Abstract
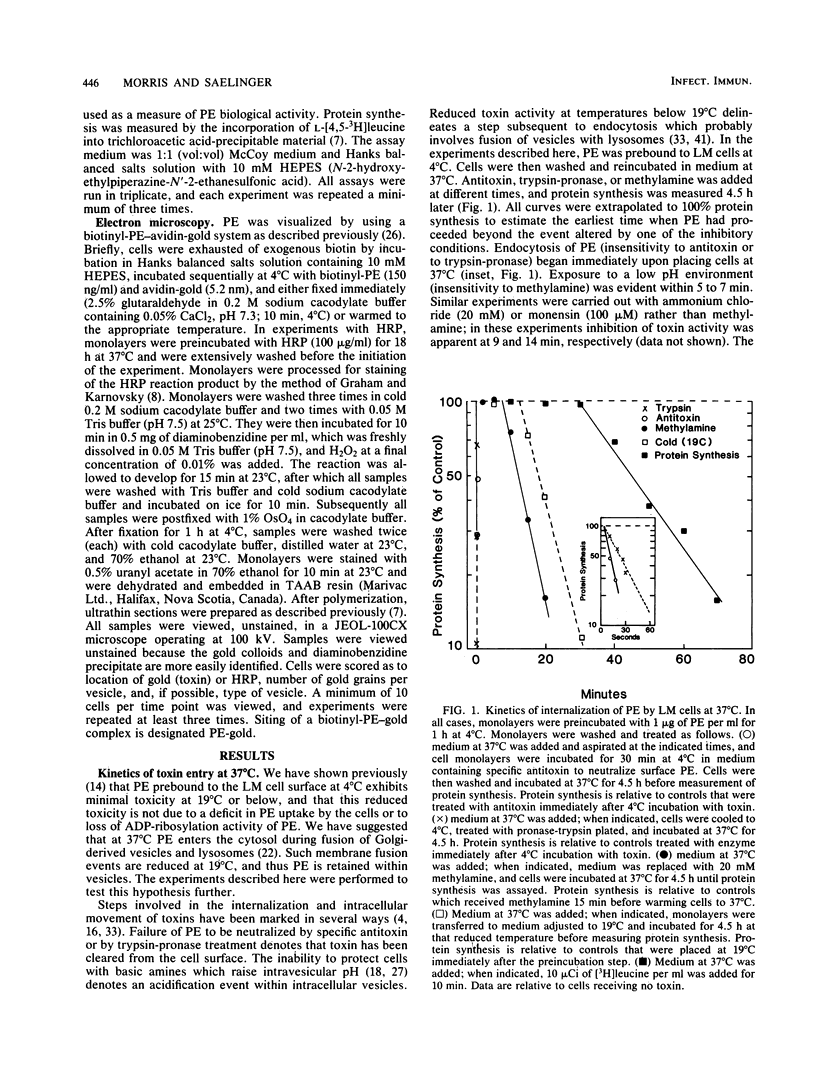
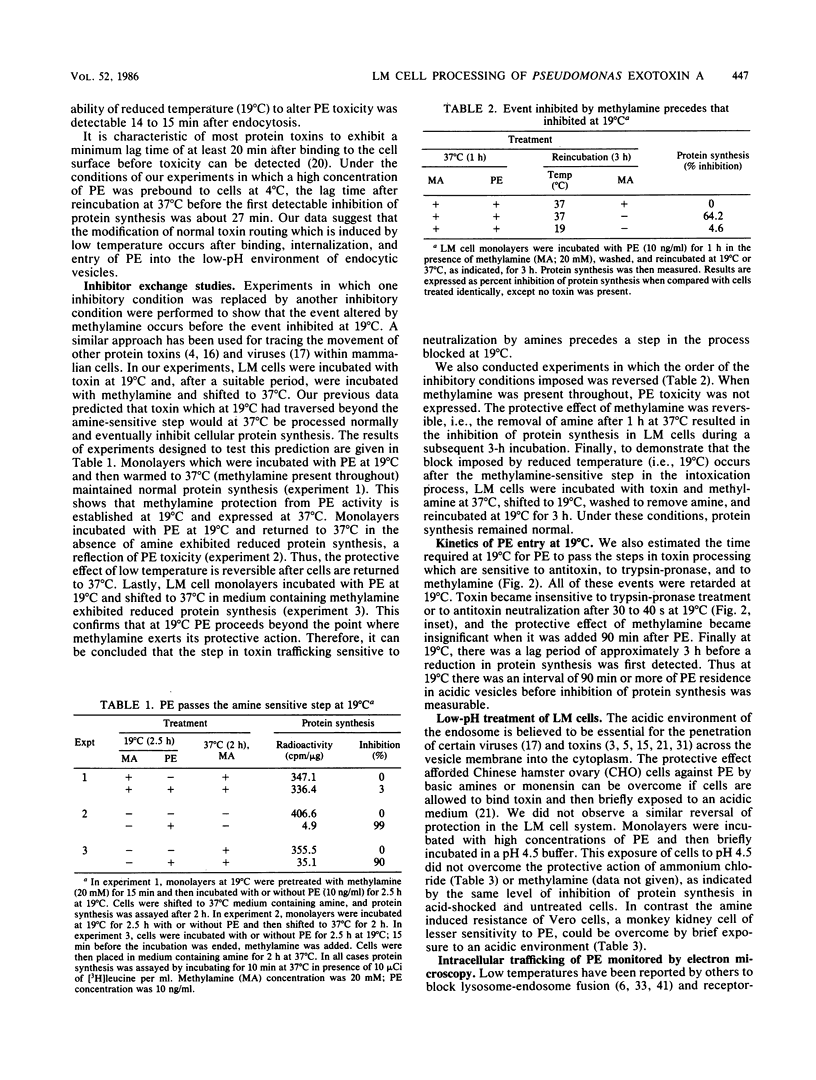
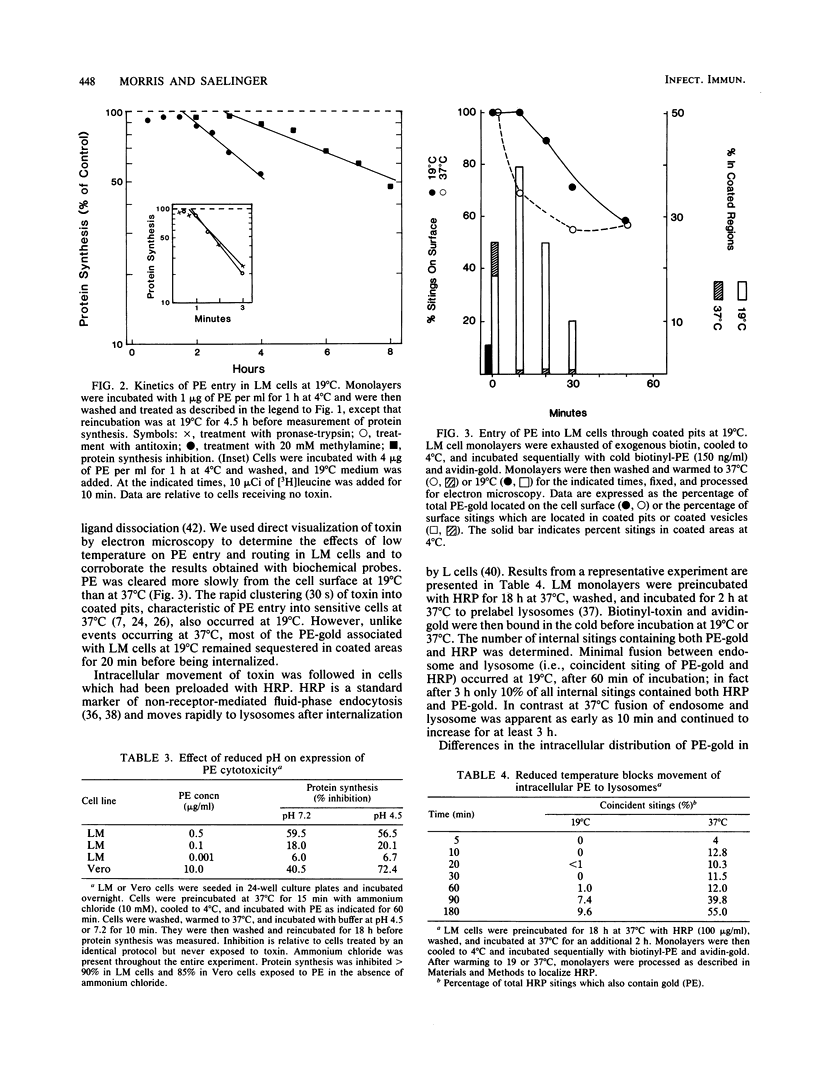
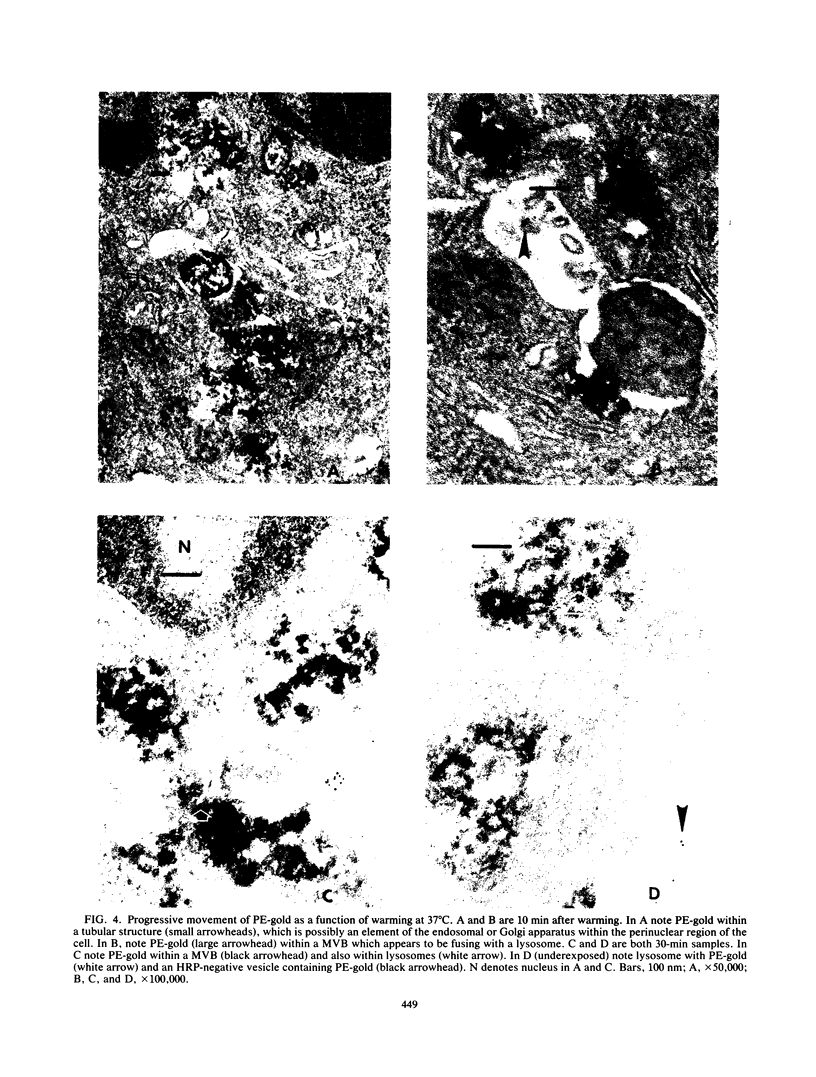
The movement of Pseudomonas exotoxin A (PE) into the cytoplasm of mouse LM fibroblasts was followed by using inhibition of protein synthesis as a biochemical index of toxin activity; biotinyl-PE and avidin-gold colloids were used for electron microscopy. At 37 degrees C both specific antitoxin and pronase-trypsin protected cells against PE toxicity when added within seconds of warming cells, whereas methylamine was protective when added during the first 7 min of endocytosis. Lowering the temperature to 19 degrees C afforded protection when the temperature transition was accomplished within 15 min of the original endocytic event. These data suggest that PE enters an acidic compartment before reaching a step blocked by shifting cells from 37 to 19 degrees C. PE expressed toxicity for LM cells at 19 degrees C, but at a concentration 1 order of magnitude higher than that required at 37 degrees C. At 19 degrees C, antitoxin or trypsin-pronase protection was rapidly ablated. In contrast cells were fully protected by methylamine for 90 min. Using electron microscopy we demonstrated that toxin moved normally (30 s) to coated areas at 19 degrees C, but remained at this site for up to 20 min before being internalized. The majority of the toxin internalized at 19 degrees C remained in endosomes or in Golgi-associated vesicles and was not delivered to lysosomes. The results suggest that, under physiological conditions (37 degrees C), PE rapidly enters cells through coated areas, moves to an acidic compartment (i.e., the