Abstract
Objective
To determine whether tolerance and enhancement of innate immune function can be induced by the gram-positive cell wall component peptidoglycan (PGN).
Design
Controlled, in vivo laboratory study
Subjects
Male mice, 8-12 wks (C57BL6/J; C3H/HeJ; B6.129-Tlr2tm1kir/J)
Interventions
Mice were given intraperitoneal injections of 1mg PGN on 2 consecutive days. Mice were then challenged with an intravenous injection of live S. aureus (1 × 108 colony-forming units) 2 days after the second pretreatment.
Measurements and Main Results
Mice pretreated with PGN had diminished plasma concentrations of TNFα and IFNγ in response to the bacterial challenge when compared to untreated controls. Plasma IL-10 after bacterial challenge was higher in PGN-pretreated mice than in controls. Clearance of bacteria after the Staphylococcal challenge was improved in mice pretreated with PGN, and mortality in response to a subsequent Staphylococcus challenge was significantly attenuated. PGN pretreatment of mice lacking intact TLR4 signaling (C3H/HeJ) or TLR2 signaling (TLR2 Knockouts) had similar effects on plasma cytokine balance, bacterial clearance, and mortality.
Conclusions
Exposure to peptidoglycan significantly attenuated inflammation and enhanced bacterial clearance after a subsequent challenge with Staphylococcus aureus. These results show that exposure to Gram-positive bacterial cell wall components can induce tolerance and enhance innate immune function and neither TLR2 nor TLR4 are necessary for this phenomenon. Further, although the altered cytokine balance is similar to that seen in septic patients, induced tolerance differs importantly from the clinical scenario of sepsis in that bacterial clearance and survival are improved compared to normal control animals.
Keywords: rodent, peptidoglycans, tolerance, bacterial infection
Introduction
Endotoxin (lipopolysaccharide, LPS) is a molecule found in the cell wall of Gram negative bacteria. Injection of endotoxin initiates a dramatic proinflammatory response that, if untreated, can result in profound hemodynamic derangements and may even progress to shock and death. Interestingly, animals that survive a sublethal exposure to endotoxin are resistant to subsequent challenges with normally lethal doses of LPS during the first few days following the initial exposure (1). Further, exposure to endotoxin may confer resistance to inflammation and injury induced by other challenges including myocardial infarction (2,3), neural ischemia (4,5), and gastric insults (6,7). Although the cytokine profile in LPS tolerant animals is similar to that seen in septic patients, innate immune function in the LPS tolerant state actually seems improved in that bacterial clearance is enhanced and mortality is suppressed after a Gram-negative bacterial challenge (8). We have previously shown that LPS pretreatment not only diminishes the inflammatory response but also appears to enhance the effectiveness of the innate immune system to clear both Gram-negative and Gram-positive bacterial challenges (9-11).
Although Gram-negative bacteria have historically been a major cause of sepsis in clinical patients, the incidence of Gram-positive bacterial sepsis has risen significantly over the last decade. During the same time, investigators have advanced our understanding of the process employed by innate immune cells to detect, and react to, a myriad of microbial organisms. It is now known that innate detection of offending microorganisms is dependent upon recognition of a small number of molecules that are common to broad classifications of microorganisms. Toll-like receptors (TLRs) and other receptors mediate the inflammatory effects and innate immune function in response to invading microorganisms. For example, Gram-negative bacteria are detected, at least in part, by the presence of LPS which elicits an innate immune response through TLR4 (12,13). In light of the recent findings that different receptors are activated by gram-positive bacterial molecules when compared to activation by gram-negative bacterial molecules, we were curious to extend our recent findings on bacterial clearance after tolerance induction. During an infection, it is likely that multiple receptors are activated by different bacterial molecules. How the host responds to different bacterial ligands and how prior exposures may influence responses to infection are basic questions that may ultimately provide insight into the pathogenesis of sepsis. We were interested in determining if tolerance could be induced by molecules from the cell wall of Gram-positive bacteria and, if so, whether this tolerant state would also be associated with an enhanced innate immune function after a live bacterial challenge. The aim of this study was to determine if peptidoglycan (PGN) could induce tolerance to a subsequent Gram-positive bacterial challenge. We pretreated mice with PGN and studied the response of the mice to a subsequent challenge with live Staphylococcus aureus, a bacterium frequently associated with nosocomial infections and a major pathogen in critically ill patients (14).
