Abstract
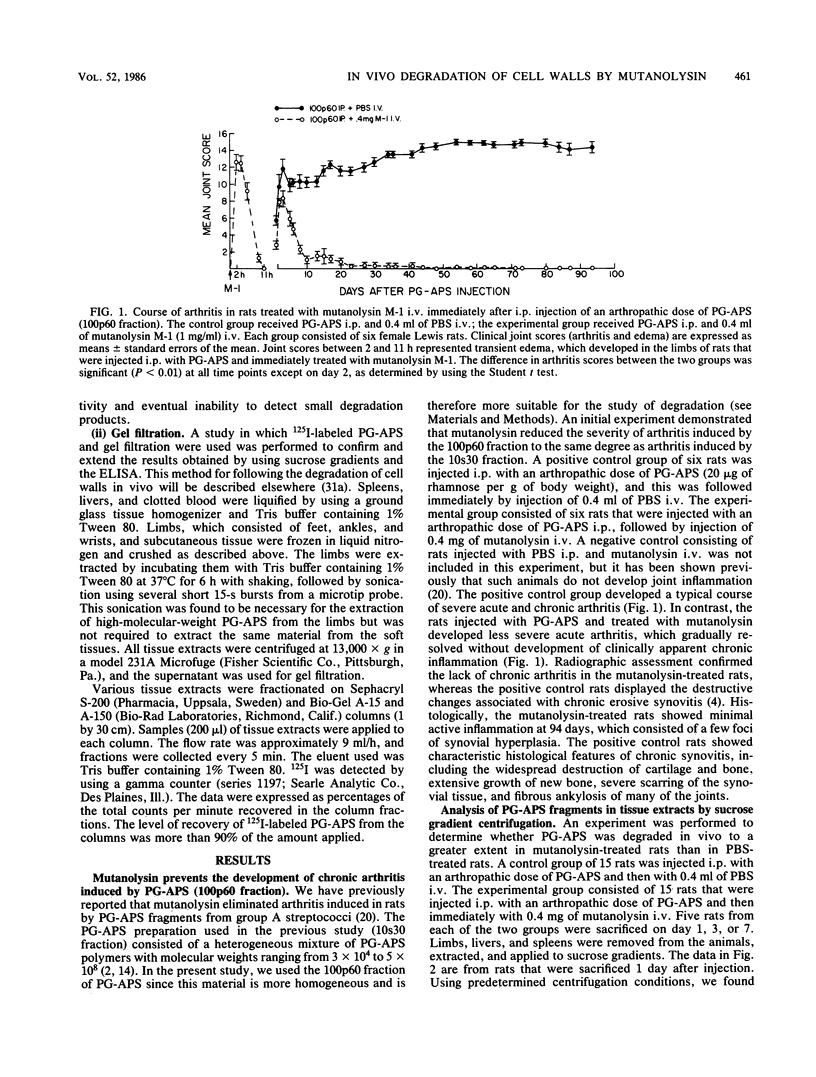
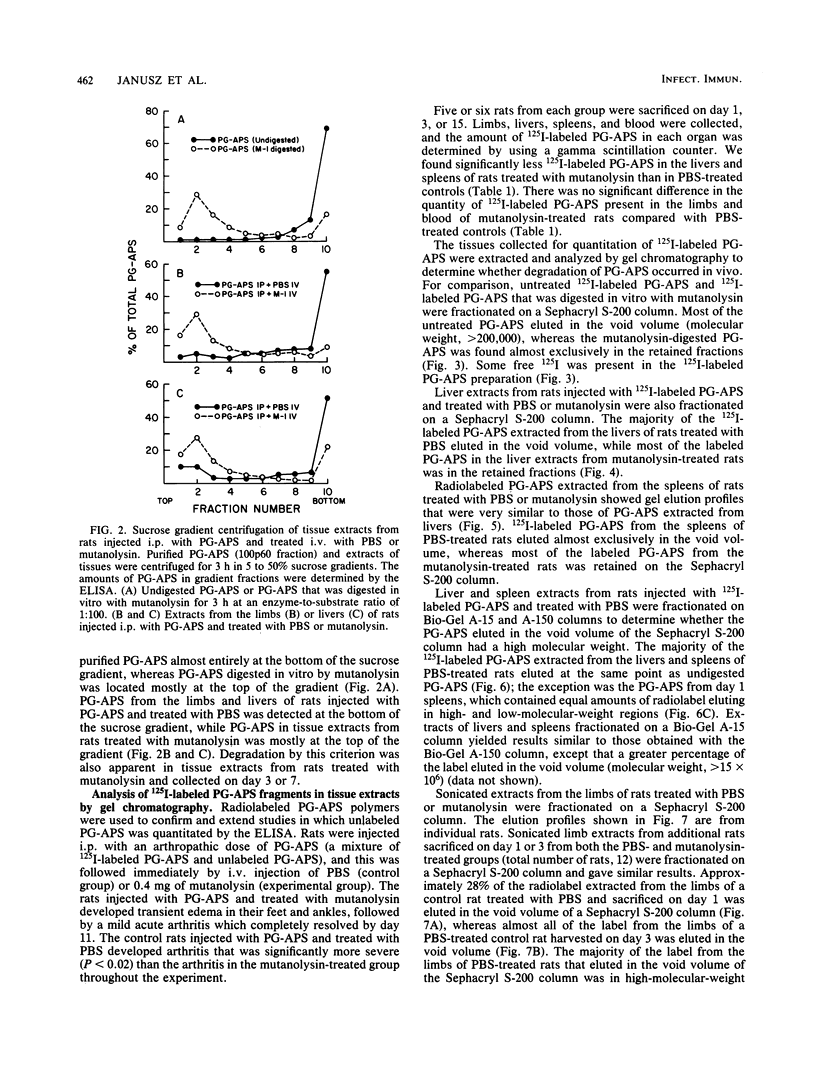
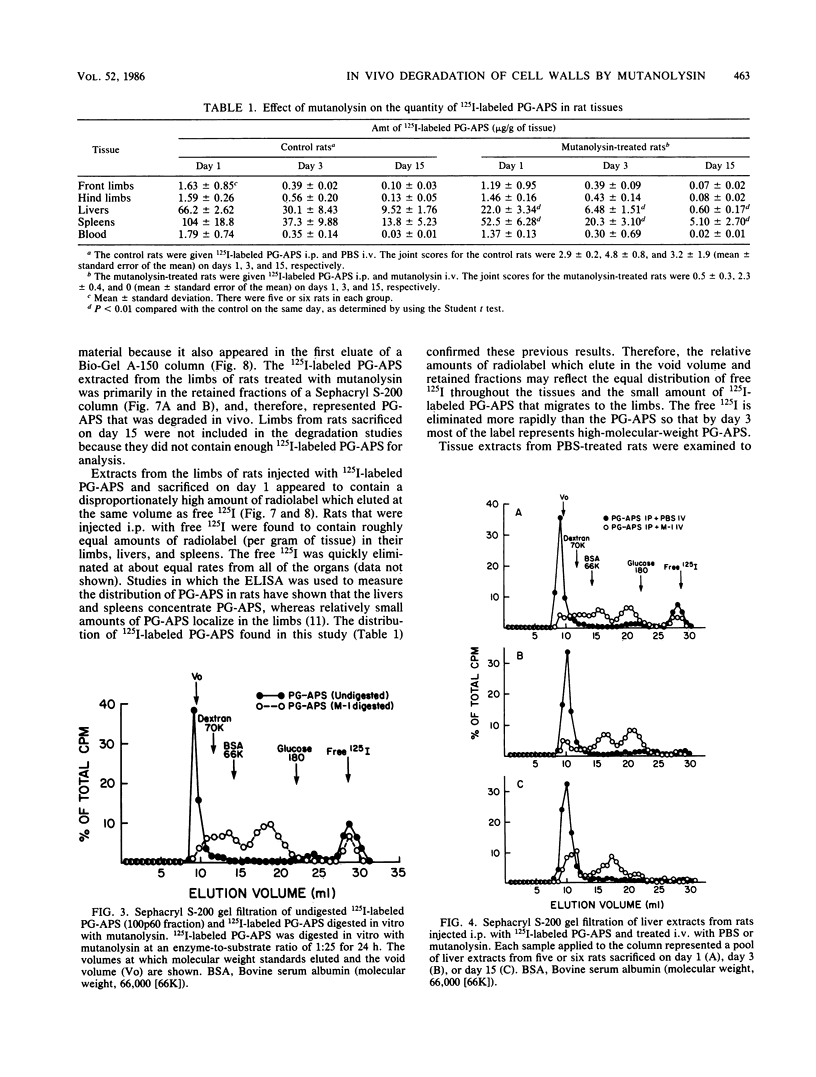
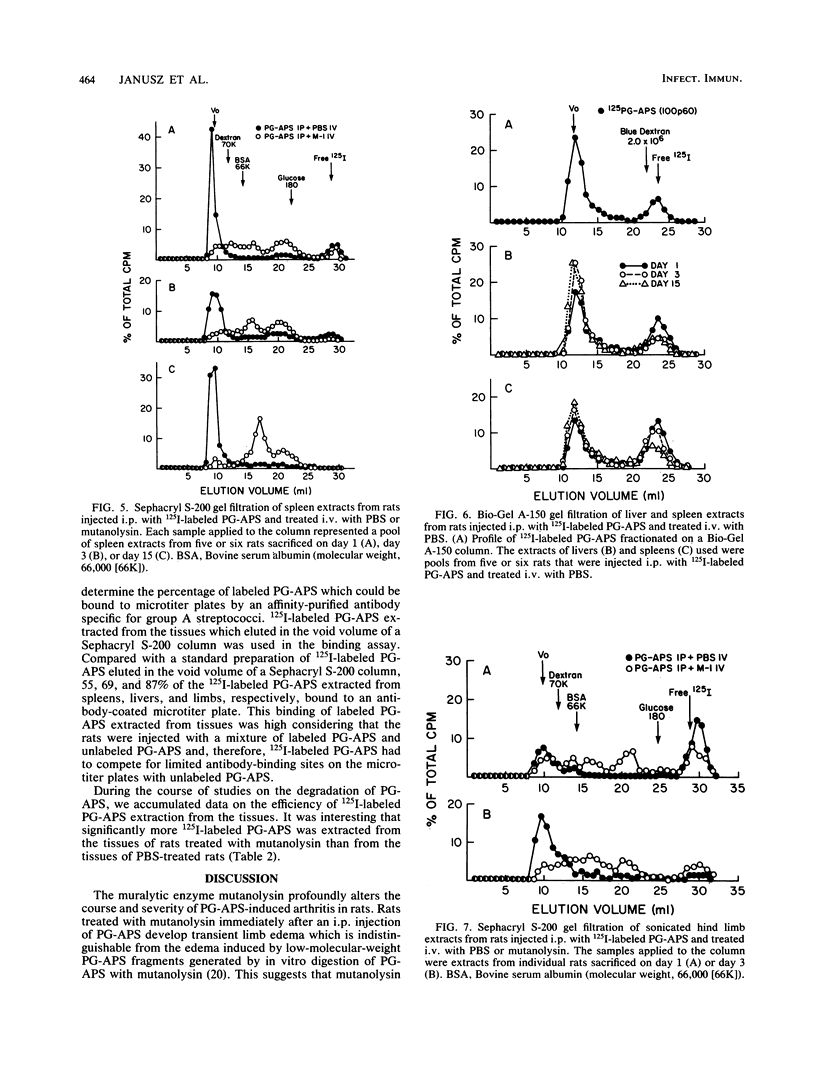
The muralytic enzyme mutanolysin can act in vivo to eliminate chronic erosive arthritis induced in rats by polymers of peptidoglycan-polysaccharide isolated from group A streptococci (PG-APS). The amounts of PG-APS in the livers and spleens of rats treated with mutanolysin were significantly reduced compared with the amounts in control rats treated with phosphate-buffered saline. However, the amounts of PG-APS in the limbs of mutanolysin- and phosphate-buffered saline-treated rats were comparable. PG-APS polymers extracted from the livers, spleens, and limbs of mutanolysin-treated rats were extensively degraded, whereas PG-APS extracted from phosphate-buffered saline-treated rats had a high molecular weight. We propose that mutanolysin abrogates arthritis in rats by degrading PG-APS polymers to a size which is no longer able to induce chronic erosive arthritis, even though the polymers are still present in the limbs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. A., Russell M. W., Mestecky J. Hepatobiliary transport of IgA immune complexes: molecular and cellular aspects. J Immunol. 