endosome), and then probably to the Golgi region en route to lysosomes. The evidence suggests that movement of toxin from endosomes or Golgi vesicles to lysosomes is blocked at 19 degrees C. We hypothesize that the active form of PE enters the cytosol, where it expresses its toxicity during fusion of Golgi-derived, toxin-laden vesicles with lysosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. Sorting and recycling of cell surface receptors and endocytosed ligands: the asialoglycoprotein and transferrin receptors. J Cell Biochem. 1983;23(1-4):107–130. doi: 10.1002/jcb.240230111. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Moehring J. M., Moehring T. J. Binding and uptake of diphtheria toxin by toxin-resistant Chinese hamster ovary and mouse cells. Mol Cell Biol. 1983 Jul;3(7):1283–1294. doi: 10.1128/mcb.3.7.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., O'Keefe D. O., Stookey M., Graves J. Identification of a cold-sensitive step in the mechanism of modeccin action. J Biol Chem. 1984 Apr 10;259(7):4083–4088. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot J. C., Sundan A., Olsnes S., Sandvig K. Entry of diphtheria toxin linked to concanavalin A into primate and murine cells. J Cell Physiol. 1985 Feb;122(2):193–199. doi: 10.1002/jcp.1041220205. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart M. D., Morris R. E., Bonventre P. F., Leppla S., Saelinger C. B. Evidence for pseudomonas exotoxin A receptors on plasma membrane of toxin-sensitive lm fibroblasts. Infect Immun. 1984 Sep;45(3):596–603. doi: 10.1128/iai.45.3.596-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Mathis L. S., Stookey M., Shia S. P., Stone D. K., Draper R. K. A Chinese hamster ovary cell mutant with a heat-sensitive, conditional-lethal defect in vacuolar function. J Cell Biol. 1984 Dec;99(6):1907–1916. doi: 10.1083/jcb.99.