Abstract
Purpose
Indolemine 2,3-dioxygenase (IDO)-mediated oxidation of tryptophan produces kynurenines (KYNs), which may play a role in cataract formation. The molecular mechanisms by which KYNs cause cellular changes are poorly understood. The effects of KYNs on mouse lens epithelial cells by overexpression of human IDO were investigated.
Methods
Lens epithelial cells (mLECs) derived from human IDO-overexpressing hemizygous transgenic (hemTg) and wild-type (Wt) mice were used. IDO activity was measured by quantifying kynurenine (KYN) by HPLC. KYN-mediated protein modifications were detected by immunocytochemistry and measured by ELISA. Cell proliferation and apoptosis were measured with commercially available kits. Cell distribution between cell cycle phases was examined with flow cytometric analysis. Immunoprecipitation followed by LC/MS was used to identify kynurenine-modified proteins.
Results
mLECs derived from hemTg animals exhibited considerable IDO immunoreactivity and enzyme activity, which were barely detectable in Wt mLECs. KYN and KYN-mediated protein modification were detected in hemTg but not in Wt mLECs; the modified proteins were myosin II and α/γ-actin. HemTg mLECs displayed reduced viability and proliferation. Cell cycle analysis of hemTg mLEC cultures showed approximately a twofold increase in cells at G2/M or in both phases, relative to Wt mLECs. Blocking IDO activity with 1-methyl-d,l-tryptophan in hemTg mLECs prevented KYN formation, KYN-mediated protein modification, and G2/M arrest.
Conclusions
Excess IDO activity in mLECs results in KYN production, KYN-mediated modification of myosin II and α/γ-actin, and cell cycle perturbation. Modification of myosin II and γ-actin by KYN may interfere with cytokinesis, leading to defective epithelial cell division and thus a decreased number of fiber cells.
Indoleamine 2,3-dioxygenase (IDO) catalyzes the oxidation of tryptophan to N-formylkynurenine (NFK), the first step in the kynurenine pathway. Through a series of reactions that involve intermediates such as kynurenine (KYN) and 3-OH kynurenine (3OHKYN), N-formylkynurenine eventually forms NAD.1,2 3OHKYN is glucosylated to form 3OHKYN-glucoside, which is a major kynurenine in the human lens.3 IDO production is markedly increased during infection or inflammation and is induced by proinflammatory cytokines, including interferon (IFN)-γ.4
Several lines of evidence point to a role for IDO in suppressing cell proliferation by depleting the essential amino acid l-tryptophan. IDO has been implicated in the inhibition of propagation of viruses5 and protozoan parasites6 in eukaryotic cells and is also associated with inhibition of T-cell-mediated immune responses in tumor cells.7,8 In the placenta, IDO expression inhibits maternal T-cell-mediated rejection of the allogeneic fetus.9 IDO-expressing dendritic cells inhibit allogeneic T-cell proliferation in vitro.10
In the eye lens, IDO is expressed in the anterior epithelium.11 Lens absorption of ultraviolet A light is attributable in part to KYNs. KYNs readily pass through cell membranes and may diffuse through the cortex into the lens nucleus. At physiological pH, KYNs undergo side-chain deamination to produce α, β-unsaturated ketoalkenes,12 which react with nucleophilic amino acids present in lens proteins and also with cysteinyl residues of glutathione3,13-16 through Michael addition, to form covalent adducts. Such adducts are present in the nuclear regions of lenses in people more than 50 years of age,17 but their concentrations are reduced in cataractous lenses from age-matched patients,18 possibly due to further degradation. Recent studies have demonstrated the deleterious effects of such modifications on lens proteins, including α-crystallin. For example, modification of His83 by deaminated KYN in αB-crystallin reduces its chaperone function.19 KYNs have been implicated in reactive oxygen species (ROS)-mediated crystallin modification, as they can spontaneously produce ROS in the presence of trace metal ions. Such reactions are implicated in cross-linking of crystallins,20 suggesting that KYNs are responsible, in part, for chemical modification and cross-linking of proteins in aging and cataractous lenses.
KYNs also exert cytotoxic effects. Several studies, including those on neuronal cells, have shown that KYNs have proapoptotic effects.21,22 Such effects may be responsible for the enhanced apoptosis in kidney tubular epithelial cells that overexpress IDO.23 Despite these findings, the molecular mechanisms by which KYNs affect cells are poorly understood. To begin to answer this question, in a recent study (Mailankot et al., manuscript submitted) we developed a transgenic (Tg) mouse model that expresses human IDO (hIDO) in the lens. The lenses from homozygous (homTg) animals had high IDO activity and had the following characteristics that were absent in lenses from wild-type animals: The lenses (1) exhibited dense nuclear cataracts within 3 months after birth and had a significantly smaller diameter, (2) had high KYN and KYN-modified protein content, and (3) had severely decreased fiber cell differentiation, and undifferentiated fiber cells at the lens nucleus contained KYN-modified proteins and were apoptotic. Lenses in the hemizygous (hemTg) animals were similar to those in wild-type mice even though they had IDO activity; the activity, however, was approximately four times lower than that in homTg animals. From the observations in homTg animals, we concluded that overexpression of IDO leads to cytotoxic effects through formation of KYNs in the lens. To test this possibility, in this study we investigated the effects of IDO overexpression in cultured mouse lens epithelial cells (mLECs).
Materials and Methods
Animals and Cell Culture
mLECs were isolated from C57BL/6 animals. HomTg mice were generated by using a transgene construct with hIDO inserted between the EcoRI sites of minigene having a chimeric promoter that consisted of a chick δ1-crystallin enhancer and the αA-crystallin promoter (Mailnakot et al., manuscript submitted). HemTg animals were generated by breeding homTg mice with C57BL/6 Wt. mLECs were isolated and cultured from capsule-epithelial explants of 2- to 3-month-old Wt and Tg mice, as previously described.24 Cells were grown on 35-mm plates in Eagle’s minimum essential medium (MEM; Sigma-Aldrich, St. Louis, MO) containing 50 μg/mL gentamicin and 20% fetal bovine serum. Cells at passages 4 to 6 were used for the entire study. The studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Treatment of mLECs
For KYN treatment, Wt mLECs were weaned into serum-free medium containing 50 μM KYN and were cultured for 3 days. In some experiments, the cells were cultured in the presence of 20 μM 1-methyl-dl-tryptophan (MT) for 3 days to block IDO activity. Cells grown in serum-free medium alone served as control cultures.
Measurement of IDO Activity and KYN Content
The cells were trypsinized, homogenized on ice in 200 μL PBS (pH 7.4), and centrifuged (14,000g, 4°C, 15 minutes). Supernatants containing the soluble proteins were added to a reaction mixture containing 50 mM sodium phosphate buffer (pH 6.5), 20 mM ascorbic acid sodium salt (Sigma-Aldrich), 200 μg/mL bovine pancreas catalase (Sigma-Aldrich), 10 μM methylene blue and 400 μM l-tryptophan (Sigma-Aldrich). The reaction was performed at 37°C for 1 hour and was stopped by adding 40 μL 30% (wt/vol) trichloroacetic acid. The samples were incubated at 65°C for 15 minutes to convert NFK to KYN and then centrifuged (14,000g, 4°C, 15 minutes). Control cultures were prepared in the same way, except that the protein extract was incubated with 20 μM MT. KYN content was estimated by reversed-phase HPLC (RP-HPLC) with standards (0.2-5.0 nanomoles of d,l-KYN [Sigma-Aldrich]) and ammonium acetate (10 mM) as solvent A and 10% methanol in ammonium acetate as solvent B. The percentage of solvent B in the gradient was 0% (10 minutes), 0% to 100% (10 minutes) and 100% to 0% (15 minutes). The flow rate was 0.8 mL/min. The column eluate was monitored for absorbance at 360 nm. Enzyme activity was expressed as nanomoles of KYN formed per milligram protein per minute. Protein was quantified by an assay kit with BSA used as the standard (Bio-Rad, Hercules, CA).
