Introduction
The lives of an estimated 10 million people in the United States are impacted by debilitating cochlear noise injury acquired in leisure or occupational settings (Kopke et al., 2007). Although noise injury has been intensively studied for decades, the only clinically successful preventive measure against noise-induced hearing loss (NIHL) remains a hearing protective device (HPD), and the only treatment is a hearing aid. We cannot yet identify people who are most at risk in noisy environments. Even if we could, there is still no practical pharmacological approach to minimizing hearing loss. HPD use is often uneven and impractical in many settings, and HPD effectiveness is simply not up to the task in some work environments. A continued broad and well-funded assault on gaps in our understanding of NIHL remains vital. Significant discoveries can emerge from studies aimed at seemingly remote facets of noise injury, and narrowly defining which facets merit study will always be a mistake. Here we briefly review recent progress in several areas. Particularly exciting recent developments include new evidence for a phagocytic function of supporting cells in removing damaged outer hair cells (OHCs), demonstration of large scale inflammatory cell invasion of the lateral wall after noise, support for strong genetic modulation of injury to the lateral wall, and the discovery of remarkable interactions between noise and age of exposure. We also update the potential role in NIHL of the cochlear lateral wall in light of newly discovered functions of gap junctions. Other recent reviews (Darrat et al., 2007; Hawkins et al., 2005; Henderson et al., 2006; Hibino et al., 2006; Kopke et al., 2007; Le Prell et al., 2007b; Ohlemiller, 2006; Talaska et al., 2007; Wangemann, 2006) offer more comprehensive accounts of findings touched upon here.
Most recent studies of noise injury have utilized mouse models. Mice are the best model for questions that are overtly genetic, and have distinct advantages for aging studies, yet they may pose limitations for physiologic and biochemical studies. Many strains exhibit pronounced vulnerability to NIHL (e.g., Ohlemiller et al., 2000a), but still may show less hair cell loss in the exposure band than guinea pigs or chinchillas (Ou et al., 2000; Wang et al., 2002b). Mice also remain subject to concerns about the comparability of mechanisms for hearing in the audiologic and ultrasonic range. Two increasingly recognized phenomena add caveats to the touted high degree of genetic control of mouse models. First is environmentally-modulated epigenetic control of transcription and translation, which can effectively alter genotype by permanently silencing genes (see below). Second is variation in the number of copies of genes, which occurs even within inbred strains such as C57BL/6 (B6) (Watkins-Chow et al., 2008). Copy number variation is thought to arise from faulty DNA repair, and can alter phenotype via gene-dose effects. Depending on how early it occurs in development, it may render most individuals chimeric for many genes. Copy number variation may require presently non-standard tests to account for some differences among nominally genetically identical mice.
No Single Archetype for Noise Injury
Two broad classes of noise exposures and their sequelae may be distinguished (Lim, 1986; Spoendlin et al., 1973). For every model well-studied from a dose-response perspective, there exists an inflection point on the noise intensity continuum, above which the extent of hearing loss abruptly accelerates. The defining anatomic change above this inflection is rupture of the reticular lamina and wholesale disruption of endolymphatic compartment. For most models, this is found for intensities above 125–130 dB SPL, although it may fall lower for temporally-skewed impact and impulse noise (Qiu et al., 2007), and for mice (Wang et al., 2002b). Above the inflection point, hair cell loss clearly increases; however, we know of only one quantitative study of changes in non-sensory and supporting cells (Wang et al., 2002b). That study in mice indicated significant redistribution in the cellular and spatial pattern of injury when scala media is breached versus when it remains intact. The assumption that scala media remains intact, and that cellular injury was principally biochemical and less overtly traumatic, attends most of the findings summarized here.
For severe exposures below the inflection point, injury to the organ of Corti, afferent neurons, stria vascularis, and spiral ligament is found, and has seemed part of a distinctly mammalian ‘template’ for noise damage. A dramatic ability of genetics to alter the cellular targets of noise was recently demonstrated in mice (Ohlemiller et al., 2007). B6 mice are notoriously more vulnerable to NIHL than are CBA/J and CBA/CaJ mice (Erway et al., 1996; Ohlemiller et al., 2000a), yet they were shown to be resistant to noise-induced reversible endocochlear potential (EP) reduction and associated cell pathology found in the lateral wall and limbus of CBAs after noise (Fig. 1). Anatomical correlates of the injury in CBAs included acute changes in Type I and II fibrocytes, as well as strial basal cells. These same cells were reduced in number by about 30% at 8 wks post-exposure in CBAs, while B6 mice showed no significant loss. Examination of F1 hybrid and N2 backcross mice indicated that strain differences could be explained assuming 1–2 large effect quantitative trait loci (QTLs), and were independent of Cdh23ahl and strial melanin expression. Such findings point to independent genetic modulation of noise injury to the organ of Corti versus lateral wall. Although permanent lateral wall injury observed in CBA mice may not directly contribute to permanent threshold shifts (PTS), the acute changes in the same cells may reflect processes that amplify NIHL at the time of exposure (see below). It is also possible that moderate cell loss in the stria and ligament reduces the long term ‘homeostatic capacity’ of the cochlea, and accelerates apparent aging. These new findings emphasize the need for caution in extrapolating observations from any single model—even beyond any single strain within a species.
Figure 1.
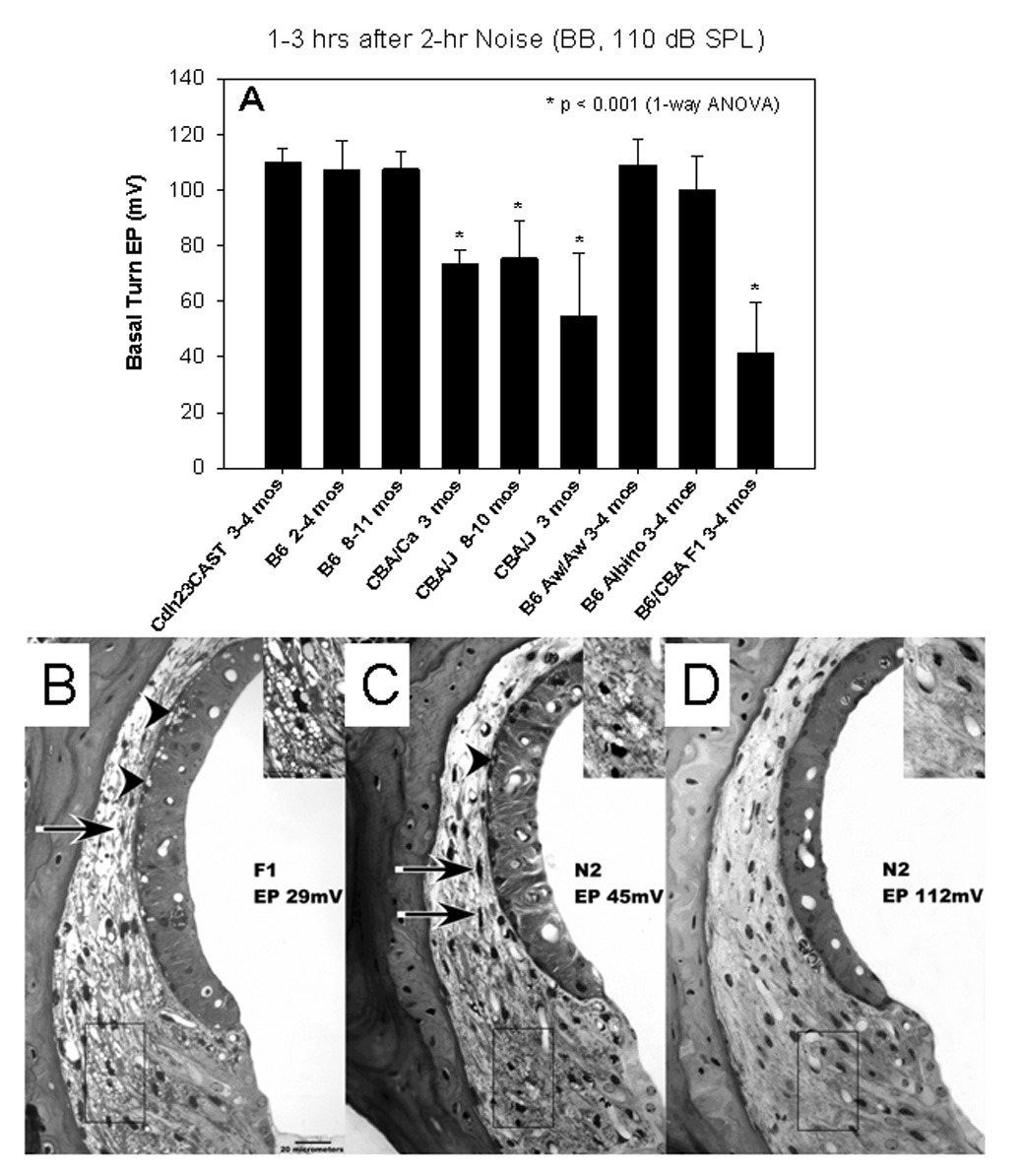
A. Comparison of endocochlear potentials (EPs, recorded in the basal turn) in different strains of mice within hours of a single noise exposure. C57BL/6J (B6) mice showed no significant EP reduction, nor did co-isogenic white-bellied agouti (Aw) mice, or congenics lacking the Cdh23ahl allele. Co-isogenic albino B6 mice showed a small but significant EP reduction compared to unexposed mice of the same strain (not shown). CBA/J and CBA/CaJ mice showed larger and highly significant EP reduction, as did B6xCBA/J F1 hybrid mice. EP reduction in the hybrids indicates that reduction is dominant between these two strains. (Adapted with permission from Ohlemiller 2006) B. Appearance of the lateral wall in the cochlear upper basal turn of example B6xCBA/J F1 hybrid mouse in the acute period after noise. The hybrids showed pathology like that in CBA/J, including shrinkage of Type I fibrocytes (arrow) and vacuolization of both Type II fibrocytes (inset) and strial basal cells (arrowheads). C.–D. F1xB6 N2 backcross mice showed EPs varying from normal to <40 mV. Their lateral wall pathology corresponded with the measured EP, so that the lateral wall in C resembled that in B, while the lateral wall in D appeared normal. Half of 42 noise-exposed N2s showed a normal EP (>90 mV), suggesting that a small number of dominant-acting genes are responsible for EP reduction in CBA/J mice. (Adapted with permission from Ohlemiller and Gagnon, 2007)
Risk Factors for NIHL
Prenatal and epigenetic factors
Environmental stress on the mother during pregnancy is known to negatively impact the development and stress responses of her offspring (Barker, 1995, 1998). Stress-related chronic elevation of glucocorticoids in utero may favor the growth of some organs and systems at the expense of others. ‘Losers’ in such a scenario may include inner ear tissues, which could end up with fewer blood vessels and progenitor cells (Barrenäs et al., 2003; 2005). Barrenas and colleagues suggest that prenatal stress in humans leads to a combination of depressed birth weight and adult stature, plus elevated hearing thresholds that may reflect sensitivity to noise. Support for this principle has come from recent work in pregnant rats subjected to different types of stressors (Kadner et al., 2006). Elevated maternal glucocorticoids may permanently impair innate protective mechanisms such as antioxidant defenses. Canlon and colleagues (Canlon et al., 2003) have presented evidence that such a process can promote noise vulnerability later in life (See Hougaard et al., 2007 for contrary result.).
