Abstract
Reducing tidal volume as a part of a protective ventilation strategy may result in hypercapnia. In this study, we focused on the influence of hypercapnia on endothelial- neutrophil responses in models of inflammatory-stimulated human pulmonary microvascular endothelial cells (HMVEC) and in an animal model of lipopolysaccharide (LPS)-induced acute lung injury. Neutrophil adhesion and adhesion molecule(s) expression and nuclear factor-κB (NF-κB) were analyzed in TNF-α and LPS-treated HMVEC exposed to either eucapnia or hypercapnia. In the in vivo limb, bronchoalveolar lavage fluid cell counts and differentials, adhesion molecule and chemokine expression were assessed in LPS-treated rabbits ventilated with either low tidal volume ventilation and eucapnia or hypercapnia. In both the in vitro and in vivo models, hypercapnia significantly increased neutrophil adhesion and adhesion molecule expression compared to eucapnia. Acitvity of NF-κB was significantly enhanced by hypercapnia in the in vitro experiments. IL-8 expression was greatest both in vitro and in vivo under conditions of hypercapnia and concomitant inflammation. CD11a expression was greatest in isolated human neutrophils exposed to hypercapnia + LPS. Our results demonstrate that endothelial-neutrophil responses per measurement of fundamental molecules of adhesion are significantly increased during hypercapnia and that hypercapnia mimics conditions of eucapnia + inflammation.
Keywords: hypercapnia, neutrophil, endothelium, adhesion molecule, lung injury
1. Introduction
Despite significant advances in understanding the pathophysiology of inflammatory-mediated lung injury, most notably the acute respiratory distress syndrome (ARDS), mortality remains significant [1]. Traditional approaches of mechanical ventilation contribute to lung injury via up-regulation of local pulmonary inflammatory responses and worsening alveolar-capillary permeability [2–6]. To prevent such ventilator-induced injury (VILI), mechanical ventilation with reduced tidal volumes has been introduced as an effective strategy for management of patients suffering from ALI/ARDS [7]. Net reductions in minute ventilation gives rise to a significant increase in carbon dioxide tension (PCO2), sometimes referred to as “permissive hypercapnia”. The underlying rationale for permissive hypercapnia is to reduce the net mechanical stress imposed on the lung at a time when alveolar-capillary integrity is breeched and more vulnerable to injury. While the concept of “permissive hypercapnia” has been embraced, comparatively little information is known about the direct effects of carbon dioxide (CO2) on lung utilizing this specific strategy, especially as it relates to inflammatory cell signaling and injury.
We have previously reported that hypercapnic conditions, with concomitant inflammation, stimulates inducible nitric oxide synthase (NOS II) gene expression, increase lung cell protein tyrosine nitration and accelerate lung cell apoptosis and impaired epithelial barrier function [8]. More recently, we demonstrated that decreasing minute ventilation via reducing respiratory rates and tidal volumes lead to in an increase in lung injury [9]. Interestingly, hypercapnia in the presence of intratracheal instilled LPS led to a significant increase in the bronchoalveolar lavage (BAL) neutrophils and myeloperoxidase (MPO), indicating that hypercapnia may mediate injury by stimulating pulmonary endothelial and neutrophil responses.
The present study serves to build on the observation that hypercapnia increases endothelial and neutrophil responses culminating in greater neutrophil adhesion. Indeed, in both in vitro and in vivo models of pulmonary inflammation, hypercapnia increased neutrophil adhesion via increased expression of adhesion molecules (ICAM-1, VCAM-1, E-selectin) and chemokines. In the in vitro limb, the transcriptional regulatory protein, NF-κB demonstrated significantly increased expression and isolated human neutrophil-derived CD11a, receptor epitope was found to be significantly activated. In the in vivo limb, BAL fluid cell counts and histological specimens retrieved from animals mechanically ventilated under conditions of hypercapnia and hypercapnia + LPS had significantly higher cell counts with a greater number polymorphonuclear cells in the differential. From these observations, we conclude that hypercapnia increases endothelial-neutrophil responses in way that might be contributory to lung injury. Hypercapnia repetitively mimicked eucapnic + inflammatory stimulated conditions.
2. Materials and Methods
All experiments conformed to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee and Institutional Review Board at the University of Alabama at Birmingham, Birmingham, AL, USA.
2.1. Cell Culture
Pulmonary human microvascular endothelial cells (HMVEC) were purchased from Cambrex (Cambrex Bio Science Baltimore, Inc., Baltimore, MD, USA) as cryopreserved fourth-passage culture and used at passages 6–12. The cells were cultured in an endothelial cell basal medium-2 (EBM-2; Cambrex) supplemented with 5% fetal bovine serum, EGM-2MV SingleQuotes (Cambrex) at 37°C in a humidified incubator saturated with a gas mixture containing 21% oxygen (O2) and 5% CO2 in nitrogen (N2). The endothelial cells were grown on 75-cm2 tissue culture flasks (Corning, Corning, NY, USA), and then subcultured with 0.025% trypsin-0.05 millimolar (mM) ethylenediamine tetraacetic acid (EDTA; GIBCO BRL). Cell viability always exceeded 95% as determined by the trypan-blue exclusion test.
2.2. Exposure of HMVEC Monolayer to Hypercapnia
First, the EBM-2 in 75-cm2 culture flasks without HMVEC were equilibrated with either normocapnic gas (21% O2 and 5% CO2 in N2) or hypercapnic gas (21% O2 and 10% CO2 in N2) for 2 h at 37°C in a humidified multi-gas incubator. Subsequently, the equilibrated medium was quickly applied onto confluent HMVEC monolayers, which were continued to be exposed to either normocapnic or hypercapnic gas for up to 4–24 h. Simultaneously, LPS (E. coli O55:B5; Sigma, St. Louis, MO, USA) dissolved in Dulbecco's phosphate-buffered saline (DPBS) was administered to the medium at a final concentration of 1 ng/ml or 10 ng/ml, and tumor necrosis factor-α (TNF: Sigma) dissolved in DPBS was administered to the medium at a final concentration of 5 ng/ml or 10 ng/ml. Under normocapnic conditions, endothelial cell viability was >95% without TNF-α or LPS and 93 ± 2% after 24 h of exposure to TNF-α or LPS. The viability of endothelial cells in hypercapnic conditions at 24 h was 94 ± 2% without TNF-α or LPS and 91 ± 3% with TNF-α or LPS. Under phase-contrast microscopy, the endothelial monolayer was morphologically normal under each experimental condition. The pH and PCO2 values in EBM-2 before application to HMVEC were respectively, 7.36 ± 0.03 and 36.5 ± 0.5 mm Hg upon exposure to eucapnic gas. The medium PCO2 was not altered during incubation with HMVEC. Qualitatively the same tendency was observed with the hypercapnic gas; i.e., the pH and PCO2 in the medium without HMVEC were, respectively, 7.0 ± 0.01 and 63.5 ± 2.4 mm Hg with no changes observed in these parameters during incubation with HMVEC.
2.3. Neutrophil Adherence to an Endothelial Cell Monolayer
HMVEC in 48-well tissue culture plates (Corning) were exposed to either normocapnic or hypercapnic gas with or without TNF-α or LPS in a humidified multi-gas incubator for 4 h. Neutrophils (PMN) were isolated from healthy adult volunteers and separated on a discontinuous gradient of Histopaque 1,077 and 1,119 (Sigma). Purity and viability of PMN preparations were monitored by trypan blue exclusion and were > 95%. PMN were then labeled with 500 µl of cell Tracker Green (Molecular Probes) and after washing 5 × 106 cells/ml introduced into each HMVEC containing well. The plates were then incubated for 1 h at 37°C under either normocapnic or hypercapnic conditions in a humidified incubator. Non-adherent neutrophils were removed by gently washing the plates twice with pre-warmed DPBS, and then collected by centrifuging at 5000 g for 5 minutes (min). Both non-adherent and adherent neutrophils were then measured and % adhesion determined as described previously [10].
