Abstract
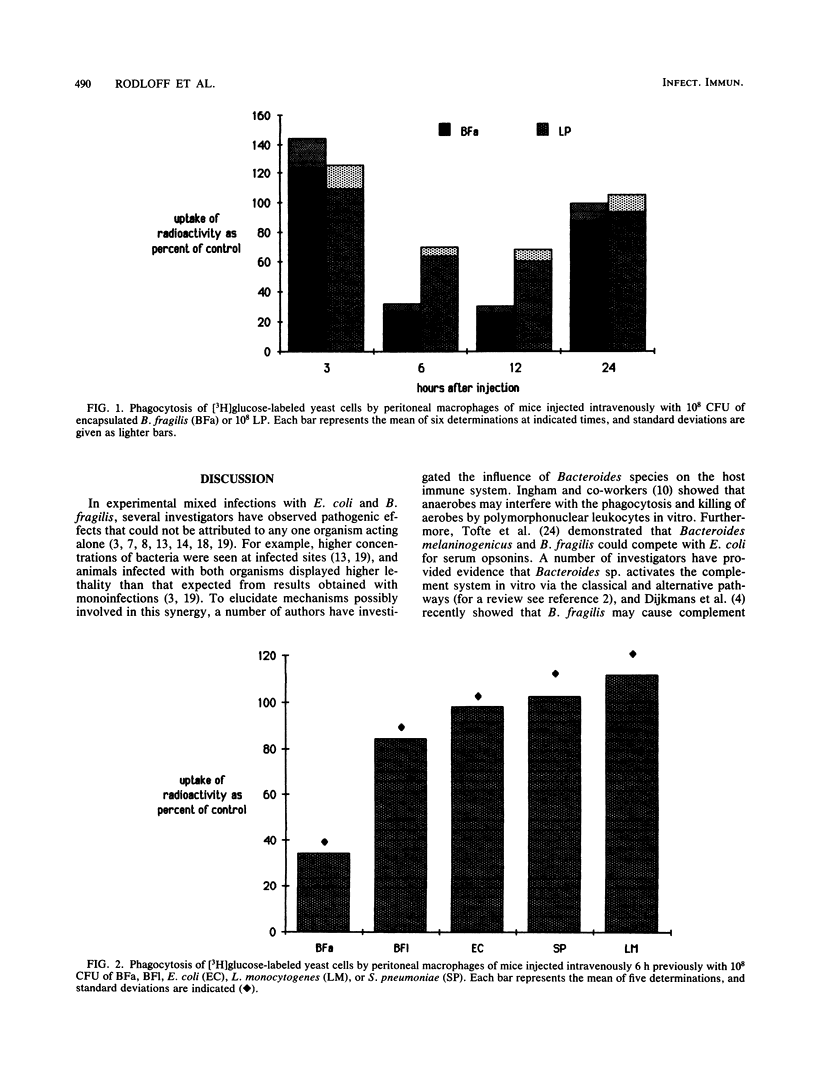
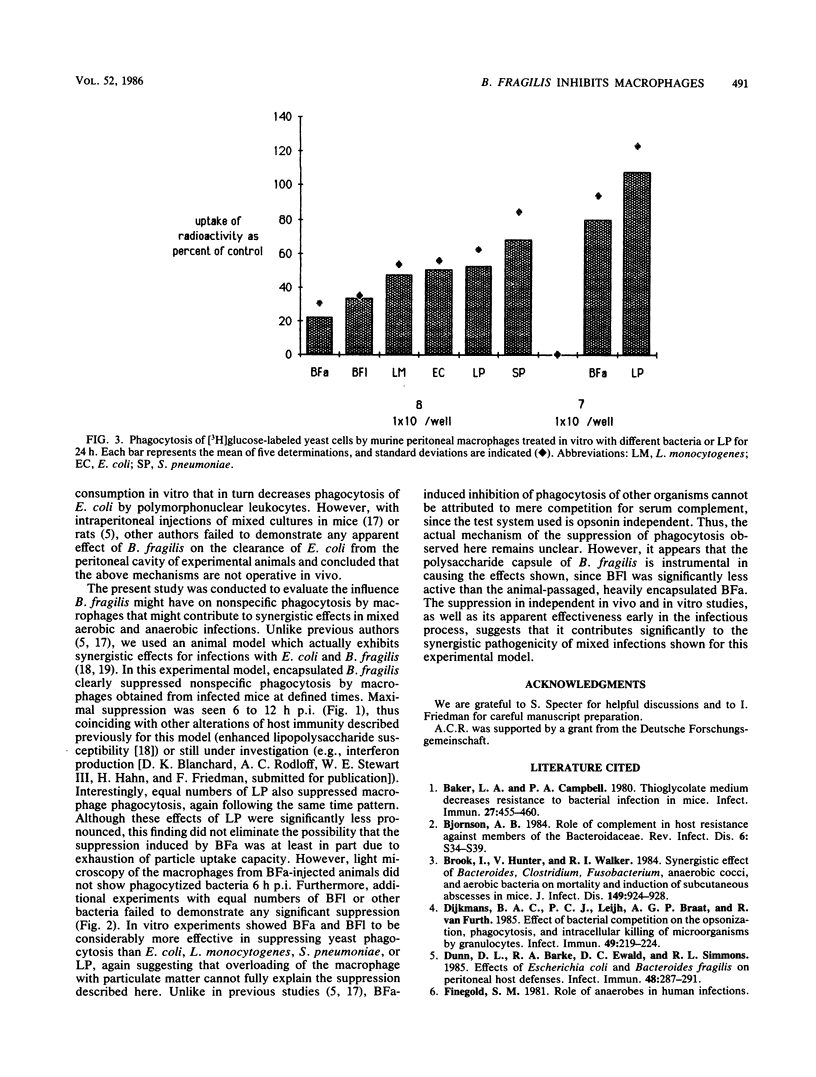
A number of investigators have provided experimental evidence for synergistic effects in mixed infections with Escherichia coli and Bacteroides fragilis. In vitro studies have suggested that competition for serum opsonins and diminished subsequent phagocytosis by polymorphonuclear leukocytes might explain these effects. In the present study we evaluated the effect of B. fragilis on macrophage phagocytosis. It was shown that peritoneal macrophages from mice injected intravenously 6 to 12 h earlier with 10(8) CFU of encapsulated B. fragilis were markedly suppressed in their phagocytic ability. Injections of laboratory-passaged, less-encapsulated B. fragilis, other bacteria, or latex particles were either not suppressive of macrophage phagocytosis or less effective. When peritoneal macrophages were treated in vitro for 24 h with the same challenge organisms prior to assessing their phagocytic capacity, encapsulated B. fragilis also proved significantly more suppressive than challenges with other organisms or latex particles. We conclude that suppression of macrophage phagocytosis by B. fragilis seems to be an important mechanism contributing to synergistic effects described for mixed aerobic and anaerobic infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. A., Campbell P. A. Thioglycolate medium decreases resistance to bacterial infection in mice. Infect Immun. 1980 Feb;27(2):455–460. doi: 10.1128/iai.27.2.455-460.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B. Role of complement in host resistance against members of the Bacteroidaceae. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S34–S39. doi: 10.1093/clinids/6.supplement_1.s34. [DOI] [PubMed] [Google Scholar]
- Brook I., Hunter V., Walker R. I. Synergistic effect of bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscesses in mice. J Infect Dis. 1984 Jun;149(6):924–928. doi: 10.1093/infdis/149.6.924. [DOI] [PubMed] [Google Scholar]
- Dijkmans B. A., Leijh P. C., Braat A. G., van Furth R. Effect of bacterial competition on the opsonization, phagocytosis, and intracellular killing of microorganisms by granulocytes. Infect Immun. 1985 Jul;49(1):219–224. doi: 10.1128/iai.49.1.219-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Ewald D. C., Simmons R. L. Effects of Escherichia coli and Bacteroides fragilis on peritoneal host defenses. Infect Immun. 1985 May;48(2):287–291. doi: 10.1128/iai.48.2.287-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Middleton R. L., Narang H. K., Codd A. A., Selkon J. B. Phagocytosis and killing of bacteria in aerobic and anaerobic conditions. J Med Microbiol. 1981 Nov;14(4):391–399. doi: 10.1099/00222615-14-4-391. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jones G. R., Gemmell C. G. Impairment by Bacteroides species of opsonisation and phagocytosis of enterobacteria. J Med Microbiol. 1982 Aug;15(3):351–361. doi: 10.1099/00222615-15-3-351. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Crabb J., Bartlett J. G. Protective efficacy of immunization with capsular antigen against experimental infection with Bacteroides fragilis. J Infect Dis. 1979 Nov;140(5):724–731. doi: 10.1093/infdis/140.5.724. [DOI] [PubMed] [Google Scholar]
- Kelly M. J. The quantitative and histological demonstration of pathogenic synergy between Escherichia coli and Bacteroides fragilis in guinea-pig wounds. J Med Microbiol. 1978 Nov;11(4):513–523. doi: 10.1099/00222615-11-4-513. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Markham R. B., Zaleznik D. F., Cisneros R. L., Kasper D. L. Evidence for T cell-dependent immunity to Bacteroides fragilis in an intraabdominal abscess model. J Clin Invest. 1982 Jan;69(1):9–16. doi: 10.1172/JCI110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov M., Finlay-Jones J. J., McDonald P. J. Effect of Bacteroides fragilis on the peritoneal clearance of Escherichia coli in mice. Infect Immun. 1981 Apr;32(1):398–399. doi: 10.1128/iai.32.1.398-399.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodloff A. C., Hahn H. Synergistic lethality in experimental infections with Escherichia coli and Bacteroides fragilis. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Oct;258(1):112–119. doi: 10.1016/s0176-6724(84)80015-2. [DOI] [PubMed] [Google Scholar]
- Rodloff A. C., Rodloff S., Fischer B., Hahn H. Intravenous injection of mice with bacteroides fragilis and Escherichia coli: role of Thioglycollate medium in the infectious process. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):405–412. [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. L., Klempner M. S., Kasper D. L., Gorbach S. L. Alterations in opsonophagocytic killing by neutrophils of Bacteroides fragilis associated with animal and laboratory passage: effect of capsular polysaccharide. J Infect Dis. 1982 Jan;145(1):72–77. doi: 10.1093/infdis/145.1.72. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L. Dissociation of bactericidal activity from other functions of activated macrophages in exudates induced by thioglycolate medium. Infect Immun. 1981 Oct;34(1):274–284. doi: 10.1128/iai.34.1.274-284.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Schmeling D., Bracke J., Kim Y., Quie P. G. Opsonization of four Bacteroides species: role of the classical complement pathway and immunoglobulin. Infect Immun. 1980 Mar;27(3):784–792. doi: 10.1128/iai.27.3.784-792.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