The prenatal environment may exert its effects partially through epigenetic modifications to DNA. In utero and early life events can modify gene expression via methylation of DNA and resulting histone repression of transcription, and also post-transcriptionally through de-methylation and activation of microRNAs (Feil, 2006; Gallou-Kabani et al., 2007). This process governs tissue differentiation during development, but only recently has its influence beyond developmentally regulated genes been recognized. Based on a mix of environment (uterine and postnatal) and stochastic processes, anyone may effectively become hemizygous or genetically null for many genes. Cell epigenotypes are passed on in somatic cell mitosis and can be passed to offspring. Even more surprisingly, epigenotype can be inherited according to rules that seem to violate Mendel’s laws (e.g., imprinting and trans-generational inheritance). Since hearing-related genes are not immune to this process, epigenetic principles seem likely to complicate human and animal studies of genetic influences on acquired hearing loss.
Environmental factors in adulthood
Oxidative stress and hypoxia/ischemia are implicated in NIHL. Environmental factors that amplify cochlear oxidative stress and impair blood flow might therefore be anticipated to exacerbate noise injury. New evidence supports such an effect by volatile industrial compounds such as toluene and ethylbenzene (Fechter et al., 2007), smoking (Uchida et al., 2005; Wild et al., 2005), and hyperlipidemia (Chang et al., 2007). These factors may interact synergistically. Their impact will further depend on genetic alleles that influence cochlear blood flow, cochlear protective systems, and the robustness of preconditioning effects (see below).
Genes known to impact NIHL
Certain alleles of several known genes promote permanent NIHL in mice. The best-studied among these is Cdh23ahl, which promotes noise injury in B6 mice, and presumably many other strains (Johnson et al., 2000; Johnson et al., 1997). Yet even when the influence of Cdh23ahl is removed from B6, it has been noted that these mice remain more noise susceptible than CBA/J or CBA/CaJ (Ohlemiller et al., 2007; Ortmann et al., 2004). Recently, another pro-NIHL locus has been isolated, Ahl3 on chromosome 17 of B6, that may help explain this residual difference (Morita et al., 2007). New discoveries of alleles that promote noise-induced temporary threshold shifts (TTS) derive from knockout (KO) mice for NFκB activation (Lang et al., 2006a), KOs for estrogen receptor β (ERβ) and aromatase (Meltser et al., 2008), and KOs for orphan glutamate receptor δ1 subunit (GluRδ1) (Gao et al., 2007). The exact mechanism by which GluRδ1 deficiency promotes TTS is unclear, although the proximate cause appears to be reversible EP reduction. NFκB deficiency may magnify excitotoxic injury to afferent dendrites (Tahera et al., 2006b), and appears to promote a degenerative process that resembles neural presbycusis (Lang et al., 2006a). ERβ and aromatase deficiency may similarly magnify excitotoxic injury, perhaps by reducing levels of brain-derived neurotrophic factor (BDNF) (Meltser et al., 2008). Mutations affecting hair cell ion channels could also impact noise vulnerability, and in fact, increased PTS has been now found for mice deficient in the BK calcium-activated K+ channel expressed by basal OHCs (Engel et al., 2006).
Genetically-based variation in noise sensitivity is established in humans (e.g., Heinonen-Guzejev et al., 2005), and recent work has revealed some genes that may be involved. Based on work in mice, genes mediating cochlear protection offer candidates for pro-NIHL alleles in humans (Fairfield et al., 2005; Ohlemiller et al., 1999; 2000b; Sugahara et al., 2003). This notion has found support in recent human genetic studies. Certain alleles of genes encoding heat shock protein HSP70 isoforms (Yang et al., 2006), and antioxidants paraoxonase and manganese superoxide dismutase (SOD2) (Fortunato et al., 2004) may promote NIHL. Some alleles of KCNE1, which encodes a potassium channel regulatory subunit, may also worsen NIHL (Van Laer et al., 2006). The major cochlear function of this protein is to impart control of K+ flow from strial marginal cells into scala media. As we will consider further, modulation of KCNE1/KCNQ1 channel complexes by ATP and Ca++ (Housley et al., 2002; Lee et al., 2008) may constitute a protective mechanism against NIHL.
The role of strial pigmentation has long been of interest because of anticipated protective effects of melanin against noise and ototoxins (Meyer zum Gottesberge, 1988). Although animal studies of this issue can be based upon direct characterization of strial melanin, human studies have had to rely on proxy measures of skin and eye color. Except in the case of albinism, skin color seems a poor indicator of the amount of strial pigment (Bonaccorsi, 1965; Tota et al., 1967 (cited in Cunningham et al., 1982)). Eye color may represent a better index of the amount of strial melanin, but not necessarily of type (Barrenäs, 1997; Bartels et al., 2001). Melanin comes in multiple forms, depending in part on what alleles are present at the agouti (A) locus. Not all melanins appear equally beneficial: Eumelanin (black melanin) may be protective, while pheomelanin (red melanin) may actually promote injury by pro-oxidant activity. The dominant A allele generally switches melanin production from eumelanin to other forms. Agouti has been assumed to be expressed in the cochlear stria, but this is has not been tested. Retrospective human studies of noise-exposed workers stratified by eye color have produced quite mixed results (Attias et al., 1985; Cunningham et al., 1982). However, a recent study of a large sample of metal workers (Da Costa et al., 2008) obtained a ~9 dB sensitivity difference between ‘dark-eyed’ and ‘fair-eyed’ subjects having a history of moderate work-related noise exposure. Animal data bearing on this issue are inconclusive, and often have relied on sub-optimal genetic controls. Commercial pigmented and albino rabbit and guinea pig models used for some studies (Borg et al., 1995; Conlee et al., 1986) differ genetically. Any apparent differences between these may therefore be unrelated to pigmentation. Albino and pigmented inbred mouse strains are subject to the same confound unless they exist as single-mutation variants within the same strain. One well-controlled study (Bartels et al., 2001) compared co-isogenic pigmentation variants within B6, both in terms of the actual amount of strial eumelanin present and susceptibility to noise. Notably, the dominant Ay agouti allele did not promote significant pheomelanin production in the stria. Moreover, the absence of melanin in C57BL/6-Tyrc-2J mice, which are albino, had no influence on the extent of PTS. Our own recent comparisons of the effects of broadband noise (110 dB SPL, 2 hr) and aging (>24 mos) on the EP in B6 and C57BL/6-Tyrc-2J albino mice (Ohlemiller et al., 2007; 2008) revealed modest but statistically significant (~10 mV) reductions for both conditions only in the albinos. While the EP probably recovers from noise, the common thread linking these findings to the recent positive results in humans may be reduced resilience of the non-pigmented stria. If this is correct, repeated moderate noise exposures may reveal differences in PTS between albino and pigmented B6. The larger principal of interest here is not whether albinism can promote NIHL. Rather, it is whether any of the >25 genes that impact the amount, type, or distribution of strial melanin can, for some allele combinations, render some individuals more vulnerable to noise. No single experiment or strain can provide a universal answer to this issue, since multiple genes will influence the outcome.
Death Comes to the Organ of Corti
Repair and partial restoration of function to the organ of Corti after noise injury requires re-establishment of boundaries and removal of dead and moribund cells. Involvement of macrophages in this process has long been recognized (Fredelius et al., 1990), although the existence of resident macrophages has been questioned. Recent experiments (Abrashkin et al., 2006) reveal a macrophage function for supporting cells—especially Deiters’ cells—in removing debris from dying OHCs. This makes sense, in that it falls to Deiters’ cells to restore the junctional integrity of the reticular lamina after OHC loss. Dying hair cells have generally been considered to follow either apoptosis or necrosis (oncosis), depending on the severity of their injury. Careful examination, however, has revealed an apparent mode of OHC death that adheres to neither of these (Bohne et al., 2007). The existence of a novel ‘third pathway’ (the authors’ term) in the organ of Corti may reflect the need to maintain ionic boundaries in the damaged organ.
Other recent discoveries pertain to the nature of apoptosis as a mechanism for sensory cell degeneration. Two major apoptotic pathways are recognized: extrinsic and intrinsic (mitochondrial) (Van De Water et al., 2004), both involving caspases. Some evidence, however, points to an additional caspase-independent mitochondrial pathway, and it has remained unclear whether, and how, this pathway may operate in the cochlea. According to recent findings (Han et al., 2006; Yamashita et al., 2004), in addition to cytochrome C release (which engages caspases) distressed mitochondria release Apoptosis-Inducing Factor (AIF) and endonuclease G (endoG) into the cytoplasm. One or both of these then migrate into the cell nucleus and initiate DNA fragmentation. The mechanism linking cell stress to the mitochondrial response may depend on dysregulation of cytosolic calcium and subsequent activation of Bcl-2-associated death promoter (BAD) (Vicente-Torres et al., 2006). BAD is thought to migrate into mitochondria and sequester anti-apoptotic proteins, leading to permeabilization of the mitochondrial membrane, followed by release of cytochrome C, AIF, and endoG. Nitric oxide (NO), produced either in the cytosol of OHCs or within mitochondria, has also recently been advocated as a key initiator of the caspase-independent mitochondrial pathway (Shi et al., 2007b).
Noise-induced Phagocyte Invasion of the Lateral Wall
Hematopoietically-derived cells are observed within the perilymphatic scala after cochlear inoculation with antigens (Ma et al., 2000; Ryan et al., 2002; Satoh et al., 2002). Only recently has the scope of noise-related invasion also been recognized. Experiments in mice by Hirose and colleagues (Hirose et al., 2005) and other groups (Tornabene et al., 2006) have revealed large scale influxes of mononuclear phagocytes, peaking about 7 days after a single damaging exposure (Fig. 2). In the Hirose et al. study, the invading cells appeared to emerge from the Type IV fibrocyte region of the spiral ligament to take up positions in superior ligament, stria vascularis, and spiral limbus. Phagocytes may be drawn to the ligament by cytokines such as CCL2 (MCP-1), IL-6, IL-1β, and TNFα (Fujioka et al., 2006; Ichimiya et al., 2000; Yoshida et al., 1999a), all known to be expressed by fibrocytes and fibroblasts upon sensing stress-related changes in their surroundings (Gallit et al., 1994; Schonherr et al., 2000). Within the cochlea, conditions to which these cells react may include hypoxia/ischemia, osmotic stress, and changes in tensile stress exerted by the collagen scaffold to which they attach (Grinnell, 2000). Capillary endothelial cells within the ligament may also secrete chemotactic factors (Shi et al., 2007a). Once released, cytokines appear to act in both autocrine and paracrine fashion to promote further cytokine release (Ichimiya et al., 2000; Yoshida et al., 1999a).
Figure 2.