2.4. Expression of Adhesion Molecules (ICAM-1, VCAM-1, E-selectin and P-selectin) on HMVEC
Expression of adhesion molecules (ICAM-1, VCAM-1, E-selectin and P-selectin) on HMVEC was determined using ELISA. The endothelial cells were exposed to normocapnic or hypercapnic gas with or without TNF-α or LPS stimulation for 4 h. The cells were washed twice with warmed phosphate-buffered saline (PBS) and fixed using 1% paraformaldehyde. Subsequently, the endothelial cells were incubated with either anti-human ICAM-1 (CD54) monoclonal antibody (R & D Systems, Inc., Minneapolis, MN, USA), anti-human VCAM-1 (CD106) monoclonal antibody (BD Biosciences, San Jose, CA, USA), anti-human E-selectin (CD62E) monoclonal antibody (R & D Systems, Inc.) or anti-human P-selectin (CD62P) monoclonal antibody (R & D Systems, Inc.). The expression of the adhesion molecules were visualized with a horseradish peroxidase (HRP)-conjugated secondary antibody followed by addition of 3,3’,5,5’-Tetramethylbenzidine (TMB) peroxidase substrate. The acid stop solution was added after being incubated for 10 min at room temperature and the expression was evaluated by the reading at 450 nanometer (nm).
2.5. Transcription Factors Assay for NF-κB
A transcription factor assay kit (TransAM NF-κB p65, Active Motif, Inc., Carlsbad, CA, USA) was utilized to quantify NF-κB activation. Nuclear extract was prepared by using an Active Motif Nuclear Extract Kit. TransAM NF-κB Kits contain 96-well plate on which has been immobilized oligonucleotide containing the NF-κB consensus site (5′-GGACTTTCC-3′). The active form of NF-κB contained in nuclear extract specially binds to this oligonucleotide. The primary antibodies used to detect NF-κB recognized an epitope on p65 that is accessible only when NF-κB is activated and bound to its target DNA. An HRP-conjugated secondary antibody was utilized and provided a sensitive colorimetric readout that was quantified by spectrophotometry. Briefly, the experiment procedure was as followed: 20 µl (18 µg) nuclear extracts from HMVEC was added to the sample wells, Jurkat nuclear extract (TransAM kit) used as a positive control was added to positive control wells. Wells with lysis buffer but without nuclear extract were identified as blanks. 100 µl of diluted NF-κB antibody was added to the wells. The plate was incubated for 1 h at room temperature and the wells were washed with washing buffer. Subsequently, 100 µl diluted HRP conjugated secondary antibody was added to all wells and incubated for 1 h at room temperature. The wells were washed 4 times and developing solution added. Stop solution was then added to the wells when a color change of medium to dark blue was observed. Finally, absorbance was read on a spectrophotometer within 5 min at 450 nm. DNA-binding activity of NF-κB was examined at 4 h after initiation of TNF-α or LPS stimulation.
2.6. Animal Model of Mechanical Ventilation
New Zealand white rabbits weighing 2.5 to 3.0 kg were anesthetized with intravenous ketamine (10 mg/kg), administered via a marginal ear vein. Anesthesia was maintained with ketamine (10 mg/kg/h) and xylazine (3 mg/kg/h). Intermittent neuromuscular blockade was maintained with intravenous administration of pancuronium bromide (0.5 mg/kg/h). A 3.5 in internal diameter endotracheal tube was placed via a tracheostomy. Ventilation was initiated with a tidal volume (VT) of 7 ml/kg, positive end-expiratory pressure (PEEP) at 3 cm H2O, with a respiratory rate to maintain a PaCO2 between 35–45 mm Hg (eucapnia), or 55–65 mm Hg (hypercapnia), and FiO2 = 0.4 for 4 h. Mean arterial pressure (mm Hg), heart rate (beats per minute), and esophageal temperature (°C) were measured throughout the study protocol and recorded at 30 min intervals. In one group of rabbits, lung injury was induced by a single intratracheal instillation of LPS (20 mg/kg; E. coli, serotype 055:B5; Sigma Chemical Corporation, St. Louis, MO, USA) administered over 20 min.
2.7. Expression of Adhesion Molecules (ICAM-1 and VCAM-1 ) in Lung Homogenates
A 100 mg section of the upper right lung lobe was homogenized in Hank’s Balanced Salt Solution (HBSS). Total protein concentration was measured using the bicinchoninic protein assay. Adhesion molecules expression was measured using ELISA. Each well of a 96-well plate was coated with lung homogenate. One mg/ml of protein from homogenized lungs was added into each well. Subsequently, these wells were incubated with either anti-rabbit ICAM-1 (CD54) monoclonal antibody or anti-rabbit VCAM-1 (CD106) monoclonal antibody. Antibodies were a kind gift from Dr. Myron I. Cybulsky, University of Toronto, Toronto, Ontario, Canada. The expression of the adhesion molecules were visualized with a HRP-conjugated secondary antibody followed by addition of TMB peroxidase substrate. The acid stop solution was added after incubated for 10 min at room temperature, and the expression was evaluated by the reading at 450 nm.
2.8. Immunohistology of Lung
Paraffin-embedded lung tissue sections 5 µm in thickness were stained for interleukin-8 (IL-8) [11]. In preparation for immunohistochemistry (IHC), sections were de-waxed and rehydrated through graded alcohols to a final wash in Tris buffered saline (TBS). Sections were treated with 0.3% hydrogen peroxide for 10 min to block endogenous peroxidase activity and washed with TBS containing 0.1% Triton X-100 (TBST) and TBS. Non-specific protein binding was blocked with 5% normal donkey serum for 2 h at room temperature. Sections were incubated with a goat polyclonal IL-8 antibody (1:40 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. Control sections were treated in parallel but incubated with normal goat serum (as a negative control) instead of the primary antibody. All sections were incubated in a moist chamber. Sections were then incubated with biotin-conjugated donkey anti-goat IgG (1:200 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min. After 30 min of incubation in an avidin-biotin complex, the reaction product was visualized by 3, 3’-diaminobenzidine (DAB; DAKO Corporation, Carpinteria, CA, USA). Finally, sections were dehydrated and cleared in ethanol and xylene, and mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA). Representative photomicrographs were acquired via a Nikon (Nikon, Tokyo 100-8331, Japan) Diaphot microscope with brightfield optics and a Nikon Coolpix 950 digital color camera. Stained sections were examined using the image analysis software, IPLab Spectrum (Scanalytics, Inc., Fairfax, VA, USA).
2.9. BALF from Mechanically Ventilated Rabbits
BALF was obtained by bronchoalveolar lavage of the left lung with 5 aliquots of 30 ml 0.9% NaCl. Cells in the BAL fluid were pelleted (800 × g, 10 min, 4°C), washed in ammonium chloride (ACK) lysing buffer to lyse red blood cells, and resuspended in Dulbecco’s Modification of Eagle’s medium (DMEM). Cell numbers were counted with a hemocytometer, and cell differentiation was measured on Cytospin preparations by using Diff-Quik staining. Total protein concentration in the BAL was measured using the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
2.10. Western Blot Analysis of E-selectin Expression
The rabbit lung homogenate of the upper right lung lobe was used for the assay of the protein expression of E-selectin. Three volumes of phosphate-buffered saline (PBS) were added to the tissues and homogenized. The samples were loaded on 10 % SDS-polyacrylamide mini-gels. Equal amounts of protein were loaded for each treatment. After electrophoresis, proteins were electrically transferred to nitrocellulose membranes. The membranes were incubated in 5% milk TBST at 4°C overnight and subsequently incubated with the primary antibodies at room temperature. The primary antibodies were polyclonal goat anti-human E-selectin antibody (R & D Systems, Inc. 1:1000 dilutions). After being washed with TBST, the membranes were incubated with secondary antibodies (HRP-conjugated donkey anti-goat IgG, Santa Cruz Biotechnology, Inc.) at room temperature for 50 min. The immunoblots were visualized using enhanced chemiluminescence detection reagents. The absorbance of the band was measured using Kodak ID Imagine software (Kodak, Rochester, NY, USA). Data were evaluated as % of absorbance levels of the sham-operated group. As a loading control, beta-actin was measured and the data expressed as a ratio (data not shown).
