Abstract
The effects of several plasmids, including cloning vectors and R factors, on the virulence of Vibrio cholerae CA401R were determined by measuring the dose-related diarrheal response in orally challenged infant mice. The plasmids were also examined for their effects on the colonization ability of strain CA401R by joint infection experiments with a spectinomycin-resistant CA401 strain as an internal standard. One V. cholerae R factor, pVH2, enhanced the diarrheal response, while R factors Rts1 and pVH1 reduced it; plasmids RP4, pRK290, Sa, pSJ8, pSJ5, and pBR328 had no effect. The ability of the plasmids to affect in vitro toxin production by CA401R was variable. Cells containing large plasmids all showed a modest decrease in colonization ability. These results showed that some plasmids affected V. cholerae virulence, but that the cloning vectors pBR328, RP4, and pRK290 did not.
Full text
PDF

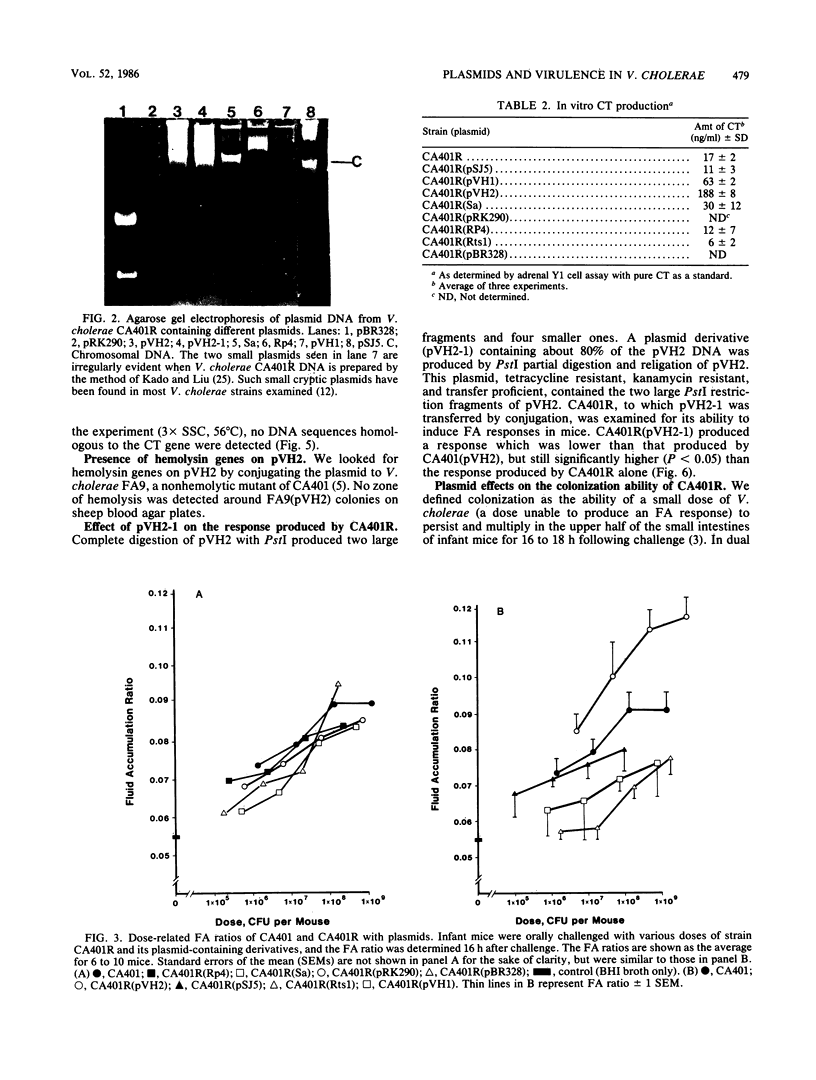



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- BHASKARAN K. Recombination of characters between mutant stocks of Vibrio cholerae, strain 162. J Gen Microbiol. 1960 Aug;23:47–54. doi: 10.1099/00221287-23-1-47. [DOI] [PubMed] [Google Scholar]
- Baselski V. S., Medina R. A., Parker C. D. Survival and multiplication of Vibrio cholerae in the upper bowel of infant mice. Infect Immun. 1978 Nov;22(2):435–440. doi: 10.1128/iai.22.2.435-440.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Parker C. D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978 Aug;21(2):518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V. S., Upchurch S., Parker C. D. Isolation and phenotypic characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1978 Oct;22(1):181–188. doi: 10.1128/iai.22.1.181-188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselski V., Briggs R., Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977 Mar;15(3):704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K., Sinha V. B. Transmissible plasmid factors and fertility inhibition in Vibrio cholerae. J Gen Microbiol. 1971 Nov;69(1):89–97. doi: 10.1099/00221287-69-1-89. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Datta A., Parker C. D., Wohlhieter J. A., Baron L. S. Isolation and characterization of the fertility factor P of Vibrio cholerae. J Bacteriol. 1973 Feb;113(2):763–771. doi: 10.1128/jb.113.2.763-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Glass R. I., Huq I., Alim A. R., Yunus M. Emergence of multiply antibiotic-resistant Vibrio cholerae in Bangladesh. J Infect Dis. 1980 Dec;142(6):939–942. doi: 10.1093/infdis/142.6.939. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Transposon-facilitated recombination in Vibrio cholerae. Mol Gen Genet. 1979 Feb 16;170(1):93–101. doi: 10.1007/BF00268584. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rabert D. K., Svinarich D. M., Whitfield H. J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect Immun. 1982 Nov;38(2):476–486. doi: 10.1128/iai.38.2.476-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J., Rollins D. M., Joseph S. W. Vibrio factors cause rapid fluid accumulation in suckling mice. Infect Immun. 1983 Jun;40(3):1083–1091. doi: 10.1128/iai.40.3.1083-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Lanier S., Baselski V. S., Parker C. D. In vivo evaluation of pathogenicity of clinical and environmental isolates of Vibrio cholerae. Infect Immun. 1980 Jun;28(3):681–687. doi: 10.1128/iai.28.3.681-687.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha V. B., Srivastava B. S. Suppression of pathogenicity by P and V plasmids in Vibrio cholerae. J Gen Microbiol. 1978 Feb;104(2):251–255. doi: 10.1099/00221287-104-2-251. [DOI] [PubMed] [Google Scholar]
- Sporecke I., Castro D., Mekalanos J. J. Genetic mapping of Vibrio cholerae enterotoxin structural genes. J Bacteriol. 1984 Jan;157(1):253–261. doi: 10.1128/jb.157.1.253-261.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Kakizawa Y., Takayasu H., Yoshikawa M. Temperature sensitivity of cell growth in Escherichia coli associated with the temperature sensitive R(KM) factor. Nature. 1968 Jul 20;219(5151):284–285. doi: 10.1038/219284a0. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Huq I. Plasmid-encoded multiple antibiotic resistance in Vibrio cholerae El Tor from Bangladesh. Lancet. 1980 Jun 7;1(8180):1247–1248. doi: 10.1016/s0140-6736(80)91701-8. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Yalow R., Berson S. A. Detection of Australia antigen and antibody by means of radioimmunoassay techniques. J Infect Dis. 1970 May;121(5):550–554. doi: 10.1093/infdis/121.5.550. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sugawara K., Watanabe M. [Virulence of rifampicin-resistant mutants of bacteria]. Nihon Saikingaku Zasshi. 1971 Jun;26(5):192–199. doi: 10.3412/jsb.26.192. [DOI] [PubMed] [Google Scholar]
- Yokota T., Kasuga T., Kaneko M., Kuwahara S. Genetic behavior of R factors in Vibrio cholerae. J Bacteriol. 1972 Jan;109(1):440–442. doi: 10.1128/jb.109.1.440-442.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]