wada G.-H, Wada Y. Proton-translocating ATPases: introducing unique enzymes coupling catalysis and proton translocation through mechanical rotation. In: Futai M, Wada Y, Kaplan J, editors. Handbook of ATPases: biochemistry, cell biology, pathophysiology. Wiley-VCH Verlag GmbH & Co., KgaA; Weinheim, Germany: 2003. pp. 237–260. [Google Scholar]
- Girvin M.E, Rastogi V.K, Abidgard F, Marklly J.L, Fillingame R.H. Solution structure of transmembrane H+-transporting subunit c of the F1F0 ATP synthase. Biochemistry. 1998;37:8817–8824. doi: 10.1021/bi980511m. doi:10.1021/bi980511m [DOI] [PubMed] [Google Scholar]
- Grubmeyer C, Cross R.L, Penefsky H.S. Mechanism of ATP hydrolysis by beef heart mitochondrial ATPase. Rate constants for elementary steps in catalysis at a single site. J. Biol. Chem. 1982;257:12 092–12 100. [PubMed] [Google Scholar]
- Hosokawa H, Nakanishi-Matsui M, Kashiwagi S, Fujii-Taira I, Hayashi K, Iwamoto-Kihara A, Wada Y, Futai M. ATP-dependent rotation of mutant ATP synthase defective in proton transport. J. Biol. Chem. 2005;280:23 797–23 801. doi: 10.1074/jbc.M502650200. doi:10.1074/jbc.M502650200 [DOI] [PubMed] [Google Scholar]
- Ida K, Noumi T, Maeda M, Fukui T, Futai M. Catalytic site of F1-ATPase of Escherichia coli. Lys155 and Lys201 of the β subunit are located near the γ-phosphate group of ATP in the presence of Mg2+ J. Biol. Chem. 1991;266:5425–5429. [PubMed] [Google Scholar]
- Iko Y, Sambongi Y, Tanabe M, Iwamoto-Kihara A, Saito K, Ueda I, Wada Y, Futai M. ATP synthase F1 sector rotation. Defective torque generation in the β subunit Ser-174 to the Phe mutant and its suppression by second mutation. J. Biol. Chem. 2001;276:47 508–47 511. doi: 10.1074/jbc.M108803200. doi:10.1074/jbc.M108803200 [DOI] [PubMed] [Google Scholar]
- Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida Y, Kinoshita K., Jr Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427:465–468. doi: 10.1038/nature02212. doi:10.1038/nature02212 [DOI] [PubMed] [Google Scholar]
- Iwamoto A, Miki J, Maeda M, Futai M. H+ATPase γ subunit from Escherichia coli: role of the conserved carboxyl terminus. J. Biol. Chem. 1990;265:5043–5048. [PubMed] [Google Scholar]
- Iwamoto A, Park M.-Y, Maeda M, Fuati M. Domain near ATP γ phosphate in the catalytic site of H+-ATPase. Model proposed from mutagenesis and inhibitor studies. J. Biol. Chem. 1993;268:3156–3160. [PubMed] [Google Scholar]
- Jiang W, Hermolin J, Fillingame R.H. The preferred stoichiometry of c subunits in the rotary motor sector of Escherichia coli ATP synthase is 10. Proc. Natl Acd. Sci. USA. 2001;98:4966–4971. doi: 10.1073/pnas.081424898. doi:10.1073/pnas.081424898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H, Futai M. Structure and function of H+ATPase: what we have learned from Escherichia coli H+-ATPase. Annu. NY Acad. Sci. 1982;402:45–64. doi: 10.1111/j.1749-6632.1982.tb25731.x. doi:10.1111/j.1749-6632.1982.tb25731.x [DOI] [PubMed] [Google Scholar]
- Kato-Yamada Y, Noji H, Yasuda R, Kinoshita K, Jr, Yoshida M. Direct observation of the rotation of ϵ subunit in F1-ATPase. J. Biol. Chem. 1998;273:19 375–19 377. doi: 10.1074/jbc.273.31.19375. doi:10.1074/jbc.273.31.19375 [DOI] [PubMed] [Google Scholar]