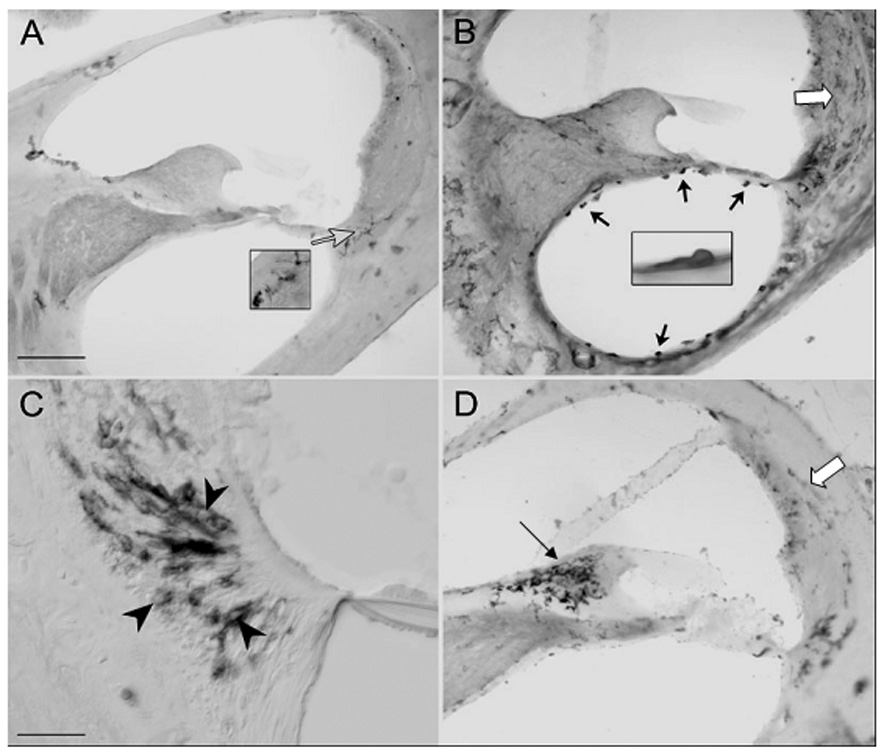
Invasion of the cochlear upper basal turn of CBA/CaJ mice by macrophages (indicated by staining for CD45) after a single noise exposure. A. Non-exposed control mouse shows low level of staining. B. 1–3 days after exposure, macrophages can be seen invading scala tympani (black arrows) and the spiral ligament. C. Within the ligament, the largest concentration of macrophages appears in the Type IV region (arrowheads), although staining in the Type I and II regions is also increased (white arrows in B,D). D. At 7 days post exposure macrophages are concentrated in spiral limbus (black arrow). Scale bar: 100 µm in A,B,D; 25 µm in C. (Adapted with permission from Hirose et al., 2005)
What are the invading phagocytes doing? Most likely, one function is removal of debris, given that the largest mobilization of phagocytes is seen in regions of the ligament where noise-related cell loss is most evident (Hirose et al., 2005; Ohlemiller et al., 2007; Wang et al., 2002b). Consistent with this interpretation is the fact that phagocytes appear in the spiral limbus of CBA-related mice, in which noise-related fibrocyte loss is pronounced (Hirose et al., 2005), but not in spiral limbus of B6 mice, in which little permanent limbus damage may occur (Ohlemiller et al., 2007; Sautter et al., 2006). Another process that may operate in parallel is for migratory hematopoietically-derived cells to serve as progenitors to replace lost fibrocytes (Lang et al., 2006b). Ohlemiller and Gagnon (2007) noted that the modest amount of cell loss in the ligament of CBA/J mice seen 8 wks after exposure seemed inconsistent with the number of pyknotic nuclei found 24 hrs after noise, and suggested that these cells are probably replaced. By contrast, little cell replacement seems to occur in the limbus despite an invasion of hematopoietically-derived cells rivaling that in the ligament. Perhaps in the limbus, phagocytosing and replacement functions do not coincide.
Even extensive fibrocyte loss in the spiral ligament is not necessarily associated with permanent EP reduction (Ohlemiller et al., 2006; Wu et al., 2003). One might therefore anticipate that manipulation of chemotactic factors that draw phagocytes to cochlear connective tissue would have little effect on eventual recovery of thresholds from noise. In fact, neither a knockout of chemokine CCL2 nor its receptor CCR2 affected threshold recovery (Sautter et al., 2006). The surprise, however, was that absence of CCR2 greatly increased hair cell loss, even though the phagocytes from which CCR2 had been eliminated were still recruited to the cochlea. Just how elimination of a receptor from phagocytes that apparently remain in the lateral wall and never enter the organ of Corti can impact hair cell survival seems difficult to explain. The authors provide two hypotheses. First and simplest, as-yet-undetected CCR2 receptors on hair cells or supporting cells may mediate a protective effect that employs a ligand other than CCL2. The second hypothesis is that the essential CCR2 receptors normally reside on one or more populations of invading macrophages, and that elimination of CCR2 removes safeguards from some harmful action of these cells. This hypothesis appears to require that macrophages migrate into the organ of Corti. As an alternative, we propose that the critical CCR2 receptors reside on spiral ligament fibrocytes where they participate in control of gap junctions. As we consider in the next section, gap junctions may serve complex functions that extend far beyond K+ regulation.
The findings of Hirose and colleagues raise the intriguing possibility that inflammation can confer protection of sensory organs. This would run counter to many earlier studies of cochlear inflammation, although most of these involved viral infection or instillation of an antigen directly into the cochlea (Ryan et al., 2002; Woolf, 1996). Reported protection against NIHL by glucocorticoids and other steroid anti-inflammatories (Sendowski et al., 2006; Tabuchi et al., 2005; Takemura et al., 2004) also argues against a beneficial role of inflammation, although steroids may also act through antioxidant and anti-apoptotic effects (summarized in Sendowski et al., 2006). One biochemical process by which leucocytes may invade the lateral wall after noise exposure was recently characterized by Shi and Nuttall (Shi et al., 2007a). Noise activates poly (ADP-ribose) polymerase-1 (PARP-1), a DNA repair enzyme, in capillary endothelial cells of the stria and ligament. PARP-1, it was suggested, may act through transcription factor NFκB to cause expression of leukocyte adhesion factors ICAM-1, PECAM-1, and P-selectin by capillary endothelial cells. The resulting slowing and adherence of leucocytes to capillary walls may serve both to impair blood flow and promote leukocyte extravasation into spiral ligament, which was interpreted to represent a purely pathological event.
Noise Injury to the Organ of Corti versus Cochlear Lateral Wall
Among the many cellular targets of noise injury, most permanent threshold shifts appear explainable in terms of hair cell loss, non-lethal injury to hair cells, or changes in the cellular makeup or conformation of the organ of Corti (Chen et al., 2003; Hamernik et al., 1989; Liberman et al., 1978; Ou et al., 2000; Wang et al., 2002b). While EP reduction may contribute to acute threshold shifts, under most exposure conditions, the EP probably recovers (Hirose et al., 2003; Ohlemiller et al., 2007). Since generation of the EP involves regulation of many ions including Na+, K+, Ca++, Cl−, and H+ (Hibino et al., 2006; Wangemann, 2006), and since the EP further requires maintenance of scala media boundaries, we submit that recovery of the EP indicates recovery of normal cochlear fluid homeostasis. Some permanent cell loss within stria, ligament, and limbus probably can probably be tolerated because of redundancy of cells, and perhaps cell functions, in these structures. The point of the foregoing is this: Based on the apparent physiological and anatomical recovery of the cochlear lateral wall, no obligate role for the lateral wall in determining the amount of noise-related PTS is indicated. Studies that assert such a link should make clear by what mechanism it is supposed to operate, and whether it is based on events at the time of exposure or permanent lateral wall dysfunction. Given that some innate protective mechanisms may decrease the EP and reduce strial K+ efflux (Housley et al., 2002; Lee et al., 2008), it is quite possible that EP reduction by noise partially offsets any harmful effects of lateral wall injury (Kanno et al., 1993). As a model—or target—for future experiments, we will argue that acute lateral wall injury can increase the severity of PTS through mechanisms dominated by ‘non-traditional’ functions of gap junctions.
Disruption of gap junctions by noise
Channels composed prominently of connexins 26 and 30 (Cx26, Cx30) link Deiters’, Hensen’s, Claudius, and outer sulcus cells to form a continuous path for K+ ions into extracellular spaces of the spiral ligament (Hibino et al., 2006; Wangemann, 2006). Once in the ligament, they are actively taken up by Type II and IV fibrocytes for conveyance to the stria vascularis via another junctional network connecting fibrocytes and strial basal cells, intermediate cells, and pericytes. Genetic impairments of mechanisms for removing K+ from the fluid immediately surrounding hair cells (Boettger et al., 2002; Boettger et al., 2003; Cohen-Salmon et al., 2002), and for moving K+ though the ligament (Delprat et al., 2005; Minowa et al., 1999), promote hair cell loss. The typically modest permanent effects of noise on the lateral wall seem unlikely to exert such dramatic effects. The acute phase of noise injury, however, could feature failure of ion regulatory mechanisms on a sufficient scale to impact the organ of Corti. Potential mechanisms for this include reversible mechanical disruption, as well as osmotic or redox-based reduction of gap junction conductance (Anand et al., 2005; Chanson et al., 2005). It could be just such processes that are ameliorated by certain chemokines, receptors, or macrophages, thus accounting for apparent protective effects of CCR2 (Sautter et al., 2006). Notably, gap junction uncouplers can produce acute lateral wall pathology resembling that caused by noise (Hirose et al., 2003; Ohlemiller et al., 2007; Spiess et al., 2002).
Functions of gap junctions beyond K+ transport may protect the organ of Corti
The effects of impaired intercellular communication in noise injury need not be limited to K+ dysregulation. Certainly, elevated extracellular K+ can alter cell membrane potentials and influence excitability, yet it is not clear just how cytotoxic K+ is, or how readily it promotes cell death in the organ of Corti. Burgeoning evidence from an active and exciting area of inquiry (Beltramello et al., 2005; Zhang et al., 2005; 2006) indicates that the role of gap junctions extends beyond intercellular K+ trafficking to encompass bidirectional movement of ATP, Ca++, cyclic AMP, and inositol triphosphate (IP3). This traffic may serve both communication and nutritive functions. Ca++ ‘waves’ may expedite movement of ATP and IP3 as part of a coordinated response to local noise injury (Gale et al., 2004; Piazza et al., 2007). Newly revealed roles for coupled connexins, connexin hemi-channels, and even cytosolic connexins (Krysko et al., 2005; Lin et al., 2003; Zhao et al., 2005) add yet more potential modes of interaction between the organ of Corti and lateral wall during noise exposure. At least for molecules less than ~1 kilodalton in size (Matsunami et al., 2006), a significant aspect of this interaction could be flow of metabolites from capillaries in the ligament and stria to the organ of Corti. Most nutrients are assumed to reach the organ through perilymphatic spaces rather than endolymph (e.g., Ito et al., 1993). Since ligament fibrocytes must be able to take up nutrients from both strial and ligament capillaries, reduction in distance and increased efficiency could be obtained by conveying these to the organ via gap junctions.
