Abstract
The N-terminal domain of outer membrane protein OprF of Pseudomonas aeruginosa forms a membrane spanning eight-stranded anti-parallel β-barrel domain that folds into a membrane channel with low conductance. The structure of this protein has been modeled after the crystal structure of the homologous protein OmpA of Escherichia coli. A number of molecular dynamics simulations have been carried out for the homology modeled structure of OprF in an explicit molecular model for the rough lipopolysaccharide (LPS) outer membrane of P. aeruginosa. The structural stability of the outer membrane model as a result of the strong electrostatic interactions compared to simple lipid bilayers is restricting both the conformational flexibility and the lateral diffusion of the porin in the membrane. Constricting side-chain interactions within the pore are similar to those found in reported simulations of the protein in a solvated lipid bilayer membrane. Because of the strong interactions between the loop regions of OprF and functional groups in the saccharide core of the LPS, the entrance to the channel from the extracellular space is widened compared to the lipid bilayer simulations in which the loops are extruding in the solvent. The specific electrostatic signature of the LPS membrane, which results in a net intrinsic dipole across the membrane, is found to be altered by the presence of OprF, resulting in a small electrically positive patch at the position of the channel.
Keywords: Outer Membrane Protein, OprF N-terminal domain, Lipopolysaccharide, Molecular Dynamics Simulation
Introduction
Pseudomonas aeruginosa is a ubiquitous environmental Gram-negative bacterium with high metabolic versatility and an exceptional ability to adapt to a wide range of ecological environments, including soil, marches, coastal habitats, plant and animal tissues.1 It is a major opportunistic human pathogen that can result in life-threatening infections in many immune-compromised patients, such as lung infections in children with cystic fibrosis,2,3 bacteraemia in burn victims, urinary-tract infections in catheterized patients, hospital-acquired pneumonia in patients on respirators, infections in cancer patients receiving chemotherapy, and keratitis and corneal ulcers in users of extended-wear soft contact lenses.4 The inherent resistance against antibiotics of P. aeruginosa makes these infections difficult to treat. Multiple organ failure caused by Gram-negative bacterial sepsis syndrome is the main reason for the high morbidity and mortality from P. aeruginosa infections. At present, there are no commercially available vaccines with which to prevent infection with this organism, and experimental vaccines have high toxicity or low and inconsistent immunogenicity. 5-7
Major outer membrane protein OmpA of Escherichia coli and major outer membrane OprF of P. aeruginosa are orthologs with significant amino acid similarity (56%, 39% identity) in their C-terminal domains. Even though the N-terminal fragments are considerably less similar (15% identity), based on secondary structure predictions a model for the N-terminal fragment of OprF have been suggested8 that was constructed using homology modeling of the primary sequence onto the experimentally determined crystal structure of the N-terminal domain of OmpA.9,10 The structure forms an eight-stranded anti-parallel β-barrel domain that is inserted in the outer membrane. These two proteins exhibit a remarkable difference in the size of the pores formed in planar lipid bilayer membranes, as indicated by conductance experiments on the N-terminal domains of both proteins. Conductance values of 0.05 to 0.08 nS have been reported for OmpA,11,12 indicating the formation of very small channels, whereas the OprF protein appears to form both small as well as much larger pores,11 resulting in conductance measurements of 0.07 nS and 0.36 nS8, respectively. The homology modeled structure showed a high structural similarity between OmpA and OprF in this domain, including the conservation of hydrophobic residues that point outward from the β-barrel, as well as the rings of aromatic residues at the interfaces of the internal lipid with the aqueous periplasm and the link region of lipid A with the core oligosaccharide of the lipopolysaccharide (LPS). Differences were observed for some of the residues inside the pore that form three water-filled cavities but present a possible barrier for ion channel formation in the case of OmpA. However, in OprF these residues are smaller, suggesting the ability for transport of ions through the pore.
One of the main virulence factors is the highly potent toxin lipopolysaccharide (LPS), the primary component of the outer membrane of P. aeruginosa, and which is composed of lipid A, a core oligosaccharide with an inner and outer core region, and an O-specific polysaccharide antigen chain. Lipid A is anchored into the hydrophobic region of the outer membrane. The inner core LPS consists of the two saccharides L-glycero-D-manno-heptose (HEP1) and 3-deoxy-D-manno-octulosonic acid (KDO). To a large extent, membrane stability is due to the multiple phosphoryl substituents in the core polysaccharide region. P. aeruginosa is among the Gram-negative bacteria with the most highly phosphorylated saccharides in the inner core region, with three phosphate groups on the C2, C4 and C6 positions of the inner core heptose. These, together with the phosphate groups on both proximal and distal lipid-A glucosamine head groups are essential for the membrane's integrity through strong ionic interactions between the negatively charged core oligosaccharide and divalent counter ions. The third component of the LPS is the O-specific antigen. These antigen chains consist of up to 20 repeating monomers of three to five saccharide subunits The O-antigens extend out from the cell surface into the cell's environment.
