Abstract
NADPH-cytochrome P450 reductase (CPR) and cytochrome-b5 (b5) together with NADH-b5 reductase (b5R) play important roles in cytochrome P450 3A-mediated drug metabolism via electron transfer. However, it is not clear whether variability in expression of these accessory proteins contributes to the known interindividual variability in CYP3A activity. CPR and b5 were measured in human liver microsomes (HLMs) by spectrophotometry and immunoblotting. HLMs from elderly (≥46 years) male donors (n = 11) averaged 27% (P = 0.034) and 41% (P = 0.011) lower CPR levels than young (≤45 years) male donors (n = 21) for spectrophotometric and immunoblot values, respectively. Similarly, HLMs from elderly male donors averaged 43% (P = 0.034) and 47% (P = 0.011) lower b5 levels than young male donors for spectrophotometric and immunoblot values, respectively. α-Lipoic acid and 6-propyl-2-thiouracil were evaluated for selectivity of inhibition of CPR and b5R activities, respectively, using recombinant enzymes and HLMs, as well as for effects on CYP3A-mediated triazolam hydroxylation in HLMs with either NADH or β-NADPH. The results indicate that both compounds are relatively nonselective inhibitors of CPR and b5R activities. Finally, we used multivariate regression analysis and showed that variability in CPR or b5 expression between HLMs does not contribute significantly to variability in CYP3A-mediated midazolam hydroxylation. Consequently, while aging is associated with decreased CPR and b5 expression in human livers, this effect does not contribute to CYP3A variability.
NADPH-cytochrome P450 reductase (CPR) is an electron transfer diflavoprotein (Porter and Kasper, 1986). It is widely expressed in all tissues and is most abundant in the endoplasmic reticulum of the liver (Simmons and Kasper, 1989). CPR is responsible for transferring electrons to many naturally occurring electron acceptors, including cytochrome P450 enzymes and cytochrome-b5 (Murataliev et al., 2004; Guengerich, 2005) as well as to some nonphysiological electron acceptors that are often used to test enzymatic activity of CPR in in vitro assays.
CPR is obligatory for catalysis of many P450 reactions. In the endoplasmic reticulum membrane, CPR transfers the electrons from NADPH into the P450 catalytic cycle (Elmore and Porter, 2002). CPR is a required electron transfer partner for the catalytic activity mediated by CYP3A isoforms. Antibodies against CPR activity significantly impair CYP3A-mediated drug metabolism in a concentration-dependent manner (Yamazaki et al., 1996). In recombinant enzyme systems, CYP3A reactions cannot proceed without CPR. Increasing concentrations of CPR enhance the metabolism of testosterone in a concentration-dependent manner (Yamazaki et al., 1999, 2002).
Compared with P450 enzymes, relatively little interindividual variation in gene expression of CPR has been found in adult human livers (Shephard et al., 1992). However, various studies have shown an aging-related decline of CPR activity or its expression in rodents (Schmucker and Wang, 1983; Guo et al., 1993; Warrington et al., 2004) but not in rhesus monkeys (Maloney et al., 1986; Schmucker and Wang, 1987). Gender-related differences in CPR activity have also been reported in mice. Guo et al. (1993) found that CPR activity was higher in male than in female mice ranging in age from 6 to 48 months. In humans, the effects of aging and gender on CPR are unclear. One study in human livers reported that CPR activity did not change with aging, while CPR protein expression measured by immunoprecipitation showed a weakly negative correlation with aging in females, but not in males (Schmucker et al., 1990). In another study, CPR activity was found to decrease with aging, while gender had no effect on CPR activity (George et al., 1995).
Cytochrome-b5 (b5) and NADH-b5 reductase (b5R) are ubiquitous electron transport proteins. In endoplasmic reticulum, b5 plays important roles in maintenance of normal cellular functions by transferring electrons to microsomal desaturases that synthesize unsaturated fatty acids, plasmalogens, and cholesterol (Vergéres and Waskell, 1995). b5R is responsible for transferring electrons from NADH to b5 in these reactions (Yamazaki et al., 1996), although NADPH can also be used as the electron donor in some cases. b5 can also accept electrons from an alternative reductase, CPR (Guengerich, 2005).
b5/b5R participates in drug metabolism, augmenting the reactions mediated by some P450 isoforms, including CYP3A4. In recombinant systems, the metabolism of testosterone, nifedipine, and midazolam, the three typical index substrates for CYP3A, is enhanced by addition of b5 and b5R (Holmans et al., 1994; Voice et al., 1999; Yamazaki et al., 1999, 2002). In human liver microsomes (HLMs), b5 antisera inhibited the metabolism of testosterone and nifedipine, suggesting that b5 is an essential component in modulation CYP3A activity (Yamazaki et al., 1996).
Little is known about the effect of aging and gender on the expression of membrane-bound b5 and b5R. Previous study has shown that b5 content and b5R activity decrease with aging in human erythrocytes (Matsuki et al., 1981).
Large interindividual variability in CYP3A activity in vivo and in vitro has been observed for many years (Parkinson et al., 2004; Cotreau et al., 2005). Both in vivo and in vitro studies have shown a gender-specific and aging-dependent decrease of CYP3A-mediated drug metabolism (Patki et al., 2004; Cotreau et al., 2005). However, variations in CYP3A activity in vitro are not completely explained by variation in CYP3A protein levels alone (Parkinson et al., 2004; Cotreau et al., 2005). Consequently, we hypothesized that variation of activity and/or protein content of CPR or b5 might account in part for high interindividual variability of CYP3A activity as well as for effects of aging and gender. Using a well characterized bank of HLMs (n = 46), we evaluated variability in CPR and b5 levels with respect to donor age and gender. In rat liver microsomes α-lipoic acid and 6-propyl-2-thiouracil (PTU) have been reported to inhibit CPR (Slepneva et al., 1995) and b5R activities (Lee and Kariya, 1986), respectively. Consequently, we evaluated the selectivities of these compounds as CPR and b5R inhibitors using both recombinant enzymes and HLMs, and we also determined the effects of these inhibitors on CYP3A-mediated triazolam hydroxylation in HLMs.