To estimate the amount of KYN formed, we counted the cells and then homogenized them in 100 μL 100% ethanol. The homogenate was kept at -20°C for 1 hour and then centrifuged (14,000g, 4°C, 15 minutes). The supernatant was removed and kept at -20°C while the pellet was re-extracted with 80% ethanol (100 μL). The homogenate was kept at -20°C for 1 hour and then centrifuged as before, and the supernatants were combined and dried in a concentrator (Speed-Vac; Savant-Thermo Scientific; Waltham, MA). Samples and standards (0.2-0.5 nanomoles of d,l-KYN) were analyzed by RP-HPLC. Sodium acetate (20 mM)/acetic acid buffer (pH 4.5) was used as solvent A and 20% methanol as solvent B. The percentage of solvent B in the gradient was 0% (30 minutes), 0% to 50% (2 minutes), 50% to 100% (8 minutes) and 100% to 0% (6 minutes). The flow rate was 0.6 mL/min. Results are expressed as nanomoles per million cells. NFK and 3OHKYN were analyzed in the same runs with the use of authentic NFK and 3OHKYN standards.
To estimate KYN content in the culture medium, lyophilized medium (100 mg) was extracted with ethanol, concentrated (Speed-Vac; Savant Thermo Scientific), and processed as just described. To estimate tryptophan in the culture medium, 24 μL of medium was brought up to 200 μL with solvent A. Samples and standards were analyzed by RP-HPLC as described for KYN estimation. Results were expressed as nanomoles per milliliter medium.
Estimation of KYN Modification in Proteins by ELISA
The estimation was performed as described previously.16 Briefly, microplate wells were coated with cell lysate in 0.05 M carbonate buffer (pH 9.7) at a concentration of 1 μg and blocked with 5% nonfat dry milk (NFDM). The wells were then incubated with a mouse anti-KYN mAb (1:1000 diluted in PBS) followed by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (1:15,000 in PBST; Promega, Madison, WI). The enzyme activity was assessed by adding 100 μL 3,3′,5,5′-tetramethylbenzidine, and the reaction was stopped with 50 μL 2 N H2SO4. The samples were read at 450 nm.
Immunocytochemistry
The cells were cultured in chambered slides 1 day before the experiments. After the cells were washed twice with PBS, they were fixed with 4% paraformaldehyde in PBS at -20°C for 15 minutes, followed by washing twice with PBS and permeabilizing with 0.1% Triton X-100 in PBS at -20°C for 5 minutes. After the slides were then washed five times with PBS to remove the detergent, they were blocked with 3% NFDM/1% BSA in PBS for 30 minutes at room temperature (RT) and washed twice for 5 minutes with PBS. The slides were incubated with the primary antibody in 0.1% BSA/PBS for 1 hour at RT and washed twice for 5 minutes with PBS. Mouse anti-αA/αB-crystallin mAb (1:100 dilution; Stressgen), mouse anti IDO mAb (1:40 strength; Chemicon), and mouse anti KYN-mAb (1:50 dilution) were used as primary antibodies. The slides were incubated with secondary antibody (anti-mouse IgG) conjugated with Texas red (1:200 dilution; Invitrogen-Molecular Probes, Eugene, OR). The secondary antibody was diluted in 0.1% BSA/PBS and applied to the slides for 1 hour at RT. After washing twice with PBS for 5 minutes, the slides were incubated with phalloidin (Invitrogen-Molecular Probes) for 30 minutes, washed twice with PBS and then incubated with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen-Molecular Probes) for 1 minute. The slides were washed twice for 5 minutes with PBS, permanently mounted, and viewed with a fluorescence microscope (Model BX60; Olympus, Lake Success, NY), and images were acquired with an attached digital camera (Spot RT Slider; Diagnostic Instruments, Inc., connected to a Macintosh computer using Spot RT Slider software, version 3.5.5). Secondary antibody contribution to immune reaction was verified by staining without the primary antibody.
Assessment of Protein Expression
To assess the protein expression in mLECs, water soluble proteins (10 μg) were subjected to SDS-PAGE on 15% gel followed by staining (Bio-Safe staining solution; Bio-Rad) and destaining in water.
For Western blot analysis, cell lysates corresponding to 40 μg protein were subjected to SDS-PAGE on a 15% Tris-HCl gel, electrophoretically transferred onto a nitrocellulose membrane, blocked with 5% nonfat dry milk, and incubated with IDO mAb (1:500 dilution; Chemicon, Temecula, CA) and anti GAPDH antibody (1:300 dilution; Millipore, Billerica, MA[b]). The membrane was subsequently incubated with goat anti-mouse HRP-conjugated antibody (1:5000 dilution; Promega, Madison, WI[b]) and developed with a kit (SuperSignal West Pico Chemiluminescence Kit; Pierce, Rockford, IL).
Assessment of Cell Proliferation
The cell proliferation was performed by adding reagent (CellTiter 96 Aqueous One Solution; Promega) directly to the wells as per the manufacturer’s instructions. Briefly, 48 hours before the assay, mLECs (400 cells/well) were dispensed into 96-well plates in triplicate and cultured. The reagent (20 μL) was added into each well and incubated for 4 hours at 37°C in a humidified, 5% CO2 atmosphere. Bioreduction of a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] by cells to a colored formazan compound was determined spectrophotometrically at 490 nm. The number of viable cells per well was calculated from a standard curve obtained with 0 to 1000 Wt mLECs/well.
Determination of Apoptosis
Cells grown in 12-well plates were analyzed for apoptosis (In Situ Cell Death Detection Kit; Roche Diagnostics, Indianapolis, IN), per the manufacturer’s instructions. The cells were counterstained with DAPI to identify the nuclei. As a negative control, the cells were incubated without terminal transferase.
Flow Cytometry
Two million cells were collected by trypsinization and washed twice with PBS. After fixing in 2 mL 90% methanol in PBS for 1 hour at -20°C, the cells were washed twice, and resuspended in 250 μL PBS. Then, 5 μL RNase stock solution (RNase-2 mg/mL, EDTA-10 mM, and sodium azide-0.1%) was added and incubated at 37°C for 30 minutes. The cells were chilled at 4°C for 10 minutes and incubated with 250 μL propidium iodide (PI) stock solution (PI, 100 μg/mL; Triton X-100, 0.1%; sodium azide, 0.1%) at 4°C for 60 minutes. They were then analyzed by flow cytometry (EPICS-XL; Beckman Coulter, Fullerton, CA). At least 20,000 events per sample were evaluated. All histograms were analyzed with flow cytometry software (EXPO32; Beckman Coulter) to determine the percentage of cells in the G1, S, and G2/M stages of the cell cycle.
Identification of KYN-Modified Proteins
Protein from mLEC lysate (200 μg) was incubated with mouse anti-KYN mAb16 (2 μg) for 1 hour at RT followed by addition of protein G-Sepharose (GE Healthcare, Piscataway, NJ) and incubation for 1 hour at RT with shaking. To check whether nonspecific binding occurred, proteins were incubated with protein G-Sepharose without the antibody. The sample mixture was centrifuged, and the pellet was washed five times for 5 minutes each with PBS. The gel pellet was dissolved in SDS-PAGE sample buffer, and proteins were subjected to electrophoresis on a 15% gel. The proteins were stained (Bio-Safe; Bio-Rad) and destained in water. The protein bands were cut out of the gel, minced, and subjected to in-gel digestion with trypsin. The resultant peptides were analyzed on a linear ion trap mass spectrometer (LTQ; Thermo Fisher Scientific, coupled with an Ettan MDLC system; GE Healthcare). The spectra were acquired by data-dependent methods: one full scan (m/z of 300-2000) was performed followed by MS/MS on the five most abundant precursor ions at 30% normalized collision energy. The dynamic exclusion criteria were set as follows: repeat count, 1; repeat duration, 45 seconds; and exclusion duration, 180 seconds. The obtained data were submitted to a protein search engine (Mascot; Matrix Science, Boston, MA) by searching the Swiss-Prot (sprot 50.3) mouse database (http://www.expasy.org; provided in the public domain by Swiss Institute of Bioinformatics, Geneva, Switzerland).