Contributions of the lateral wall to permanent injury of the organ of Corti and NIHL seem more likely to reflect acute disruption of extended functions of gap junctions than K+ regulation. K+ may be indirectly involved, insofar as acute elevation of K+ in the extracellular space may contribute to gap junction uncoupling. Some noted changes in lateral wall function, such as reduction in activity of Ca++- and Na+/K+-ATPases, shown to occur in the acute phase after noise (Cheng et al., 2008; Hsu et al., 2000), are probably correlated--rather than causal--events that coincide with reversible EP reduction. A potential limitation for two-way communication schemes utilizing gap junctions is discontinuity of the syncytium between the outer sulcus cells and Type II fibrocytes. Presumably only molecules actively taken up the ‘downstream’ cell could travel much of the distance between stria and organ of Corti.
Type IV fibrocytes in PTS
Type IV fibrocytes may be dramatically reduced in number by noise (Wang et al., 2002b), and some investigators have attributed to these a significant role in hearing loss. This is certainly plausible. Among all fibrocytes, these cells in particular can be decimated by noise, potentially giving rise to mechanisms for hair cell loss described above. There is disagreement, however, regarding whether K+ moves principally through the organ of Corti or scala tympani. Outer sulcus cells, whose root processes interdigitate with Type II fibrocytes, seem ideal for transporting K+ from the lateral organ of Corti. Type IV fibrocytes reside more inferiorly within highly porous bone, and seem well-positioned to take up K+ from perilymph. The extent of participation of Type IV cells in essential gap junction signaling functions is unknown. We and others (Gao et al., 2007) have emphasized their possibly redundant role, and the lack of clear evidence that their loss promotes hearing loss. Type IV fibrocytes do not appear to express Na-K-Cl cotransporter at the same levels as do Type II fibrocytes (Kikuchi et al., 2000a; 2000b), and may not be as critical for K+ transport.
Opposite effects of the same cascades
Apparent contradictions may cloud the role in NIHL of signaling cascades that are protective only under narrow conditions, or that operate in organ of Corti and lateral wall with opposite effects. Transcription factor NFκB and nitric oxide offer good recent examples (Fujioka et al., 2006; Masuda et al., 2006). Noise-related activation of NFκB can upregulate inducible nitric oxide synthase (iNOS). The resulting NO generation may preserve cochlear blood flow in the lateral wall, but may also promote peroxynitrite production and nitrosative stress in both the lateral wall and organ of Corti (Shi et al., 2007b). By contrast, NFκB activation within radial afferent dendrites may aid Ca++ regulation and reduce excitotoxic injury (Tahera et al., 2006b). As we also considered (Shi et al., 2007a), noise-related NFκB activation in the lateral wall would be expected to promote vasodilation, but in doing so apparently also promotes leucocyte adhesion and inflammation. The good guy/bad guy status of NFκB and NOS may be clarified using KO mouse models. At least on certain genetic backgrounds (Lang et al., 2006a), the absence of NFκB appears deleterious. To our knowledge, unambiguous effects on NIHL of eliminating one or more NOS isoforms have not yet been demonstrated.
Protective Mechanisms
Noise exposure engages a number of innate protective and repair mechanisms. These operate at multiple levels, encompassing brain-coordinated protective systems such as middle ear reflexes and cochlear efferents, paracrine signaling among inner ear cells, and subcellular biochemical cascades. Genetic polymorphisms that impact the efficacy of innate protections, as well as their enhancement by preconditioning effects, probably account for some inter-individual variation in noise susceptibility. Recent developments in this area include new evidence for protection by lateral efferent neurons, new details regarding mechanisms of sound conditioning, and a new form of preconditioning.
Cochlear efferent systems
Evidence indicates that the strength of the medial olivocochlear (MOC) efferent reflex can account for some inter-individual differences in noise susceptibility (Maison et al., 2000) (See Wagner et al., 2005 for contrary result.). In keeping with this, genetically-engineered enhancement of the MOC system appears protective against both TTS and PTS in mice (Maison et al., 2002). New evidence (Darrow et al., 2007) now implicates the lateral (inner hair cell afferent) olivocochlear (LOC) system in resistance to TTS. It is suggested that LOC activity reduces excitotoxic injury to afferent dendrites, thought to represent a major component of TTS.
There remains disagreement as to whether resistance to noise injury would have presented significant selective pressure to steer the evolution of cochlear efferent systems (Kirk et al., 2003). Protection from noise may only be a by-product of other selective pressures, most likely detection of signals in noise. However, the same objection applies to protective subcellular cascades (e.g., antioxidants, heat shock proteins) that have been shown to mitigate NIHL. These arose in response to the survival needs of eukaryotic cells long predating the cochlea, yet are engaged in the cochlea by noise stress. Complex paracrine signaling systems, such as purinergic mechanisms we consider next, are more difficult to divine from an evolutionary drive perspective, since they may engage competing—even opposing—processes. Regardless of how and why they evolved, these systems and cascades appear to have found new utility against noise, and mutations that impair their function may have new consequences.
Purinergic regulation and signaling
Noise exposure promotes the release into endolymph of adenosine and adenosine triphosphate (ATP) (Ford et al., 1997; Munoz et al., 2001). These act in paracrine manner on cells lining scala media, both by direct gating of ion currents, and via G-protein-coupled cascades. Adenosine receptors are widely distributed in the organ of Corti, spiral ganglion, and lateral wall (Vlajkovic et al., 2007), and are upregulated by noise (Ramkamur et al., 2004). Application of adenosine analog R-phenylisopropylaenosine (R-PIA) has been shown to reduce NIHL in chinchillas (Hu et al., 1997), and may also effect protection by activating antioxidant systems (Ramkamur et al., 2001). The primary source of noise-elicited endolymphatic ATP release appears to be marginal cells of stria vascularis (Munoz et al., 2001; White et al., 1995). Like adenosine receptors, ATP receptors are widely distributed around the luminal surfaces of scala media (Lee et al., 2001; Mockett et al., 1994; Mockett et al., 1995). Because ATP can be toxic, endolymph also contains enzymes (ectonucleotidases) that hydrolyze ATP to ADP (Vlajkovic et al., 2002). These are up-regulated by noise in parallel with ATP levels (Vlajkovic et al., 2004; 2006). We have already considered that Ca++ waves transmitted through gap junctions within the organ of Corti (and perhaps lateral wall) facilitate rapid ATP movement, potentially as part of a response to noise injury (Gale et al., 2004; Piazza et al., 2007). ATP in the organ may in part act directly on mechanics to reduce cochlear amplification (Bobbin et al., 2005). Another way to reduce the responsiveness of the cochlea is to reduce the currents that drive hair cell receptor potentials. Additional protective effects of ATP are thought to arise from its activation of a K+ shunt conductance that lowers the input resistance of scala media and reduces the EP (Munoz et al., 1995; 1999; Thorne et al., 2004). While the current shunt runs through many cell types, a particularly active sink for K+ appears to be the outer sulcus cells (Lee et al., 2001). K+ release from strial marginal cells is also subject to ATP-mediated negative feedback acting through a G-protein-triggered cascade (Marcus et al., 1998; 2005). In sum, purinergic regulatory systems in the cochlea appear to constitute a protective mechanism that is probably genetically modifiable (Van Laer et al., 2006). They may also fine-tune both cochlear micromechanics and fluid homeostasis to maintain constancy of hearing sensitivity.
Preconditioning phenomena
Pharmacologic approaches to preventing NIHL have been, with few exceptions, only somewhat successful. This may reflect a tendency of exogenous agents to throw complex protective feedback loops out of balance. For this reason, any single agent may both promote and impair innate protective mechanisms (See Endo et al., 2005 for similar complication in a transgenic mouse.). Another approach is preconditioning. That is, engaging innate protection using mild stress, thereby conferring protection against later, more severe stress. Preconditioning has the potential to activate multiple endogenous mechanisms in a coordinated manner and thus produce clearer net benefits. Preconditioning engaged by moderate heat, ischemia, hypoxia, or hyperoxia has been found effective against injury in many tissues, including brain, heart, and retina (Dirnagl et al., 2003; Gidday, 2006; Pasupathy et al., 2005; Ran et al., 2005). In the cochlea, preconditioning protection accrues from mild heat (Yoshida et al., 1999b), sound (Canlon, 1997; Niu et al., 2002; Yoshida et al., 2000), and restraint (Wang et al., 2002a). Canlon and colleagues (Tahera et al., 2006a; Tahera et al., 2007; Tahera et al., 2006b) have proposed that glucocorticoids mediate protection by sound and restraint, although most data have been obtained in the context of neuronal injury during TTS. New evidence now adds mild hypoxia (4 hr 8% O2) to the list of effective preconditioning methods in mice (Gagnon et al., 2007). Protection from PTS by hypoxia was shown to differ between inbred strains, such that CBA/J and CBA/CaJ mice benefited, while B6 did not. Strain effects were suggested to be due to differences in the activation of hypoxia-inducible factor 1α (HIF-1α). Hypoxia, heat stress, loud sound, etc., are not immediately convertible to practical therapies, of course. However, pharmacologic mimicry of these by applying agonists for upstream events may ultimately have advantages over some other agents.
Noise Injury as a Contributor to Presbycusis
Degeneration due to noise and aging converges on the same three principal cochlear structures, namely organ of Corti, neurons, and stria vascularis. Unlike aging, the generally accepted picture of noise injury has been one of a stable lesion that may interact with aging in a additive, less than additive (Boettcher, 2002; Mills et al., 1997), or sometimes super-additive way (Miller et al., 1998). Overall similarity of the main focus of noise injury and sensory presbycusis—the organ of Corti—has led us and others to propose overlap of mechanisms. To our knowledge, no one has proposed similar overlap for noise and strial presbycusis, although it cannot be ruled out that subtle strial injury is unstable and eventually promotes EP reduction. The great recent surprise has been that early noise exposure in CBA/CaJ mice—even exposure that does not cause measurable NIHL—can lead later in life to accelerated loss of spiral ganglion cells (Kujawa et al., 2006). This neuronal loss, which occurs even in the absence of hair cell loss (Fig. 3), dominates late-life hearing loss, and effectively creates neural presbycusis. The authors propose that early exposure in mice (<4 mos of age) may irreparably alter trophic interactions needed for neuronal survival, or promotes acute or chronic excitotoxicity (See also Pujol et al., 1991). These findings are certain to force a re-examination of the stability of NIHL as a function of age and genetics. Future developments along this line of experimentation may re-energize research in presbycusis.
Figure 3.