Materials and Methods
Reagents. PTU, cytochrome c (bovine heart), KCN, α-lipoic acid, β-NADPH, NADH, NADP+, isocitrate dehydrogenase, dl-isocitrate, and 50 mM potassium phosphate buffer, pH 7.5, were purchased from Sigma-Aldrich (St. Louis, MO). Human b5 antisera and purified recombinant human b5R were provided by Dr. L. Trepanier (University of Wisconsin-Madison, Madison, WI). Methods used to generate these reagents and establish antibody selectivity have been published previously (Kurian et al., 2004). Potassium phosphate monobasic, acetonitrile, and methanol were obtained from Thermo Fisher Scientific (Waltham, MA). Laemmli's SDS sample reducing buffer, Tris-glycine-SDS running buffer, transfer buffer, and Tris-buffered saline (TBS)/Tween 20 buffer were purchased from Boston BioProducts (Worcester, MA). Polyvinylidene difluoride membranes and Tris-glycine-SDS polyacrylamide gradient gels (4–15% and 10–20%) were from Bio-Rad (Hercules, CA). Purified recombinant human CPR, b5, and BenchMark protein ladder were from Invitrogen (Carlsbad, CA). Rabbit polyclonal primary antibody to CPR was purchased from Abcam Inc. (Cambridge, MA). Goat anti-rabbit IgG horseradish peroxidase (HRP)-labeled secondary antibody was from PerkinElmer Life and Analytical Sciences (Waltham, MA). SuperSignal West Pico chemiluminescent substrate (for HRP) was from Pierce Chemical (Rockford, IL). Potassium ferricyanide was from MP Biomedicals (Irvine, CA).
Human Liver Microsomes. Liver samples were obtained from the International Institute for the Advancement of Medicine (Exton, PA) or from the Liver Tissue Procurement and Distribution System (University of Minnesota, Minneapolis, MI). The majority of livers were from organ transplant donors with a history of head trauma or cerebral ischemia. The others were either from biopsy or autopsy specimens of apparently healthy tissue. All livers did not have known liver diseases. Donors included 32 males and 14 females with ages ranging from 2 to 75 years. They were 40 whites, four African-Americans, and two Hispanics. A history of cigarette smoking and alcohol consumption was reported for 15 and 21 donors, respectively. Specific details of each liver donor were provided previously (Hesse et al., 2004). Microsomes were prepared through ultracentrifugation and stored at –80°C until use as described previously (von Moltke et al., 1993).
Measurement of CPR Activity. Concentrations of CPR in the set of 46 HLMs were determined using an activity assay that spectrophotometrically measured the rate of CPR-mediated reduction of cytochrome c by β-NADPH (Venkatakrishnan et al., 2000). The assays were performed using standard 1-cm disposable cuvettes (Thermo Fisher Scientific) in a total reaction volume of 1 ml. Each reaction contained 330 mM KH2PO4, pH 7.6, 1 mM KCN, 50 μM cytochrome c, and 15 to 35 μg of microsomal protein. Reactions were initiated by the addition of 10 μl of a 4.2 mM solution of β-NADPH to the sample cuvette. The increase in absorbance at wavelength 550 nm was recorded at room temperature for 3 min using a dual-beam spectrophotometer (Uvikon, Kontron, Zürich). The rate of reduction of cytochrome c was determined as the slope of the linear part of the absorbance-time curve. Calibration curves were generated using 0.14 to 7 pmol of purified recombinant human CPR. The concentrations of CPR in HLMs were calculated using the calibration curve. Each experiment was performed in duplicate and repeated twice. Assays without HLMs or without β-NADPH were used as negative controls. Intra- and interassay coefficients of variation were less than 10%.
Measurement of b5 Concentrations by Spectrophotometry. Concentrations of cytochrome b5 in 46 HLMs were determined by measuring the differential absorbance between NADH-reduced and oxidized microsomes (Venkatakrishnan et al., 2000). Microsomes were diluted to 1 mg/ml in 100 mM KH2PO4, pH 7.4, and divided between two 1-ml cuvettes. The absorbance of oxidized b5 was measured at 410 nm. By addition of 5 μl of 20 mM β-NADH to the sample cuvette, b5 was reduced and the absorbance was recorded at 425 nm. The concentration of b5 (C) was calculated using the following equation based on the Beer-Lambert law: C = A/(ε · L), where A is the difference in absorbance between oxidized and reduced b5, L is the pathway length (1 cm), and ε is the extinction coefficient (185 mM–1 · cm–1). Assays without HLMs or without addition of NADH were used as negative controls. Intra- and interassay coefficients of variation were less than 10%.
Immunoquantitation of Microsomal CPR and b5 Protein Content. Microsomal CPR and b5 protein content were measured by quantitative immunoblot analysis as follows. In brief, CPR and b5 were separated on gradient denaturing polyacrylamide gels (4–12% and 10–20% gels, respectively). After electrophoresis, protein was transferred onto polyvinylidene difluoride membranes for 1 h (CPR) or 2 h (b5) at 25 V. Blots were blocked with 5% nonfat milk in TBS/Tween 20 buffer for 1 h, washed in TBS/Tween 20 buffer, and then incubated with primary antibody for 1 h. After further washes and incubation in the HRP-conjugated secondary antibody for 1 h, chemiluminescent substrate (West Pico; Pierce Chemical) for 5 min at room temperature, labeled bands were imaged by cooled charge-coupled device camera (Kodak Image Station 440; Eastman Kodak, Rochester, NY). A calibration curve was generated by plotting the band intensity versus various concentrations of purified recombinant CPR or b5. The concentrations of CPR or b5 in HLMs were calculated based on the band intensities and the slope value of the calibration curve. Each protein was measured twice. The interassay variability was less than 15%.
