Abstract
Oxidative stress has been implicated in the etiology of neurodegenerative disease, cancer and aging. Indeed, the reactive oxygen and nitrogen species generated by inflammatory cells that created oxidative stress is thought to be one of the major factor by which chronic inflammation contributes to neoplastic transformation as well as many other diseases. We have recently reported that mice lacking nuclear factor-erythroid 2-related factor 2 (Nrf2) are more susceptibility to dextran sulfate sodium (DSS)-induced colitis and colorectal carcinogenesis. Nrf2 is a basic leucine zipper redox-sensitive transcriptional factor that plays a center role in ARE (antioxidant response element)-mediated induction of phase II detoxifying and antioxidant enzymes. We found that increased susceptibility of Nrf2 deficient mice to DSS-induced colitis and colorectal cancer was associated with decreased expression of antioxidant/phase II detoxifying enzymes in parallel with upregulation of pro-inflammatory cytokines/biomarkers. These findings suggest that Nrf2 may play an important role in defense against oxidative stress possibly by activation of cellular antioxidant machinery as well as suppression of pro-inflammatory signaling pathways. In addition, in vivo and in vitro data generated from our laboratory suggest that many dietary compounds can differentially regulate Nrf2-mediated antioxidant/anti-inflammatory signaling pathways as the first line defense or induce apoptosis once the cells have been damaged. In this review, we will summarize our thoughts on the potential cross-talks between Nrf2 and NF-κB pathways. Although the mechanisms involved in the cross-talk between these signaling pathways are still illusive, targeting Nrf2-antioxidative stress signaling is an ideal strategy to prevent or treat oxidative-stress related diseases.
Keywords: Nrf2, NF-κB, DSS, AOM, apoptosis
1. Introduction
Nrf2 is an important cytoprotective transcription factor. When challenged by oxidants or electrophiles, Nrf2 induces the transcription of diverse antioxidant enzymes, phase II detoxification enzymes, phase III efflux transporters. In combine, these cytoprotective genes can rapidly and coordinately neutralize, detoxify and remove those invading xenobiotics. Accumulating evidences show that Nrf2 is also the promising target for cancer chemoprevention.
2. Nrf2 signaling (I): the hinge and latch model
At unstressed condition, Nrf2 is sequestered in the cytoplasm by a cytosolic repressor Keap1 (Kelch-like ECH-associated protein 1) [1]. Recently, Keap1 is also characterized as a Cullin 3-dependent ubiquitination substrate adaptor protein [2–5]. So Nrf2 is not only physically sequestered in the cytoplasm, but also constantly degraded. At the oxidative condition, Nrf2 is released from Keap1 repression, translocates to the nucleus, forms heterodimer with small Maf (musculoaponeurotic fibrosarcoma) proteins, recognizes and binds to a cis-acting enhancer called antioxidant response element (ARE), and eventually recruits the whole transcription machinery including the RNA polymerase II to transcribe phase II/III genes [1].
In an old model of Nrf2 signaling [1], Keap1 is depicted as the primary redox sensor. The redox signals are subsequently relayed to Nrf2 in the process of Nrf2 releasing. In this model, there are some tacit assumptions. First, oxidants can induce Nrf2 releasing from Keap1. Second, free Nrf2 automatically translocates into the nucleus. The intensity of oxidative signals is encoded by the amount of Nrf2 proteins released from Keap1. New experimental observation in Keap1/Nrf2 studies paints a quite different picture.