2.11. Lung Homogenate for IL-8
Concentrations of IL-8 in the lung homogenate were measured using ELISA. Total protein concentration of lung homogenate was measured using the BCA protein assay. Each well of a 96-well plate was coated with 150 µl diluted lung homogenate fluid, which is 0.5 mg/ml. Blocking buffer (PBS containing 1% BSA and 0.02% azide) was added to block nonspecific protein binding. These wells were incubated with goat anti-human IL-8 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The concentrations of IL-8 were visualized with a horseradish peroxidase-conjugated secondary antibody followed by the addition of 3,3′,5,5′-tetramethylbenzidine peroxidase substrate. The acid stop solution was added after incubated for 15 min at room temperature, and the expression was evaluated by the reading at 450 nm.
2.12. Culture Media ELISA of IL-8
The human IL-8 sandwich ELISA kit (Cell Sciences, Canton, MA) was used for the in vitro quantitative determination of interleukin-8 (IL-8) in HMVEC culture medium. A monoclonal antibody specific for IL-8 has been coated onto the wells of the micro-titer strips provided in this kit. First, 100 µl of sample, diluted standard and control were added into the plate. Then, 50 µl of diluted biotinylated detection antibody were added to all wells. After incubate for 1 hour at room temperature, the plate was washed and incubated with 100 µl of HRP-Streptavidin conjugate for 30 min. Finally, TMB was added and let the color develop for 10 min. The absorbance was read at 450 nm immediately after the addition of sulfuric acid.
2.13. Flow Cytometry
Human neutrophils were isolated from blood with cold histopaque (Sigma), followed by aliquoting 1 × 106 cells per well into two 48 wells plates. The Krebs buffer pre-balanced with 5% or 10% CO2 were added in each plate as control, and LPS or FMLP solved in 5% or 10% CO2 pre-balanced Krebs buffer were added into different wells in each plate. The two plates were incubated in 5% CO2 balanced and 10% CO2 balanced cell culture incubator for 30 min separately. Then cells were transferred to FACS tubes, pallet and resuspended in FACS buffer (PBS containing 1% BSA and 0.1% NaN3). The cells were initially incubated in FACS buffer containing 10% human Ab serum (ICN Biomedicals) for 20 min at 4°C. Cells were then washed two times with cold PBS and incubated with conjugated or anti-human CD11a activation epitope unconjugated Abs (1 mg/ml, Abcam, Cambridge, MA) for 30 min, and then incubated with fluorochrome-conjugated secondary Alexa fluo 488 goat anti-mouse Ab (Invitrogen/Molecular Probes) for an additional 30 min. Cells were washed three times with PBS before each step of immunostaining. Cells were then fixed using 500 µl of 1% paraformaldehyde in PBS and analyzed in a FACScan cytometer (BD Biosciences). Single-color FACS analysis was performed by gating on the typical forward and side light scatter characteristics of analyzed cells. Nonspecific staining was determined for each Ab using isotype controls. Samples were considered negative if the percentage of positive cells was 2% or less. Percentage of cells that were Ab positive was calculated by comparison with the corresponding isotype control. A total of at least 5,000 events were scanned for each experimental condition. Flow cytometry data were analyzed with CellQuest software.
2.14. Statistical Analysis
The data are presented as means ± SEM. Data were analyzed by one-way ANOVA followed by Tukey-Kramer’s multiple group comparison of the means for parametric data and by the Kruskal-Wallis ANOVA and Dunn’s multiple group comparison of the means for nonparametric data. A P - value of ≤ 0.05 was considered statistically significant.
3. Results
3.1. Neutrophil Adherence to an Endothelial Cell Monolayer
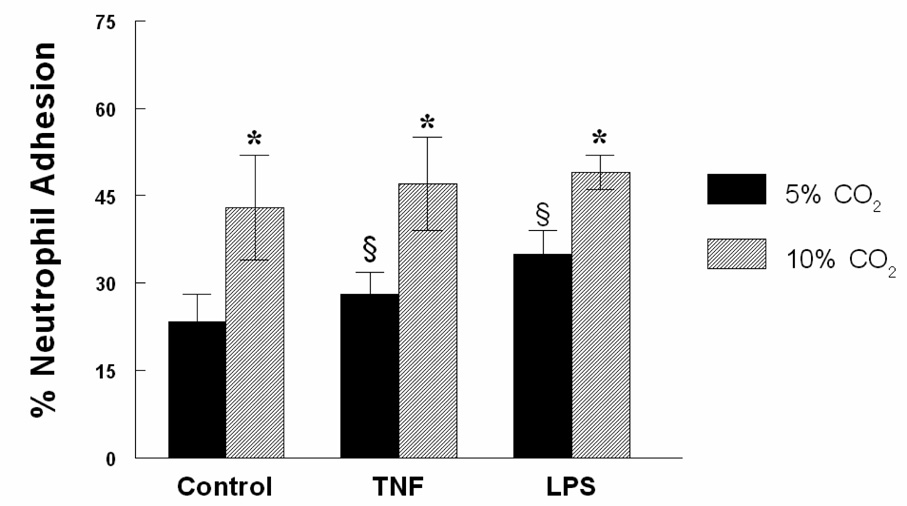
Human neutrophil adherence to a cultured HMVEC monolayer was measured in response to control treatment, TNF-α or LPS exposure and in the presence of either eucapnia or hypercapnia. Hypercapnia significantly increased neutrophil adhesion in HMVEC incubated for 4 h compared to eucapnia controls (43 ± 9 % vs. 23.4 ± 4.7 %, P < 0.05) (Figure 1). Hypercapnia-exposed cells treated with TNF-α and LPS resulted in increased neutrophil adhesion compared to cells compared to their eucapnic counterparts. All hypercapnia groups demonstrated significant increases in adhesion when compared to their respective eucapnic group. Cells exposed to hypercapnia alone displayed essentially equivalent degrees of adhesion when compared to both TNF-α and LPS-treated groups.
Figure 1. Hypercapnia stimulates neutrophil adhesion to HMVEC.
HMVEC were exposed to TNF-α or LPS (4 h) under eucapnic or hypercapnic conditions and then neutrophil adhesion assessed. Data are mean values ± SEM , P < 0.05. (n=6 per condition);* Significant difference compared with eucapnia alone (P < 0.05). § Significant difference from the eucapnia alone (P< 0.05).
3.2. Expression of Adhesion Molecules on Endothelial cells
Expression of VCAM-1, E-selectin and P-selectin significantly increased under conditions of hypercapnia compared to eucapnia in HMVEC (Table 1). Expression of ICAM-1, VCAM-1 and E-selectin were significantly increased by the addition of either LPS or TNF-α relative to control under both eucapnic and hypercapnic exposure. Within each treatment group, expression of these adhesion molecules was higher in the presence of hypercanpia compared to eucapnia (Table 1). Interestingly, whereas TNF-α or LPS had no effect on P-selectin expression relative to control, hypercapnia significantly increased its expression relative to eucapnia.
Table 1.
Effects of Hypercapnia on Adhesion Molecule Expression in Human Pulmonary Microvascular Cells Treated with TNF-α (10 ng/ml) or LPS (1 ng/ml) and Incubated for 24 hours.
| ICAM-1 | 5 % CO2 | 10 % CO2 | |
|---|---|---|---|
| Control | 0.74 ± .13 | 0.80 ± .11 | |
| TNF-α | 1.06 ± .19 | 1.58 ± .10a,b | |
| LPS | 1.07 ± .10c | 1.39 ± .17a,b | |
| VCAM-1 | |||
| Control | 0.23 ± .06 | 0.41 ± .02a | |
| TNF-α | 0.44 ± 05c | 0.71 ± .06a,b | |
| LPS | 0.42 ± .09c | 0.74 ± .19a, b | |
| E-selectin | |||
| Control | 0.30 ± .03 | 0.46 ± .03a | |
| TNF-α | 0.87 ± .08c | 1.15 ± .17a,b | |
| LPS | 0.86 ± .09c | 1.35 ± .09a,b | |
| P-selectin | |||
| Control | 0.27 ± .02 | 0.43 ± .04a | |
| TNF-α | 0.31 ± .03 | 0.46 ± .03a | |
| LPS | 0.27 ± .02 | 0.42 ± .01a |
ICAM -1 - intercellular adhesion molecule; VCAM-1 - vascular cell adhesion molecule; E- selectin – endothelial selectin; P-selectin – platelet selectin; TNF-α tumor necrosis factor alpha; LPS – lipopolysaccharide; Data shown as mean ± standard deviation. n = 6 per condition.