Statistics
The results were analyzed by using one-way analysis of variance (ANOVA), followed by the Fisher protected least significant difference test (Statview 5.0 software; SAS Institute, Inc., Cary, NC). The level of significance was set at P < 0.05.
Results
Isolation and Characterization of mLECs
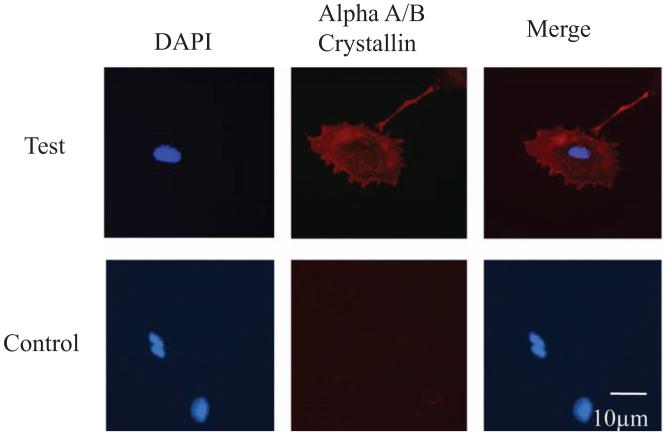
Initially, we tried to isolate and culture mLECs from homTg animals. But despite repeated efforts, the isolated cells failed to proliferate and died within 7 to 10 days of isolation, possibly because of the cytotoxic effect of the high KYN. We then successfully isolated mLECs from hemTg animals. Wt and hemTg cells showed immunoreactivity for αA/αB-crystallin, confirming that they are mLECs (Fig. 1). Incubating cells with only the secondary antibody showed no immunoreactivity, confirming that the staining was specific for α-crystallins.
Figure 1.
Characterization of mLECs. Isolated Wt and hemTg mLECs were immunostained for αA/αB-crystallin (red) in the presence (test) and absence (control) of mouse anti αA/αB-crystallin monoclonal antibody. The cells were counterstained with DAPI (blue). The images are representative of three independent cultures from three mice.
IDO Expression
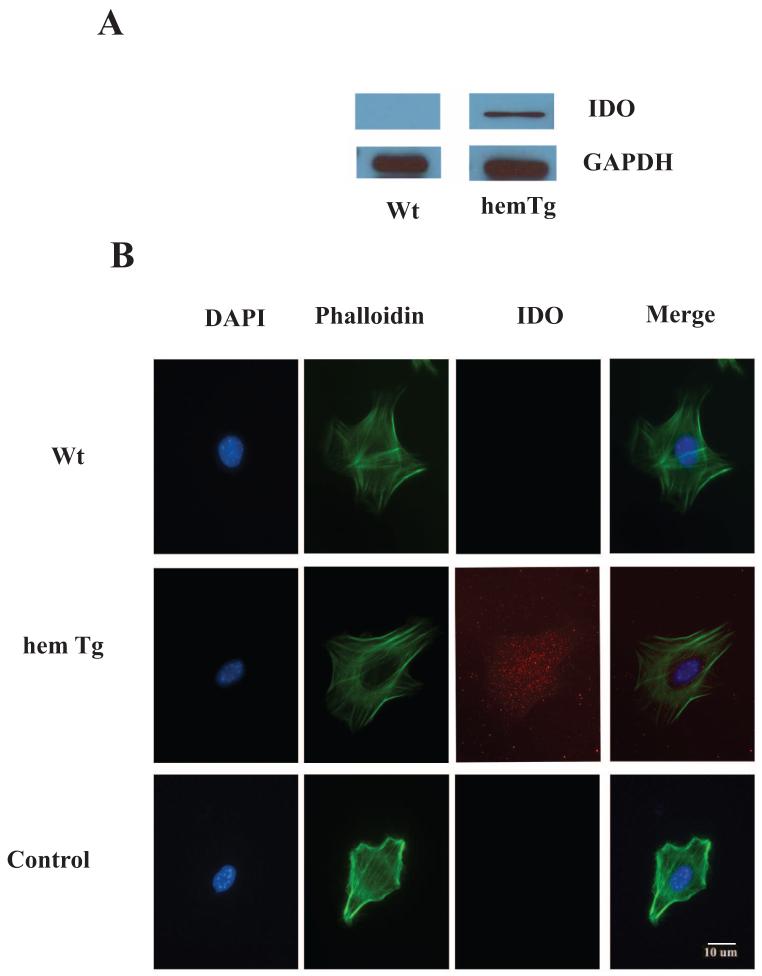
IDO activity in mLECs was measured by estimating the amount of KYN formed during incubation of mLEC proteins with 1 mM l-tryptophan. In cells from hemTg animals, the rate of KYN formation was estimated to be 0.15 ± 0.02 nanomoles/mg protein/min, whereas mLECs from Wt animals showed no detectable activity. Based on the KYN measured, we estimated that 2.0% of added d,l-tryptophan was converted into KYN in 1 hour. To confirm that the observed KYN formation was due to the IDO activity, we incubated cell lysates either at 65°C for 15 minutes or with the IDO inhibitor, 20 μM MT (37°C, 60 minutes). Both treatments abolished the enzyme activity. Western blot using anti-IDO mAb with GAPDH as the loading control detected IDO in hemTg mLECs but not in Wt mLECs (Fig. 2A). Immunocytochemical studies showed IDO throughout the cytoplasm in hemTg mLECs (Fig. 2B). The Wt mLECs showed no immunoreactivity. These findings further confirmed overexpression of IDO in mLECs of hemTg animals.
Figure 2.
IDO expression in Wt and hemTg mLECs. (A) Western blot analysis. IDO was detected by using mouse anti-IDO monoclonal antibody in hemTg mLECs but not in Wt mLECs. GAPDH was used as the loading control. (B) Immunocytochemistry for IDO. IDO was detected in hemTg mLECs by using the monoclonal antibody and Texas red-conjugated secondary antibody. The cells were also stained for F-actin with phalloidin (green) and counterstained with DAPI (blue). Data are representative of results in three independent experiments.
KYN and KYN-Modified Proteins
Using RP-HPLC we determined that the KYN concentration in hemTg mLECs was 0.11 ± 0.01 nanomoles/106 cells. KYN was not detected in Wt mLECs. The formation of KYN led to modification of cellular proteins, as detected by ELISA (0.34 ± 0.01 OD vs. 0.02 ± 0.01, hemTg versus Wt, respectively). No immunoreactivity was present when the primary antibody was preincubated with KYN-modified RNase A, confirming that the immunoreactivity in the ELISA resulted from KYN-modified proteins.
Immunocytochemistry was performed to localize KYN-modified proteins. KYN-modified proteins were present throughout the cytoplasm (Fig. 3). The immunoreaction was markedly reduced when the antibody was preincubated with KYN-modified RNase A. Wt mLECs were negative for immunostaining. Together, these data suggest that overexpression of hIDO results in high KYN and KYN modification of proteins in mLECs.
Figure 3.