As a result of the presence of anionic LPS, the outer membrane of Gram-negative bacteria presents an effective barrier to many hydrophobic compounds, including many antibiotics. Although the highly anionic nature of LPS deters the binding of hydrophobic compounds, there are a few cationic antibiotics, such as gentamicin for P. aeruginosa, that have been found to increase the permeability of the outer membrane,12 presumably by the disruption of the outer membrane which subsequently facilitates the entry of themselves as well as other molecules, including lysozyme and other, hydrophobic compounds that inhibit specific metabolic processes. Nevertheless, among Gram-negative bacteria, P. aeruginosa has a notoriously high intrinsic drug resistance that has been linked to the high phosphate content of the inner core LPS.13 In particular the three phosphates groups on saccharide residue HEP1 are specific for P. aeruginosa.14 The exceptional antibiotic resistance of P. aeruginosa is not only due to the strong outer membrane integrity. The outer membrane of Gram-negative bacteria contains a variety of porins, water-filled protein channels making the membrane semi-permeable. In fact, P. aeruginosa has been found to have a size exclusion limit six times larger than E. coli. This will allow antibiotics to diffuse across the outer membrane, and will require the microbe to have additional resistance mechanisms. At least two secondary resistance mechanisms in P. aeruginosa have been identified. One is a periplasmic beta-lactamase that hydrolyzes the slow influx of beta-lactams such as imipenem and panipenem, making it the major secondary defense against antibiotic agents. Other systems for intrinsic antibiotic resistance are multi-drug efflux systems in the outer membrane, such as the MexAB-OprM RND efflux system, that excrete antimicrobial agents that have entered the periplasm through the non-specific porins.15 The low permeability of the outer membrane requires the existence of specialized pathways allowing the transport of substrates required for growth. P. aeruginosa uses three classes of trans-membrane porins: general porins such as OprF, specific porins such as OprP/O, OprD and OprB, and gated porins such as OprC, OprH, FpvA, and PupA/B.
Because of the biomedical interest in OprF, the current work is concerned with providing a characterization of the role of the LPS outer membrane on the structure and the dynamics of the trans-membrane domain of this protein. Simulation studies of outer membrane proteins of Gram-negative bacteria have been reported using simple lipid bilayers,16 which is the membrane environment used in most in vitro experimental studies of outer membrane proteins. Simulation studies focused on the dynamics and function of outer membrane proteins in vivo require a representation of the asymmetric outer membrane environment that includes the lipopolysaccharide layer to be more comparable to physiological conditions. This makes simulations much more complex because of several factors: a) the limited experimental data available allowing the construction of a molecular model for a single LPS molecule, b) the need for a parameterization of the oligosaccharide, c) the complicated setup/equilibration procedure as a result of the highly charged LPS molecules that need to be neutralized by counter ions, and d) the increase in computational requirements as a result of the much larger molecular system. The primary molecular structures of LPS molecules, including the O-specific chains, have been determined for a number of Gram-negative bacteria.18,19 However, very little is known about the structure and dynamic behavior of bacterial LPS membranes, or about the interaction of LPS membranes with membrane proteins. A few molecular modeling studies have been reported of a single LPS molecule in vacuum using a simplified force field17 and in aqueous solution,18 of solvated lipid-A22,23 and of a small, non-periodic assembly of LPS molecules in aqueous solution.19 Our recent molecular simulation studies, using NWChem20,21 of the rough LPS membrane of P. aeruginosa are the first and only reported studies to date for a complete, periodic LPS outer membrane.22-25
Methods
OprF Molecular Model
Experimental evidence suggests that OprF folds into two distinct conformations, an abundant closed channel form, and a rare open channel form.11,26 A similar existence of open and closed conformations had also been found for the outer membrane protein OmpA of E. coli.27,28 In the current work, the homology modeled closed channel structure of the N-terminal domain of P. aeruginosa OprF8 was used to carry out molecular dynamics simulations to characterize the role of the structure and asymmetric electrostatic character of the membrane on the dynamical and electrostatic behavior of the protein. The C-terminal domain in this conformation is believed to associate with the peptidoglycan in the periplasm.29
The starting structure of the OprF amino terminal domain used in this study was modeled by Brinkman et al.8 on the known crystal structure of the N-terminal domain of OmpA of E. coli,9,10 which has 15% identity. The structural similarity of the two proteins is suggested by the many functional and sequence relationships.30 An independent homology-based model by Khalid et al.31 was found to be very similar to the model by Brinkman et al.,8 except in the flexible loop regions, and both lead to stable computer simulations. The resulting models for OmpA and OprF have structural similarity in the conservation of hydrophobic residues exposed to the outside of the barrel and two rings of aromatic residues that interact with the head groups of the Lipid-A and phospholipid (PL) membrane layers. The N-terminal domains form eight-stranded β-barrels, resulting in very small trans-membrane channels. There is a difference between the two membrane spanning domains with respect to residues inside the resulting pore that can be linked to the difference in channel function between the two proteins. The molecular structure of the OprF protein embedded in the LPS membrane is illustrated in Figure 1. Indicated are the four loop regions L1 – L4 that interact with the polysaccharide region of the membrane.
Figure 1.

Molecular representation of the simulated system. Protein OprF of Pseudomonas aeruginosa is embedded in the outer membrane formed by the inner (phosphatidylethanolamine) and outer (lipopolysaccharide) leaflets. The graphical representation of the membrane is clipped in the plane normal to the membrane in order to expose the secondary structure of OprF (yellow and orange ribbons). Ca2+ atoms are represented by van der Waals spheres in pink.
Lipopolysaccharide Membrane Model
For the description of the outer membrane a model was used with an explicit description of the P. aeruginosa specific lipopolysaccharide and phospholipid layers of the microbial membrane matrix.23,25 The model of the lipopolysaccharide was constructed from chemical composition information from NMR spectroscopy studies reported in the literature. Figure 2 depicts the chemical composition of the rough LPS used in this study. The dynamics and energetic properties of the asymmetric LPS membrane of P. aeruginosa constructed from this molecular model reproduce experimental findings for area per head group, carbon-deuterium order parameters and electrostatic potential across the membrane.32,33 The model has also been used in studies of the interaction of this membrane with mineral surfaces and solvated recalcitrant ions.23
Figure 2.
Molecular Structure of the Lipopolysaccharide of Pseudomonas aeruginosa, consisting of the hydrophobic Lipid-A, the inner code saccharide region, and the strain-specific outer core saccharides.