P-value (P < .05 is considered statistically significant between eucapnia and hypercapnia groups using a repeated measure analysis of variance
represents statistical significance between control and treated cells in the hypercapnia group
represents statistical significance between control and treated cells in the eucapnia group
3.3. Transcription Factors Assay for NF-κB
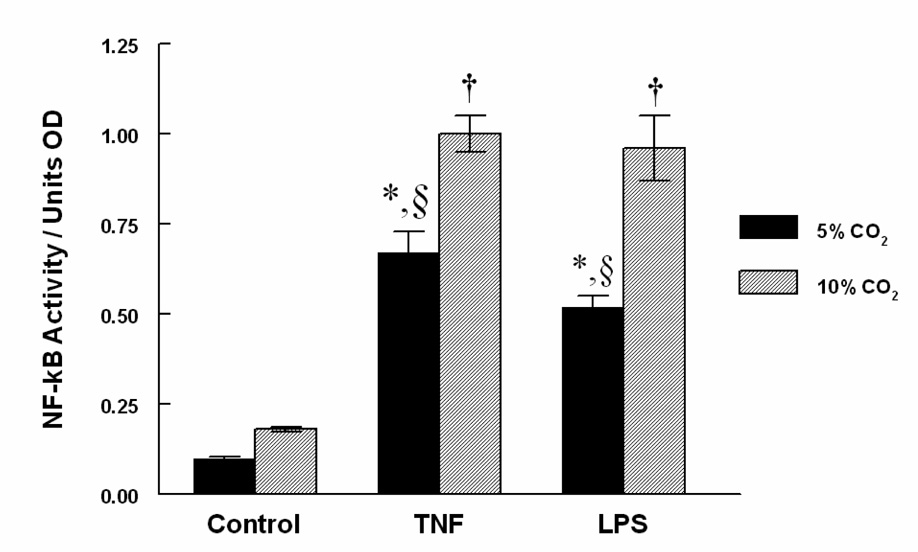
Activity of NF-κB, a transcriptional regulating protein that mediates up-regulation of adhesion molecule expression was measured. Figure 2 demonstrates that TNF-α or LPS exposed cells increased NF-κB relative to control and consistent with data in Table 1. These effects were significantly amplified by hypercapnia relative to eucapnia. No significant difference existed between eucapnia and hypercapnic groups that were untreated, although a trend towards increased activity in the hypercapnia alone group was observed.
Figure 2. Hypercapnia stimulates NF-κB activity in HMVEC.
HMVEC were exposed to TNF-α or LPS under eucapnic or hypercapnic conditions for 4h and NF-κB activation then measured. NF-κB was significantly amplified in HMVEC incubated under conditions of hypercapnia for 4 h. Data are mean values ± SEM. For each condition, n = 6. § Significant difference from the eucapnia group in the same treatment group (P < 0.05).* Significant difference compared with eucapnia alone (P < 0.05). † Significant difference from the hypercapnia alone (P < 0.05).
3.4. Expression of Adhesion Molecules in Lung Homogenates
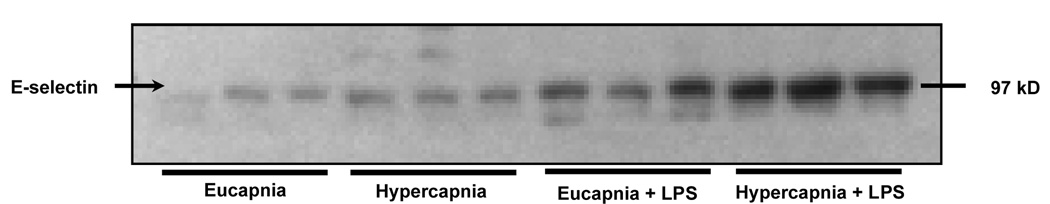
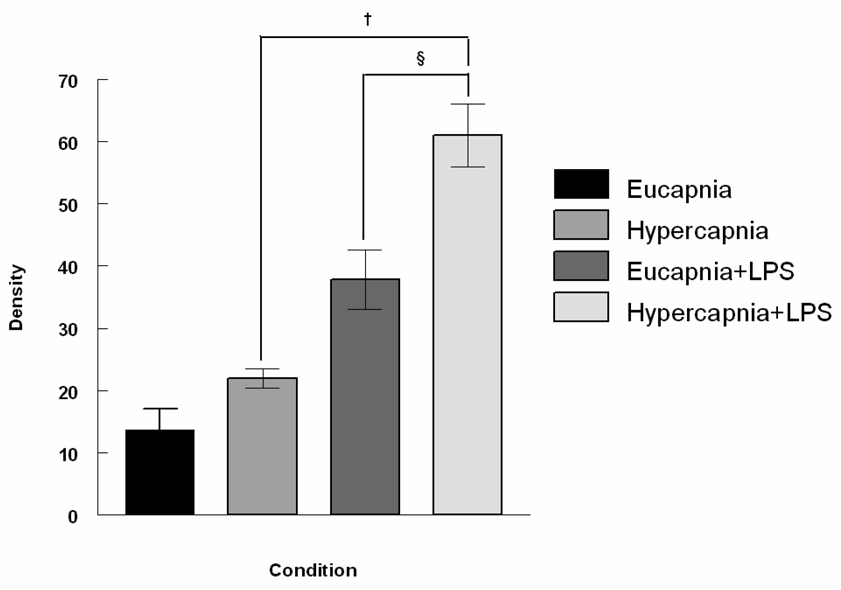
To evaluate the potential of hypercapnia to exacerbate inflammatory reactions in the lung, rabbits were mechanically ventilated for 4 hours and exposed to either normocapnia or hypercania under basal conditions or during inflammation stimulated by intratracheal instillation of LPS. Table 2 demonstrates that influence of the conditions on ICAM-1 and VCAM-1 expression in lung homogenates. Compared to eucapnia, hypercapnia significantly increased ICAM-1 and VCAM-1 expression in lung tissues of LPS-treated rabbits (1.50 ± 0.10 vs. 1.03 ± 0.20 and 0.61 ± 0.10 vs. 0.35 ± 0.05, respectively, P < 0.05). Hypercapnia alone significantly increased VCAM-1 expression in lung tissues without LPS administration. Lastly, in vivo E-selectin expression measured via Western blot analysis increased significantly in both groups treated with LPS, but was greatest in the LPS-treated hypercapnic groups (Figure 3A and 3B). Consistent with other findings, hypercapnia significantly increased E-selectin expression.
Table 2.
Effects of Hypercapnia on Adhesion Molecule Expression in LPS–Treated and Mechanically Ventilated Rabbit
| ICAM-1 | Eucapnia | Hypercapnia | |
|---|---|---|---|
| − LPS | 0.74 ± .13 | 0.80 ± .11 | |
| + LPS | 1.03 ± .20c | 1.50 ± .10a,b | |
| VCAM-1 | |||
| − LPS | 0.23 ± .06 | 0.41 ±.02a | |
| + LPS | 0.35 ± .05c | 0.61 ± .10a,b |
ICAM-1 - intercellular adhesion molecule; VCAM-1 - vascular cell adhesion molecule; LPS - lipopolysaccharide; Data shown as mean ± standard deviation. n = 3 per condition.
P < .05 is considered statistically significant between eucapnia and hypercapnia groups using a repeated measure analysis of variance
represents statistical significance between control and treated cells in the hypercapnia group
represents statistical significance between control and treated cells in the eucapnia group
Figure 3.
Figure 3A Effect of hypercapnia on the LPS-induced expression of E-selectin in the mechanically ventilated rabbit lung. Lung tissues from four groups: eucapnia, hypercapnia, eucapnia + LPS and hypercapnia + LPS were analyzed for E-selectin expression via Western Blot analysis. For each condition, n=3.
Figure 3B. Densitometry of Western Blot analysis of E-selectin expression in the mechanically ventilated rabbit lung. The expression of E-selectin was greatest in LPS- treated animals exposed to hypercapnia. Data are mean values of E-selectin/β-actin ± SEM. For each condition, n=3. § Significant difference between hypercapnic groups (P < 0.05). † Significant difference between LPS-treated groups (P < 0.05).