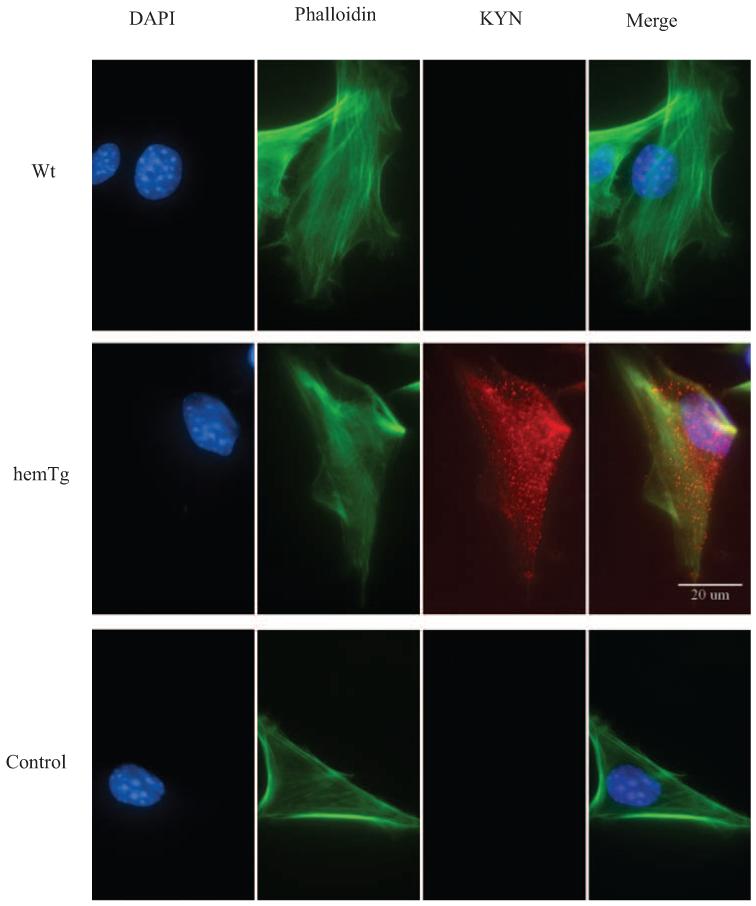
Immunostaining for KYN-modified proteins. KYN-modified proteins were detected using a monoclonal antibody and Texas-red conjugated secondary antibody. Cells were also stained for F-actin by phalloidin (green) and nuclei with DAPI (blue). KYN-modified proteins (red) were detected in KYN treated mLECs but not in untreated cells. Data are representative of results in three independent experiments.
KYN and Tryptophan Content of Culture Medium
KYN was detected in hemTg mLEC culture medium (0.47 ± 0.07 nanomoles of KYN per milliliter) but was absent in Wt mLEC culture medium (Fig. 4A). To determine whether mLECs from hemTg mice used tryptophan from the culture medium at a higher rate than cells from Wt mice, we measured tryptophan in the media by RP-HPLC. The tryptophan content was reduced by 0.5 nanomoles/mL (∼2%) culture media in hemTg mLECs when compared to Wt (29 ± 1.92 vs. 28.5 ± 1.9 nanomoles/mL; Wt versus hemTg, respectively; Fig. 4B) This difference correlated with measured intra- and extracellular (in the medium) KYN in hemTg mLE. At 100% confluence, the average number of cells per 100-mm plate in 10 mL medium was 2 × 106. The amount of KYN formed from 2 × 106 hemTg mLECs was ∼4.8 nanomoles. Thus, even after growth of cells to confluence, the culture medium had sufficient tryptophan.
Figure 4.
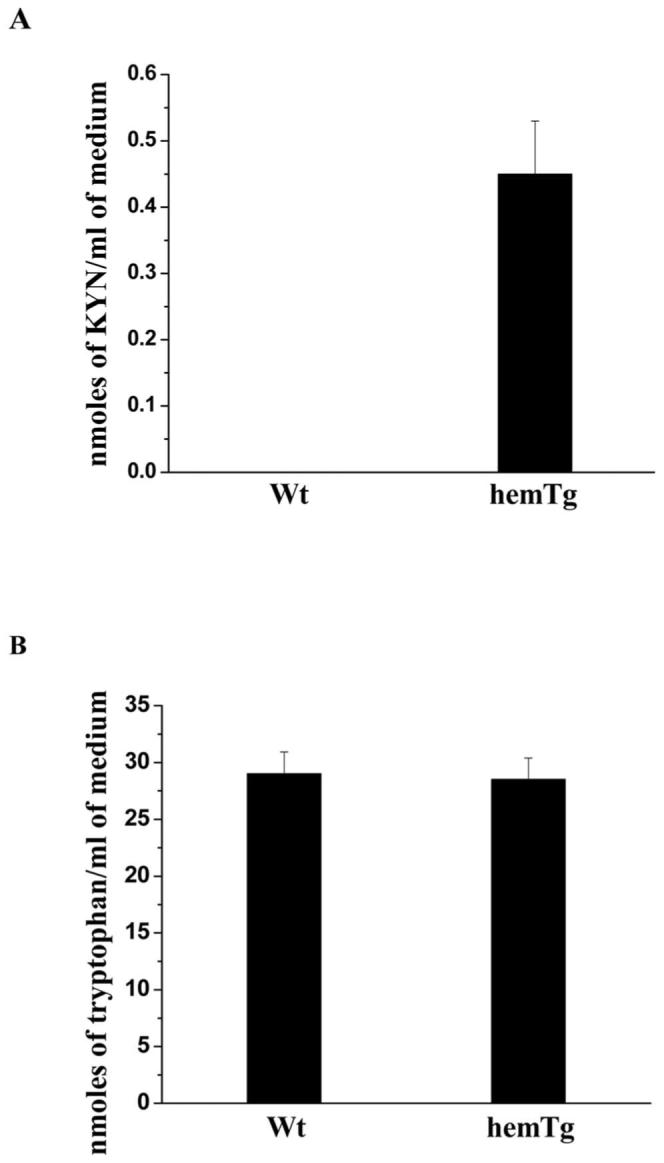
KYN and l-tryptophan in culture medium. Wt and hemTg mLECs were cultured for 3 days, the cell-free medium was collected, lyophilized and processed for measurement for IDO activity. KYN and l-tryptophan were quantified by HPLC. (A) KYN in the medium. KYN was detected in the hemTg mLEC culture medium but not in that of Wt mLECs. (B) l-Tryptophan in the medium. l-tryptophan content decreased slightly in culture medium from hemTg mLECs but not in that of Wt mLECs. Results shown are the mean ± SD of three independent experiments.