Recently, two Keap1 binding motifs, i.e. an ETGE motif [6] and a DLG motif [7] have been characterized in the Neh2 domain of Nrf2. In an isothermal calorimetric measurement, two phases of Nrf2/Keap1 binding, i.e. a high affinity phase I binding, mediated by the ETGE motif and a low affinity phase II binding mediated by the DLG motif, has been distinguish [8]. Accordingly, an elegant “Hinge and Latch” model is proposed by Yamamoto’s group [9] to vividly illustrate how Keap1 functions as a substrate adaptor protein. At unstressed condition, Keap1 exists in homodimer mediated by its BTB (Broad complex, Tramtrack and Bric a brac) domain. Nrf2 first forms high affinity binding with Keap1 via the ETGE motif. This strong binding functions as the “hinge”. Nrf2 can also form weak binding with Keap1 via the DLG motif, as the “latch”. Linking the DLG and the ETGE motifs is an alpha helix. Six out of seven lysine residues of this alpha helix locate at the same side. Recently, the crystal structure of the Nrf2-binding Kelch domain of Keap1 has been resolved [10]. The geometric distance between the two Kelch domains matches perfectly with the length of this alpha helix [10]. So when the “latch” is in position, the alpha helix is straightened up to maximally expose those lysine residues, presumably as ubiquitination acceptors. At the oxidative condition however, an intermolecular disulfide bond is formed between Cys273 and Cys 288 of Keap1 dimer. Consequently, the profound conformation change in Keap1 dimer disrupts the latch-binding. As a result, the randomly floating alpha helix will not in appropriate orientation for effective ubiquitination. The Nrf2 ubiquitination is impeded. The oxidants however, may fail to disrupt Keap1/Nrf2 binding mediated by the ETGE motif. Recently, Mesecar’s group reported that overdose sulforaphane (SFN) treatments failed to disrupt Keap1/Neh2 binding, no matter the treatment of SFN was on Keap1 first, on Neh2 first or on Keap1/Neh2 complex [11]. So oxidants uncouple two Keap1 mediated repressions: whereas oxidants indeed impede Nrf2 degradation, oxidants fail to disrupt Keap1/Nrf2 binding [12]. The observation that oxidants failed to disrupt Keap1/Nrf2 binding put a big question mark on this old Nrf2 signaling model.
3. Nrf2 signaling (II): graded nuclear translocation of Nrf2
The second question is whether free Nrf2 automatically translocates into the nucleus? The answer is a definite no. Recently we characterized in Nrf2 a nuclear export signal (NES) in the leucine zipper domain (NESzip) [13] and another NES motif located in the Neh5 transactivation domain (NESTA) [14]. In addition, we also identified a bipartite nuclear localization signal (NLS) in the basic region (bNLS) [13]. It is the combined activities of multiple NLS/NES motifs that determine the subcellular localization of Nrf2. When full length wild type Nrf2 was expressed, a mixed distribution pattern was observed [14]. So the jointed nuclear exporting activities mediated by NESTA and NESzip motifs can counter balance the nuclear importing activity mediated by the bNLS motif. When the NESTA motif was disabled by L183A mutation, the distribution of mutant was converted to a nuclear distribution [14]. When the NESzip motif was disabled by L544A mutation, the distribution of mutant was also converted into a nuclear distribution [14]. We naturally ask a question: does the disabling of NESTA or NESzip motif occurs at physiological condition? Embedded in the NESTA motif there is a cysteine residue (Cys 183). Our tandem mass spectrometry analysis showed that this cysteine residue is a redox-reactive cysteine (Li et al, unpublished data). Sulfhydryl modification at this cysteine residue may generate spatial hindrance to nuclear exporting protein CRM1 (chromosome region maintenance 1). In other word, this NESTA motif is a conditional NES that can be switched off by oxidants. The disabling of this NESTA motif triggers Nrf2 nuclear translocation.
We further examined the kinetics of Nrf2 nuclear translocation mediated by the NESTA motif. We treated EGFP-NESTA expressing cells with different dosages of sulforaphane (SFN). We used two parameters to describe nuclear translocation: the maximal nuclear accumulation (Amax) and half time to achieve maximal nuclear accumulation (t1/2). The Amax values were positively correlated with SFN concentrations. The t1/2 values were inversely correlated with SFN concentrations [14]. In other words, the higher the concentration of oxidants, more Nrf2 proteins translocate into the nucleus at higher speed. This graded nuclear translocation of Nrf2 showed that Nrf2 can not only transducer the presence of oxidative stress, but also the intensity of oxidative stress.