Materials and Methods
Tolerance Model
Male, 6-8 week old wild type C57BL/6J, C3H/HeJ, and TLR2 Knockout (B6.129-Tlr2tm1kir/J) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in a monitored, light-dark cycled environment and provided with standard lab chow and water ad libitum. Peptidoglycan (PGN), derived from Staphylococccus aureus, was purchased from Sigma Chemical (St. Louis, MO). Mice were injected with PGN (1mg in 0.1ml of saline i.p.) once daily for 2 consecutive days. Control mice received normal saline (0.1 ml) in the same regimen. All mice were injected at the same time each morning. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch and met National Institutes of Health guidelines for the use of experimental animals in research.
Staphylococcus-induced challenge
Staphylococcus aureus (strain 14458, American Type Culture Collection, Rockville, MD) was inoculated into nutrient broth and allowed to replicate overnight in a shaking incubator at 37°C. The resulting bacterial culture was washed with 10ml of sterile 0.9% saline. Viable numbers of colony-forming units (cfu) were determined by plating serial dilutions overnight on nutrient agar. Bacteria were suspended in sterile 0.9% saline at a final concentration of 1 × 109 cfu/ml. Mice were challenged with 0.1ml of this suspension (1 × 108 cfu; i.v.) 2 days after the second dose of PGN.
Bacterial Clearance
The mice were sacrificed under isoflurane 6 hours after intravenous injection of Staphylococcus aureus. Spleen and liver samples were aseptically excised, weighed and homogenized in sterile saline using sterile tissue grinders. Serial dilutions of tissue homogenates were plated on nutrient agar and incubated overnight at 37°C. Bacterial colony-forming units were counted to assess bacterial burden.
Enzyme-linked Immunosorbent Assay (ELISA)
Blood samples were collected 6 hours after the Staphylococcus challenge and plasma cytokine concentrations were measured by ELISA. IFN-γ, IL-10, and TNFα ELISA kits (eBiosciences, San Diego, CA) were used to measure cytokine concentrations in plasma according to the manufacturer’s instructions. Briefly, standards or experimental samples were added to 96-well plates coated with monoclonal antibody against the cytokine of interest and incubated for 2 hours. After washing, horseradish peroxidase-conjugated, cytokine-specific antibody was added to each well, incubated for 2 hours, and washed. Substrate solution (TMB, Sigma Chemical, St. Louis, MO) was added and incubated for 30 minutes, and the reaction was terminated by the addition of stop solution (2N H2SO4). Cytokine concentrations were determined by measuring optical density at 450 nM using a microtiter plate reader (Dynatech Laboratories, Chantilly, VA).
Data Analysis
Results are presented as mean ± SEM. Sample data comparing 2 groups were analyzed by unpaired t test while data from multiple groups were analyzed by ANOVA and post-hoc Tukey’s test. Survival curves were analyzed by log-rank test. A value of p<0.05 was considered statistically significant.
Results
Pretreatment with peptidoglycan (PGN) induced a cytokine response but suppressed the inflammatory response to subsequent bacterial challenge
Pretreatment of mice with PGN resulted in elevated plasma concentrations of TNFα and IL-10, but not IFNγ, at 6 hours after injection (Table 1). In the absence of further challenge, these cytokines were not detectable 48 hours later. However, mice challenged with S. aureus 48 hours after pretreatment with PGN had suppressed IFNγ (p<0.05) and TNFα (p<.01) and increased IL-10 (p<.001) responses when compared to saline pre-treated control mice (Figure 1).
Table I.