Effect of Exogenous KYN
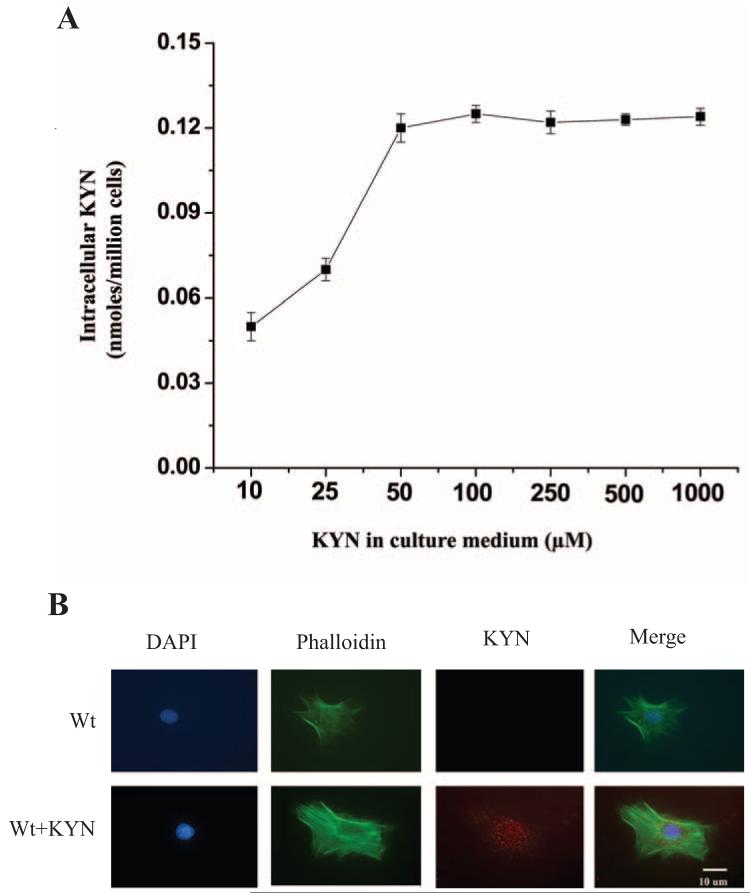
To study the effect of KYN, Wt mLECs were cultured with 10 to 1000 μM KYN for 3 days. When the extracellular KYN concentration was low, the intracellular KYN concentration increased to 50 μM (Fig. 5A). However, increasing the extracellular KYN concentration beyond 50 μM did not further increase intracellular KYN. These data suggest that KYN uptake by mLECs is regulated. Similar to the findings in hemTg mLECs shown in Figure 3, immunostaining of Wt mLECs incubated with KYN showed KYN modifications in the cytoplasm, but more at the perinuclear region (Fig. 5B).
Figure 5.
Effect of exogenous KYN on intracellular KYN and KYN modification in mLECs. (A) mLECs from Wt were incubated with 0 to 1.0 mM KYN for 3 days. Cells were lysed, protein precipitated, and KYN estimated in the protein-free supernatants by HPLC. Results are the mean ± SD of results in three independent experiments. (B) Wt mLECs were incubated with 50 μM KYN for 3 days and immunostained for KYN-modified proteins. KYN-modified proteins (red) were detected in KYN-treated mLECs but not in untreated cells. Cells were also stained for F-actin by phalloidin (green) and nuclei with DAPI (blue). Images are representative of three independent experiments.
Effect of 1-Methyl d,l-tryptophan (MT) Treatment
To further confirm that the effects seen in mLECs from hemTg were due to IDO-mediated KYN formation, we incubated mLECs from hemTg with an inhibitor of IDO, MT. At 3 days of treatment, 20 μM MT completely inhibited IDO. As a result, neither KYN nor significant KYN modifications were detected (data not shown).
Cell Proliferation, Apoptosis, and Cell Cycle Analysis
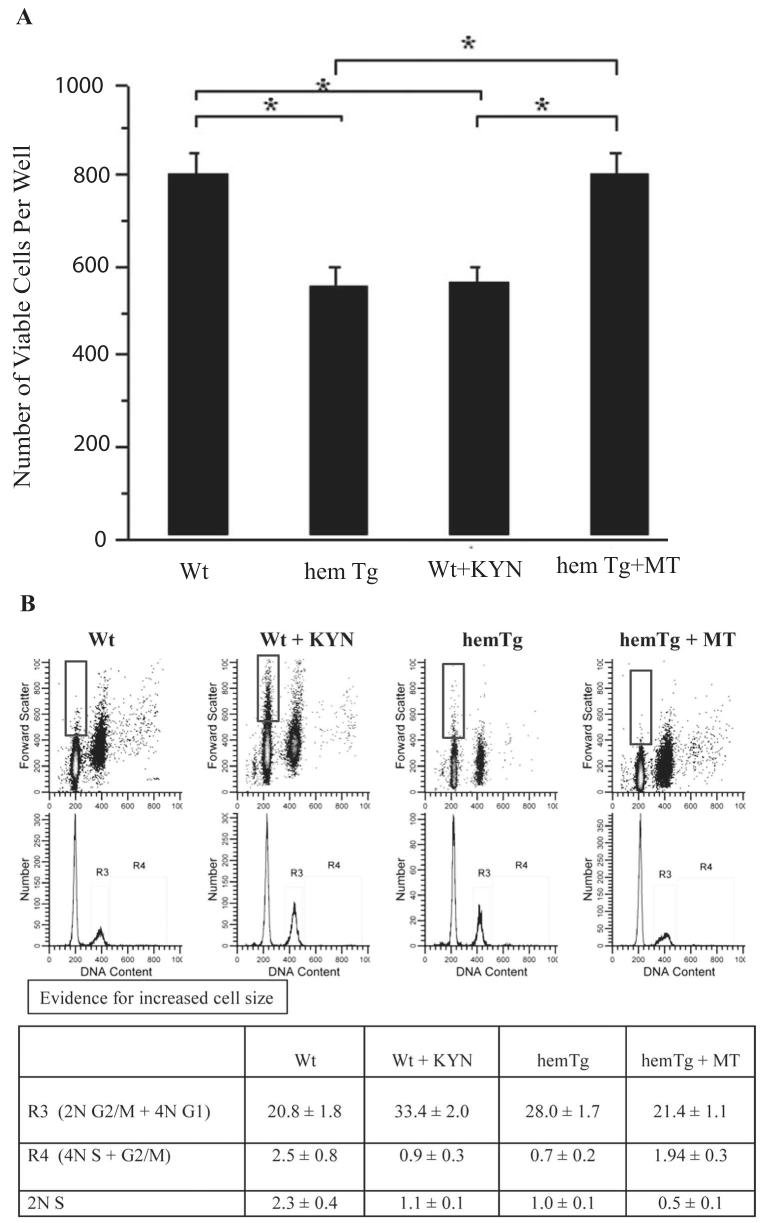
An MTS assay was used to determine the cell proliferation rate. After 48 hours of culturing, the number of viable cells increased by twofold in Wt mLECs (∼800 cell/well from the initial ∼400 cells/well), but the increase was only 1.3-fold in hemTg mLECs (P < 0.0001; Fig. 6A). Similar to hemTg mLECs, KYN-treated Wt mLECs also showed only ∼1.3-fold increase in viable cells. Of interest, treatment with MT enhanced cell proliferation in hemTg mLECs, the number of viable cells increased by ∼2-fold, similar to Wt mLECs.
Figure 6.
Cell proliferation and cell cycle analysis. (A) MTT assay for cell proliferation. The number of viable hemTg mLECs was lower than Wt mLECs. Treatment of Wt mLECs with KYN reduced the number of viable cells, and MT treatment of hemTg mLECs increased the number. *P < 0.0001. Results shown are mean ± SD of three independent experiments. (B) Flow cytometric analysis. hemTg mLECs and KYN-treated Wt mLECs showed an increased number of G2/M and 4N G1 cells. The cell cycle perturbation in hemTg mLECs was normalized by MT treatment. R3, G2/M + 4N G1; R4, 4N S+G2/M.
To determine whether apoptosis leads to reduction in viable cells, we performed TUNEL staining in culturing cells for 3 days. The number of TUNEL-positive cells did not significantly differ between hemTg and Wt mLECs (data not shown). No significant apoptosis was found in KYN-treated Wt mLECs also. In addition, FACS analysis of TUNEL-positive cells, in which we included both adherent and floating cells, did not show a difference in either untreated or KYN-treated cells (data not shown). These results suggest that the reduction in the number of viable cells in hemTg mLECs did not result from enhanced apoptosis.