3.5. Neutrophil Infiltration in BAL Fluid
Exposure of LPS-treated animals to hypercapnia resulted in the greatest increase in lung cell counts and percentage of neutrophils measured in the BALF (Table 3). Specifically, pulmonary cells recovered by BALF were significantly greater in the LPS-treated animal receiving ventilation with hypercapnia compared with LPS-treated animals receiving ventilation with eucapnia. Similarly, recovered PMNs from the BALF were maximal in the LPS-treated animals receiving hypercapnia ventilation. In contrast, the % of macrophages were maximal in the BALF of animals untreated and ventilated under eucapnic conditions (98%) and were found to decrease under conditions of LPS treatment and hypercapnic ventilation (34.5%).
Table 3.
Cell Counts and Differentials Retrieved from BALF of LPS-Treated and Mechanically Ventilated Rabbits (n = 3 animals/condition)
| Condition | Cell Count (# cells) | Cell Differential (%) | |
|---|---|---|---|
| PMN | Macrophage | ||
| Eucapnia | 2.42 × 106 | 2 | 98 |
| Hypercapnia | 5.12 × 106a | 7.5a | 92.5a |
| Eucapnia + LPS | 6.0 × 106c | 31c | 69c |
| Hypercapnia + LPS | 1.1 × 107a,b | 65.5a,b | 34.5a,b |
LPS – lipopolysaccharide; PMN – polymononuclear cells.
P-value (P < .05 is considered statistically significant between eucapnia and hypercapnia groups using a repeated measure analysis of variance
represents statistical significance between control and treated cells in the hypercapnia group
represents statistical significance between control and treated cells in the eucapnia group
3.6. Lung Histology
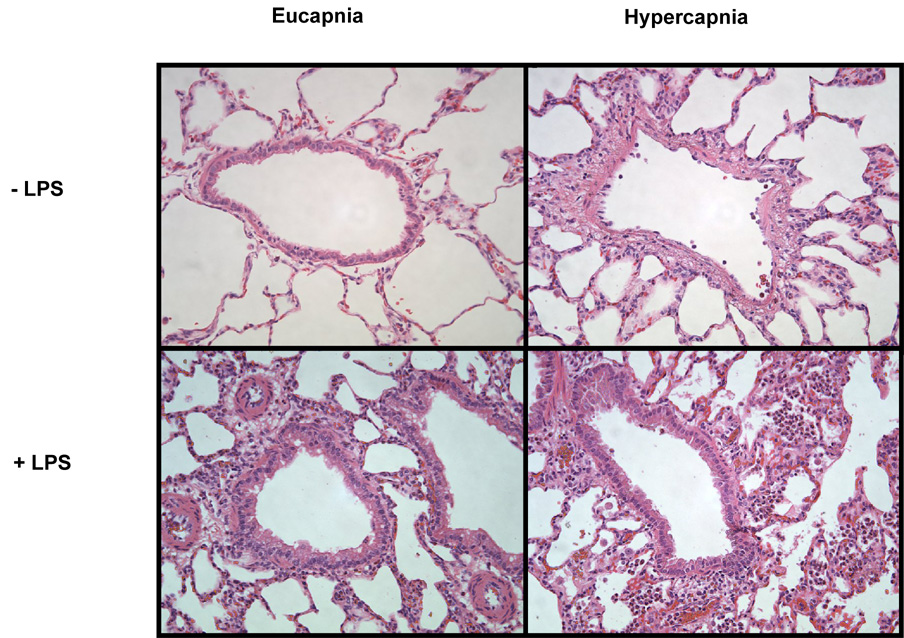
Significant differences were observed after evaluation of lung histology (Figure 4). Groups receiving ventilation with hypercapnia displayed extensive PMN cell and macrophage infiltration. The greatest degrees of histology change were observed in LPS-treated animals receiving hypercapnia ventilation compared with LPS-treated animals receiving eucapnia ventilation that only demonstrated moderate PMN infiltration and moderate alveolar transudation accompanying septical edema along with increased epithelial height. Animals receiving hypercapnia ventilation with concomitant LPS-induced inflammation exhibited significant greater alveolar transudation, septal edema, and extravasation of red blood cells. A visible histological change was also observed between animals receiving hypercapnia or eucapnia alone where mild PMN and macrophage infiltration accompanied by mild alveolar transudation occurred in the hypercapnic group. Animals receiving eucapnic ventilation alone were observed to have few PMN, few macrophages, and no alveolar or capillary damage visible.
Figure 4. Hypercapnia induces pulmonary inflammatory histological changes.
LPS-treated animals receiving hypercapnia ventilation were observed undergo the greatest degree of inflammatory change. A bronchoalveolar specimen (20x). Eucapnia - LPS – few PMNs, few macrophages, and no alveolar or capillary damage was visible; Hypercapnia - LPS – mild PMNs and macrophage infiltration accompanied by mild alveolar transudation occurred; Eucapnia + LPS − moderate PMNs infiltration, moderate alveolar transudation accompanying septial edema along with increased epithelial height; Hypercapnia + LPS − significant alveolar transudation, septal edema, and extravasation of red blood cells. Shown are representative sections for each condition tested. Original magnification, × 20.
3.7. IL-8 Expression in Lung
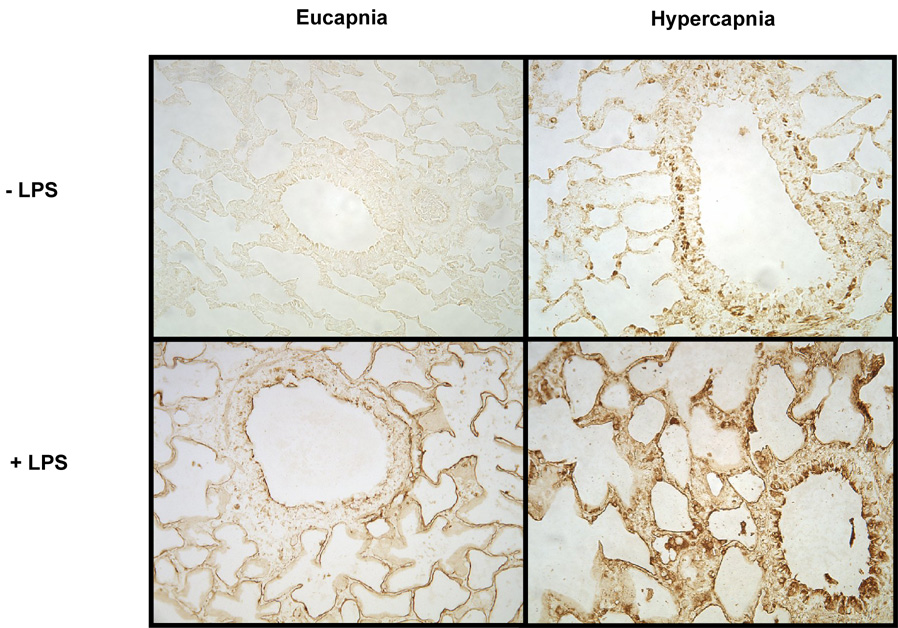
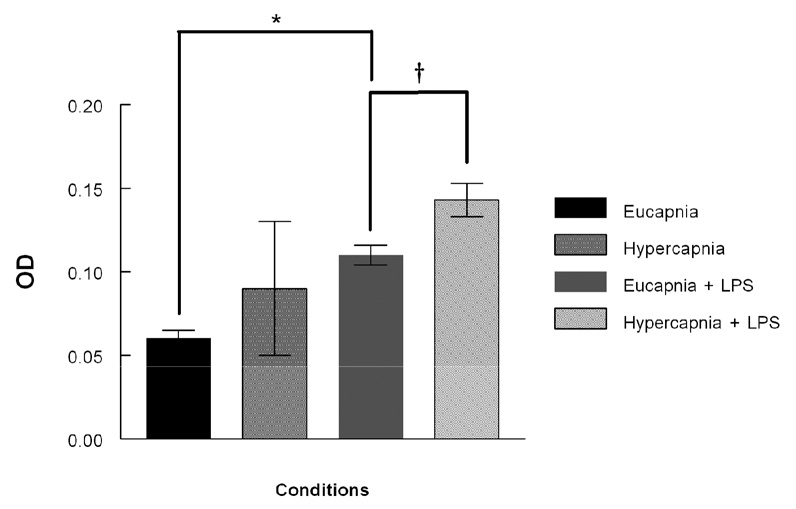
Lungs were analyzed by immunohistochemistry (IHC) for expression of the pro-inflammatory cytokine IL-8, a potent chemoattractant and activator of PMN and which is a general index of PMN influx. IL-8 expression was present to the greatest extent under hypercapnic + LPS conditions (Figure 5A). Hypercapnia alone resulted in enhanced staining lung tissue, but to a much lesser degree compared to eucapnia. ELISA analyses of lung homogenates from ventilated animals were utilized to supplement IL-8 IHC. These analyses demonstrated that animals ventilated with hypercapnia in the presence of LPS had the greatest increase in IL-8 expression compared to the animals ventilated with under eucapnic + LPS conditions (0.14 ± .01 versus 0.11 ± 0.006, respectively, P < 0.05) (Figure 5B).