Cytokine induction at 6 & 48 hours after i.p. injection of peptidoglycan or saline (control).
| IFNγ (pg/ml) |
IL-10 (pg/ml) |
TNFα (pg/ml) |
|
|---|---|---|---|
| Control | N.D. | N.D. | N.D. |
| PGN — 6hrs | N.D. | 418 ± 135 | 123 ± 44 |
| PGN — 48hrs | N.D. | N.D. | N.D. |
Data shown are Mean ± SEM. N.D. = None detected. Lower limit of detection for the IFNγ assay was 8 pg/ml.; 30 pg/ml for the IL-10 assay; and 8 pg/ml for the TNFα assay.
Figure 1.
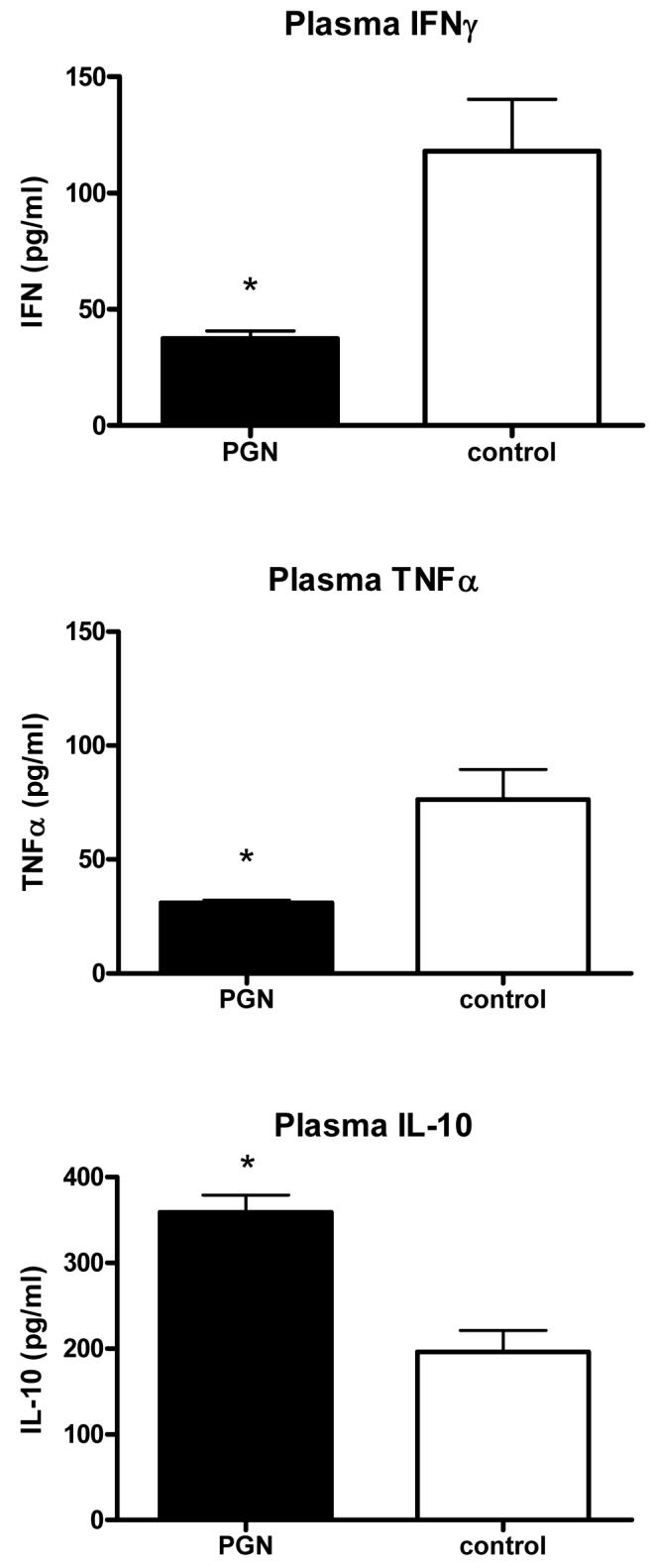
Mice were pretreated with peptidoglycan (PGN). The mice were subsequently challenged with live Staphylococcus aureus. Plasma samples were collected from the mice 6 hours later. Pretreatment with PGN was associated with decreased induction of IFNγ and TNFα and higher induction of IL-10 after S. aureus challenge when compared to the control mice (* indicates p < 0.05 vs control, N = 5 per group).