Cell cycle analysis was performed with flow cytometry. HemTg mLECs showed a markedly increased R3 fraction, suggesting a delay in G2/M, or both phases (Fig. 6B). The percentage of hemTg mLECs in the G2/M phase was ∼1.7-fold greater compared with Wt mLECs. In both cases, the number of cells in sub-G1 phase was not significant, supporting the idea that increased cell death did not contribute to reduced cell proliferation. Compared with untreated Wt cells, the number of KYN-treated Wt cells in the R3 fraction increased by twofold but did not increase in the sub-G1 phase. These results suggest that exogenous KYN brings about changes in Wt mLECs similar to those in hemTg mLECs and imply that intracellular KYN results in delayed entrance into the G2/M, or both phases. MT-treated hemTg mLECs showed a nearly 50% reduction in cells at G2/M when compared with cells without such treatment. Although delayed entrance into the G2 and or M phase is the simplest description of these results, it is also possible that there are other cell cycle effects (e.g., G1 delay). We noted a marked increase in cell size in KYN-treated G1 cells (Fig. 7), suggesting prolonged growth in this phase. Taken together, these data strongly support the idea that IDO-mediated KYN formation in hemTg mLECs produces significant cell cycle delays that lead to reduced cell proliferation.
Figure 7.
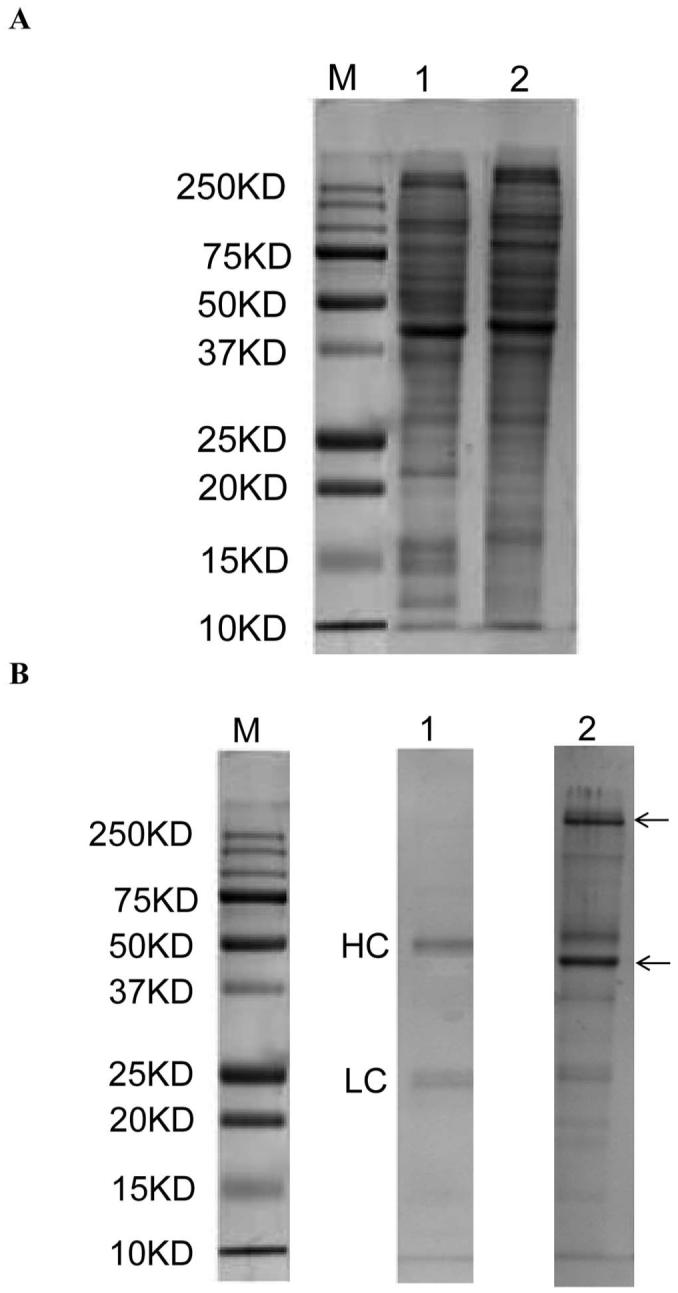
KYN-modified proteins in mLECs. (A) SDS-PAGE of mLECs lysates. Cell lysate of hemTg mLECs showed high-molecular-weight proteins that were absent in Wt mLECs. (B) SDS-PAGE of immunoprecipitated KYN-modified proteins. Two major KYN-modified proteins (arrows) were detected in hemTg mLECs lysate. Lane M: molecular weight marker; lane 1:Wt mLECs; lane 2: hemTg mLECs. HC, IgG heavy chain; LC, IgG light chain.
KYN-Modified Proteins
Because our evidence did not point to tryptophan deficiency, we postulated that the observed cell cycle delay might be due to KYN modification of key proteins. SDS-PAGE of cell lysates from hemTg and Wt mLECs is shown in Figure 7A. The hemTg mLEC lysates had a few high molecular weight proteins that were absent in Wt. These are possibly KYN-mediated cross-linked proteins. Immunoprecipitation of these cell lysate proteins revealed two distinct protein bands in the hemTg samples that were not present in Wt lysates (Fig. 7B). Incubation of hemTg lysate without the primary antibody or incubation without lysate showed no bands from cellular proteins, except the bands corresponding to heavy chain and light chain of antibody. The two proteins in hemTg were trypsin digested and analyzed by LC/MS. The mass spectrometry data are shown in Table 1. The two proteins were identified as myosin II and α/γ-actin.
Table 1.
KYN-Antibody Binding Proteins Identified by LC/MS
| Protein ID | Protein Name | Sequence Mass | Coverage % | Mascot Score | Unique Peptide |
|---|---|---|---|---|---|
| Band 1 | |||||
| MYH9_MOUSE (Q8VDD5) | Myosin-9 (Myosin heavy chain, nonmuscle IIa) | 226,086 | 63 | 11,549 | 150 |
| MYH10_MOUSE (Q61879) | Myosin-10 (Myosin heavy chain, nonmuscle IIb) | 228,855 | 33 | 2,025 | 59 |
| FLNA_MOUSE (Q8BTM8) | Filamin-A (α-filamin) | 280,887 | 28 | 1,916 | 61 |
| FLNB_MOUSE (Q80X90) | Filamin-B (β-filamin) | 277,579 | 5 | 192 | 11 |
| Band 2 | |||||
| ACTG_MOUSE (P63260) | Actin, cytoplasmic 2 (γ-actin) | 41,766 | 74 | 13,619 | 26 |
| ACTC_MOUSE (P68033) | Actin, α cardiac | 41,992 | 64 | 7,593 | 22 |
| HSP47_MOUSE (P19324) | 47-kDa heat shock protein precursor | 46,560 | 41 | 608 | 15 |
| VIME_MOUSE (P20152) | Vimentin | 53,524 | 34 | 341 | 19 |
Discussion
Although studies have demonstrated that IDO has antiproliferative effects,5,6,9,10 the processes involved are not well defined. In the human lens, the downstream products of IDO (i.e., KYNs), can serve as UV filters. However, recent studies on KYN modifications of lens proteins point to IDO’s role in cataractogenesis.15,16,25 Our previous study (manuscript submitted) in transgenic mice overexpressing hIDO in the lens showed that KYN presence and KYN modification of proteins can harm the lens: enhanced formation of KYN in IDO overexpression results in fiber cell apoptosis, poor fiber cell differentiation, and cataract development. However, due to experimental design limitations in that study, we were only able to demonstrate deleterious effects of KYNs on fiber cells, but not on epithelial cells. Thus, the present study was undertaken to address the effect of KYN on epithelial cells. The major findings of the present work were that (1) IDO overexpression in mLECs resulted in accumulation of KYN and KYN-modified proteins in cells, (2) KYN and KYN modification of proteins correlated with cell accumulation in the G2/M or in both phases of the cell cycle, (3) the effects of IDO overexpression on cell cycle were preventable by inhibiting IDO with a selective inhibitor, and (4) myosin II and α/γ-actin were present as KYN-modified proteins in hemTg mLECs.