Figure 5.
Figure 5A. Immunohistochemistry of IL-8 from bronchopulmonary segments of mechanically ventilated animals. Animals exposed to hypercapnia + LPS demonstrated the greatest increase in IL-8 staining. Shown are representative sections for each condition tested. Original magnification, × 20.
Figure 5B. Lung homogenate IL-8 expression in the mechanically ventilated rabbit measured with ELISA. The expression of IL-8 was greatest in LPS- treated animals exposed to hypercapnia. Data are mean values ± SEM. For each condition, n=3. † Significant difference between hypercapnia and eucapnia in LPS-treated groups (P < 0.05). * Significant difference between eucapnia alone and eucapnia plus LPS (P < 0.05). OD – optical density, ELISA – enzyme linked immunosorbent assay.
3.8. IL-8 Production in HMVEC Culture Media
The presence of IL-8 in HMVEC culture media was measured by specific ELISA. LPS-stimulated HMVEC exposed to hypercapnia demonstrated a significant increase in IL-8 than the LPS-treated cells with exposure to eucapnia, as measured by specific ELISA standardized to total protein (1.73 ± 0.3 versus 1.09 ± 0.1 ng/ml; P<0.01) (Figure 6).
Figure 6. The Effect of Hypercapnia on IL-8 production (ELISA) in HMVEC culture media.
The IL-8 production in LPS stimulated HMVEC exposed to hypercapnia was greater than the LPS treated cells with exposure to eucapnia. Data are mean values ± SEM. For each condition, n=3. § Significant difference from the eucapnia group in the same treatment group (P < 0.05).* Significant difference compared with eucapnia alone (P < 0.05). † Significant difference from the hypercapnia alone (P < 0.05). ELISA – enzyme linked immunosorbent assay.
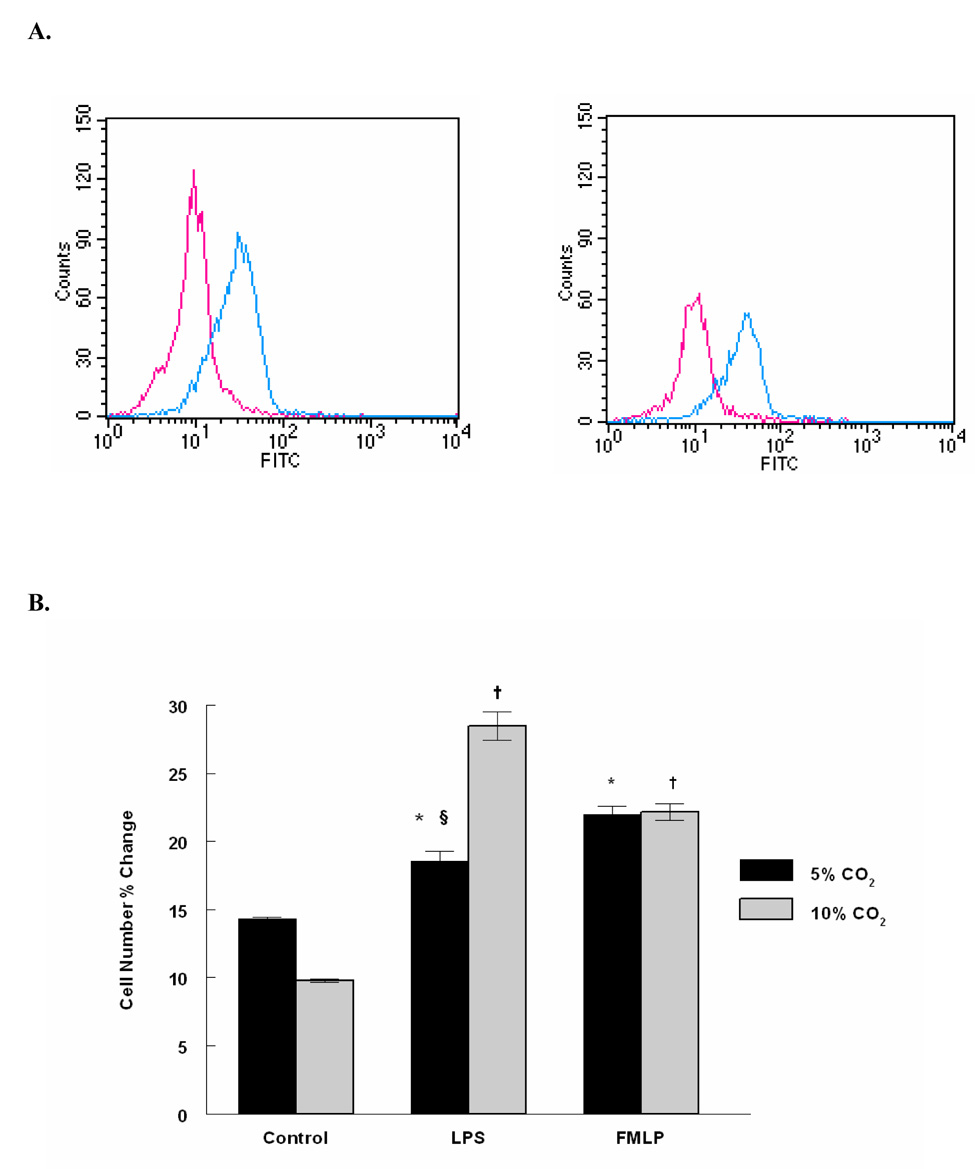
3.9. CD11a Activation Epitope Expression on Human Neutrophils (FACS Analysis)
To assess if hypercapnia may also stimulate neutrophil adhesivity, human neutrophils were exposed to eucapnia or hypercapnia in the presence and absence of LPS, Neutrophil activation was assessed by measuring expression of a CD11a activation eptiope by FACS. Figure 7 shows that LPS stimulates CD11a activation and that hypercapnia potentiates this response compared to eucapnia. Interestingly, FMLP (used as a positive control) dependent CD11a activation was unaffected by hypercapnia.
Figure 7. FACS Analysis of the Effect of Hypercapnia on the CD11a Activation Epitope Expression on LPS Treated Human Neutrophils.
Human neutrophils were subjected to single color immunostaining followed by flow cytometry analysis. A, PMNs exposed to hypercapnia + LPS represent 28.45 ± 1.0% of the positive population (right), PMNs exposed to eucapnia + LPS represent 18.55 ± 0.7% of the positive population (left). B, Gated positive percentage population comparison between different treatments. Results are calculated as the mean value of two experiments ± SD. A representative experiment is shown (n = 2). § Significant difference from the eucapnia group in the same treatment group (P < 0.05). * Significant difference compared with eucapnia alone (P < 0.05). † Significant difference from the hypercapnia alone (P < 0.05).