Molecular System Setup
The molecular system was constructed by placing 60 LPS molecules and 150 PL molecules on two two-dimensional regular grids with a spacing of 4 nm between the two planes, and with the OprF model placed in the center. Each LPS and PL molecule was randomly rotated around the molecular axis perpendicular to the plane membrane. Ca2+ counter ions were added to electrically neutralize the system. The positions of the counter ions were optimized by steepest descent energy minimization. Subsequently, the PL and clusters of LPS with the closest Ca2+ ions were iteratively moved towards the OprF until all intermolecular distances fell below 0.3 nm. The resulting structure was used in a molecular dynamics simulation using anisotropic pressure scaling at 1.025×109 Pa to collapse the membrane to the experimental density. During this process, restraint potentials were used to maintain the overall orientation of the PL and LPS molecules perpendicular to the plane of the membrane. These simulations were run for 5 ns at a temperature of 50 K, using a time step of 1 fs and a charge group based potential cutoff of 1.4 nm. The low temperature and length of the simulations are needed to gradually reach the correct density of the system with the large number of restraints required to maintain the LPS and phospholipid molecules in the correct orientation. After the experimental membrane density was reached, the system was solvated and thermalized in 0.5 ns time segments at 50, 100, 150, 200 and 298 K, using the restraint potentials. This was followed by equilibration for an additional 3 ns at 298.15 K without the further use of restraint potentials. Because of the slow diffusion of LPS compared to PL bilayers, the use of this procedure is required to allow for the adjustment of LPS and PL molecular orientations in the field of the membrane protein.33
Simulation Protocol
All simulations were performed with the molecular dynamics module of the NWChem program,20,21 and the analyses of molecular trajectories were carried out using the data-intensive trajectory analysis capabilities of the DIANA program.34 The force field used for the simulations is the AMBER95 force field, augmented with an extension of the GLYCAM parameters35 for the saccharide groups of the LPS, and the SPC/E water model.36 Simulations were performed in the isothermal isobaric ensemble, using weak coupling37 to an external temperature of 298.15 K and a pressure of 1 atm, with anisotropic pressure scaling. The time step used was 2 fs. Bonds to hydrogen atoms were constrained using the SHAKE procedure.38 The cutoff radius for the interatomic interactions was 1.0 nm, with long range electrostatic corrections evaluated using the smooth particle-mesh Ewald method.39
The analyses of the molecular trajectories were carried out using the data-intensive analysis tools of the DIANA program.34 Secondary structure analysis of the membrane protein was performed using DSSP.40 The extent to which a pore was formed during the simulations was determined with the program HOLE.41 The program VMD42 was used for the graphical visualization of the molecular systems.
Results and Discussion
Structural stability
Two 10 ns molecular simulations were performed to investigate the conformational stability of the OprF in the LPS membrane. The two simulations differed in the number of water molecules placed in the pore of the initial structure. In the first simulation (I) no water molecules were placed in the pore created by the β-barrel. To further examine the integrity and flexibility of the pore, in a second simulation (II), seven water molecules were placed in the available space of the pore region. Simulations of the LPS membrane of 10 ns length have been shown to be adequate to obtain converged values for energetic, electrostatic and structural properties such as order parameters.33
The RMSD with respect to the initial structure as a function of simulation time is a measure of the structural flexibility, and is depicted in Figure 3 for the two simulations. The RMSD for simulation I reaches 0.18 nm for the protein backbone atoms and 0.15 nm if only the β-barrel backbone atoms are taken into account. These results are slightly smaller than the values of 0.27 and 0.16 nm, respectively, for a simulation of OprF without added water molecules in the pore carried out in a lipid bilayer, as reported by Khalid and coworkers.31 A significant difference, however, is that Khalid et al. report the RMSD to reach a plateau in about 2 ns, whereas the current simulation in an LPS membrane takes at least 6 ns to reach convergence. This is clearly a result of the significantly lower diffusion rate in an LPS membrane compared to lipid bilayers. It is important to point out that the system was equilibrated for nearly 6 ns before collecting the trajectory to which this analysis is applied. The observed convergence of the RMSD is a measure of the time required to sample the available phase space rather than a measure of the time needed to reach equilibrium. The RMSD for protein and β-barrel backbone atoms in simulation II are 0.18 nm and 0.11 nm, respectively, and much lower than the values of 0.33 nm and 0.21 nm, respectively, reported by Khalid et al. for their simulation of OprF with 18 added water molecules in the pore carried out in a lipid bilayer. This large difference in RMSD between the two membrane environments is a result of the relative rigidity of the LPS membrane caused by the strong electrostatic interactions in the polysaccharide layer, leading to a significant decrease in lateral mobility. This clearly affects the flexibility of the trans-membrane protein as measured by the RMSD. The simulations in the LPS membrane show a slightly larger difference between the RMSD for the protein backbone atoms and those for the β-barrel backbone atoms than was found by Khalid et al. for the simulations in the lipid bilayer. This also is expected, as the loop regions are constrained by the polysaccharide chains in the case of the LPS membrane, but exposed to the aqueous environment and, therefore, more mobile in the case of the lipid bilayer simulations.
Figure 3.
Root-mean-square deviations (RMSD) of backbone atoms N, C, Cα of OprF simulations II (A) and I (B) from the model structure.8 RMSD for all OprF residues are represented in black and for β-strand residues only in grey. Rotational and translational superimposition of pairs of structures was applied using Cα atoms of β-strand residues.