- Le N.P, Omote H, Wada Y, Al-Shawi M.K, Nakamoto R, Futai M. Escherichia coli ATP synthase α subunit Arg-376: the catalytic site arginine does not participate in the hydrolysis/synthesis reaction, but is required for promotion to steady state. Biochemistry. 2000;39:2778–2783. doi: 10.1021/bi992530h. doi:10.1021/bi992530h [DOI] [PubMed] [Google Scholar]
- Löbau S, Weber J, Wilke-Mounts S, Senior A.E. F1-ATPase, roles of three catalytic site residues. J. Biol. Chem. 1997;272:3648–3656. doi: 10.1074/jbc.272.6.3648. doi:10.1074/jbc.272.6.3648 [DOI] [PubMed] [Google Scholar]
- Lolkema J.S, Boekema E.J. The A-type ATP synthase subunit K of Methanopyrus kandleri is deduced from its sequence to form a monomeric rotor comprising 13 hairpin domains. FEBS Lett. 2003;543:47–50. doi: 10.1016/s0014-5793(03)00398-3. doi:10.1016/S0014-5793(03)00398-3 [DOI] [PubMed] [Google Scholar]
- Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus. Science. 2005;308:659–662. doi: 10.1126/science.1111199. doi:10.1126/science.1111199 [DOI] [PubMed] [Google Scholar]
- Miki J, Maeda M, Mukohata Y, Futai M. The γ subunit of ATP synthase from spinach chloroplasts: primary structure deduced from cDNA sequence. FEBS Lett. 1988;232:221–226. doi: 10.1016/0014-5793(88)80421-6. doi:10.1016/0014-5793(88)80421-6 [DOI] [PubMed] [Google Scholar]
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979;206:1148–1159. doi: 10.1126/science.388618. doi:10.1126/science.388618 [DOI] [PubMed] [Google Scholar]
- Mitome N, Suzuki T, Hayashi S, Yoshida M. Thermophilic ATP synthase has a decamer c-ring: induction of noninteger 10:3 H+/ATP ratio and permissive elastic coupling. Proc. Natl Acad. Sci. USA. 2004;101:12 159–12 164. doi: 10.1073/pnas.0403545101. doi:10.1073/pnas.0403545101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Iwamoto A, Hanada H, Maeda M, Futai M. One-step purification of Escherichia coli H+-ATPase (F0F1) and its reconstitution into liposomes with neurotransmitter transporters. J. Biol. Chem. 1991;266:22 141–22 146. [PubMed] [Google Scholar]
- Murata T, Yamato I, Kakimura Y, Leslie A.G, Walker J.E. Structure of the rotor of the V-type Na+-ATPase from Enterococcus hirae. Scinece. 2005;308:654–659. doi: 10.1126/science.1110064. doi:10.1126/science.1110064 [DOI] [PubMed] [Google Scholar]
- Nakamoto R.K, Shin K, Iwamoto A, Omote H, Maeda M, Futai M. Escherichia coli F0F1 ATPase residues involved in catalysis and coupling. Annu. NY Acad. Sci. 1992;671:335–344. doi: 10.1111/j.1749-6632.1992.tb43807.x. doi:10.1111/j.1749-6632.1992.tb43807.x [DOI] [PubMed] [Google Scholar]
- Nakamoto R.K, Maeda M, Futai M. The γ subunit of the Escherichia coli ATP synthase. Mutations in the carboxyl terminal region restore energy coupling to the amino-terminal mutant γMet23Lys. J. Biol. Chem. 1993;268:867–872. [PubMed] [Google Scholar]