Microsomal CYP3A Activity and Protein Content. Midazolam 1-hydroxylation activity (a validated CYP3A-specific index activity) as well as CYP3A4 protein content (by quantitative immunoblotting) was determined in the same set of 46 HLMs. These data and the analytical methods have been reported in detail previously (He et al., 2006).
Inhibitory Selectivities of α-Lipoic Acid and PTU. The selectivities of α-lipoic acid and PTU as chemical inhibitors of CPR and b5R were tested using both purified recombinant enzyme systems and HLMs. CPR activity was measured spectrophotometrically as described above. Concentrations of α-lipoic acid (up to 2 mM) or PTU (up to 4 mM) prepared in methanol were added to 2-ml polypropylene tubes and evaporated to dryness in a 40°C vacuum oven. The reaction was started by addition of 1 ml of the reaction mixture containing either 40 to 60 μg of microsomal protein or 2 nM purified recombinant CPR.
Inhibition of b5R activity by α-lipoic acid and PTU was determined from b5R-mediated reduction of potassium ferricyanide by NADH. The measurements were performed based on the methods described previously, with some modification (Badwey et al., 1983). Various concentrations (up to 4 mM) of α-lipoic acid or PTU in methanol solution were evaporated to dryness in a 40°C vacuum oven before addition of the reaction mixture containing 50 mM KH2PO4, pH 7.6, 0.5 mM KCN, 0.5 mM potassium ferricyanide, and 40 to 60 μg of microsomal protein or 0.2 μg/ml purified recombinant b5R. Reactions were started by addition of 10 μl of a 20 mM solution of β-NADH to the sample cuvette. The inhibition by PTU required a preincubation of PTU with HLMs or b5R enzyme at 25°C for 15 min. The decreased absorbance at wavelength 420 nm was recorded at room temperature for 1 min using a dual-beam spectrophotometer (Uvikon). The rate of reduction of potassium ferricyanide was determined as the slope of the linear part of the absorbance-time curve.
Each assay was performed in duplicate using individually prepared HLMs from four different donors. For purified recombinant CPR or b5R experiments, assays were performed in triplicate. Activities were expressed as the percentage of control activity (no inhibitor added). Intra- and interassay coefficients of variation were both less than 15%.
Effect of α-Lipoic Acid and PTU on Triazolam Hydroxylation by HLMs. Measurement of rates of triazolam 1- and 4-hydroxylations by HLMs was performed based on the method described previously with minor modification (von Moltke et al., 1996). Reaction volume was 250 μl, triazolam concentration was 250 μM, concentration of HLM protein was 0.1 mg/ml, and incubation time was 20 min. Control incubations with no cofactor, no protein, and no substrate were performed concurrently as negative control. For evaluation of effects of PTU (up to 4 mM) and α-lipoic acid (up to 40 mM), HLMs from four different individuals (0.1–0.25 mg/ml) were used. PTU was preincubated with HLMs at 37°C for 15 min. NAD+ (0.5 mM), NADP+ (0.5 mM), or NAD+ plus NADP+ (0.5 mM each) were evaluated as cofactors.
Statistical Analysis. Descriptive statistics (mean and S.D.) of microsomal protein contents of CPR or b5 were calculated for the entire set of HLMs as well as HLMs stratified by donor gender and age. For the purpose of stratification of HLMs by age, groups included those donors ≤45 years old (young) and those donors ≥46 years old (elderly). This resulted in HLMs from 21 young male, 11 elderly male, seven young female, and seven elderly female donors. Statistical tests (SigmaStat 3.0; Systat Software, Inc., San Jose, CA) included analysis of variance (one-way and two-way) on rank transformed data with post hoc analysis by Student-Newman-Keuls multiple pairwise comparisons testing, Spearman rank order correlation, and simple and multiple linear regression on rank transformed data. A two-tailed P value of <0.05 was considered to be statistically significant. IC50 values (inhibitor concentration resulting in 50% decrease in CPR, b5R, or CYP3A activity) were determined from the curve-fits of the concentration-inhibition data using GraphPad Prism software (GraphPad Software, Inc., San Diego CA).