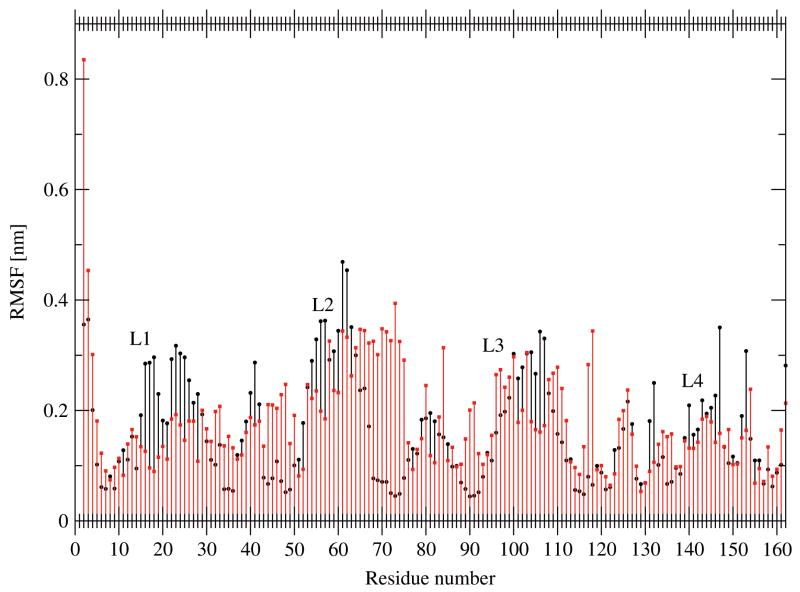
The time averaged root mean square fluctuation of the Cα atoms is a measure of the relative flexibility of each residue and provides a means to assess the relative flexibility of different regions of the protein. This RMSF is depicted in Figure 4 for the OprF simulations as the average over 10 ns. The RMSF pattern observed in the LPS simulations is very similar to the results of Khalid et al. Notable differences are in loop L1, which is more flexible, and the β-barrel backbone, which appears to be slightly less flexible. The lower RMSF for the β-barrel backbone can be explained by the overall higher rigidity of the LPS membrane compared to a lipid bilayer. The pronounced conservation of the protein secondary structure, as shown in Figure 5 for simulation II, is another indication of the strong stabilizing effect of the membrane on the protein structure.
Figure 4.
Root-mean-square atom-positional fluctuations (RMSF) of Cα atoms of OprF simulations I (red) and II (black) as function of residue sequence number and calculated over a period of 10 ns. Rotational and translational superimposition of pairs of structures was applied using Cα atoms of β-strand residues.
Figure 5.
Secondary structure of OprF in the single model structure (A) and as function of time for simulation II. Secondary structure definition: 3-helix (blue), β-strand (red), bend and turn (yellow), coil (white).40 MD structures were sampled at every 5 ps.
Protein dynamics
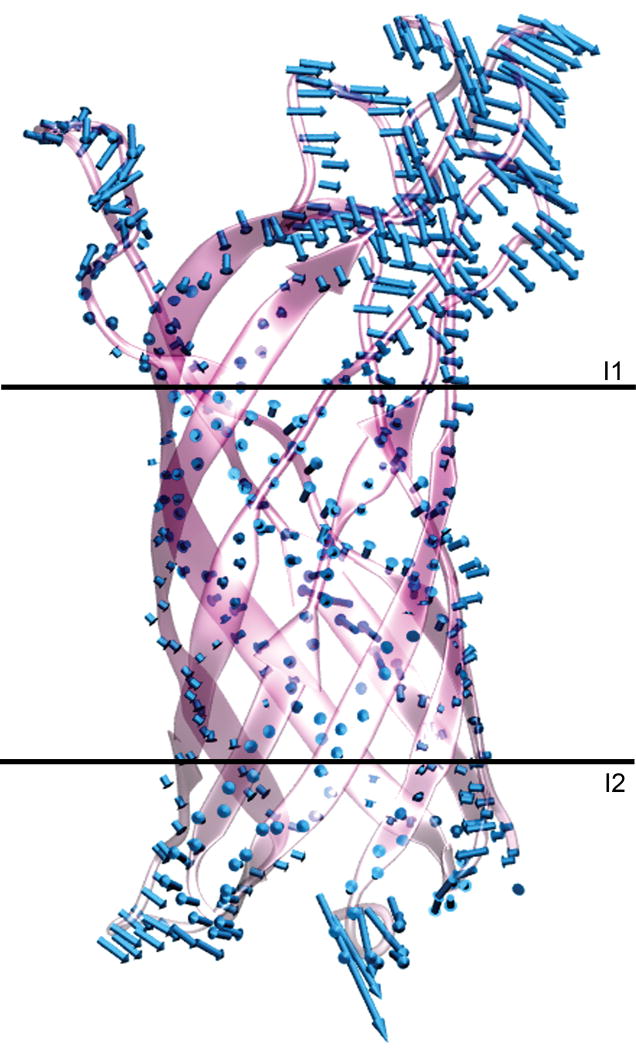
To further characterize the protein mobility a principal component analysis was carried out for the Cα atoms of simulation II. The results for this analysis over the last 10 ns of the simulation are illustrated in Figure 6. The primary low frequency motion can be described by a small number of eigenmodes. From the relative amplitudes of the eigenvalues shown in Figure 6A, the first four eigenmodes include 75% of the overall motion in the protein. The first eigenvector alone contributes 45% of this motion, and includes predominantly motions of the loop regions of the protein. The highest amplitude motions of the first eigenvector are shown in Figure 7, and illustrate the relative rigidity of the β-barrel backbone. Interestingly, most of the motion is for the loops in the polysaccharide region of the membrane, indicating the relatively high degree of flexibility. This is due to the strong electrostatic interactions between the loop residues and the charged groups in the LPS saccharide layer. These interactions are the cause of the observation that, whereas the porin core is more restricted in the LPS membrane, the loops that extend into the saccharide layer of the LPS membrane are more flexible than they were found to be in the aqueous environment close to a lipid bilayer membrane. The projection onto the first eigenvector shows a continuous increase, indicating an opening motion of the loop regions throughout the length of the simulation. This flexibility is consistent with observed water channels extending deep into the membrane,22 the rapid reorientation of the polysaccharide chains of this membrane upon contact with solid surfaces,25 and the thermodynamically favorable uptake of solvated cations deep into the polysaccharide region of the membrane.23
Figure 6.