1982 May;128(5):2183–2186. [PubMed] [Google Scholar]
- Chetty C., Klapper D. G., Schwab J. H. Soluble peptidoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation. Infect Immun. 1982 Dec;38(3):1010–1019. doi: 10.1128/iai.38.3.1010-1019.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorbaru R., Petit J. F., Lederer E., Zissman E., Bona C., Chedid L. Presence and subcellular localization of two distinct mitogenic fractions in the cells of Nocardia rubra and Nocardia opaca: preparation of soluble mitogenic peptidoglycan fractions. Infect Immun. 1976 Apr;13(4):1084–1090. doi: 10.1128/iai.13.4.1084-1090.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Davies P., Page R. C., Allison A. C. Changes in cellular enzyme levels and extracellular release of lysosomal acid hydrolases in macrophages exposed to group A streptococcal cell wall substance. J Exp Med. 1974 May 1;139(5):1262–1282. doi: 10.1084/jem.139.5.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Stimpson S. A., Cromartie W. J., Schwab J. H. Reactivation of streptococcal cell wall-induced arthritis by homologous and heterologous cell wall polymers. Arthritis Rheum. 1985 Dec;28(12):1402–1411. doi: 10.1002/art.1780281213. [DOI] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Zabriskie J. B., Lachman L. B., Chen Y. S. Streptococcal cell walls and synovial cell activation. Stimulation of synovial fibroblast plasminogen activator activity by monocytes treated with group A streptococcal cell wall sonicates and muramyl dipeptide. J Exp Med. 1982 Jun 1;155(6):1702–1718. doi: 10.1084/jem.155.6.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz M. J., Chetty C., Eisenberg R. A., Cromartie W. J., Schwab J. H. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin. J Exp Med. 1984 Nov 1;160(5):1360–1374. doi: 10.1084/jem.160.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Allen J. B., Schwab J. H. In vivo changes in complement induced with peptidoglycan-polysaccharide polymers from streptococcal cell walls. Infect Immun. 1982 Jan;35(1):377–380. doi: 10.1128/iai.35.1.377-380.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky K. B., MacRae E. K., Lambris J. D. Capping of complement receptors on human neutrophils induced by group A streptococcal cell walls. J Immunol. 1983 Apr;130(4):1674–1677. [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Claflin J. L., Schroer K., Mestecky J. Immunoglobulin A-mediated hepatobiliary transport constitutes a natural pathway for disposing of bacterial antigens. Infect Immun. 1983 Dec;42(3):1041–1048. doi: 10.1128/iai.42.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D. Am J Pathol. 1983 Jul;112(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Cromartie W. J., Schwab J. H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986 May;52(2):390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Vermeulen M. W., Gray G. R. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984 Nov;46(2):476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Yoshino S., Cromartie W. J., Schwab J. H. Inflammation induced by bacterial cell wall fragments in the rat air pouch. Comparison of rat strains and measurement of arachidonic acid metabolites. Am J Pathol. 1985 Nov;121(2):327–336. [PMC free article] [PubMed] [Google Scholar]