- Nakamoto R.K, Al-shawi M.E, Futai M. The ATP synthase γ subunit. Suppressor mutagenesis reveals three helical regions involved in energy coupling. J. Biol. Chem. 1995;270:1402–1406. doi: 10.1074/jbc.270.23.14042. doi:10.1074/jbc.270.3.1402 [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Futai M. Stochastic proton pumping ATPases: from single molecules to diverse physiological roles. IUBMB Life. 2006;58:318–322. doi: 10.1080/15216540600702255. [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Kashiwagi S, Hosokawa H, Cipriano D.J, Futai M. Stochastic high-speed rotation of the Escherichia coli ATP synthase F1 sector: the ϵ subunit sensitive rotation. J. Biol. Chem. 2006;281:4126–4131. doi: 10.1074/jbc.M510090200. doi:10.1074/jbc.M510090200 [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Kashiwagi S, Ubukata T, Iwamoto-Kihara A, Wada Y, Futai M. ATP synthase F1 sector β subunit: a key domain for driving stochastic rotation. J. Biol. Chem. 2007;282:20 698–20 704. doi: 10.1074/jbc.M700551200. doi:10.1074/jbc.M700551200 [DOI] [PubMed] [Google Scholar]
- Nishio K, Iwamoto-Kihara A, Yamamoto A, Wada Y, Futai M. Subunit rotation of ATP synthase embedded in membranes: α or β subunit rotation relative to the c subunit ring. Proc. Natl Acad. Sci. USA. 2002;99:13 448–13 452. doi: 10.1073/pnas.202149599. doi:10.1073/pnas.202149599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji H, Yasuda R, Yoshida N, Kinoshita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. doi:10.1038/386299a0 [DOI] [PubMed] [Google Scholar]
- Omote H, Maeda M, Futai M. Effects of mutations of conserved Lys-155 and Thr-156 residues in the phosphate-binding glycine-rich sequence of the F1-ATPase β subunit of Escherichia coli. J. Biol. Chem. 1992;267:20 571–20 576. [PubMed] [Google Scholar]
- Omote H, Park M.-Y, Maeda M, Futai M. The α/β subunit interaction in H+-ATPase (ATP synthase). An Escherichia coli α subunit mutation (Arg296→Cys) restores coupling efficiency to the deleterious β subunit mutant (Ser174→Phe) J. Biol. Chem. 1994;269:10 265–10 269. [PubMed] [Google Scholar]
- Omote H, Le N.P, Park M.-Y, Maeda M, Futai M. β subunit Glu-185 of Escherichia coli H+-ATPase (ATP synthase) is an essential residue for cooperative catalysis. J. Biol. Chem. 1995;270:25 656–25 660. doi: 10.1074/jbc.270.43.25656. doi:10.1074/jbc.270.43.25656 [DOI] [PubMed] [Google Scholar]
- Omote H, Sambonmatsu N, Saito K, Sambongi Y, Iwamoto-Kihara A, Yanagida T, Wada Y, Futai M. The γ subunit rotation and torque generation in F1-ATPase from wild-type or uncoupled mutant Escherichia coli. Proc. Natl Acad. Sci. USA. 1999;96:7780–7784. doi: 10.1073/pnas.96.14.7780. doi:10.1073/pnas.96.14.7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pänke O, Gumbiowski K, Junge W, Engelbrecht S. F-ATPase: specific observation of the rotating c subunit oligomer of EF0EF1. FEBS Lett. 2000;472:34–38. doi: 10.1016/s0014-5793(00)01436-8. doi:10.1016/S0014-5793(00)01436-8 [DOI] [PubMed] [Google Scholar]
- Park M.-Y, Omote H, Maeda M, Futai M. Conserved Glu-181 and Arg-182 residues of the Escherichia coli H+-ATPase (ATP synthase) β subunit are essential for catalysis: properties of 33 mutants between βGlu-161 and βLys-201 residues. J. Biochem. 1994;116:1139–1145. doi: 10.1093/oxfordjournals.jbchem.a124640. [DOI] [PubMed] [Google Scholar]
- Pedersen P.L, Carafoli E. Ion motive ATPase. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987;12:146–150. doi:10.1016/0968-0004(87)90071-5 [Google Scholar]