Results
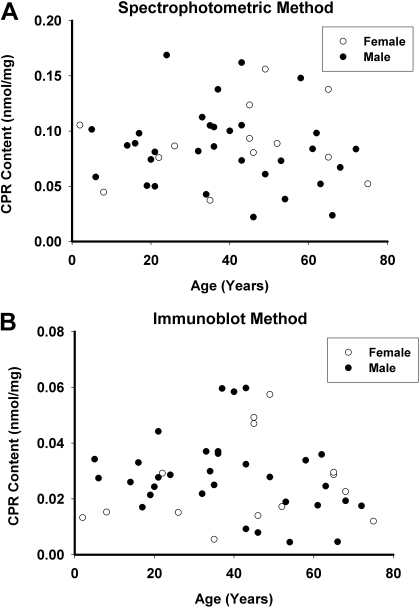
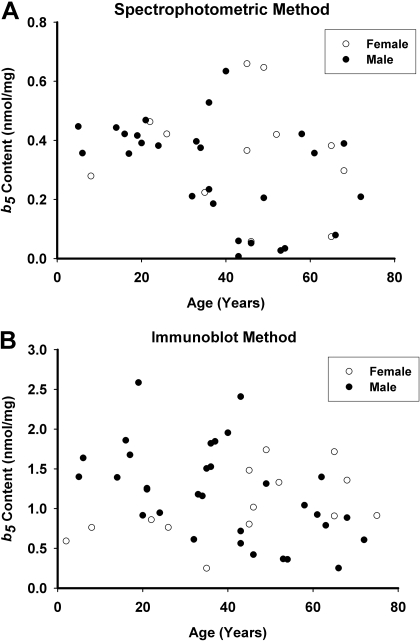
CPR and b5 Expression in the HLMs Bank. CPR concentrations in the set of HLMs measured by spectrophotometry ranged from 0.022 to 0.170 nmol/mg protein (0.086 ± 0.035 nmol/mg protein) (Fig. 1A), while immunoblotting yielded somewhat lower results, ranging from 0.005 to 0.060 nmol/mg protein (0.028 ± 0.015 nmol/mg protein) (Fig. 1B). CPR levels measured by the two assay methods were relatively well correlated [Spearman correlation coefficient (Rs) = 0.60, P < 0.001]. Concentrations of b5 in the HLMs measured by spectrophotometry ranged from 0.007 to 0.66 nmol/mg protein (0.32 ± 0.18 nmol/mg protein) (Fig. 2A), while immunoblotting results were higher, ranging from 0.25 to 2.59 nmol/mg protein (1.15 ± 0.56 nmol/mg protein) (Fig. 2B). Levels of b5 assayed by these two methods were somewhat poorly correlated (Rs = 0.41, P = 0.01).
Fig. 1.
CPR content in HLMs. CPR content in the set of 46 HLMs was measured by spectrophotometry (A) and immunoblotting (B). Results are expressed as CPR content (nanomoles per milligram of microsomal protein) versus donor age in years. Data from female donors are represented as open circles, and data from male donors are represented as filled black circles.
Fig. 2.
Cytochrome b5 content in HLMs. b5 content in the set of 46 HLMs was measured by spectrophotometry (A) and immunoblotting (B). Results are expressed as b5 content (nanomoles per milligram of microsomal protein) versus donor age in years. Data from female donors are represented as open circles, and data from male donors are represented as filled black circles.
Effect of Aging and Gender on CPR and b5 Content of HLMs. CPR and b5 content data were stratified by liver donor gender and age and then analyzed by two-way analysis of variance. As shown in Table 1 and Fig. 1, microsomal CPR concentrations measured by either assay method were not associated with either aging (P > 0.05) or gender (P > 0.05). However, within the set of HLMs from male donors, elderly male donors (n = 11) averaged 27% (P = 0.034) and 41% (P = 0.011) lower CPR levels than young male donors (n = 21) for spectrophotometric and immunoblot values, respectively. There were no differences in microsomal CPR content measured by spectrophotometric or immunoblot methods between young and elderly females (P > 0.05).
TABLE 1.
NADPH-cytochrome P450 reductase and cytochrome-b5 content in a set of 46 HLMs
Values are mean ± S.D. The young group included liver donors ≤45 years old, and the elderly group included donors ≥46 years old [n = 21 (young males), n = 11 (elderly males), n = 7 (young females), and n = 7 (elderly females)].
| Assay Method | Young Males | Elderly Males | Young Females | Elderly Females |
|---|---|---|---|---|
| nmol/mg | ||||
| NADPH-cytochrome P450 reductase content | ||||
| Spectrophotometric | 0.095 ± 0.033* | 0.069 ± 0.037* | 0.082 ± 0.031 | 0.095 ± 0.039 |
| Immunoblot | 0.033 ± 0.014* | 0.019 ± 0.011* | 0.025 ± 0.017 | 0.026 ± 0.016 |
| Cytochrome-b5 content | ||||
| Spectrophotometric | 0.35 ± 0.16* | 0.20 ± 0.16* | 0.40 ± 0.15 | 0.31 ± 0.22 |
| Immunoblot | 1.44 ± 0.54* | 0.76 ± 0.39* | 0.79 ± 0.37 | 1.28 ± 0.35 |
P < 0.05 for young males versus elderly males by Student-Newman-Keuls test.
Regarding microsomal b5 content (Table 1; Fig. 2), mean spectrophotometric values were 30% lower (P = 0.047) in the elderly donor group compared with the young donor group (0.24 ± 0.19 versus 0.36 ± 0.16 nmol/mg protein, respectively). Although immunoblot-derived b5 content also averaged 30% lower in the elderly donor group compared with the young donor group (0.96 ± 0.45 versus 1.38 ± 0.57 nmol/mg protein, respectively), the difference did not reach statistical significance (P > 0.05). Within the set of HLMs from male donors, elderly male donors averaged 43% (P = 0.034) and 47% (P = 0.011) lower microsomal b5 levels than young male donors for spectrophotometric and immunoblot values, respectively. There were no differences in microsomal b5 content by either spectrophotometric or immunoblot methods between young and elderly females (P > 0.05).