Principal component analysis. (A) Amplitude (top panel) and magnitude (bottom panel) of eigenvalues calculated from the covariance matrix of Cα coordinates of the MD simulation of OprF. (B) Eigenvector components for the atomic displacement along the first eigenvector.
Figure 7.
Porcupine plot representing the highest amplitude motions along the first eigenvector. The horizontal lines I1 and I2 identify the boundaries of the hydrophobic acyl chain region.
Pore Radius
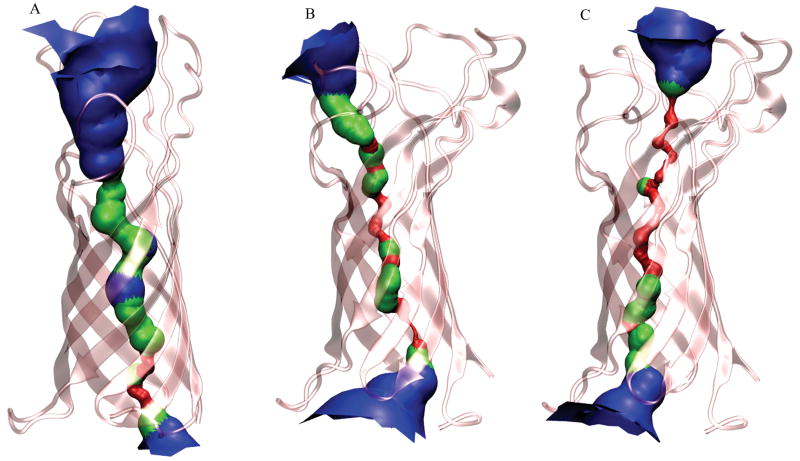
The most recent experimental studies indicate that OprF of P. aeruginosa exists in an open and closed conformation.11,26 A wide range of values for conductance of OprF under different experimental conditions have been reported.8,43-45 To examine the extent to which a pore can be formed in the closed conformation used in this simulation study, the pore radius profile evaluated from the trajectory generated of the simulation II is shown in Figure 8. Relative to the pore radius calculated for the homology modeled structure, the pore appears to be more constricted near the phosphorylated Lipid-A head groups. This region is marked by very strong electrostatic interactions compared to a lipid bilayer. Visual inspection shows the narrowing of the pore to be the result of side chain interactions in the internal channel, and not caused by an overall contraction of the β–barrel structure of the protein. In fact, visual inspection shows that the diameter of the β–barrel structure increases slightly during the simulation as compared to the initial structure. The decrease of the pore diameter in the center of the protein is particularly noticeable in four regions and can be directly related to side-chains interactions through hydrogen bonds (residues S47/D73, E8/K121, K14/N115/D135, S20/Y51/H95) or to side chain conformational rearrangement to decrease exposition to the oligosaccharide charged groups (residue L69). A similar small narrowing of the OprF pore was also observed by Khalid and coworkers in their simulations of this membrane protein in a lipid bilayer.31 In these simulations, residues Y51/H95 and E8/K121 were identified as the most obvious constricting regions and were shown to prevent water molecules from entering and traversing the pore, respectively. In the LPS simulation, the effect of these residues on the decrease of the pore diameter appears to be less significant than that of residues S47/D73, E8/K121 and K14/N115/D135. The narrowest region of the pore corresponds to the location of residues K14/N115/D135 with an average diameter smaller than the radius of a water molecule. The two other regions where the pore diameter differs significantly from the homology model correspond to residues S47/D73 and L69. A widening of the pore entrance to the periplasm is the result of displacement of residues Met1, Gln2 and His77. The calculation of the pore diameter of OprF was found to be somewhat sensitive to the choice of coordinates defining the initial sampling point within the channel. This is illustrated in Figure 9 where the calculated pore diameter depicted for a number of HOLE calculations starting from different initial positions, and indicates a pore topography with multiple narrow paths, making an exhaustive sampling difficult. This is also reflected in the uncertainties in the conductance estimates than can be evaluated based on the HOLE geometries, which for the OprF simulations in this study are found to be around 26 pS or less. While in the same order of magnitude, this is less than reported experimental values of 65-75 pS found for OprF in planar lipid bilayers.43,45
Figure 8.
Pore radius profile along the Z-axis for the MD-derived ensemble of structures (gray), for the homology model (black) and the final MD structure of OprF using two different seed positions for the HOLE sampling algorithm (blue and red). Minimum radius of a water molecule is 0.15 nm (dashed line).
Figure 9.
Representation of the internal surface of the pore channel of the homology model (A) and the 10-ns structure (B, C) of OprF colored according to the pore radius: radius < 0.115 nm in red, 0.115 nm > radius < 0.230 nm in green and radius > 0.230 nm in blue).
Electrostatic Effects
The asymmetric nature of LPS membranes introduces a potential gradient across the membrane with a negative surface charge density on the LPS side and a positive charge density on the PE side of the membrane.32 This potential plays a crucial role in many membrane-related processes, particularly binding of charged species and voltage-dependent gating of porin channels.22,24 Our goal is to investigate whether the presence of OprF alters the electrostatic potential of the LPS membrane and how the latter affects the molecular dynamics of OprF by comparison to a lipid bilayer. Experimental studies attribute values of -85 and -123 mV to the trans-membrane potential of Paracoccus denitrificans and E. coli respectively.46,47 The LPS membrane model for P. aeruginosa used here has an average trans-membrane potential of -150 mV.33 Differences in the chemical composition of the LPS among these species account for the variation in the trans-membrane potential. P. aeruginosa has the most highly phosphorylated inner core region among Gram-negative bacteria which is expected to result in a more electronegative surface potential.