Based on those observations, we propose a new Nrf2 signaling model. At unstressed condition, Nrf2 is constantly degraded via Keap1-mediated ubiquitination. Nrf2 degradation may be counter balanced by constitutive Nrf2 translation. As a consequence, very small pool size of free Nrf2 proteins exists. At the oxidative condition, Keap1-mediated ubiquitination is impeded. Nrf2 translation is elevated as reported by Chen’s group at Arizona [15]. As a result, the pool of free Nrf2 proteins expands. But the influx of Nrf2 is still determined by the intensity of oxidative stress. In this new model, Nrf2 is the primary redox sensor. Keap1 still modulates the redox sensitivity of Nrf2 by controlling the availability of free Nrf2 proteins. This new model may compromise discrepancies that cannot be explained by the old model.
4. Nrf2 mediated cancer chemoprevention
Nrf2 is an important anti-neoplastic factor and the key target of cancer chemoprevention. We compared the anti-neoplastic effect between Nrf2 wild type (WT) and knockout (KO) mice in 7,12-dimethylbenz(a)anthracene (DMBA)-12-O-tetradecanoylphorbol-13-acetate (TPA)- induced skin carcinogenesis model [16]. Topic application of a single dose DMBA (200 nmol) followed by TPA treatment (8 nmol, twice a week) for 25 weeks elicited significantly higher tumor incidence in Nrf2 KO mice in comparison with WT mice [16]. Pretreatment of sulforaphane (daily topical application of 100 nmol) for 2 weeks significantly inhibited carcinogenesis in WT mice (p<0.05) but not in KO mice (p>0.05) [16]. It is also very noteworthy that in tumor samples from both Nrf2 WT and KO mice, the expression of Nrf2 and Nrf2 regulated gene heme oxygenase 1 (HO-1) were shut off [16]. The mechanism(s) of Nrf2 shut off is/are currently intensively investigated.
5. Chronic inflammation promotes neoplastic transformation
Accumulated evidence showed that chronic inflammation promotes neoplastic transformation. Recently we developed a chronic inflammation mice model. We used dextran sulfate sodium (DSS) to induce colonic colitis [17]. Feeding of 1% DSS in drinking water for 1 week induce colonic colitis. In comparison with Nrf2 WT mice, the colonic colitis observed in Nrf2 KO mice appeared to be more severe, including lost of colonic crypt, massive infiltration of inflammatory cells and anal bleeding. In addition, immunocytochemical staining of nitrotyrosine, a biomarker of inflammation, was more intense in Nrf2 KO mice. One reason why DSS-induced colonic colitis is more severe in Nrf2 KO mice is the lower induction of phase II protective gene, such as HO-1, GST (Glutathione-S-Transferase), UDP-glucuronosyltransferase (UGT) 1A1, in Nrf2 KO mice. Concomitantly, more intense induction of inflammatory biomarkers, such as cytokine interleukin (IL)-1β, IL-6 and TNFα (tumor necrosis factor-alpha) as well as proinflammatory enzymes inducible nitric oxide synthetase (iNOS) and cycloxygenase 2 (COX2), was observed in Nrf2 KO mice in comparison with WT mice. Since these pro-inflammatory biomarkers are all effecter genes regulated by the NFκB pathway, these observation is consistent with previous observation of anti-inflammatory function of Nrf2. Ablation of Nrf2 seems to accelerate NFκB mediated pro-inflammatory reaction.