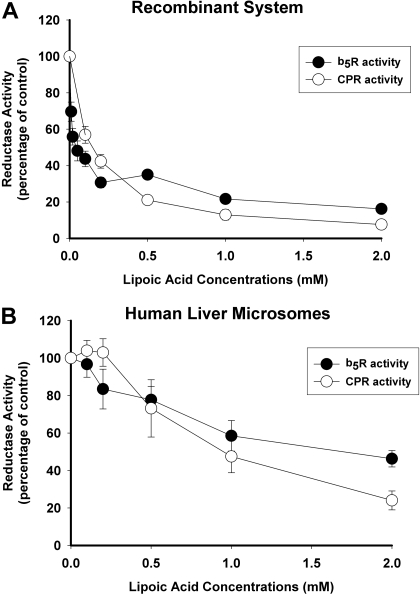
Inhibitory Selectivities of α-Lipoic Acid and PTU. α-Lipoic acid and PTU were evaluated as selective inhibitors of CPR and b5R, respectively, using both recombinant enzymes and HLMs. As shown in Fig. 3A, both CPR and b5R activities by recombinant enzymes were inhibited equally well by α-lipoic acid with maximal effects (92 and 84% reduction in activity, respectively) observed at 2 mM α-lipoic acid concentration. Calculated IC50 values were 0.14 ± 0.02 mM (mean ± S.E.) for inhibition of recombinant CPR and 0.05 ± 0.05 mM for inhibition of recombinant b5Rby α-lipoic acid. Similar results were obtained with HLMs, although the extent of inhibition was somewhat less reaching only 76 and 54% reduction in CPR and b5R activities, respectively, at 2 mM α-lipoic acid concentration (Fig. 3B).
Fig. 3.
Inhibition of CPR and b5R activity by α-lipoic acid. Reductase activities were measured in the absence and presence of increasing concentrations of α-lipoic acid. CPR activities were measured using recombinant NADPH-cytochrome P450 reductase (A) and HLMs (B), whereas b5R activities were measured using recombinant cytochrome b5 reductase (A) and HLMs (B). Results are expressed as the percentage of control activities (incubations without α-lipoic acid) and represent the mean ± S.D. of either three replicates (for recombinant enzyme) or four separate experiments using HLMs from four different donors.
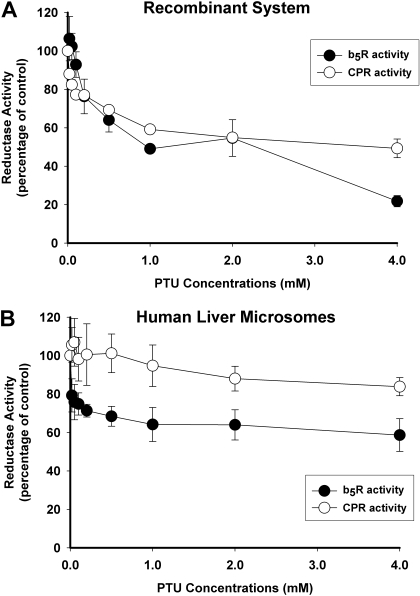
PTU also inhibited both CPR and b5R activities in purified recombinant enzymes with decreases of 51 and 78%, respectively, observed at 4 mM PTU concentration (Fig. 4A). PTU was also somewhat less inhibitory in HLMs, with only 16% reduction in CPR activity and 41% reduction in b5R activity at the highest PTU concentration evaluated (4 mM) (Fig. 4B).
Fig. 4.
Inhibition of CPR and b5R activity by PTU. Reductase activities were measured in the absence and presence of increasing concentrations of PTU. CPR activities were measured using recombinant NADPH-cytochrome P450 reductase (A) and HLMs (B), whereas b5R activities were measured using recombinant cytochrome b5 reductase (A) and HLMs (B). Results are expressed as the percentage of control activities (incubations without PTU) and represent the mean ± S.D. of either three replicates (for recombinant enzyme) or four separate experiments using HLMs from four different donors.
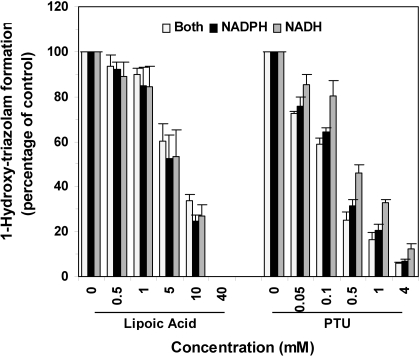
Inhibition of Triazolam Hydroxylation in HLMs by α-Lipoic Acid and PTU. The effects of α-lipoic acid (up to 40 mM) and PTU (up to 4 mM) on triazolam hydroxylation activities were evaluated in HLMs in the presence of NADH alone, β-NADPH alone, or a mixture of both NADH and β-NADPH. As shown in Fig. 5, both α-lipoic acid and PTU inhibited the 1-hydroxylation of triazolam in a concentration-dependent manner. Similar results were obtained for effects on 4-hydroxytriazolam formation (data not shown). IC50 values for α-lipoic acid inhibition of 1-hydroxytriazolam formation were 4.71 ± 0.04 (estimate ± S.E. of the estimate), 4.72 ± 0.03, and 6.22 ± 0.03 mM for NADH alone, β-NADPH alone, or a mixture of both NADH and β-NADPH, respectively. IC50 values for PTU inhibition were 0.43 ± 0.03, 0.20 ± 0.02, and 0.15 ± 0.02 mM for NADH alone, β-NADPH alone, or a mixture of both NADH and β-NADPH, respectively. Although the type of cofactor included did not influence the extent of inhibition of 1-hydroxytriazolam formation by α-lipoic acid, PTU inhibited CYP3A activity to a greater extent in the presence of β-NADPH compared with incubations that excluded β-NADPH at all PTU concentrations evaluated (P < 0.05, Student-Newman-Keuls test) (Fig. 5).
Fig. 5.
Inhibition of triazolam 1-hydroxylation in HLMs by α-lipoic acid and PTU. Triazolam 1-hydroxylation activities in HLMs were measured in the absence and presence of increasing concentrations of α-lipoic acid (left) or PTU (right). Cofactors included either NADH alone (black bar), β-NADPH alone (hatched bar), or a mixture of NADH plus β-NADPH (open bars). Results are expressed as the percentage of control activities and represent the mean ± S.D. of four experiments using HLMs from four different donors. *, P < 0.05 by Student-Newman-Keuls test for NADH alone incubation versus β-NADPH alone and NADH/β-NADPH incubations measured at the same inhibitor concentration. Similar results were obtained for 4-hydroxylation of triazolam (data not shown).