Since OprF has a total charge of -4e at neutral pH, the protein does not significantly change the trans-membrane potential of the LPS membrane. However, the arrangement of Ca2+ counter-ions in the membrane matrix due to the presence of the protein induces local changes of the electrostatic potential on the surface of the membrane as shown in Figure 10A. In the absence of proteins, shown in Figure 10B, the surface potential of the LPS membrane is predominantly electronegative with very few electropositive potential patches.22,48 The presence of OprF induces an increase of charge polarization on the membrane surface as well as an increase of the positive potential in the area of protein insertion. Although the electrostatic surface potential will be highly dependent on the initial position of the counter-ions, the multiple-step protocol used to construct and equilibrate the LPS membrane ensures a stable, homogenous distribution of counter-ions around the LPS molecules.33 Furthermore, as experimental studies indicate, divalent cations bind specifically to carboxylate and phosphate groups of the inner core and lead to a decrease of the fluidity of the LPS membrane.21,27,55 Thus, these counter-ions exhibit extremely low mobility in the LPS membrane whose dynamics can be adequately described by the present ensemble of structures.
Figure 10.
Electrostatic potential of the LPS membrane. Electrostatic potential mapped onto a two-dimensional slice along the x-y plane of the LPS membrane in the presence (A) and absence (B) of OprF. The electrostatic potential is within the range of -200 to 200 mV. Red corresponds to a negative potential, blue to a positive potential and white to neutral areas of the LPS membranes. The structure of the LPS membrane was obtained from a previous simulation.33
Comparison of average structures obtained from of MD simulations of OprF in a LPS membrane and a lipid bilayer indicates that the secondary structure pattern is similar between the two ensembles.31 However, in presence of the phospholipid bilayer, OprF pore wall exhibits a more electronegative potential than in presence of the LPS membrane as illustrated in Figure 11. Furthermore, the residues located in the extra-cellular loops and entrance of the pore exhibit fairly diverging conformations. In the LPS membrane, OprF is fully immersed in the lipid matrix and the loops on the extra-cellular side are surrounded by electronegative functional groups of the inner and outer core as well as by Ca2+ cations. Interaction between these groups and oppositely charged groups in the protein leads to the opening of the extra-cellular entrance to the pore as seen in Figure 7. Conversely, the extra-cellular loops of OprF embedded in a phospholipid bilayer will be exposed to the solvent. In the absence of the oligosaccharide chains and cations, these oppositely charged residues favors hydrogen bond and electrostatic interactions among themselves. As result the extra-cellular loops adopt a closed conformation, restricting the entrance to the pore, as illustrated in Figure 11. It is difficult to assess the biological implications of these structural differences given the experimental evidence that the N-terminal β-barrel domain is unlikely to allow the diffusion of organic molecules of even a small size.11 However, these findings suggest that the oligosaccharide portion of LPS membrane may play an essential role in the gating mechanism of OprF.
Figure 11.
OprF structure from MD simulations in the LPS membrane (A and C) compared to simulations by Khalid et al.31 in a lipid bilayer environment (B and D. Electrostatic potential mapped onto the solvent accessible surfaces of backbone atoms of OprF in a LPS membrane (A) and phospholipids bilayer (B). The residues Arg143, Lys63 and Glu19 are represented in CPK. Cartoon representation of 10 structures of OprF sampled along 10 ns of simulation in a LPS membrane (C) and in a lipid bilayer (D). Basic and acidic residues are represented in sticks (C, D). The electrostatic potential is within the range of -100 to 100 mV. It was calculated for and mapped onto the average structure from the set of structures shown in C and D. Red corresponds to a negative potential, blue to a positive potential and white to neutral areas of the LPS membranes.
Conclusion
Previous molecular modeling and simulation studies of transmembrane protein domains have focused on the structural and dynamical properties of these proteins in lipid bilayer environments. This study presents the first molecular dynamics simulation of a trans-membrane domain of an outer membrane protein in an explicit molecular model for the lipopolysaccharide-phospholipid asymmetric outer membrane environment of the microbial cell envelope. Simulations have been carried out for OprF in an LPS membrane, and compared to a simulation study by the Sansom group of this protein in simulations of this protein in a lipid bilayer that used the same homology modeled protein structure.31 The structure of the protein is stable over the time span of the simulations. The lower lateral diffusion in the LPS membrane results in a lower mobility of the β–barrel structure of the protein relative to the simulations in a lipid bilayer, but the loop regions have similar mobility in the two membrane environments as measure by the Cα RMSF. The presence of the polysaccharide component of the LPS results in a conformation of the pore that is more closed at the extracellular entrance, compared to the initial structure that was homology modeled based on a high resolution crystal structure of OprF homolog OmpA of Escherichia coli. The stability of the protein in the current simulations suggest that homology models can be used as initial structures for simulations of outer membrane proteins for which experimental X-ray or NMR structures are not available.
Acknowledgments
This work was supported in part by the NIH National Institute for Allergies and Infectious Diseases. The development of trajectory analysis tools used in this work was supported by the US Department of Energy's Office of Advanced Scientific Computing Research. Computational resources were provided by the Environmental Molecular Sciences Laboratory at Pacific Northwest National Laboratory. The authors thank Professor Fiona S. L. Brinkman for providing the coordinates of the homology model of OprF, and Professor Syma Khalid for providing simulation data from the lipid bilayer OprF simulations and for stimulating discussions, and Dr. Roberto D. Lins for critical reading of the manuscript. Pacific Northwest National Laboratory is operated for DOE by Battelle.
