Abstract
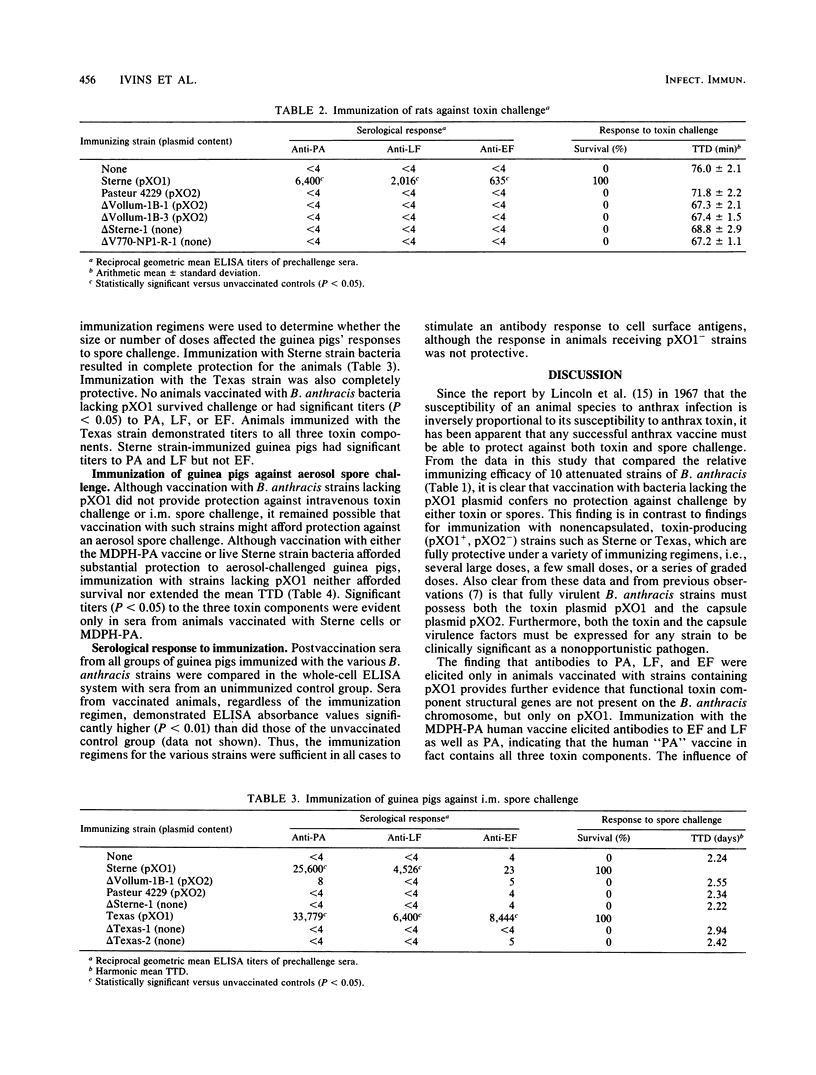
Live, attenuated strains of Bacillus anthracis lacking either the capsule plasmid pXO2, the toxin plasmid pXO1, or both were tested for their efficacy as vaccines against intravenous challenge with anthrax toxin in Fischer 344 rats and against aerosol or intramuscular challenge with virulent anthrax spores in Hartley guinea pigs. Animals immunized with toxigenic, nonencapsulated (pXO1+, pXO2-) strains survived toxin and spore challenge and demonstrated postimmunization antibody titers to the three components of anthrax toxin (protective antigen, lethal factor, and edema factor). Immunization with two nontoxigenic, encapsulated (pXO1-, pXO2+), Pasteur vaccine strains neither provided protection nor elicited titers to any of the toxin components. Therefore, to immunize successfully against anthrax toxin or spore challenge, attenuated, live strains of B. anthracis must produce the toxin components specified by the pXO1 plasmid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALL F. A., TAYLOR M. J., THORNE C. B. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J Bacteriol. 1962 Jun;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman P. S., Gold H., Plotkin S. A., Fekety F. R., Werrin M., Ingraham N. R. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962 Apr;52(4):632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEARMON I. A., Jr, KLEIN F., LINCOLN R. E., MAHLANDT B. G., FERNELIUS A. L. Immunological studies of anthrax. I. An index to determine quantitative immunization. J Immunol. 1961 Sep;87:233–239. [PubMed] [Google Scholar]
- Ezzell J. W., Ivins B. E., Leppla S. H. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect Immun. 1984 Sep;45(3):761–767. doi: 10.1128/iai.45.3.761-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985 Aug;49(2):291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAINES B. W., KLEIN F., LINCOLN R. E. QUANTITATIVE ASSAY FOR CRUDE ANTHRAX TOXINS. J Bacteriol. 1965 Jan;89:74–83. doi: 10.1128/jb.89.1.74-83.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton P., Carman J. A., Melling J. Anthrax: the disease in relation to vaccines. Vaccine. 1984 Jun;2(2):125–132. doi: 10.1016/0264-410x(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Lincoln R. E., Walker J. S., Klein F., Rosenwald A. J., Jones W. I., Jr Value of field data for extrapolation in anthrax. Fed Proc. 1967 Sep;26(5):1558–1562. [PubMed] [Google Scholar]
- Mikesell P., Ivins B. E., Ristroph J. D., Dreier T. M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983 Jan;39(1):371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUZISS M., MANNING L. C., LYNCH J. W., BARCLAYE, ABELOW I., WRIGHT G. G. Large-scale production of protective antigen of Bacillus anthracis in anaerobic cultures. Appl Microbiol. 1963 Jul;11:330–334. doi: 10.1128/am.11.4.330-334.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROESSLER W. G., KAUTTER D. A. Modifications to the Henderson apparatus for studying air-borne infections. Evaluations using aerosols of Listeria monocytogenes. J Infect Dis. 1962 Jan-Feb;110:17–22. doi: 10.1093/infdis/110.1.17. [DOI] [PubMed] [Google Scholar]
- Ristroph J. D., Ivins B. E. Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun. 1983 Jan;39(1):483–486. doi: 10.1128/iai.39.1.483-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H., GALLOP R. C. The chemical basis of the virulence of Bacillus anthracis. VI. An extracellular immunising aggressin isolated from exudates of infected guinea-pigs. Br J Exp Pathol. 1956 Apr;37(2):144–155. [PMC free article] [PubMed] [Google Scholar]
- STANLEY J. L., SMITH H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol. 1961 Sep;26:49–63. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- Uchida I., Sekizaki T., Hashimoto K., Terakado N. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J Gen Microbiol. 1985 Feb;131(2):363–367. doi: 10.1099/00221287-131-2-363. [DOI] [PubMed] [Google Scholar]