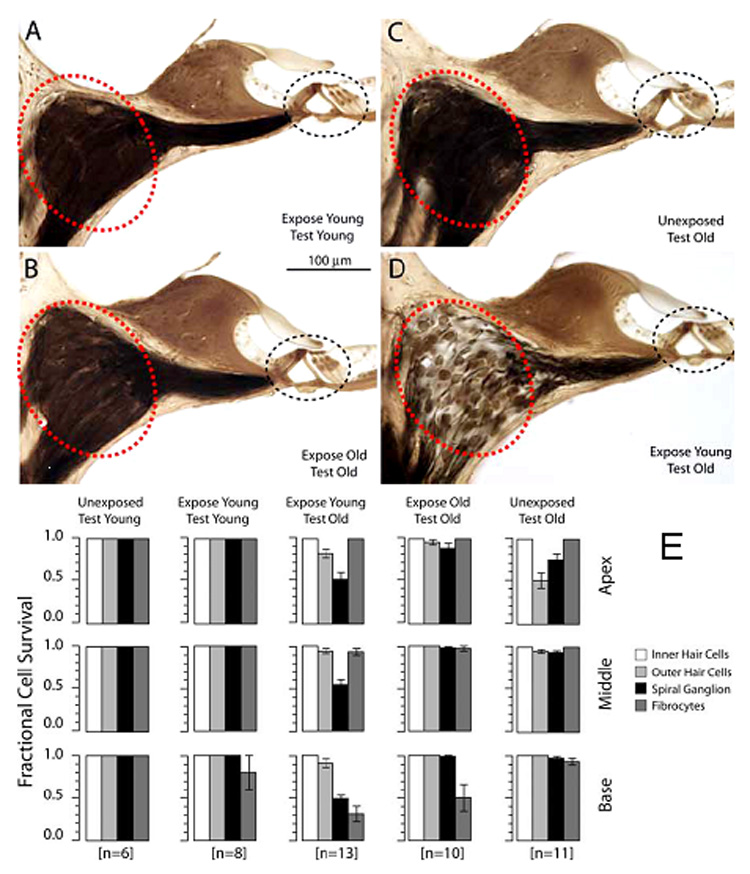
A.–D. Thick plastic sections from example cochlear upper basal turn of CBA/CaJ mice that were noise-exposed one time, either when they were young (4 wks) or old (96–98 wks). Mouse exposed when young and then allowed to age (D) shows greater neuronal loss than mouse examined young (A) or exposed and examined old (B). (Compare large circled areas in A,B,D.). C shows an old non-exposed control. Organ of Corti (small circled areas) appears normal in all cases. E. Quantitation of trends indicated in A–D. While Type IV fibrocytes were reduced in number after exposure at any age, spiral ganglion cells were notably reduced only in mice that were exposed young and allowed to age, despite the survival of inner hair cells. (Adapted with permission from Kujawa and Liberman, 2006)
New Pharmacologic Interventions
Recent studies have tested the protective ability of a wide variety of compounds against permanent NIHL. Given that benefit accrues from application of many types of antioxidants, growth factors, calcium antagonists, and anti-apoptotics, there is hope that some combination of these can ultimately be shown practical in humans, assuming they can be applied orally, and in time. As can be gleaned from Table I, most of the newer studies (>2005) have focused on antioxidants. The efficacy of any single antioxidant may be limited by several factors, including limited access to cellular compartments, action against only few forms of ROS, interference with redox-based signaling, or a tendency to throw innate ROS protections out of balance. In addition, the mechanisms of noise injury encompass parallel pathways that are not limited to oxidative stress. Thus, therapies combining multiple antioxidants, or antioxidants plus other agents, may have advantages over single-agent approaches. At present, we know of two trials using combination therapies that have demonstrated clear additive or multiplicative interactions (Choi et al., 2008; Le Prell et al., 2007a).
Table 1.
Therapeutics shown successful against NIHL 2005–2008
| Agent | Type | Route | Subjects | Type of noise | Reference |
|---|---|---|---|---|---|
| Salicylate | *Antioxidant | IP | Mice | BBN, 113 dB, 3.5 hr | Adelman et al., 2008 |
| D-methionine | Antioxidant | IP | Mice | 4 kHz OBN, 110 dB, 4 hr | Samson et al., 2008 |
| Salicylate + Trolox | Antioxidant, Anti-nitrosative | IP, SC | Guinea pigs | 4 kHz OBN, 120 dB, 5 hr | Yamashita et al., 2005 |
| Tempol + Creatine | Antioxidants | Oral | Guinea pigs | 4 kHz OBN, 120 dB, 5 hr | Minami et al., 2007 |
| Tempol or 3-aminobenzamide | Antioxidant PARS inhib. | IP | Mice | 4 kHz, 128 dB, 4 hr | Murashita et al., 2006 |
| Vit. E + Idebenone | Antioxidants | IP, IM | Guinea pigs | 6 kHz, 120 dB, 40 min | Fetoni et al., 2008 |
| N-acetyl-cysteine | Antioxidant | IP, oral | Chinchillas | Various High-Kurtosis | Bielefeld et al., 2007 |
| Idebenone | Antioxidant | IP | Guinea pigs | 6 kHz, 120 dB, 40 min | Sergi et al., 2006 |
| Vit. C | Antioxidant | Diet | Guinea pigs | 4 kHz OBN, 114 dB, 6 hr | McFadden et al., 2005 |
| Edaravone | Antioxidant | Perilymph | Guinea pigs | 4 kHz ?BN, 130 dB, 4 hr | Tanaka et al., 2005 |
| Vit. A+C+E + Mg++ | **Antioxidants + Ca++ inhib. | IP | Guinea pigs | 4 kHz OBN, 120 dB, 5 hr | Le Prell et al., 2007 |
| Hydroxy-phenyl- | **Antioxidants | IP | Chinchillas | 4 kHz OBN, 105 dB, 6 hr | Choi et al., 2008 |
| N-tert-butylnitrone + | + Energy enhancer | ||||
| N-acetyl-cysteine + | |||||
| Acetyl-carnitine | |||||
| N-acetyl-cysteine | Antioxidant | IP | Chinchillas | 4 kHz OBN, 105 dB, 6 hr | Coleman et al., 2007a |
| Acetyl-carnitine | Energy enhancer | ||||
| T-817MA | Antioxidant and Neuroprotectant | Oral | Guinea pigs | 4 kHz OBN, 120 dB, 5 hr | Yamashita et al., 2008 |
| BN 82270 | Antioxidant, Calpain inhibitor | Perilymph | Guinea pigs | 6 kHz, 120 dB, 30 min | Wang et al., 2007a |
| Cyclosporin A | Calcineurin | IP | Guinea pigs | 2 kHz, 120 dB, 10 min | Uemaetomari et al., 2005 |
| FK506 | Inhibitors | Mice | 4 kHz, 128 dB, 4 hr | ||
| Trimethadione | T-type Ca++ | IP | Mice | BBN, 110 dB, 30 min | Shen et al., 2007 |
| Ethosuximide | channel blockers | ||||
| Caroverine | Glutamate antagonist | SC | Rats | Impulse Noise 160 dB peak 50 pulses |
Duan et al., 2006 |
| Geranylgeranyl-acetone | Induces heat shock proteins | Oral | Guinea pigs | 4 kHz OBN, 130 dB, 3 hr | Mikuriya et al., 2005 |
| Human insulin-like growth factor-1 | Growth factor | Round window | Rats | BBN, 120 dB, 2 hr | Iwai et al., 2006 |
| Amitriptyline | Induces neurotrophic factor | IP | Guinea pigs | 4 kHz OBN, 117 dB, 24 hr | Shibata et al., 2007 |
| Retinoic acid | Anti-apoptotic | Oral | Mice | BBN, 122 dB, 3 hr/d 3 days | Ahn et al., 2005 |
| AM-111 | Anti-apoptotic | IP, Round window | Chinchillas | Impulse Noise 155 dB peak 150 pulses |
Coleman et al., 2007b |
| D-JNKI-1 peptide | Anti-apoptotic | Round window | Guinea pigs | 6 kHz, 130 dB, 15 min | Wang et al., 2007b |
Also intended to de-sensitize OHCs
Added benefit from combination
BBN:Broadband noise; OBN:Octave-band noise
Conclusions
Unclear assumptions regarding exactly how events in the cochlear lateral wall are supposed to mediate PTS have rendered some recent noise exposure results difficult to interpret. Noise can permanently affect both the organ of Corti and lateral wall, but the extent of PTS is probably established by injury to the organ of Corti alone. Therefore, any exacerbation of PTS by events in the lateral wall must be assumed either to occur acutely at the time of exposure, or through ongoing effects of permanent injury to the spiral ligament and stria vascularis. Such a long term influence has the potential to produce unstable injury, which is counter to most conceptions of NIHL. As a framework for new experiments, we propose that the lateral wall does indeed modulate noise injury to the organ of Corti. However, it is not the extent of permanent injury to the lateral wall, but rather the degree of acute disruption of gap junctions connecting the organ and spiral ligament that sets the amount of PTS. In accord with mounting evidence, gap junctions are taken to mediate two-way passage of signaling molecules and nutrients needed to maintain the organ of Corti, and protect it from noise injury. The role of K+ dysregulation may primarily be to disrupt gap junction conductance.
New developments undermine some long-held assumptions about NIHL in mammals. It appears necessary to re-examine generalizations about exactly which cochlear cell types are killed or impaired by noise. We cannot use guinea pigs—or even other mouse strains—to infer necessarily what genes are expressed or what pathways are activated in, say, the CBA/CaJ mouse cochlea after noise. It is also necessary to reconsider the role inflammation triggered by noise exposure. Exposure results in young animals challenge the presumed stability of PTS, and the independence of noise- and age-related pathology. If we add to these the potential confounds posed by developmental stress, inadvertent preconditioning, epigenetic effects, and gene copy number, a ‘complete’ set of controls for any experiment becomes infeasible, and the caveats attending all findings become too many to list. Which models are most relevant to human NIHL? Which conditions? They all are. We simply need to recognize the incompleteness of any one model and experiment.