- Rondelez Y, Tresset G, Nakashima T, Kato-Yamada Y, Fujita H, Takeuchi S, Noji H. Highly coupled ATP synthesis by F1-ATPase single molecules. Nature. 2005;433:773–777. doi: 10.1038/nature03277. doi:10.1038/nature03277 [DOI] [PubMed] [Google Scholar]
- Sabbert D, Engelbrecht S, Junge W. Intersubunit rotation in active F-ATPase. Nature. 1996;381:623–625. doi: 10.1038/381623a0. doi:10.1038/381623a0 [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y, Futai M. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science. 1999;286:1722–1724. doi: 10.1126/science.286.5445.1722. doi:10.1126/science.286.5445.1722 [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Ueda I, Wada Y, Futai M. A biological molecular motor, proton-translocating ATP synthase: multi-diciplinary approach for a unique membrane enzyme. J. Bioenerg. Biomemb. 2000;32:441–448. doi: 10.1023/a:1005656706248. doi:10.1023/A:1005656706248 [DOI] [PubMed] [Google Scholar]
- Schulenberg B, Aggeler R, Murray J, Capaldi R.A. The γϵ-c subunit interface in the ATP synthase of Escherichia coli. Cross-linking of the subunit to the c subunit ring does not impair enzyme function, that of γ to c subunit leads to uncoupling. J. Biol. Chem. 1999;274:34 233–34 237. doi: 10.1074/jbc.274.48.34233. doi:10.1074/jbc.274.48.34233 [DOI] [PubMed] [Google Scholar]
- Seelert H, Poetsch A, Dencher N.A, Engel A, Stahlberg H, Müller D.J. Proton-powered turbine of a plant motor. Nature. 2000;405:418–419. doi: 10.1038/35013148. doi:10.1038/35013148 [DOI] [PubMed] [Google Scholar]
- Senior A.E, Al-Shawi M.K. Further examination of seventeen mutations in the Escherichia coli F1-ATPase β subunit. J. Biol. Chem. 1992;267:21 471–21 478. [PubMed] [Google Scholar]
- Senior A.E, Wilke-Mounts S, Al-Shawi M.K. Lysine 155 in the β subunit is a catalytic residue of Escherichia coli F1 ATPase. J. Biol. Chem. 1993;268:6989–6994. [PubMed] [Google Scholar]
- Senior A.E, Nadanaciva S, Weber J. The molecular mechanism of ATP synthesis by F1F0 ATP synthase. Biochim. Biophys. Acta. 2002;1553:188–211. doi: 10.1016/s0005-2728(02)00185-8. doi:10.1016/S0005-2728(02)00185-8 [DOI] [PubMed] [Google Scholar]
- Shimabukuro K, Yasuda R, Muneyuki E, Hara K.Y, Kinoshida K, Jr, Yoshida M. Catalysis and rotation of F1 motor: cleavage of ATP at the catalytic site occurs in 1 ms before 40° substep rotation. Proc. Natl Acad. Sci. USA. 2003;100:14 731–14 736. doi: 10.1073/pnas.2434983100. doi:10.1073/pnas.2434983100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Nakamoto R.K, Maeda M, Futai M. F0F1-ATPase γ subunit mutations perturb the coupling between catalysis and transport. J. Biol. Chem. 1992;267:20 835–20 839. [PubMed] [Google Scholar]
- Singh S, Turina P, Bustamante C.J, Keller D.J, Capaldi R.A. Topographical structure of membrane-bound Escherichia coli F0F1 ATP synthase in aqueous buffer. FEBS Lett. 1996;397:30–34. doi: 10.1016/s0014-5793(96)01127-1. doi:10.1016/S0014-5793(96)01127-1 [DOI] [PubMed] [Google Scholar]
- Sone N, Yoshida M, Hirata H, Kagawa Y. Purification and properties of a dicyclohexylcarbodiimide-sensitive adenosine triphosphatase from a thermophilic bacterium. J. Biol. Chem. 1975;250:7917–7923. [PubMed] [Google Scholar]