References
- 1.Goldberg JB, Pier GB. The role of the CFTR in susceptibility to Pseudomonas aeruginosa infections in cystic fibrosis. Trends in Microbiology. 2000;8(11):514–520. doi: 10.1016/s0966-842x(00)01872-2. [DOI] [PubMed] [Google Scholar]
- 2.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, Nadel JA, Prince A, Basbaum CB. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(3):967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, Nagai S, Izumi T. The potential of various lipopolysaccharides to release IL-8 and G-CSF. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;278(4):L658–L666. doi: 10.1152/ajplung.2000.278.4.L658. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SK, Masinick S, Garrett M, Hazlett LD. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infection and Immunity. 1997;65(7):2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatano K, Boisot S, Desjardins D, Wright DC, Brisker J, Pier GB. Immunogenic and Antigenic Properties of a Heptavalent High-Molecular-Weight O-Polysaccharide Vaccine Derived from Pseudomonas aeruginosa. Infection and Immunity. 1994;62(9):3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemachandra S, Kamboj K, Copfer J, Pier G, Green LL, Schreiber JR. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal Pseudomonas sepsis. Infection and Immunity. 2001;69(4):2223–2229. doi: 10.1128/IAI.69.4.2223-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pier GB. Promises and pitfalls of Pseudomonas aeruginosa lipopolysaccharide as a vaccine antigen. Carbohydrate Research. 2003;338(23):2549–2556. doi: 10.1016/s0008-6215(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 8.Brinkman FSL, Bains M, Hancock REW. The amino terminus of Pseudomonas aeruginosa outer membrane protein OprF forms channels in lipid bilayer membranes: Correlation with a three-dimensional model. 2000;182:5251. doi: 10.1128/jb.182.18.5251-5255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Journal of Bacteriology. 2000;182(23):6863–6863. [Google Scholar]
- 9.Pautsch A, Schulz GE. High-resolution structure of the OmpA membrane domain. Journal of Molecular Biology. 2000;298(2):273–282. doi: 10.1006/jmbi.2000.3671. [DOI] [PubMed] [Google Scholar]
- 10.Pautsch A, Schulz GE. Structure of the outer membrane protein A transmembrane domain. Nature Structural Biology. 1998;5(11):1013–1017. doi: 10.1038/2983. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara E, Nestorovich EM, Bezrukov SM, Nikaido H. Pseudomonas aeruginosa porin OprF exists in two different conformations. Journal of Biological Chemistry. 2006;281(24):16220–16229. doi: 10.1074/jbc.M600680200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin NL, Beveridge TJ. Gentamicin Interaction with Pseudomonas aeruginosa Cell-Envelope. Antimicrobial Agents and Chemotherapy. 1986;29(6):1079–1087. doi: 10.1128/aac.29.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh AG, Matewish MJ, Burrows LL, Monteiro MA, Perry MB, Lam JS. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Molecular Microbiology. 2000;35(4):718–727. doi: 10.1046/j.1365-2958.2000.01741.x. [DOI] [PubMed] [Google Scholar]
- 14.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda N, Sakagawa E, Ohya S. Outer-Membrane Proteins Responsible for Multiple-Drug Resistance in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 1995;39(3):645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baaden M, Meier C, Sansom MSP. A molecular dynamics investigation of mono and dimeric states of the outer membrane enzyme OMPLA. Journal of Molecular Biology. 2003;331(1):177–189. doi: 10.1016/s0022-2836(03)00718-6. [DOI] [PubMed] [Google Scholar]
- 17.Kastowsky M, Gutberlet T, Bradaczek H. Molecular Modeling of the 3-Dimensional Structure and Conformational Flexibility of Bacterial Lipopolysaccharide. Journal of Bacteriology. 1992;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obst S, Kastowsky M, Bradaczek H. Molecular dynamics simulations of six different fully hydrated monomeric conformers of Escherichia coli re-lipopolysaccharide in the presence and absence of Ca2+ Biophysical Journal. 1997;72(3):1031–1046. doi: 10.1016/S0006-3495(97)78755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotra LP, Golemi D, Amro NA, Liu GY, Mobashery S. Dynamics of the lipopolysaccharide assembly on the surface of Escherichia coli. Journal of the American Chemical Society. 121(38):1999. 8707–8711. [Google Scholar]
- 20.Straatsma TP, McCammon JA. Load balancing of molecular dynamics simulation with NWChem. IBM Systems Journal. 2001;40(2):328–341. [Google Scholar]
- 21.Straatsma TP, Philippopoulos M, McCammon JA. NWChem: Exploiting parallelism in molecular simulations. Computer Physics Communications. 2000;128(1-2):377–385. [Google Scholar]
- 22.Lins RD, Straatsma TP. Computer simulation of the rough lipopolysaccharide membrane of Pseudomonas aeruginosa. Biophysical Journal. 2001;81(2):1037–1046. doi: 10.1016/S0006-3495(01)75761-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lins RD, Vorpagel ER, Guglielmi M, Straatsma TP. Characterization of Uranyl Uptake by the Rough Lipopolysaccharide Membrane of Pseudomonas aeruginosa. Biomacromolecules. 2008;9(1):29–35. doi: 10.1021/bm700609r. [DOI] [PubMed] [Google Scholar]
- 24.Shroll RM, Straatsma TP. Molecular structure of the outer bacterial membrane of Pseudomonas aeruginosa via classical simulation. Biopolymers. 2002;65(6):395–407. doi: 10.1002/bip.10279. [DOI] [PubMed] [Google Scholar]