Acknowledgements
Thanks to Drs. Sharon Kujawa and Keiko Hirose for sharing their work, Keiko Hirose for comments on the manuscript, and Drs. Elizabeth Keithley and Josef Miller for helpful discussions. Thanks also to Ashley Dahl for administrative assistance. Supported by NIH R01 DC008321 (KKO), R01 DC03454 (KKO), and P30 DC04665 (R. Chole).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang C-H, Crumling MA, Swiderski DL, Beyer LA, Gong T-WL, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hearing Res. 2006;218:20–29. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Adelman C, Freeman S, Paz Z, Sohmer H. Salicylic acid injection before noise exposure reduces permanent threshold shift. Audiol. Neurotol. 2008;13:266–272. doi: 10.1159/000115436. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Kang HH, Kim Y-J, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochemical and Biophysical Research Communications. 2005;335:485–490. doi: 10.1016/j.bbrc.2005.07.114. [DOI] [PubMed] [Google Scholar]
- Anand RJ, Hackam DJ. The role of gap junctions in health and disease. Crit. Care Med. 2005;33 Suppl.:S535–S538. doi: 10.1097/01.ccm.0000194035.40266.b2. [DOI] [PubMed] [Google Scholar]
- Attias J, Pratt H. Auditory-evoked potential correlates of susceptibility to noise-induced hearing loss. Audiology. 1985;24:149–156. doi: 10.3109/00206098509081548. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of disease. Eur, J. Clin. Invest. 1995;25:457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Barker DJP. In utero programming of chronic disease. Clin. Sci. 1998;95:115–128. [PubMed] [Google Scholar]
- Barrenäs M-L, Bratthall A, Dahlgren J. The thrifty phenotype hypothesis and hearing problems. Brit. Med. J. 2003;327:1199–1200. doi: 10.1136/bmj.327.7425.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrenäs M-L, Bratthall A, Dahlgren J. The association between short stature and sensorineural hearing loss. Hearing Res. 2005;205:123–130. doi: 10.1016/j.heares.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Barrenäs M. Hair cell loss from acoustic trauma in chloroquine-treated red, black and albino guinea pigs. Audiology. 1997;36:187–201. [PubMed] [Google Scholar]
- Bartels S, Ito S, Trune DR, Nuttall AL. Noise-induced hearing loss: the effect of melanin in the stria vascularis. Hearing Res. 2001;154:116–123. doi: 10.1016/s0378-5955(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nature Cell Biology. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, Kopke RD, Jackson RL, Coleman JKM, Liu J, Henderson D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Oto-Laryngol. 2007;127:914–919. doi: 10.1080/00016480601110188. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Salt AN. ATP-γ-S shifts the operating point of outer hair cell transduction towards scala tympani. Hearing Res. 2005;205:35–43. doi: 10.1016/j.heares.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Boettcher FA. Susceptibility to acoustic trauma in young and aged gerbils. J. Acoust. Soc. Am. 2002;112:2948–2955. doi: 10.1121/1.1513364. [DOI] [PubMed] [Google Scholar]
- Boettger T, Hubner C, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lasking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–877. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Pape H-C, Volkl H, Hubner C, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hearing Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi P. Il colore dell'iride come "test" di valutazione quantiatativia, nell'uomo. della concentrazzione di melania nella stria vascolare. 1965;64:725–738. [PubMed] [Google Scholar]
- Borg E, Canlon B, Engstrom B. Noise-induced hearing loss: Literature review and experiments in rabbits. Morphological and physiological features, exposure parameters and temporal factors, variability and interactions. Scand. Audiol. 1995;40 Suppl.:1–147. [PubMed] [Google Scholar]
- Canlon B. Protection against noise trauma by sound conditioning. Ear, Nose, Throat J. 1997;76:248–255. [PubMed] [Google Scholar]
- Canlon B, Erichsen S, Nemlander E, Chen M, Hossain A, Celsi G, Ceccatelli S. Alterations in intrauterine environment by glucocorticoids modifies the developmental programme of the auditory system. Eur. J. Neurosci. 2003;17:2035–2041. doi: 10.1046/j.1460-9568.2003.02641.x. [DOI] [PubMed] [Google Scholar]
- Chang N-C, Yu ML, Ho K-Y, Ho CK. Hyperlipidemia in noise-induced hearing loss. Otolaryngology-Head and Neck Surgery. 2007;137:603–606. doi: 10.1016/j.otohns.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Chanson M, Derouette J-P, Roth I, Foglia B, Scerri I, Dudez T, Kwak BR. Gap junctional communication in tissue inflammation and repair. Biochem. Biophys. Acta. 2005;1711:197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Chen G-D, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hearing Res. 2003;177:81–90. doi: 10.1016/s0378-5955(02)00802-x. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Liu S-H, Young Y-H, Hsu CJ, Lin-Shiau SY. Protection from noise-induced temporary threshold shift by D-Methionine is associated with preservation of ATPase activities. Ear Hear. 2008;29:65–75. doi: 10.1097/AUD.0b013e31815d635b. [DOI] [PubMed] [Google Scholar]
- Choi CH, Chen K, Vasquez-Weldon A, Jackson RL, Floyd RA, Kopke RD. Effectiveness of 4-hydroxy phenyl N-tert-butylnitrone (4-OHPBN) alone and in combination with other antioxidant drugs in the treatment of acute acoustic trauma in chinchilla. Free Radical Biology and Medicine. 2008;44:1772–1784. doi: 10.1016/j.freeradbiomed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin J-P, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junctional network causes hearing impairment and cell death. Curr. Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JKM, Littlesunday C, Jackson R, Meyer T. AM-111 protects against permanent hearing loss from impulse noise trauma. Hearing Res. 2007a;226:70–78. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Coleman JKM, Kopke RD, Liu J, Ge X, Harper EA, Jones GE, Cater TL, Jackson RL. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hearing Res. 2007b;226:104–113. doi: 10.1016/j.heares.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Abdul-Baqi KJ, McCandless GA, Creel DJ. Differential susceptibility to noise-induced permanent threshold shift between albino and pigmented guinea pigs. Hearing Res. 1986;23:81–91. doi: 10.1016/0378-5955(86)90177-2. [DOI] [PubMed] [Google Scholar]
- Cunningham DR, Norris ML. Eye color and noise-induced hearing loss: a population study. Ear Hear. 1982;3:211–214. doi: 10.1097/00003446-198207000-00005. [DOI] [PubMed] [Google Scholar]
- Da Costa DA, Castro JC, Macedo MEG. Iris pigmentation and susceptibility to noise-induced hearing loss. Int. J. Audiol. 2008;47:115–118. doi: 10.1080/14992020701704776. [DOI] [PubMed] [Google Scholar]
- Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr. Opin. Otolaryngol. Head Neck Surg. 2007;15:358–363. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J. Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprat B, Ruel J, Guitton MJ, Hamard G, Lenoir M, Pujol R, Puel JL, Brabet P, Hamel CPJA. Deafness and cochlear fibrocyte alterations in mice deficient for the inner ear protein otospiralin. Molecular & Cellular Biology. 2005;25:847–853. doi: 10.1128/MCB.25.2.847-853.2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. TINS. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Duan M, Chen Z, Qiu J, Ulfendahl M, Laurell G, Borg E, Ruan R. Low-dose, long-term caroverine administration attenuates impulse noise-induced hearing loss in the rat. Acta. Otolaryngol. 2006;126:1140–1147. doi: 10.1080/00016480500540519. [DOI] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Iguchi F, Kita T, Okano T, Sha S-H, Schacht J, Shiga A, Kim T-S, Ito J. Elevation of superoxide dismutase increases acoustic trauma from noise exposure. Free Radical Biology and Medicine. 2005;38:492–498. doi: 10.1016/j.freeradbiomed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Engel J, Braig C, Ruttiger L, Kuhn S, Zimmermann U, Blin N, Sausbier M, Kalbacher H, Munkner S, Rohbock K, Ruth P, Winter H, Knipper M. Two classes of outer hair cells along the tonotopic axis of the cochlea. Neurosci. 2006;143:837–849. doi: 10.1016/j.neuroscience.2006.08.060. [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau Y-W, Davis RR, Kreig EF. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hearing Res. 1996;93:181–187. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Fairfield DA, Lomax MI, Dootz GA, Chen S, Galecki TA, Benjamin IJ, Dolan DF, Altschuler RA. Heat shock factor 1-deficient mice exhibit decreased recovery of hearing following noise overstimulation. J. Neurosci. Res. 2005;81:589–596. doi: 10.1002/jnr.20417. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Gearhart C, Fulton S, Campbell J, Fisher J, Na K, Cocker D, Nelson-Miller A, Moon P, Pouyatos B. Promotion of noise-induced cochlear injury by toluene and ethylbenzene in the rat. Toxicolog. Sci. 2007;98:542–551. doi: 10.1093/toxsci/kfm109. [DOI] [PubMed] [Google Scholar]
- Feil R. Environmental and nutritional effects on the epigenetic regulation of genes. Mutation Res. 2006;600:46–57. doi: 10.1016/j.mrfmmm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Ferraresi A, La Greca C, Rizzo D, Sergi B, Tringali G, Piacentini R, Troiani D. Antioxidant protection against acoustic trauma by coadministration of idebenone and vitamin E. NeuroReport. 2008;19:277–281. doi: 10.1097/WNR.0b013e3282f50c66. [DOI] [PubMed] [Google Scholar]
- Ford MS, Maggirwar SB, Rybak LP, Whitworth C, Ramkamur V. Expression and function of adenosine receptors in the chinchilla cochlea. Hearing Res. 1997;105:130–140. doi: 10.1016/s0378-5955(96)00204-3. [DOI] [PubMed] [Google Scholar]
- Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, Vitale DF, La Manna P, Saulino C, Marcelli V, Sacchetti L. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin. Chem. 2004;50:2012–2018. doi: 10.1373/clinchem.2004.037788. [DOI] [PubMed] [Google Scholar]
- Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of Corti after acoustic overstimulation. Acta. Otolaryngol. 1990;109:76–82. doi: 10.3109/00016489009107417. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006;83:575–583. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- Gagnon PM, Simmons DD, Bao J, Lei D, Ortmann AJ, Ohlemiller KK. Temporal and genetic influences on protection against noise-induced hearing loss by hypoxic preconditioning in mice. Hearing Res. 2007;226:79–91. doi: 10.1016/j.heares.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr. Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gallit J, Clark RA. Wound repair in the context of extracellular matrix. Curr. Op. Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Gross M-S, Junien C. Nutri-epigenomics: Lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin. Chem. Lab. Med. 2007;45:321–327. doi: 10.1515/CCLM.2007.081. [DOI] [PubMed] [Google Scholar]
- Gao J, Maison SF, Wu X, Hirose K, Jones SM, Bayazitov I, Tian Y, Mittleman G, Matthews DB, Zakharenko SS, Liberman MC, Zuo J. Orphan glutamate receptor δ1 subunit required for high-frequency hearing. Molec. Cell. Biol. 2007;27:4500–4512. doi: 10.1128/MCB.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature Rev. Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen-matrix contraction. growth-factor signalling and mechanical loading. Trends in Cell Biology. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hearing Res. 1989;38:199–212. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Han W, Shi X, Nuttall AL. AIF and endoG translocation in noise exposure induced hair cell death. Hearing Res. 2006;211:85–95. doi: 10.1016/j.heares.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Schacht J. Sketches of Otohistory. Part 10: Noise-induced hearing loss. Audiol. Neurotol. 2005;10:305–309. doi: 10.1159/000087347. [DOI] [PubMed] [Google Scholar]