- Sone N, Yoshida M, Hirata H, Kagawa Y. Adenosine triphosphate synthesis by an electrochemical gradient in vesicles reconstituted from purified adenosine triphosphatase and phospholipids of a thermophilic bacterium. J. Biol. Chem. 1977;252:2956–2960. [PubMed] [Google Scholar]
- Stahlberg H, Müller D.J, Suda K, Fotiadis D, Engel A, Meier T, Matthey U, Dimroth P. Bacterial Na+-ATP synthase has an undecameric rotor. EMBO Rep. 2001;21:1–5. doi: 10.1093/embo-reports/kve047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock D, Leslie A.G.W, Walker J.E. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. doi:10.1126/science.286.5445.1700 [DOI] [PubMed] [Google Scholar]
- Stock D, Gibbons C, Arechaga I, Leslie A.G.W, Walker J.E. The rotary mechanism of ATP synthesis. Curr. Opin. Str. Biol. 2000;10:672–679. doi: 10.1016/s0959-440x(00)00147-0. doi:10.1016/S0959-440X(00)00147-0 [DOI] [PubMed] [Google Scholar]
- Takeyasu K, Omote H, Nettikadan S, Tokumasu F, Iwamoto A, Futai M. Molecular imaging of Escherichia coli F0F1-ATPase in reconstituted membranes using atomic force microscopy. FEBS Lett. 1996;392:110–113. doi: 10.1016/0014-5793(96)00796-x. doi:10.1016/0014-5793(96)00796-X [DOI] [PubMed] [Google Scholar]
- Tanabe M, Nishio K, Iko Y, Sambongi Y, Iwamoto-Kihara A, Omote H, Ueda I, Wada Y, Futai M. Rotation of a complex of the γ subunit and c ring of Escherichia coli ATP synthase: the rotor and stator are interchangeable. J. Biol. Chem. 2001;276:15 269–15 274. doi: 10.1074/jbc.M100289200. doi:10.1074/jbc.M100289200 [DOI] [PubMed] [Google Scholar]
- Ueno H, Suzuki T, Kinoshita K, Jr, Yoshida Y. ATP-driven stepwise rotation of F0F1-ATP synthase. Proc. Natl Acad. Sci. USA. 2005;102:1333–1338. doi: 10.1073/pnas.0407857102. doi:10.1073/pnas.0407857102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Long J.C, Zhang D, Vik S.B. A novel labeling approach supports the five-transmembrane model of subunit a of the Escherichia coli ATP synthase. J. Biol. Chem. 1999;274:17 353–17 357. doi: 10.1074/jbc.274.24.17353. doi:10.1074/jbc.274.24.17353 [DOI] [PubMed] [Google Scholar]
- Wada Y, Sambongi Y, Futai M. Biological nanomotor, ATP synthase F0F1: from catalysis to γϵc10–12 subunit rotation. Biochim. Biophys. Acta. 2000;1459:499–505. doi: 10.1016/s0005-2728(00)00189-4. doi:10.1016/S0005-2728(00)00189-4 [DOI] [PubMed] [Google Scholar]
- Walker J.E, Saraste M, Gay N.J. Nucleotide sequence, regulation, and structure of ATP-synthase. Biochim. Biophys. Acta. 1984;768:164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Kinoshita K, Jr, Noji H, Yoshida M. F1-ATPase: F1-ATPase is a highly efficient molecular motor that rotates with discrete 120° steps. Cell. 1998;93:1117–1124. doi: 10.1016/s0092-8674(00)81456-7. doi:10.1016/S0092-8674(00)81456-7 [DOI] [PubMed] [Google Scholar]
- Yasuda R, Noji H, Yoshida M, Kinoshita K, Itoh H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature. 2001;410:898–904. doi: 10.1038/35073513. doi:10.1038/35073513 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Sone N, Hirata H, Kagawa Y. A highly stable adenosine triphosphatase from a thermophilic bacterium. Purification, properties and reconstitution. J. Biol. Chem. 1975;250:7910–7916. [PubMed] [Google Scholar]