4. Discussion
Hypercapnia is commonly observed clinically as a consequence of a protective ventilation strategy. Our previous studies have demonstrated that ventilation with moderate hypercapnia (PaCO2 ~55–60mmHg) contribute to lung injury as evidenced by increased reactive nitrogen species, BALF neutrophils and myeloperoxidase. In the present study, there were significant increases in neutrophil counts in the BALF obtained from rabbits ventilated with reduced tidal volumes. Moreover, increased neutrophil accumulation in the lung was observed by histologic evaluation in hypercapnic versus eucapnic exposed animals. These findings corresponds with the results reported by Hanna et al, who observed that the BAL neutrophil count was significantly higher in the hypercapnia group than in eucapnia animals [12]. To further evaluate the effects of hypercapnia and specifically test the hypothesis that hypercapnia exacerbates inflammatory-mediated lung injury, we determined the effects of increased CO2 tensions (55–60 mm Hg) in modulating endothelial-neutrophil interactions in the lung in the absence and presence of inflammation. Using complimentary in vitro and in vivo approaches, our data demonstrate that hypercapnia heightens endothelial-neutrophil interactions under basal conditions and essentially mimics the conditions of eucapnia
Circulating neutrophils play a significant role in the early development of ALI. One of the proximal events in vascular inflammation is activation of endothelial cells resulting in increased expression of a variety of adhesion molecules [13–16] that then facilitate the attachment of peripheral blood leukocytes to the endothelial cell surface. Many studies have demonstrated that proinflammatory stimuli including TNF-α , LPS and interleukin-1beta (IL-1β) up-regulate endothelial adhesion molecules. To establish whether hypercapnia-dependent stimulation of neutrophil adhesion was modulated by the endothelial adhesion molecules, their expression was determined in defined in vitro studies utilizing TNF-α or LPS-activation of HMVEC at different CO2 tensions and an in vivo model established previously using LPS-administered rabbits with a fixed reduced tidal volume (7 ml/kg). Concomitant with increased PMN adhesion and infiltration into the lung, hypercapnia was observed to have a consistent effect of mimicking conditions of eucapnia and inflammation,,but in some cases when combined with both TNF-α and LPS, induced increases in ICAM-1, VCAM-1 and E-selectin expression in vitro and in vivo. These data suggest that hypercapnia promotes neutrophil adhesion in the acute inflammatory-mediated lung injury through enhancing the expression of endothelial adhesion molecules, including ICAM-1, VCAM-1 and E-selectin, but not P-selectin, which are consistent with intravital microscopy studies demonstrating an absence of neutrophils rolling in capillaries , the principal site of neutrophil emigration [17].
In the absence of activation with TNF-α or LPS, there was no significant effect of hypercapnia alone on ICAM-1 expression, however, there was a significant increase in the expression of VCAM-1, E-selectin and P-selectin, which was consistent with results in vivo demonstrating that VCAM-1 expression was significantly increased in the animals receiving a hypercapnic ventilation strategy compared to those receiving ventilation with eucapnia. The observation indicates that hypercapnia alone may increase the sensitivity of neutrophil-induced endothelial cell injury in the lung, but the magnitude of the hypercapnia effects maybe less, but no insignificant compared to in the presence of an inflammatory stimulus. The change of endothelial adhesion molecules observed in vitro experiments corresponds with the in vivo results of neutrophil counts and lung histology of inflammatory animal models.
The detailed mechanisms by which hypercapnia amplify endothelial activation remain unclear. Data presented herein suggest an important role for NF-κB, a critical transcription factor that regulates inflammatory gene expression [14,15,18–20] and which is extensively implicated in mediated ALI. In response to inflammatory stimuli, NF-κB translocates into the nucleus and activates transcription of multiple κB-dependent genes, including E-selectin, ICAM-1 and VCAM-1 [21]. In addition, expression of IL-8 is also thought to be mainly regulated by the NF-κB-related pathway [22]. Since hypercapnia increased adhesion molecules and IL-8 (discussed below), we tested its effects on modulating the DNA binding activity of NF-κB in TNF-α or LPS-treated HMVEC. In the presence of TNF-α or LPS, NF-κB DNA binding activity was significantly up-regulated by hypercapnia indicating that higher CO2 tensions increase early endothelial responses to inflammatory stimuli. It is possible that hypercapnia contributes to the cross-talk between inflammatory signaling allowing a greater activation of NF-κB dependent pathways. In contrast to our findings, other investigators reported hypercapnia acidosis attenuated LPS-induced NF-κB activation, leading to down-regulation of ICAM-1 and IL-8 [23]. The reasons underlying these contradictory findings are not clear, but may lie in the use of endothelial cells from different anatomic sites of the lung vasculature (arterial vs. microvascular), in experimental protocols (hypercapnia exposure times of 0– 3 versus 4 hrs) and in the varying concentrations of CO2 on which the lung cells are exposed [24].
IL-8 is a major mediator orchestrating migration of neutrophils from the peripheral circulation to the pulmonary compartment [25]. Moreover, exposure of neutrophils to IL-8 induces superoxide production and neutrophil degranulation [26,27]. IL-8 production from activated endothelial cells is crucial for neutrophil accumulation in inflamed tissue [28], and clinical studies have suggested the involvement of IL-8 in the inflammatory disorders including ARDS [23]. In our study, IL-8 expression via immunostaining in lung sections and ELISA from lung homogenates was increased in LPS-treated animals ventilated under conditions of hypercapnia. In the groups without LPS, hypercapnia alone enhanced IL-8 expression in the lung tissue, but to a much lesser degree. These in vivo results are consistent with in vitro observations that HMVEC exposed to hypercapnia demonstrated the greatest increase of IL-8 in the presence of LPS, which may suggest that a feed-forward mechanism where hypercapnia amplifies NF-κB signaling and IL-8 production which in turn further amplifies neutrophil accumulation and ALI. Several studies involving LPS induced IL-8 expression in pulmonary artery endothelial cells and neutrophils with exposure to the hypercapnia have been conducted [23,29]. Our results conform to the findings reported by Doyle et al, who observed that CO2 increased cytokine-induced neutrophil chemoattractant factor-1 secretion in LPS-activated rat alveolar macrophages [30]. However, the results of several other studies are in conflict with our observations. Although it is not easy to elucidate the precise rationale for the disparity in results between the present study and those mentioned above [23,29], one possibility is that cultured cell species in vitro used in previous studies differ from the HMVEC line and the lung tissues of the ventilated animal in vivo used in the present study. Contrary to IL-8, interleukin-10 (IL-10), also known as human cytokine synthesis inhibitory factor, is an anti-inflammatory cytokine. In order to elucidate it’s response under conditions of hypercapnic acidosis, we measured IL-10 using ELISA of the cell culture media (data not shown). Concentrations of IL-10 were extremely low (between 1 – 2 pg/ml) and demonstrated no significant differences in HMVEC culture media exposed to both eucapnia and hypercapnic conditions stimulated with or without TNF-α and LPS.
The general conclusions from our study are consistent with the observations of other studies in which LPS-induced neutrophil adhesion was reduced with hypercapnic acidosis in the presence of anti-ICAM-1 blocking antibody [23], suggesting that ICAM-1 plays an important role in hypercapnia-induced neutrophil adhesion. Pulmonary PMN migration can occur independently of CD18 [31], whether or not migration depends on CD18 varies with the intrapulmonary stimulus [32–35]. Endotoxin derived from Gram-negative bacteria including LPS elicits airway PMN that requires CD18 and cause the up-regulation of ICAM-1 within pulmonary vessels [36–38]. Previous studies have shown that hypercapnia promotes CD11b / CD18-mediated neutrophil adhesion to the endothelium [39,40], and acidosis may stimulate neutrophil migration [41]. In our study, we found that hypercapnia also increased the expression of CD11a activation epitope on neutrophils consistent with their increased pulmonary infiltration. The current study does suffer from certain limitations. The in vitro limb does not mimic the stresses encountered due to pulmonary flow and repetitive inspiration and expiration that would occur during routine mechanical ventilation. In an effort to overcome this, we ventilated rabbits in a manner that reproduces a general “protective” ventilatory approach, with reduced tidal volume. Titration of PEEP in our model was lower than that commonly recommended by the ARDSnet. Moreover, antibody availability to detect and/or block various molecules are limited in the rabbit.