- Heinonen-Guzejev M, Vuorinen HS, Mussalo-Rauhamaa H, Heikkila K, Koskenvou M, Kaprio J. Genetic component of noise sensitivity. Twin Res. & Human Genet. 2005;8:245–249. doi: 10.1375/1832427054253112. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hibino H, Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology. 2006;21:336–344. doi: 10.1152/physiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Barrenas M-L, Kristiansen GB, Lund SP. No evidence for enhanced noise induced hearing loss after prenatal stress or dexamethasone. Neurotoxicol. Teratol. 2007;29:613–621. doi: 10.1016/j.ntt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJM. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol. Neuro-Otol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- Hsu CJ, Shau W-Y, Chen Y-S, Liu T-C, Lin-Shiau SY. Activities of Na+,K+-ATPase and Ca2+-ATPase in cochlear lateral wall after acoustic trauma. Hearing Res. 2000;142:203–211. doi: 10.1016/s0378-5955(00)00020-4. [DOI] [PubMed] [Google Scholar]
- Hu BH, Zheng XY, McFadden S, Henderson D. The protective effects of R-PIA on noise-induced hearing loss. Hearing Res. 1997;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Yoshida K, Hirano T, Suzuki M, Mogi G. Significance of spiral ligament fibrocytes with cochlear inflammation. Int. J. Ped. Otorhinolaryngol. 2000;56:45–51. doi: 10.1016/s0165-5876(00)00408-0. [DOI] [PubMed] [Google Scholar]
- Ito M, Spicer SS, Schulte BA. Immunohistochemical localization of brain type glucose transporter in mammalian inner ears: Comparison of developmental and adult stages. Hearing Res. 1993;71:230–238. doi: 10.1016/0378-5955(93)90039-4. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim T-S, Tabata Y, Ito J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–533. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least 10 inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Kadner A, Pressimone VJ, Lally BE, Salm AK, Berrebi AS. Low-frequency hearing loss in prenatally stressed rats. NeuroReport. 2006;17:635–638. doi: 10.1097/00001756-200604240-00015. [DOI] [PubMed] [Google Scholar]
- Kanno H, Ohtani I, Hara A, Kusakari J. The effect of endocochlear potential suppression upon susceptibility to acoustic trauma. Acta. Otolaryngol. 1993;113:26–30. doi: 10.3109/00016489309135762. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Research Reviews. 2000a;32:163–166. doi: 10.1016/s0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T. Potassium recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med. Elecron Microsc. 2000b;33:51–56. doi: 10.1007/s007950070001. [DOI] [PubMed] [Google Scholar]
- Kirk EC, Smith DW. Protection from acoustic trauma is not a primary function of the medial olivocochlear system. J. Assoc. Res. Otolaryngol. 2003;4:445–465. doi: 10.1007/s10162-002-3013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke RD, Jackson RL, Coleman JKM, Liu J, Bielefeld EC, Balough BJ. NAC for noise: From the bench top to the clinic. Hearing Res. 2007;226:114–125. doi: 10.1016/j.heares.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Leybaert L, Vandenbeele P, Herde KD. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis. 2005;10:459–469. doi: 10.1007/s10495-005-1875-2. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise: Evidence of a misspent youth. J. Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Zhou D, Smythe NM, Spicer SS, Schmiedt RA. Nuclear factor κB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J. Neurosci. 2006a;26:3541–3550. doi: 10.1523/JNEUROSCI.2488-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Ebihara Y, Schmiedt RA, Minamiguchi H, Zhou D, Smythe NM, Liu L, Ogawa M, Schulte BA. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: Mesenchymal cells and fibrocytes. J. Comp. Neurol. 2006b;496:187–201. doi: 10.1002/cne.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radical Biology and Medicine. 2007a;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hearing Res. 2007b;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Purinergic signaling in the inner ear. Hearing Res. 2008;235:1–7. doi: 10.1016/j.heares.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J. Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kiang N-YS. Acoustic trauma in cats: Cochlear pathology and auditory nerve activity. Acta Otolaryngol. 1978;358:1–63. [PubMed] [Google Scholar]
- Lim DJ. Effects of noise and ototoxic drugs at the cellular level in the cochlea: a review. American Journal of Otolaryngology. 1986;7:73–99. doi: 10.1016/s0196-0709(86)80037-0. [DOI] [PubMed] [Google Scholar]
- Lin JH-C, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M. Connexin mediates gap junction-independent resistance to cellular injury. The Journal of Neuroscience. 2003;23:430–441. doi: 10.1523/JNEUROSCI.23-02-00430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C-L, Billings P, Harris JP, Keithley EM. Characterization of an experimentally induced inner ear immune response. The Laryngoscope. 2000;110:451–456. doi: 10.1097/00005537-200003000-00024. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic trauma with a noninvasive assay of olivocochlear reflex strength. J. Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J. Neurosci. 2002;22:10838–10846. doi: 10.1523/JNEUROSCI.22-24-10838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Liu J, Lee JH, Scherer EQ, Scofield MA, Wangemann P. Apical membrane P2Y4 puinergic receptor controls K+ secretion by strial marginal cell epithelium. Cell Comm. Signal. 2005;3:13. doi: 10.1186/1478-811X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Sunose H, Liu J, Bennett T, Shen Z, Scofield MA, Ryan AF. Protein kinase C mediates P2U purinergic receptor inhibition of K+ channel in apical membrane of strial marginal cells. Hearing Res. 1998;115:82–92. doi: 10.1016/s0378-5955(97)00180-9. [DOI] [PubMed] [Google Scholar]
- Masuda M, Nagashima R, Kanzaki S, Fujioka M, Ogita K, Ogawa K. Nuclear factor-kappa B nuclear translocation in the cochlea of mice following acoustic overstimulation. Brain Res. 2006;1068:237–247. doi: 10.1016/j.brainres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Matsunami T, Suzuki T, Hisa Y, Takata K, Takamatsu T, Oyamada M. Gap junctions mediate glucose transport between GLUT1-positive and -negative cells in the spiral limbus of the rat cochlea. Cell Comm. Adhesion. 2006;13:93–102. doi: 10.1080/15419060600631805. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Woo JM, Michalak N, Ding D. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hearing Res. 2005;202:200–208. doi: 10.1016/j.heares.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson EM, Hultcrantz M, Charitidi K, Gustafsson J-A, Canlon B. Estrogen receptor B protects against acoustic trauma in mice. Journal of Clinical Investigation. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM. Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. 1988;1:238–249. doi: 10.1111/j.1600-0749.1988.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Mikuriya T, Sugahara K, Takemoto T, Tanaka K, Takeno K, Shimogori H, Nakai A, Yamashita H. Geranylgeranylacetone, a heat shock protein inducer, prevents acoustic injury in the guinea pig. Brain Res. 2005;1065:107–114. doi: 10.1016/j.brainres.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Miller JM, Dolan DF, Raphael Y, Altschuler RA. Interactive effects of aging with noise induced hearing loss. Scand. Audiol. 1998;27:53–61. [PubMed] [Google Scholar]
- Mills JH, Boettcher FA, Dubno JR. Interaction of noise-induced permanent threshold shift and age-related threshold shift. J. Acoust. Soc. Am. 1997;101:1681–1686. doi: 10.1121/1.418152. [DOI] [PubMed] [Google Scholar]
- Minami SB, Yamashita D, Ogawa K, Schacht J, Miller JM. Creatine and tempol attenuate noise-induced heaing loss. Brain Res. 2007;1147:83–89. doi: 10.1016/j.brainres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, Ogura M, Asada Y, Watanabe K, Yamanaka H, Gotoh S, Nishi-Takeshima M, Sugimoto T, Kikuchi T, Takasaka T, Noda T. Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafnesss. Science. 1999;285:1408–1411. doi: 10.1126/science.285.5432.1408. [DOI] [PubMed] [Google Scholar]
- Mockett BG, Housley GD, Thorne PR. Fluorescence imaging of extracellular purinergic sites and putative ecto-ATPase sites on isolated cochlear hair cells. J. Neurosci. 1994;14:1692–1707. doi: 10.1523/JNEUROSCI.14-11-06992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Bo X, Housley GD, Thorne PR, Burnstock G. Autoradiographic labelling of P2 purinoceptors in the guinea-pig cochlea. Hearing Res. 1995;84:177–193. doi: 10.1016/0378-5955(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Morita Y, Hirokawa S, Kikkawa Y, Nomura T, Yonekawa H, Shiroishi T, Takahashi S, Kominami R. Fine mapping of Ahl3 affecting both age-related and noise-induced hearing loss. Biochemical and Biophysical Research Communications. 2007;355:117–121. doi: 10.1016/j.bbrc.2007.01.115. [DOI] [PubMed] [Google Scholar]
- Munoz DJB, Thorne PR, Housley GD. P2X receptor-mediated changes in cochlear potentials arising from exogenous adenosine 5′-triphosphate in endolymph. Hearing Res. 1999;138:56–64. doi: 10.1016/s0378-5955(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Munoz DJB, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentration of ATP in the endolymph during sound exposure and hypoxia. Acta. Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- Munoz DJB, Thorne PR, Housley GD, Billett TE, Battersby JM. Extracellular adenosine 5′-triphosphate (ATP) in the endolymph compartment influences cochlear function. Hearing Res. 1995;90:106–118. doi: 10.1016/0378-5955(95)00152-3. [DOI] [PubMed] [Google Scholar]
- Murashita H, Tabuchi K, Hashino T, Tsuji S, Hara A. The effects of tempol, 3-aminobenzamide and nitric oxide synthase inhibitors on acousitc injury of the mouse cochlea. Hearing Res. 2006;214:1–6. doi: 10.1016/j.heares.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Niu X, Canlon B. Protective mechanisms of sound conditioning. Adv. Otorhinolaryngol. 2002;59:96–105. doi: 10.1159/000059246. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hearing Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Endocochlear potential (EP) reduction in albino coisogenics to C57BL/6 inbred mice after 24 months of age Abstr. Assn. Res. Otolaryngol. 2008;31:219. [Google Scholar]
- Ohlemiller KK, Wright JS, Heidbreder AF. Vulnerability to noise-induced hearing loss in 'middle-aged' and young adult mice: A dose-response approach in CBA, C57BL, and BALB inbred strains. Hearing Res. 2000a;149:239–247. doi: 10.1016/s0378-5955(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Lett JM, Gagnon PM. Cellular correlates of age-related endocochlear potential reduction in a mouse model. Hearing Res. 2006;220:10–26. doi: 10.1016/j.heares.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding D-L, Lear PM, Ho Y-S. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol. 2000b;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding D-L, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-Superoxide Dismutase gene (SOD1) increases susceptibility to noise-induced hearing loss. Audiol. Neuro-Otol. 1999;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- Ortmann AJ, Faulkner KF, Gagnon PM, Ohlemiller KK. Removal of the Ahl allele from the C57BL/6 background does not improve noise resistance. Abstr., Assn. Res. Otolaryngol. 2004;27:168. [Google Scholar]
- Ou HC, Bohne BA, Harding GW. Noise damage in the C57BL/CBA mouse cochlea. Hearing Res. 2000;145:111–122. doi: 10.1016/s0378-5955(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Pasupathy S, Homer-Vanniasinkam S. Ischaemic preconditioning protects against ischaemia/reperfusion injury: Emerging concepts. Eur. J. Vas. Endovasc. Surg. 2005;29:106–115. doi: 10.1016/j.ejvs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca++ wave propagation in the organ of Corti. Cell Calcium. 2007;41:77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Pujol R, Rebillard G, Puel J-L, Lenoir M, Eybalin M, Recasens M. Glutamate Neurotoxicity in the cochlea: a possible consequence of ischaemic or anoxic conditions occuring in ageing. Acta Otolaryngol. 1991;476:32–36. doi: 10.3109/00016489109127253. [DOI] [PubMed] [Google Scholar]