Effect of CPR or b5 on CYP3A Activity in HLMs. CYP3A-mediated 1-hydroxymidazolam formation rates and CYP3A protein contents of the 46 HLMs used here have been reported in detail previously (He et al., 2006). In this study, we used simple linear and multivariate regression approaches to evaluate the possible contribution of variable CPR and/or b5 levels (by spectrophotometric and immunoblot methods) to the observed variability in CYP3A-mediated catalysis (1-hydroxymidazolam formation) with respect to CYP3A protein content. By simple linear regression, both CYP3A protein content (R2 = 0.80, P < 0.001) and CPR protein content (R2 = 0.20, P = 0.002) were identified as potential predictors of CYP3A activity. However, incorporation of both these dependent variables into a multiple linear regression model indicated that CYP3A protein content accounted for the majority of observed CYP3A activity variability with a standardized regression coefficient of 0.88 (P < 0.001) compared with 0.02 (P = 0.76) for CPR protein content. Furthermore, the regression coefficient for this multivariate model (R2 = 0.80, P < 0.001) was unchanged compared with the regression coefficient for the simple regression model that used CYP3A protein alone. Further evaluation of various other models incorporating CPR and/or b5 (spectrophotometric and immunoblot measures) in addition to CYP3A protein also failed to improve prediction of CYP3A activity over CYP3A protein alone. We also evaluated whether CYP3A protein content was correlated with either of the CPR, or b5 measures. We found a weak but statistically significant covariation between CYP3A protein and CPR protein content (Rs = 0.48, P < 0.001) but not between CYP3A protein and CPR spectrophotometric content or either b5 measure (Rs <0.20, P > 0.05).
Discussion
The initial goal of this study was to determine CPR and b5 expression in 46 HLMs using two different methods (spectrophotometry and immunoblotting) and then to investigate the effects of aging and gender on the expression of these enzymes. The results indicate that expression of CPR and b5 in HLMs both decline with aging in males in that CPR levels were 27 to 41% lower in elderly males and b5 levels were 43 to 47% lower in elderly males. Although we did not observe a similar age related difference in female livers, the number of female HLMs (seven young, seven elderly) available to us was somewhat smaller than the number of male donor HLMs (21 young, 11 elderly). Consequently, a lack of effect of aging on the expression of CPR and b5 in female HLMs cannot be concluded with any certainty and will need to be confirmed by study of a larger number of female livers.
Although CPR levels measured by spectrophotometry were reasonably well correlated with immunoblot levels (Rs = 0.60), the CPR levels measured by immunoblotting were consistently lower than levels determined by the spectrophotometric method (Table 1). In contrast, b5 levels measured by immunoblotting were consistently higher than those determined by the spectrophotometric method, and b5 levels measured by these two methods were less well correlated (Rs = 0.41). Although reasons for these discrepancies are not clear, we speculate that there may have been some inactivation of accessory proteins during liver sample collection and/or microsome preparation of HLMs, leading to lower b5 spectrophotometric levels relative to immunoblot levels. Lower CPR immunoblot levels relative to spectrophotometric levels may be the result of less efficient antibody binding to CPR in HLMs versus purified recombinant CPR. Despite these inconsistencies, we found similar differences in b5 and CPR levels between the young male and elderly male HLMs regardless of assay method.
A second goal of the study was to determine whether α-lipoic acid and PTU could be used as selective chemical inhibitors of CPR and b5R enzymatic activities, respectively. α-Lipoic acid is a naturally occurring disulfide compound found in many foods, such as meats and vegetables (especially spinach) and is often used as a dietary supplement for antioxidant effects. α-Lipoic acid has been shown previously to inhibit CPR activity both in human (Dudka, 2006) and rat (Slepneva et al., 1995) liver microsomes possibly through interaction with critical sulfhydryl groups in the CPR molecule (Yelinova et al., 1993). Our results confirmed that α-lipoic acid inhibited CPR activity in both recombinant enzymes and HLMs. However, we also observed inhibition of b5R activity by α-lipoic acid. Although the extent of inhibition of b5R activity at 2 mM α-lipoic acid was somewhat less (by approximately 10% in recombinant enzymes and 20% in HLMs) than for inhibition of CPR activity, the magnitude of the difference would be insufficient to allow this compound to discriminate between the contributions of CPR and b5R to an observed reductase activity. As far as we are aware, no other study has reported the selectivity of α-lipoic acid as a CPR inhibitor. Of note was that the inhibitory effects of α-lipoic acid on CPR and b5R were less in HLMs than in recombinant enzymes (Fig. 1). This may be due to the presence of alternative electron transfer routes in HLMs (but not in recombinant enzymes), which would compensate for inhibition of either CPR or b5R activity by α-lipoic acid.
PTU is a drug used in the treatment of hyperthyroidism via inhibition of thyroperoxidase (Streetman and Khanderia, 2003). PTU has been reported to selectively inhibit b5R activity without reducing CPR activity in rat liver microsomes at concentrations up to 10 mM (Lee and Kariya, 1986), possibly via interaction with the NADH binding site of the enzyme. We preincubated enzymes with PTU before NADH addition to enhance the inhibitory effect on b5R (Lee and Kariya, 1986). Despite this, PTU inhibition of HLMs was limited, showing maximal inhibition of b5R activity of approximately 40% in HLMs over 1 to 4 mM concentrations, with somewhat more (78% inhibition) in recombinant enzyme at 4 mM concentration. PTU was also found to inhibit CPR activity in recombinant enzyme (51% decrease at 4 mM), but it only had minimal effect on HLMs (<20% decrease at 4 mM). Consequently, we conclude that PTU is not an efficacious or selective b5R inhibitor in HLMs. The discrepancy in our results from those of Lee and Kariya (1986) who found good reductase discrimination may relate to species differences in human (this study) versus rat (previous study) reductase enzymes. Again, the lower inhibitory effects of PTU on CPR and b5R in HLMs compared with recombinant enzymes might be the result of alternative electron transfer routes in HLMs.