The formation of KYN in IDO-overexpressing cells was not unexpected. Therefore, the effects on the cell cycle could be directly due to KYN. We ruled out effects from other downstream metabolites of the KYN pathway, NFK and 3OKKYN, as these were not detected in hemTg mLECs. KYN formation and KYN modification in both the cytosolic and nuclear components lends credence to the possibility that KYN directly affected the cell cycle. Of interest, the cell cycle delay was not associated with apoptosis, and IDO expression has been shown to reduce T-cell proliferation without apoptosis.26 It is possible that KYN may not induce apoptosis in epithelial cells, unlike fiber cells. This is supported by the fact that epithelial cell apoptosis was not evident in the lenses of homTg animals that had significantly more KYN than those from hemTg (Mailankot et al., manuscript submitted). These observations suggest that epithelial cells may have robust defense mechanism(s) to deal with excess KYN.
In addition to resistance to internally formed KYN, mLECs may also be resistant to exogenous KYN. We observed that adding up to 1 mM KYN to the medium did not induce cell death. In response to external KYN, intracellular KYN was regulated, as a gradual increase in KYN concentration from 50 μM to 1 mM did not produce a corresponding increase in intracellular KYN. The KYN concentration remained constant after it reached ∼0.12 nanomoles per 106 cells. This suggests that KYN entry into cells is regulated. Thus, lens epithelial cells may be protected from both external and internal KYN, albeit at the expense of cell cycle perturbations that result in decreased proliferation and eventually, improper fiber cell differentiation.
The methyl derivative of tryptophan MT is a pharmacologic competitive inhibitor of IDO and has been used in various studies to inhibit tryptophan depletion by IDO.9,27-30 Inhibition of cell cycle arrest by MT confirmed that the arrest at G2, M, or both in hemTg mLECs is due to enhanced IDO activity. Enhanced intracellular KYN formation as a result of increased IDO activity implies that l-tryptophan, an essential amino acid, the substrate for IDO, is not a limiting factor. To compensate for the depletion of intracellular tryptophan, cells may increase their tryptophan uptake. Our data that l-trypotphan concentration is reduced in cultures of mLECs from hemTg support this possibility. Because the culture medium had sufficient tryptophan, it is unlikely that l-tryptophan depletion by increased IDO activity resulted in cell cycle delay or arrest in G1 in hemTg mLECs as observed in other experiments.
Normal lens development and growth depends on the tight spatial and temporal regulation of lens epthelial cell proliferation and differentiation. The proliferative potential of lens epithelial cells decreases with age.31,32 We observed an increase in KYN-modified proteins in mLECs. It is possible that KYN-modified proteins are removed from cells through the ubiquitin-proteasome pathway. If the function of this system is compromised during age-associated cataract formation, as reported previously,33 KYN-modified proteins may accumulate. If the modified proteins are involved directly or indirectly in cell cycle regulation, such accumulation would lead to decreased proliferation of lens epithelial cells. The observation that the proliferative ability of lens epithelial cells decreases with age34,35 supports this idea.
Although the effect of KYN on G2/M arrest was apparent, less obvious is how KYN or KYN modification interferes with the cell cycle. The cell cycle is regulated by cyclin-dependent kinases.36 The regulation of cyclin-dependent kinase inhibitors during differentiation of lens epithelial cells suggests that these proteins control cell cycle exit.37 Whether KYN affected these cyclin-dependent kinases and their inhibitors by direct chemical modification or indirectly through modification of downstream signaling molecules remains to be investigated.
Our finding that α/γ-actin and myosin II are KYN-modified proteins in hemTg mLEC lysate provides a molecular basis for impairments in cell cycle and proliferation. Modification of these two proteins could adversely affect cytokinesis in cells, as this process requires both actin and myosin (together with other structural and regulatory proteins) to form a contractile ring at the equatorial cortex and induce the furrowing that eventually results in cell division into two daughter cells.38,39 Thus, KYN modification of these proteins may have inhibited cytokinesis and consequently cell proliferation.
Whether effects on cell cycle and cell proliferation contribute to cataract formation in humans is uncertain, but indirect evidence supports the view. In TGF-β-induced anterior subcapsular cataracts in mice, LEC proliferation is deregulated.40 Enhanced calcium levels in the lens and aqueous humor, which is considered to cause lens opacification, cause decreased proliferation of human LEC41; H2O2, which causes cataract in organ cultured lenses also reduces lens epithelial cell proliferation. These observations strongly suggest that decreased proliferation of epithelial cells is associated with cataract formation. If a similar mechanism is induced by KYN modification of proteins, it might contribute to cataract formation in humans.
Human lenses have relatively low levels of IDO activity. One study reported IDO activity equivalent to 0.85 ± 0.49 nanomoles of KYN formed per hour per lens in 26- to 80-year-old human lenses.11 If the average wet weight of an adult human lens is assumed to be ∼200 mg and the protein content to be 60%, the reported IDO activity would translate to ∼0.12 to 0.2 picomoles of KYN formed per minute per milligram protein. The hemTg animals we developed had IDO activity equivalent to 0.15 ± 0.02 nanomoles/min/mg protein of KYN formed in the lens. Therefore, the IDO activity in hemTg animal lenses is ∼750 to 1200 times higher than human lenses. Such high activity of IDO may not occur even in senile cataractous human lenses. However, in conditions in which interferon-γ, an inducer of IDO expression, is elevated in the anterior eye (e.g., in uveitis42), IDO activity could increase and lead to KYN modification and G2/M arrest in lens epithelial cells. It has been suggested that interferon- γ production in LECs may be involved in cataract development.43 It will be of interest to determine the effect of interferon-γ on IDO activity in LECs.
In summary, our study demonstrates that enhanced IDO activity results in KYN-mediated cell cycle arrest in lens epithelial cells. We believe that this antiproliferative effect could contribute to the cataract formation in IDO-overexpressing homozygous transgenic animals that we have previously observed. In the human lens, KYN formation and KYN modifications could therefore play a role in age-related cataract formation.
Acknowledgments
Supported by National Eye Institute (NEI) Grants R01EY-016219 and R01EY-09912, the Carl F. Asseff, MD, Professorship (RHN), NEI Grant P30EY-11373 (Visual Sciences Research Center of CWRU), Research to Prevent Blindness, the Ohio Lions Eye Research Foundation, and National Cancer Institute Grants R01CA-073413 (JWJ) and P30CA-043703 (Case Comprehensive Cancer Center Core Facilities).