In summary, our data support the actions of “permissive” hypercapnia increases neutrophil adherence in relevant in vitro and in vivo models of pulmonary inflammation. Interestingly, NF-κB activation may play a prominent role in increasing the expression of molecules that promote neutrophil attraction and adhesion. These findings corroborate our previous findings where “permissive” hypercapnia was associated with amplified lung injury. Where to maintain the partial pressure of arterial CO2 is both a worthy question and controversial clinical issue in the current era of “protective” ventilation approaches. This study will hopefully lend insight into the continued debate of finding the proper balance between reducing ventilator-induced mechanical stress via reduced minute ventilation and the potential inflammatory consequences of moderate to high partial pressures of CO2 when hypercapnia is induced in this manner.
Acknowledgements
The authors wish to acknowledge the outstanding technical assistance provided by Ms. Fen Zhou and Mr. Phillip Chumley. We are also indebted to Dr. Myron I. Cybulsky of University of Toronto, Toronto, Ontario, Canada for generously providing us with the anti-Rabbit ICAM-1 / VCAM-1 antibodies, and Dr. Y C. T. Huang of Duke University Medical Center for kindly providing us with the IL-8 antibody methodology.
This work was supported by a National Institutes of Health Grant, #HL067982 to John D. Lang, Jr., M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fulkerson WJ, MacIntyre N, Stamler J, Crapo JD. Pathogenesis and treatment of the adult respiratory distress syndrome. Arch Intern Med. 1996;156:29–38. [PubMed] [Google Scholar]
- 2.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 3.Haitsma JJ, Uhlig S, Goggel R, Verbrugge SJ, Lachmann U, Lachmann B. Ventilator-induced lung injury leads to loss of alveolar and systemic compartmentalization of tumor necrosis factor-alpha. Intensive Care Med. 2000;26:1515–1522. doi: 10.1007/s001340000648. [DOI] [PubMed] [Google Scholar]
- 4.Ranieri VM, Suter PM, Tortorella C, De TR, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrigge H, Zinserling J, Stuber F, von ST, Hering R, Wetegrove S, et al. Effects of mechanical ventilation on release of cytokines into systemic circulation in patients with normal pulmonary function. Anesthesiology. 2000;93:1413–1417. doi: 10.1097/00000542-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Lang JD, Jr, Chumley P, Eiserich JP, Estevez A, Bamberg T, Adhami A, et al. Hypercapnia induces injury to alveolar epithelial cells via a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L994–L1002. doi: 10.1152/ajplung.2000.279.5.L994. [DOI] [PubMed] [Google Scholar]
- 9.Lang JD, Figueroa M, Sanders KD, Aslan M, Liu Y, Chumley P, et al. Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med. 2005;171:147–157. doi: 10.1164/rccm.200302-305OC. [DOI] [PubMed] [Google Scholar]
- 10.Chacko BK, Chandler RT, Mundhekar A, Khoo N, Pruitt HM, Kucik DF, et al. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: modulation of leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2005;289:H908–H915. doi: 10.1152/ajpheart.00781.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kotani M, Kotani T, Li Z, Silbajoris R, Piantadosi CA, Huang YC. Reduced inspiratory flow attenuates IL-8 release and MAPK activation of lung overstretch. Eur Respir J. 2004;24:238–246. doi: 10.1183/09031936.04.00128703. [DOI] [PubMed] [Google Scholar]
- 12.Billert H, Drobnik L, Makowski A. The influence of acute hypercapnia on the quantity and oxidative metabolism of bronchoalveolar lavage-derived leukocytes in the mechanically ventilated rabbit. Med Sci Monit. 2003;9:BR8–BR15. [PubMed] [Google Scholar]
- 13.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 14.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 15.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med. 1998;25:404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 16.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 17.Kuebler WM, Kuhnle GE, Groh J, Goetz AE. Contribution of selectins to leucocyte sequestration in pulmonary microvessels by intravital microscopy in rabbits. J Physiol. 1997;501(Pt 2):375–386. doi: 10.1111/j.1469-7793.1997.375bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L246–L255. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- 19.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 20.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 21.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 22.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 23.Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, et al. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]B activation. Am J Respir Cell Mol Biol. 2003;29:124–132. doi: 10.1165/rcmb.2002-0126OC. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Zhou D, Vicencio AG, Ryu J, Xue J, Kanaan A, et al. Effect of carbon dioxide on neonatal mouse lung: a genomic approach. J Appl Physiol. 2006;101:1556–1564. doi: 10.1152/japplphysiol.01031.2005. [DOI] [PubMed] [Google Scholar]
- 25.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard EJ, Yoshimura T. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]) Am J Respir Cell Mol Biol. 1990;2:479–486. doi: 10.1165/ajrcmb/2.6.479. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 28.Huber AR, Kunkel SL, Todd RF, III, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 29.Coakley RJ, Taggart C, Greene C, McElvaney NG, O'Neill SJ. Ambient pCO2 modulates intracellular pH, intracellular oxidant generation, and interleukin-8 secretion in human neutrophils. J Leukoc Biol. 2002;71:603–610. [PubMed] [Google Scholar]
- 30.Lang CJ, Dong P, Hosszu EK, Doyle IR. Effect of CO2 on LPS-induced cytokine responses in rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;289:L96–L103. doi: 10.1152/ajplung.00394.2004. [DOI] [PubMed] [Google Scholar]
- 32.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- 32.Doerschuk CM, Winn RK, Coxson HO, Harlan JM. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–2333. [PubMed] [Google Scholar]
- 33.Hellewell PG, Young SK, Henson PM, Worthen GS. Disparate role of the beta 2-integrin CD18 in the local accumulation of neutrophils in pulmonary and cutaneous inflammation in the rabbit. Am J Respir Cell Mol Biol. 1994;10:391–398. doi: 10.1165/ajrcmb.10.4.7510985. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Quinlan WM, Doyle NA, Graham L, Sligh JE, Takei F, et al. The roles of CD11/CD18 and ICAM-1 in acute Pseudomonas aeruginosa-induced pneumonia in mice. J Immunol. 1996;157:5016–5021. [PubMed] [Google Scholar]
- 35.Ramamoorthy C, Sasaki SS, Su DL, Sharar SR, Harlan JM, Winn RK. CD18 adhesion blockade decreases bacterial clearance and neutrophil recruitment after intrapulmonary E. coli, but not after S. aureus. J Leukoc Biol. 1997;61:167–172. doi: 10.1002/jlb.61.2.167. [DOI] [PubMed] [Google Scholar]
- 36.Tang WW, Yi ES, Remick DG, Wittwer A, Yin S, Qi M, et al. Intratracheal injection of endotoxin and cytokines. IX. Contribution of CD11a/ICAM-1 to neutrophil emigration. Am J Physiol. 1995;269:L653–L659. doi: 10.1152/ajplung.1995.269.5.L653. [DOI] [PubMed] [Google Scholar]
- 37.Freeman BD, Correa R, Karzai W, Natanson C, Patterson M, Banks S, et al. Controlled trials of rG-CSF and CD11b-directed MAb during hyperoxia and E. coli pneumonia in rats. J Appl Physiol. 1996;80:2066–2076. doi: 10.1152/jappl.1996.80.6.2066. [DOI] [PubMed] [Google Scholar]
- 38.Beck-Schimmer B, Schimmer RC, Warner RL, Schmal H, Nordblom G, Flory CM, et al. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 1997;17:344–352. doi: 10.1165/ajrcmb.17.3.2861. [DOI] [PubMed] [Google Scholar]
- 39.Serrano CV, Jr, Fraticelli A, Paniccia R, Teti A, Noble B, Corda S, et al. pH dependence of neutrophil-endothelial cell adhesion and adhesion molecule expression. Am J Physiol. 1996;271:C962–C970. doi: 10.1152/ajpcell.1996.271.3.C962. [DOI] [PubMed] [Google Scholar]
- 40.Botha AJ, Moore FA, Moore EE, Peterson VM, Goode AW. Base deficit after major trauma directly relates to neutrophil CD11b expression: a proposed mechanism of shock-induced organ injury. Intensive Care Med. 1997;23:504–509. doi: 10.1007/s001340050365. [DOI] [PubMed] [Google Scholar]
- 41.Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, et al. Extracellular acidification induces human neutrophil activation. J Immunol. 1999;162:4849–4857. [PubMed] [Google Scholar]