Based on DSS-induced colonic colitis model, we went further to test the role of Nrf2 in an azoxymethane (AOM)-DSS induced colonic carcinogenesis mice model (Khor et al, Cancer Prevent Res. in press). Mice were subcutaneously injected with a single dose of AOM (10 mg/kg body weight). Two weeks after the AOM injection, the mice were feed with 1% DSS in drinking water for 1 week. Tumorigenesis was examined after 20 weeks. The tumor incidence was significantly higher in Nrf2 KO mice (92.9%) in comparison with WT mice (53.3%). In addition, 80% of tumors are adenocarcinomas in Nrf2 KO mice in comparison with 29% in WT mice. The higher tumor incidence in Nrf2 KO mice was paralleled with severe colonic colitis including prolapsed rectum and anal bleeding. The nitrotyrosine staining was more intense in colon samples from Nrf2 KO mice in comparison with the WT samples. Induction of HO-1, UGT1A1 and NQO1 (NAD(P)H:quinone oxidoreductase 1) was diminished in Nrf2 KO mice. The induction of arachidonic acid (AA) metabolites, which is involved in pro-inflammatory response, is more intense in KO mice. The induction of COX2 and 5-lipoxygenase (5-LOX) as well as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), the major metabolites of COX2 and 5-LOX pathways, were significantly higher in NO mice than in the WT mice. The present study highlighted the important role of Nrf2 in the protection against colonic inflammation as well as carcinogenesis.
6. Potential crosstalk between Nrf2 and NFκB pathways
These data obtained from animal studies underlined the possibility that Nrf2 mediated anti-tumorigenic effect may be achieved by activation of antioxidant machinery as well as suppression of pro-inflammatory pathways mediated by NFκB signaling. Previously, we observed that lipopolysaccharide (LPS) induced NFκB activation could be attenuated by diverse Nrf2 activators, such as phenethyl isothiocyanate (PEITC), SFN and curcumin (CUR) [18]. Dietary administrations of SFN and dibenzoylmethane (DBM) alone or in combination could significantly inhibited the development of intestinal adenomas in ApcMin mice in parallel with decreased PGE2, LTB4 and COX2 activities [19]. Furthermore, PEITC and SFN treatment was found to be able to inhibit IKK/IκB phosphorylation and p65 NFκB subunit nuclear translocation, consequently alleviating NFκB signaling [20]. However, definite evidence showing that Nrf2 can directly inhibit NFkB signaling is still wanting. On the contrary, it was reported recently that NFκB could directly repress Nrf2 signaling at the transcription level. NFκB competes against Nrf2 for transcription co-activator CREB binding protein (CBP) [21]. In addition, NFκB recruits histone deacetylase 3 (HDAC3) to cause local hypoacetylation to hamper Nrf2 signaling [21]. Further studies are needed to unravel complicate interplay between Nrf2 and NFkB pathways.
7. PEITC and curcumin treatments induce apoptotic death
Previously, cancer chemopreventive compound PEITC was reported to be able to delete preneoplastic cells through induction of apoptosis [22, 23]. Treatment of tumeric curcumin was also found to be able to induce apoptosis in prostate cancer cells [24]. Indeed, we observed that treatment of PEITC and curcumin could inhibit the growth of human PC-3 prostate xenografts in nude mice [25]. The growth inhibitory effect may, at least partially, be attributed to induced apoptosis. Using an in situ apoptosis detection kit (Chemicon), we detected significantly higher percentage of apoptotic cells in mice samples treated with PEITC alone (5 μmol), curcumin alone (6 μmol), or PEITC (2.5 μmol) and curcumin (3 μmol) in combine. Consistent with the observed induced apoptosis, significantly high induction of cleaved caspase 3 and poly(ADP-ribose)polymerase (PARP) were observed in tumor samples [25].
Therefore treatments with cancer chemopreventive compounds can not only block the initiation of carcinogenesis, but also suppress the promotion and progression of carcinogenesis, at least partially by induction of apoptosis.
8. Perspectives
Previous MS-MS studies have identified reactive cysteine residues in Keap1, now Nrf2 also seems to possess reactive cysteine(s). What is the functional significance? The existence of reactive cysteines in Nrf2, especially those cysteines located in functional motifs of Nrf2, implies that Nrf2 per se is a redox-sensitive probe. It awaits further experimental examination of the relative sensitivity and functional roles of those cysteine residues, both in Keap1 and in Nrf2.