Reductase-specific inhibitory antibodies have been used in previous work to demonstrate the contribution of CPR and b5R to microsomal CYP3A activities (Yamazaki et al., 1996). However these antibodies are not commercially available and as such would have less utility than chemical inhibitors.
We also evaluated effects of α-lipoic acid and PTU on CYP3A activity in HLMs. Different cofactors were used with the rationale that incubation mixtures containing β-NADPH alone would be more susceptible to CPR inhibitors (i.e., α-lipoic acid), while those containing NADH alone would be more susceptible to b5R inhibitors (i.e., PTU). Consistent with our previous finding of a lack of reductase selectivity, α-lipoic acid inhibited CYP3A activity regardless of the cofactor included. In contrast, PTU was found to inhibit CYP3A activity to a greater extent in the presence of β-NADPH compared with incubations that lacked β-NADPH. However, this result is consistent with PTU being a CPR-selective inhibitor rather than being a b5R-selective inhibitor, as we had originally hypothesized. Regardless, these data provide further evidence that α-lipoic acid and PTU are not reductase selective inhibitors in HLMs.
Finally, we used simple linear and multivariate regression approaches to evaluate whether variable CPR and/or b5 expression in a HLM bank contributes to the observed variability of CYP3A activity in addition to that contributed by CYP3A protein content. This might occur perhaps through enhancement of CYP3A catalytic function via more efficient electron transfer in microsomes containing higher amounts of CPR or b5 relative to CYP3A protein content. However, our data indicate that variable CPR or b5 expression across the HLMs bank does not account for CYP3A activity variability in addition to that already explained by CYP3A protein levels. We also found a weak but statistically significant correlation between CYP3A protein and CPR protein content but not between CYP3A protein and b5 protein content. This finding is consistent with coregulation of gene expression of CYP3A with CPR but not with b5.
In conclusion, the results of this study indicate that hepatic CPR and b5 activity and content decrease with aging particularly in male livers. However, these changes in expression and activity of the accessory proteins are not sufficient to account for aging-related decreases in CYP3A activity in vitro (Parkinson et al., 2004; Patki et al., 2004) and in vivo (Cotreau et al., 2005).
Acknowledgments
We thank Charles L. Crespi (BD Biosciences, Woburn, MA), Scott R. Obach (Pfizer Inc., Groton, CT), Richard I. Shader (Department of Pharmacology, Tufts University, Boston, MA), and Larry H. Cohen (Millennium Pharmaceuticals Inc., Cambridge, MA) for helpful suggestions.
This work was supported by National Institute of General Medical Sciences, National Institutes of Health [Grants R01 GM061834 and R21 GM074369]; National Institute on Aging [Grant AG17880]; National Institute on Allergy and Infectious Disease, National Institutes of Health [Grant AI58784]; and National Institute of General Medical Sciences, National Institutes of Health [Grant R01 GM61753].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.023424.
ABBREVIATIONS: CPR, NADPH-cytochrome P450 reductase; b5, cytochrome-b5; b5R, NADH-b5 reductase; HLM, human liver microsome; PTU, 6-propyl-2-thiouracil; HRP, horseradish peroxidase; TBS, Tris-buffered saline.
References
- Badwey JA, Tauber AI, and Karnovsky ML (1983) Properties of NADH-cytochrome-b5 reductase from human neutrophils. Blood 62 152–157. [PubMed] [Google Scholar]
- Cotreau MM, von Moltke LL, and Greenblatt DJ (2005) The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet 44 33–60. [DOI] [PubMed] [Google Scholar]
- Dudka J (2006) Decrease in NADPH-cytochrome P450 reductase activity of the human heart, Liver and lungs in the presence of alpha-lipoic acid. Ann Nutr Metab 50 121–125. [DOI] [PubMed] [Google Scholar]
- Elmore CL and Porter TD (2002) Modification of the nucleotide cofactor-binding site of cytochrome P-450 reductase to enhance turnover with NADH in vivo. J Biol Chem 277 48960–48964. [DOI] [PubMed] [Google Scholar]
- George J, Byth K, and Farrell GC (1995) Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol 50 727–730. [DOI] [PubMed] [Google Scholar]
- Guengerich FP (2005) Reduction of cytochrome b5 by NADPH-cytochrome P450 reductase. Arch Biochem Biophys 440 204–211. [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang M, Tian G, Burger J, Gochfeld M, and Yang CS (1993) Age- and gender-related variations in the activities of drug-metabolizing and antioxidant enzymes in the white-footed mouse (Peromyscus leucopus). Growth Dev Aging 57 85–100. [PubMed] [Google Scholar]
- He P, Court MH, Greenblatt DJ, and von Moltke LL (2006) Factors influencing midazolam hydroxylation activity in human liver microsomes. Drug Metab Dispos 34 1198–1207. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, and Court MH (2004) Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14 225–238. [DOI] [PubMed] [Google Scholar]