- 25.Shroll RM, Straatsma TP. Molecular basis for microbial adhesion to geochemical surfaces: Computer simulation of Pseudomonas aeruginosa adhesion to goethite. Biophysical Journal. 2003;84(3):1765–1772. doi: 10.1016/S0006-3495(03)74984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nestorovich EM, Sugawara E, Nikaido H, Bezrukov SM. Pseudomonas aeruginosa porin OprF - Properties of the channel. Journal of Biological Chemistry. 2006;281(24):16230–16237. doi: 10.1074/jbc.M600650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara E, Nikaido H. OmpA Protein of Escherichia coli Outer-Membrane Occurs in Open and Closed Channel Forms. Journal of Biological Chemistry. 1994;269(27):17981–17987. [PubMed] [Google Scholar]
- 28.Sugawara E, Steiert M, Rouhani S, Nikaido H. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. Journal of Bacteriology. 1996;178(20):6067–6069. doi: 10.1128/jb.178.20.6067-6069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawling EG, Brinkman FSL, Hancock REW. Roles of the carboxy-terminal half of Pseudomonas aeruginosa major outer membrane protein OprF in cell shape, growth in los-osmolarity medium, and peptidoglycan association. Journal of Bacteriology. 1998;180(14):3556–3562. doi: 10.1128/jb.180.14.3556-3562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodruff WA, Hancock REW. Pseudomonas aeruginosa Outer-Membrane Protein-F - Structural Role and Relationship to the Escherichia coli OmpA Protein. Journal of Bacteriology. 1989;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalid S, Bond PJ, Deol SS, Sansom MSP. Modeling and simulations of a bacterial outer membrane protein: OprF from Pseudomonas aeruginosa. Proteins-Structure Function and Bioinformatics. 2006;63(1):6–15. doi: 10.1002/prot.20845. [DOI] [PubMed] [Google Scholar]
- 32.Soares TA, Straatsma TP. Towards Simulations of Outer Membrane Proteins in Lipopolysaccharide Membranes. AIP Conference Proceedings; 2007. pp. 1375–1378. [Google Scholar]
- 33.Soares TA, Straatsma TP. Assessment of the Convergence of Molecular Dynamics Simulations of Lipopolysaccharide Membranes. Molecular Simulation. 2008 accepted for publication. [Google Scholar]
- 34.Straatsma TP. Data Intensive Analysis of Biomolecular Simulations. AIP Conference Proceedings; 2007. pp. 1379–1382. [Google Scholar]
- 35.Woods RJ, Dwek RA, Edge CJ, Fraserreid B. Molecular Mechanical and Molecular Dynamical Simulations of Glycoproteins and Oligosaccharides. 1. GLYCAM-93 Parameter Development. Journal of Physical Chemistry. 1995;99(11):3832–3846. [Google Scholar]
- 36.Berendsen HJC, Grigera JR, Straatsma TP. The Missing Term in Effective Pair Potentials. Journal of Physical Chemistry. 1987;91(24):6269–6271. [Google Scholar]
- 37.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-Dynamics with Coupling to an External Bath. Journal of Chemical Physics. 1984;81(8):3684–3690. [Google Scholar]
- 38.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of Cartesian equations of motion of a system with constraints - Molecular dynamics of n-alkanes. Journal of Computational Physics. 1977;23(3):327–341. [Google Scholar]
- 39.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A Smooth Particle Mesh Ewald Method. Journal of Chemical Physics. 1995;103(19):8577–8593. [Google Scholar]
- 40.Kabsch W, Sander C. Dictionary of Protein Secondary Structure - Pattern-Recognition of Hydrogen-Bonded and Geometrical Features. Biopolymers. 1983;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 41.Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MSP. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. Journal of Molecular Graphics & Modelling. 1996;14(6):354–360. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 42.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 43.El Hamel C, Freulet MA, Jaquinod M, De E, Molle G, Orange N. Involvement of the C-terminal part of Pseudomonas fluorescens OprF in the modulation of its pore-forming properties. Biochimica Et Biophysica Acta-Biomembranes. 2000;1509(1-2):237–244. doi: 10.1016/s0005-2736(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 44.Freulet-Marriere MA, El Hamel C, Chevalier S, De E, Molle G, Orange N. Evidence for association of lipopolysaccharide with Pseudomonas fluorescens strain MF0 porin OprF. Research in Microbiology. 2000;151(10):873–876. doi: 10.1016/s0923-2508(00)01154-2. [DOI] [PubMed] [Google Scholar]
- 45.Saint N, El Hamel C, De E, Molle G. Ion channel formation by N-terminal domain: a common feature of OprFs of Pseudomonas and OmpA of Escherichia coli. Fems Microbiology Letters. 2000;190(2):261–265. doi: 10.1111/j.1574-6968.2000.tb09296.x. [DOI] [PubMed] [Google Scholar]
- 46.Seydel U, Eberstein W, Schroder G, Brandenburg K. Electrostatic Potential Barrier in Asymmetric Planar Lipopolysaccharide Phospholipid-Bilayers Probed with the Valinomycin-K+ Complex. Zeitschrift Fur Naturforschung C-a Journal of Biosciences. 1992;47(9-10):757–761. doi: 10.1515/znc-1992-9-1020. [DOI] [PubMed] [Google Scholar]
- 47.Wiese A, Schroder G, Brandenburg K, Hirsch A, Welte W, Seydel U. IInfluence of the Lipid Matrix on Incorporation and Function of LPS-Free Porin from Paracoccus denitrificans. Biochimica Et Biophysica Acta-Biomembranes. 1994;1190(2):231–242. doi: 10.1016/0005-2736(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 48.Soares TA, Straatsma TP, Lins RD. Influence of the B-band O-antigen Chain in the Structure and electrostatics of the Lipopolysaccharide Membrane of Pseudomonas aeruginosa. Journal of the Brazilian Chemical Society. 2008;19(2):312–320. [Google Scholar]