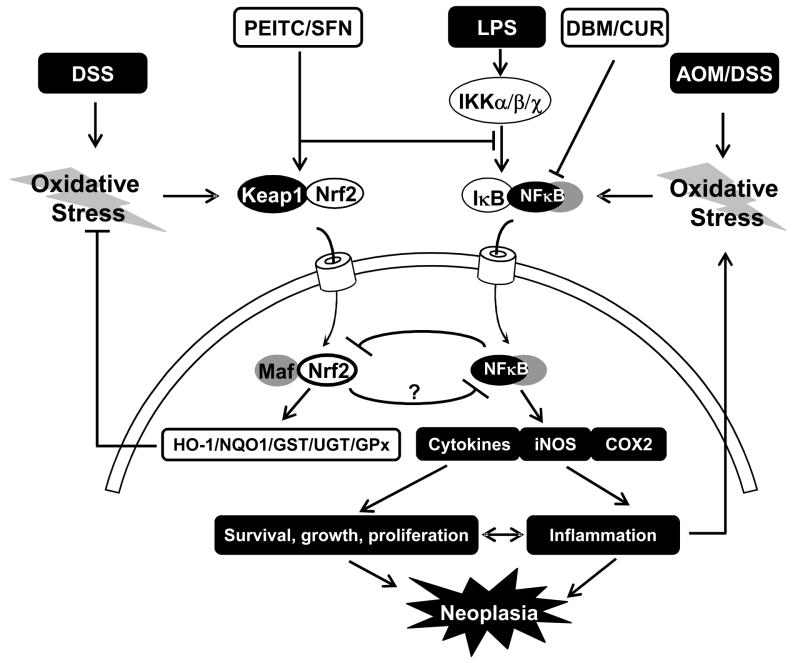
Figure 1.
Chronic colon colitis model. AOM/DSS treatments induce colon colitis via activating NFκB-mediated pro-inflammatory pathway. Prolonged colon colitis promotes neoplastic transformation. Pretreatment with chemopreventive compounds such as PEITC, SFN, DBM and CUR alone or in combine can considerably ameliorate colon carcinogenesis either by the induction of Nrf2-mediated antioxidant response to attenuate oxidative stress or by suppressing NFκB-mediated inflammatory response.
Acknowledgments
We thank Dr. Jefferson Chan for his general gift of Nrf2 knockout mice. We thank all collaborators in Drs. Allan Conney and C.S. Yang’s laboratory. We thank Drs. Hong Li and Tong Liu for their assistance in MS-MS analysis. We thank all current and former members of Dr. Kong’s laboratory for their contribution and comment.
Supported by NIH Grants CA-073674, CA-094828 and CA-118947.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–71. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–20. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 7.Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–50. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–20. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 10.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A. 2005;102:10070–5. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Jain MR, Chen C, Yue X, Hebbar V, Zhou R, Kong AN. Nrf2 Possesses a redox-insensitive nuclear export signal overlapping with the leucine zipper motif. J Biol Chem. 2005;280:28430–8. doi: 10.1074/jbc.M410601200. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Yu SW, Kong AN. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J Biol Chem. 2006;281:27251–63. doi: 10.1074/jbc.M602746200. [DOI] [PubMed] [Google Scholar]
- 15.Purdom-Dickinson SE, Sheveleva EV, Sun H, Chen QM. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol Pharmacol. 2007;72:1074–81. doi: 10.1124/mol.107.035360. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong AN. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–6. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 17.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 18.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–70. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 19.Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, Reddy BS, Huang MT, Newmark HL, Kong AN. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–44. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 21.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–27. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhary LR, Hruska KA. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J Cell Biochem. 2003;89:1–5. doi: 10.1002/jcb.10495. [DOI] [PubMed] [Google Scholar]
- 25.Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, Xu C, Gopalakrishnan A, Reddy B, Zheng X, Conney AH, Kong AN. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–21. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]