- Holmans PL, Shet MS, Martin-Wixtrom CA, Fisher CW, and Estabrook RW (1994) The high-level expression in Escherichia coli of the membrane-bound form of human and rat cytochrome b5 and studies on their mechanism of function. Arch Biochem Biophys 312 554–565. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Bajad SU, Miller JL, Chin NA, and Trepanier LA (2004) NADH cytochrome b5 reductase and cytochrome b5 catalyze the microsomal reduction of xenobiotic hydroxylamines and amidoximes in humans. J Pharmacol Exp Ther 311 1171–1178. [DOI] [PubMed] [Google Scholar]
- Lee E and Kariya K (1986) Propylthiouracil, a selective inhibitor of NADH-cytochrome b5 reductase. FEBS Lett 209 49–51. [DOI] [PubMed] [Google Scholar]
- Maloney AG, Schmucker DL, Vessey DS, and Wang RK (1986) The effects of aging on the hepatic microsomal mixed-function oxidase system of male and female monkeys. Hepatology 6 282–287. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Tamura M, Takeshita M, and Yoneyama Y (1981) Age-dependent decay of cytochrome b5 and cytochrome b5 reductase in human erythrocytes. J Biochem 194 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murataliev MB, Feyereisen R, and Walker FA (2004) Electron transfer by diflavin reductases. Biochim Biophys Acta 1698 1–26. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, and Carroll KM (2004) The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol 199 193–209. [DOI] [PubMed] [Google Scholar]
- Patki KC, von Moltke LL, Harmatz JS, Hesse LM, Court MH, and Greenblatt DJ (2004) Effect of age on in vitro triazolam biotransformation in male human liver microsomes. J Pharmacol Exp Ther 308 874–879. [DOI] [PubMed] [Google Scholar]
- Porter TD and Kasper CB (1986) NADPH-cytochrome P-450 oxidoreductase: flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry 25 1682–1687. [DOI] [PubMed] [Google Scholar]
- Schmucker DL and Wang RK (1983) Age-dependent alterations in rat liver microsomal NADPH-cytochrome c (P-450) reductase: a qualitative and quantitative analysis. Mech Ageing Dev 21 137–156. [DOI] [PubMed] [Google Scholar]
- Schmucker DL and Wang RK (1987) Effects of aging on the properties of rhesus monkey liver microsomal NADPH-cytochrome c (P-450) reductase. Drug Metab Dispos 15 225–232. [PubMed] [Google Scholar]
- Schmucker DL, Woodhouse KW, Wang RK, Wynne H, James OF, McManus M, and Kremers P (1990) Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clin Pharmacol Ther 48 365–374. [DOI] [PubMed] [Google Scholar]
- Shephard EA, Palmer CN, Segall HJ, and Phillips IR (1992) Quantification of cytochrome P450 reductase gene expression in human tissues. Arch Biochem Biophys 294 168–172. [DOI] [PubMed] [Google Scholar]
- Simmons DL and Kasper CB (1989) Quantitation of mRNAs specific for the mixed-function oxidase system in rat liver and extrahepatic tissues during development. Arch Biochem Biophys 271 10–20. [DOI] [PubMed] [Google Scholar]
- Slepneva IA, Sergeeva SV, and Khramtsov VV (1995) Reversible inhibition of NADPH-cytochrome P450 reductase by alpha-lipoic acid. Biochem Biophys Res Commun 214 1246–1253. [DOI] [PubMed] [Google Scholar]
- Streetman DD and Khanderia U (2003) Diagnosis and treatment of Graves disease. Ann Pharmacother 37 1100–1109. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, von Moltke LL, Court MH, Harmatz JS, Crespi CL, and Greenblatt DJ (2000) Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab Dispos 28 1493–1504. [PubMed] [Google Scholar]
- Vergéres G and Waskell L (1995) Cytochrome b5, its functions, structure and membrane topology. Biochimie 77 604–620. [DOI] [PubMed] [Google Scholar]
- Voice MW, Zhang Y, Wolf CR, Burchell B, and Friedberg T (1999) Effects of human cytochrome b5 on CYP3A4 activity and stability in vivo. Arch Biochem Biophys 366 116–124. [DOI] [PubMed] [Google Scholar]
- von Moltke LL, Greenblatt DJ, Harmatz JS, Duan SX, Harrel LM, Cotreau-Bibbo MM, Pritchard GA, Wright CE, and Shader RI (1996) Triazolam biotransformation by human liver microsomes in vitro: effects of metabolic inhibitors and clinical confirmation of a predicted interaction with ketoconazole. J Pharmacol Exp Ther 276 370–379. [PubMed] [Google Scholar]
- von Moltke LL, Greenblatt DJ, Harmatz JS, and Shader RI (1993) Alprazolam metabolism in vitro: studies of human, monkey, mouse and rat liver microsomes. Pharmacology 47 268–276. [DOI] [PubMed] [Google Scholar]
- Warrington JS, Greenblatt DJ, and von Moltke LL (2004) Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. J Pharmacol Exp Ther 309 720–729. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakajima M, Nakamura M, Asahi S, Shimada N, Gillam EM, Guengerich FP, Shimada T, and Yokoi T (1999) Enhancement of cytochrome P-450 3A4 catalytic activities by cytochrome b(5) in bacterial membranes. Drug Metab Dispos 27 999–1004. [PubMed] [Google Scholar]
- Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, et al. (2002) Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif 24 329–337. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakano M, Imai Y, Ueng YF, Guengerich FP, and Shimada T (1996) Roles of cytochrome b5 in the oxidation of testosterone and nifedipine by recombinant cytochrome P450 3A4 and by human liver microsomes. Arch Biochem Biophys 325 174–182. [DOI] [PubMed] [Google Scholar]
- Yelinova VI, Weiner LM, Slepneva IA, and Levina AS (1993) Reversible modification of cysteine residues of NADPH-cytochrome P-450 reductase. Biochem Biophys Res Commun 193 1044–1048. [DOI] [PubMed] [Google Scholar]