- Qiu W, Davis B, Hamernik RP. Hearing loss from interrupted, intermittent, and time varying Gaussian noise exposures: the applicability of the equal energy hypothesis. The Journal of the Acoustical Society of America. 2007;121:1613–1620. doi: 10.1121/1.2434692. [DOI] [PubMed] [Google Scholar]
- Ramkamur V, Hallam DM, Nie Z. Adenosine, oxidative stress and cytoprotection. Japanese Journal of Pharmacology. 2001;86:265–274. doi: 10.1254/jjp.86.265. [DOI] [PubMed] [Google Scholar]
- Ramkamur V, Whitworth C, Pingle SC, Hughes LF, Rybak LP. Noise induces A1 adenosine receptor expression in the chinchilla cochlea. Hearing Res. 2004;188:47–56. doi: 10.1016/S0378-5955(03)00344-7. [DOI] [PubMed] [Google Scholar]
- Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxic preconditioning in the brain. Dev. Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: Basic mechanisms and options for therapy. Acta Otolaryngol. 2002;548:38–43. doi: 10.1080/00016480260094965. [DOI] [PubMed] [Google Scholar]
- Samson J, Wiktorek-Smagur A, Politanski P, Rajkowska E, Pawlaczyk-Kowalska M, Dudarewicz A, Sha S-H, Schacht J, Sliwinska-Kowalska M. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attentuation by D-methionine. Neurosci. 2008;152:146–150. doi: 10.1016/j.neuroscience.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor Necrosis Factor-α, an initiator, and etanercept, an inhibitor of cochlear inflammation. The Laryngoscope. 2002;112:1627–1634. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Sautter NB, Shick EH, Ransohoff RM, Charo IF, Hirose K. CC chemokine receptor 2 is protective against noise-induced hair cell death: studies in CX3CR1+/GFP mice. J. Assoc. Res. Otolaryngol. 2006;7:361–372. doi: 10.1007/s10162-006-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Hausser H-J. Extracelular matrix and cytokines: a functional unit. Developmental Immunol. 2000;7:89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendowski I, Abaamrane L, Raffin F, Cros A, Clarencon D. Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs. Hearing Res. 2006;221:119–127. doi: 10.1016/j.heares.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Sergi B, Fetoni AR, Paludetti G, Ferraresi A, Navarra P, Mordente A, Troiani D. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. NeuroReport. 2006;17:857–861. doi: 10.1097/01.wnr.0000221834.18470.8c. [DOI] [PubMed] [Google Scholar]
- Shen H, Zhange B, Shin J-H, Lei D, Du Y, Gao X, Dai C, Ohlemiller KK, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers on noise-induced hearing loss. Hearing Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hearing Res. 2007a;224:1–14. doi: 10.1016/j.heares.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Shi X, Han W, Yamamoto H, Omelchenko I, Nuttall AL. Nitric oxide and mitochondrial status in noise-induced hearing loss. Free Rad. Res. 2007b;41:1313–1325. doi: 10.1080/10715760701687117. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Osumi Y, Yagi M, Kanda S, Kawamoto K, Kuriyama H, NIshiyama T, Yamashita T. Administration of amatriptyline attenuates noise-induced hearing loss via glial cell line-derived neurotrophic factor (GDNF) induction. Brain Res. 2007;1144:74–81. doi: 10.1016/j.brainres.2007.01.090. [DOI] [PubMed] [Google Scholar]
- Spiess AC, Lang H, Schulte BA, Spicer SS, Schmiedt RA. Effects of gap junction uncoupling in the gerbil cochlea. Laryngoscope. 2002;112:1635–1641. doi: 10.1097/00005537-200209000-00020. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Brun JP. Relation of structural damage to exposure time and intensity in acoustic trauma. Acta Otolaryngol. 1973;75:220–226. doi: 10.3109/00016487309139699. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Inouye S, Izu H, Katoh Y, Katsuki K, Takemoto T, Shimogori H, Yamashita H, Nakai A. Heat shock transcription factor HSF1 is required for survival of sensory hair cells against acoustic overexposure. Hearing Res. 2003;182:88–96. doi: 10.1016/s0378-5955(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Murashita H, Tobita T, Oikawa K, Tsuji S, Uemaetomari I, Hara A. Dehydroepiandrosterone sulfate reduces acoustic injury of the guinea-pig cochlea. J. Pharmacol. 2005;99:191–194. doi: 10.1254/jphs.scz050443. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B. Glucocorticoid receptor and nuclear factor-κB interactions in restraint stress-mediated protection against acoustic trauma. Endocrinol. 2006a;147:4430–4437. doi: 10.1210/en.2006-0260. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Salman H, Canlon B. Sound conditioning protects hearing by activating the hypothalamic-pituitary-adrenal axis. Neurobiol. Disease. 2007;25:189–197. doi: 10.1016/j.nbd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Bian Z, Stierna P, Hansson AC, Canlon B. NF-κB mediated glucocorticoid response in the inner ear after acousitc trauma. J. Neurosci. Res. 2006b;83:1066–1076. doi: 10.1002/jnr.20795. [DOI] [PubMed] [Google Scholar]
- Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hearing Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Talaska AE, Schacht J. Mechanisms of noise damage to the cochlea. Audiol. Med. 2007;5:3–9. [Google Scholar]
- Tanaka K, Takemoto T, Sugahara K, Okuda T, Mikuriya T, Takeno K, Hashimoto M, Shimogori H, Yamashita H. Post-exposure administration of edaravone attenuates noise-induced hearing loss. Eur. J. Pharmacol. 2005;522:116–121. doi: 10.1016/j.ejphar.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Munoz DJB, Housley GD. Puinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the guinea pig. J. Assoc. Res. Otolaryngol. 2004;5:58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley KM. Immune cell recruitment following acoustic trauma. Hearing Res. 2006;222:115–124. doi: 10.1016/j.heares.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Tota G, Bocci G. Importance of colour of the iris on the evaluation of resistance to auditory fatigue. 1967;42:183–192. [PubMed] [Google Scholar]
- Uchida Y, Nakashima T, Ando F, Niino N, Shimokada H. Is there a relevant effect of noise and smoking on hearing? A population-based aging study. Int. J. Audiol. 2005;44:86–91. doi: 10.1080/14992020500031256. [DOI] [PubMed] [Google Scholar]
- Uemaetomari I, Tabuchi K, Hoshino T, Hara A. Protective effect of calcineurin inhibitors on acoustic injury of the cochlea. Hearing Res. 2005;209:86–90. doi: 10.1016/j.heares.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, Polak M, Malgrange B, Lefebvre PP, Staecker H, Balkany TJ. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol. Neurotol. 2004;25:627–632. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Carlsson PI, Ottschytsch N, Bondeson M-L, Konings A, Vandevelde A, Dieltjens N, Fransen E, Snyders D, Borg E, Raes A, Van Camp G. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Human Mutation. 2006;27:786–795. doi: 10.1002/humu.20360. [DOI] [PubMed] [Google Scholar]
- Vicente-Torres MA, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J. Neurosci. Res. 2006;83:1564–1572. doi: 10.1002/jnr.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajkovic SM, Thorne PR, Sevigny J, Robson SC, Housley GD. NTPDase1 and NTPDase2 immunolocalization in mouse cochlea: Implications for regulation of P2 receptor signaling. J. Histochem. Cytochem. 2002;50:1435–1441. doi: 10.1177/002215540205001102. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Abi S, Wang CJH, Housley GD, Thorne PR. Differential distribution of adenosine receptors in rat cochlea. Cell Tiss. Res. 2007;328:461–471. doi: 10.1007/s00441-006-0374-2. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Vinayagamoorthy A, Thorne PR, Robson SC, Wang CJH, Housley GD. Noise-induced up-regulation of NTPDase3 expression in the rat cochlea: Implications for auditory transmission and cochlear protection. Brain Res. 2006;1104:55–63. doi: 10.1016/j.brainres.2006.05.094. [DOI] [PubMed] [Google Scholar]
- Vlajkovic SM, Housley GD, Munoz DJB, Robson SC, Sevigny J, Wang CJH, Thorne PR. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases1 and 2 in rat cochlea. Neurosci. 2004;126:763–773. doi: 10.1016/j.neuroscience.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Wagner W, Heppelmann G, Kuehn M, Tisch M, Vonthein R, Zenner H-P. Olivocochlear activity and temporary threshold shift-susceptibility in humans. The Laryngoscope. 2005;115:2021–2028. doi: 10.1097/01.MLG.0000181463.16591.A7. [DOI] [PubMed] [Google Scholar]
- Wang J, Ruel J, Ladrech S, Bonny C, van de Water T, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Molec. Pharm. 2007a;71:654–666. doi: 10.1124/mol.106.028936. [DOI] [PubMed] [Google Scholar]
- Wang J, Pignol B, Chabrier P-E, Saido T, Lloyd R, Tang Y, Lenoir M, Puel JL. A novel dual inhibitor of calpains and lipid peroxidation (BN82270) rescues the cochlea from sound trauma. Neuropharmacol. 2007b;52:1426–1437. doi: 10.1016/j.neuropharm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hearing Res. 2002a;165:96–102. doi: 10.1016/s0378-5955(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002b;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006;576.1:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins-Chow DE, Pavan WJ. Genomic copy number and expression variation within the C57BL/6J inbred mouse strain. Genome Res. 2008;18:60–66. doi: 10.1101/gr.6927808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PN, Thorne PR, Housley GD, Mockett B, Billett TE, Burnstock G. Quinacrine staining of marginal cells in the stria vascularis of the guinea-pig cochlea: A possible source of extracellular ATP? Hearing Res. 1995;90:97–105. doi: 10.1016/0378-5955(95)00151-1. [DOI] [PubMed] [Google Scholar]
- Wild DC, Brewster MJ, Banerjee AR. Noise-induced hearing loss is exacerbated by long-term smoking. Clin. Otolaryngol. 2005;30:517–520. doi: 10.1111/j.1749-4486.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- Woolf NK. Chapter 6. The role of viral infection in the development of otopathology:Labyrinthitis and autoimmune disease. In: Van De Water T, Popper AN, Fay RR, editors. Clinical Aspects of Hearing. Vol. 7. New York: Springer; 1996. pp. 155–198. [Google Scholar]
- Wu T, Marcus DC. Age-related changes in cochlear endolymphatic potassium and potential in CD-1 and CBA/CaJ mice. J. Assoc. Res. Otolaryngol. 2003;4:353–362. doi: 10.1007/s10162-002-3026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita D, Miller JM, Jiang H-Y, Minami SB, Schacht J. AIF and EndoG in noise-induced hearing loss. NeuroReport. 2004;15:2719–2722. [PubMed] [Google Scholar]
- Yamashita D, Jiang H, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neurosci. 2005;134:633–642. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Shiotani A, Kanzaki S, Nakagawa M, Ogawa K. Neuroprotective effects of T-817MA against noise-induced hearing loss. Neurosci. Res. 2008;61:38–42. doi: 10.1016/j.neures.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Yang M, Tan H, Yang Q, Wang F, Yao H, Wei Q, Tanguay RM, Wu T. Association of hsp70 polymorphisms with risk of noise-induced hearing loss in Chinese automobile workers. Cell Stress Chaperones. 2006;11:233–239. doi: 10.1379/CSC-192R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Ichimiya I, Suzuki M, Mogi G. Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hearing Res. 1999a;137:155–159. doi: 10.1016/s0378-5955(99)00134-3. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC. Sound conditioning reduces noise-induced permanent threshold shift in mice. Hearing Res. 2000;148:213–219. doi: 10.1016/s0378-5955(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J. Neurosci. 1999b;19:10116–10124. doi: 10.1523/JNEUROSCI.19-22-10116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc. Nat. Acad. Sci. USA. 2005;102:15201–15206. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H-B, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J. Membrane Biol. 2006;209:177–186. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Nat. Acad. Sci. USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]