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion M., Schlesinger P., Brooks R. M., Moehring J. M., Moehring T. J., Sly W. S. Defective acidification of endosomes in Chinese hamster ovary cell mutants "cross-resistant" to toxins and viruses. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5315–5319. doi: 10.1073/pnas.80.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983 Sep;41(3):998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Manhart M. D., Saelinger C. B. Receptor-mediated entry of Pseudomonas toxin: methylamine blocks clustering step. Infect Immun. 1983 May;40(2):806–811. doi: 10.1128/iai.40.2.806-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Saelinger C. B. Diphtheria toxin does not enter resistant cells by receptor-mediated endocytosis. Infect Immun. 1983 Nov;42(2):812–817. doi: 10.1128/iai.42.2.812-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
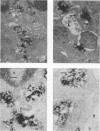
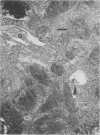
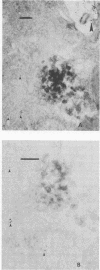
- Morris R. E., Saelinger C. B. Visualization of intracellular trafficking: use of biotinylated ligands in conjunction with avidin-gold colloids. J Histochem Cytochem. 1984 Jan;32(1):124–128. doi: 10.1177/32.1.6690597. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. O., Draper R. K. Characterization of a transferrin-diphtheria toxin conjugate. J Biol Chem. 1985 Jan 25;260(2):932–937. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Halban P., Amherdt M., Ravazzola M., Vassalli J. D., Perrelet A. Nonconverted, amino acid analog-modified proinsulin stays in a Golgi-derived clathrin-coated membrane compartment. J Cell Biol. 1984 Dec;99(6):2187–2192. doi: 10.1083/jcb.99.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger C. B., Morris R. E., Foertsch G. Trafficking of Pseudomonas exotoxin A in mammalian cells. Eur J Clin Microbiol. 1985 Apr;4(2):170–174. doi: 10.1007/BF02013592. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Sandvig K., Sundan A., Olsnes S. Evidence that diphtheria toxin and modeccin enter the cytosol from different vesicular compartments. J Cell Biol. 1984 Mar;98(3):963–970. doi: 10.1083/jcb.98.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Pool R. R., Jr, Sachdeva M., Maurey K. M., Oliver C. Evidence for both prelysosomal and lysosomal intermediates in endocytic pathways. J Cell Biol. 1984 Jan;98(1):108–115. doi: 10.1083/jcb.98.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus W. Mannose-specific binding sites for horseradish peroxidase in various cells of the rat. J Histochem Cytochem. 1983 Jan;31(1):78–84. doi: 10.1177/31.1.6833741. [DOI] [PubMed] [Google Scholar]
- Tycko B., Keith C. H., Maxfield F. R. Rapid acidification of endocytic vesicles containing asialoglycoprotein in cells of a human hepatoma line. J Cell Biol. 1983 Dec;97(6):1762–1776. doi: 10.1083/jcb.97.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deurs B., Nilausen K. Pinocytosis in mouse L-fibroblasts: ultrastructural evidence for a direct membrane shuttle between the plasma membrane and the lysosomal compartment. J Cell Biol. 1982 Aug;94(2):279–286. doi: 10.1083/jcb.94.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981 Mar 25;256(6):2615–2617. [PubMed] [Google Scholar]