Footnotes
Disclosure: M. Mailanko, None; D. Smith, None; S. Howell, None; B. Wang, None; J.W. Jacobberger, None; T. Stefan, None; R.H. Nagaraj, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Streete IM, Jamie JF, Truscott RJ. Lenticular levels of amino acids and free UV filters differ significantly between normals and cataract patients. Invest Ophthalmol Vis Sci. 2004;45:4091–4098. doi: 10.1167/iovs.04-0178. [DOI] [PubMed] [Google Scholar]
- 3.Hood BD, Garner B, Truscott RJ. Human lens coloration and aging. Evidence for crystallin modification by the major ultraviolet filter, 3-hydroxy-kynurenine O-beta-d-glucoside. J Biol Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- 4.Taylor LM, Aquilina AJ, Jamie JF, Truscott RJ. UV filter instability: consequences for the human lens. Exp Eye Res. 2002;75:165–175. doi: 10.1006/exer.2002.2012. [DOI] [PubMed] [Google Scholar]
- 5.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162:957–964. [PubMed] [Google Scholar]
- 6.Nagineni CN, Pardhasaradhi K, Martins MC, Detrick B, Hooks JJ. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immunun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune TM, Pogue SL. Inhibition of tumor cell growth by interferon-gamma is mediated by two distinct mechanisms dependent upon oxygen tension: induction of tryptophan degradation and depletion of intracellular nicotinamide adenine dinucleotide. J Clin Invest. 1989;84:863–875. doi: 10.1172/JCI114247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Maza LM, Peterson EM. Dependence of the in vitro antiproliferative activity of recombinant human gamma-interferon on the concentration of tryptophan in culture media. Cancer Res. 1988;48:346–350. [PubMed] [Google Scholar]
- 9.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 10.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takikawa O, Littlejohn TK, Truscott RJ. Indoleamine 2,3-dioxygenase in the human lens, the first enzyme in the synthesis of UV filters. Exp Eye Res. 2001;72:271–277. doi: 10.1006/exer.2000.0951. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez S, Garner B, Sheil MM, Truscott RJ. Characterisation of the major autoxidation products of 3-hydroxykynurenine under physiological conditions. Free Radic Res. 2000;32:11–23. doi: 10.1080/10715760000300021. [DOI] [PubMed] [Google Scholar]
- 13.Aquilina JA, Carver JA, Truscott RJ. Oxidation products of 3-hydroxykynurenine bind to lens proteins: relevance for nuclear cataract. Exp Eye Res. 1997;64:727–735. doi: 10.1006/exer.1996.0258. [DOI] [PubMed] [Google Scholar]
- 14.Garner B, Shaw DC, Lindner RA, Carver JA, Truscott RJ. Nonoxidative modification of lens crystallins by kynurenine: a novel post-translational protein modification with possible relevance to ageing and cataract. Biochim Biophys Acta. 2000;1476:265–278. doi: 10.1016/s0167-4838(99)00234-4. [DOI] [PubMed] [Google Scholar]
- 15.Staniszewska MM, Nagaraj RH. 3-hydroxykynurenine-mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J Biol Chem. 2005;280:22154–22164. doi: 10.1074/jbc.M501419200. [DOI] [PubMed] [Google Scholar]
- 16.Staniszewska M, Nagaraj RH. Detection of kynurenine modifications in proteins using a monoclonal antibody. J Immunol Methods. 2007;324:63–73. doi: 10.1016/j.jim.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound UV filters in normal human lenses: the concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest Ophthalmol Vis Sci. 2007;48:1718–1723. doi: 10.1167/iovs.06-1134. [DOI] [PubMed] [Google Scholar]
- 18.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound and free UV filters in cataract lenses: the concentration of UV filters is much lower than in normal lenses. Exp Eye Res. 2007;85:219–225. doi: 10.1016/j.exer.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Aquilina JA, Truscott RJ. Kynurenine binds to the peptide binding region of the chaperone alphaB-crystallin. Biochem Biophys Res Commun. 2001;285:1107–1113. doi: 10.1006/bbrc.2001.5288. [DOI] [PubMed] [Google Scholar]
- 20.Parker NR, Jamie JF, Davies MJ, Truscott RJ. Protein-bound kynurenine is a photosensitizer of oxidative damage. Free Radic Biol Med. 2004;37:1479–1489. doi: 10.1016/j.freeradbiomed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neuro Chem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee MW, Park SC, Chae HS, et al. The protective role of HSP90 against 3-hydroxykynurenine-induced neuronal apoptosis. Biochem Biophys Res Commun. 2001;284:261–267. doi: 10.1006/bbrc.2001.4938. [DOI] [PubMed] [Google Scholar]
- 23.Mohib K, Guan Q, Diao H, Du C, Jevnikar AM. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol. 2007;293:F801–F812. doi: 10.1152/ajprenal.00044.2007. [DOI] [PubMed] [Google Scholar]
- 24.Staniszewska MM, Nagaraj RH. Upregulation of glyoxalase I fails to normalize methylglyoxal levels: a possible mechanism for biochemical changes in diabetic mouse lenses. Mol Cell Biochem. 2006;288:29–36. doi: 10.1007/s11010-005-9115-1. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez S, Aquilina JA, Jamie JF, Sheil MM, Truscott RJ. Novel protein modification by kynurenine in human lenses. J Biol Chem. 2002;277:4867–4873. doi: 10.1074/jbc.M107529200. [DOI] [PubMed] [Google Scholar]
- 26.Frumento G, Rotondo R, Tonetti M, Ferrara GB. T cell proliferation is blocked by indoleamine 2,3-dioxygenase. Transplant Proc. 2001;33:428–430. doi: 10.1016/s0041-1345(00)02078-9. [DOI] [PubMed] [Google Scholar]
- 27.Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535:207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo Y, Boyd CA, Spyropoulou I, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87–98. doi: 10.1016/j.jri.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Logan GJ, Smyth CM, Earl JW, et al. HeLa cells cocultured with peripheral blood lymphocytes acquire an immuno-inhibitory phenotype through up-regulation of indoleamine 2,3-dioxygenase activity. Immunology. 2002;105:478–487. doi: 10.1046/j.1365-2567.2002.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao BG, Liu X, Link H. Antigen-specific T cell functions are suppressed over the estrogen-dendritic cell-indoleamine 2,3-dioxygenase axis. Steroids. 2004;69:653–659. doi: 10.1016/j.steroids.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Pendergrass WR, Penn PE, Li J, Wolf NS. Age-related telomere shortening occurs in lens epithelium from old rats and is slowed by caloric restriction. Exp Eye Res. 2001;73:221–228. doi: 10.1006/exer.2001.1033. [DOI] [PubMed] [Google Scholar]
- 32.Wolf NS, Li Y, Pendergrass W, Schmeider C, Turturro A. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res. 2000;70:683–692. doi: 10.1006/exer.2000.0835. [DOI] [PubMed] [Google Scholar]
- 33.Shang F, Gong X, Palmer HJ, Nowell TR, Jr, Taylor A. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Exp Eye Res. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- 34.Hawse JR, Hejtmancik JF, Huang Q, et al. Identification and functional clustering of global gene expression differences between human age-related cataract and clear lenses. Mol Vis. 2003;9:515–537. [PMC free article] [PubMed] [Google Scholar]
- 35.Ruotolo R, Grassi F, Percudani R, et al. Gene expression profiling in human age-related nuclear cataract. Mol Vis. 2003;9:538–548. [PubMed] [Google Scholar]
- 36.Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev of Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 37.Parker SB, Eichele G, Zhang P, et al. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 38.Guertin DA, Trautmann S, McCollum D. Cytokinesis in eukaryotes. Microbiol Mol Biol Rev. 2002;66:155–178. doi: 10.1128/MMBR.66.2.155-178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson RW, Snyder JA. Localization of myosin II to chromosome arms and spindle fibers in PtK1 cells: a possible role for an actomyosin system in mitosis. Protoplasma. 2005;225:113–122. doi: 10.1007/s00709-005-0085-7. [DOI] [PubMed] [Google Scholar]
- 40.Lovicu FJ, Ang S, Chorazyczewska M, McAvoy JW. Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci. 2004;26:446–455. doi: 10.1159/000082286. [DOI] [PubMed] [Google Scholar]
- 41.Matsushima H, Mukai K, Yoshida S, Obara Y. Effects of calcium on human lens epithelial cells in vitro. Jap J Ophthalmol. 2004;48:97–100. doi: 10.1007/s10384-003-0029-8. [DOI] [PubMed] [Google Scholar]
- 42.Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 43.Nagai N, Ito Y, Okamura H. Involvement of interleukin 18 in cataract development in hereditary cataract UPL rats. J Biochem. 2007;142:597–603. doi: 10.1093/jb/mvm168. [DOI] [PubMed] [Google Scholar]