Pretreatment with peptidoglycan was associated with enhanced bacterial clearance and improved survival in response to a Staphylococcus aureus challenge
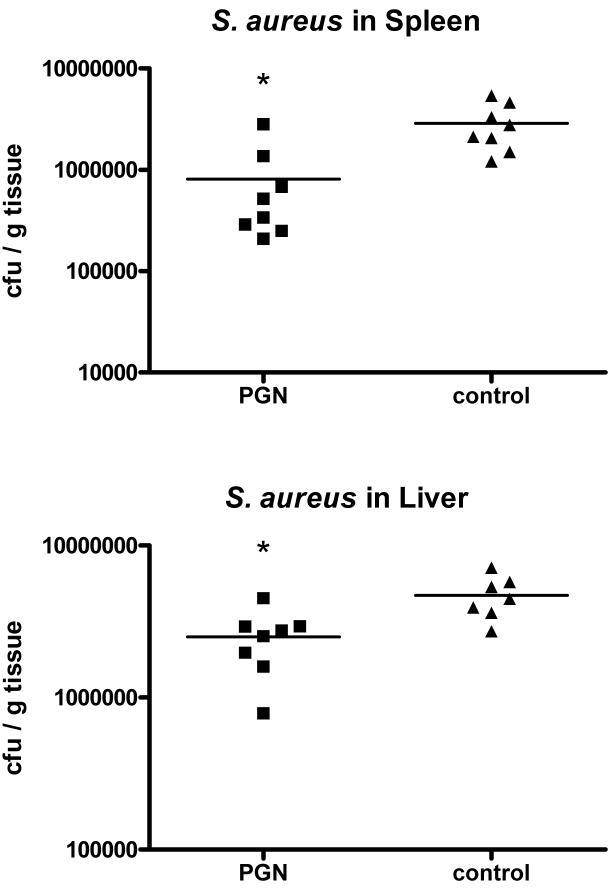
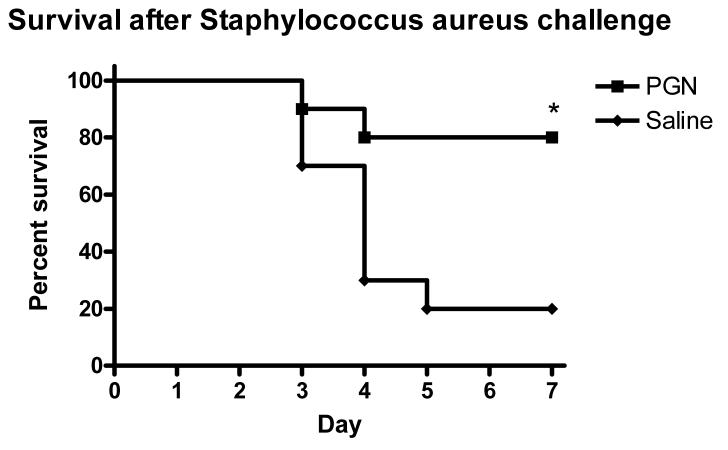
To test the immunological significance of PGN pretreatment and the effect of the altered cytokine profile in response to S. aureus challenge, bacterial clearance was studied in mice that were challenged with S. aureus 48 hours after pretreatment with PGN. Mice pretreated with PGN had 3-fold lower concentrations of bacterial colony-forming units in spleen (p<0.01) and about 50% lower cfu in liver (p<0.01) tissues when compared to saline-treated control animals (Figure 2). To further determine the immunological consequences of the effects of pretreatment with PGN, survival was observed for 7 days in mice after challenge with a highly lethal inoculum of S. aureus. Only 2 of 10 mice pretreated with PGN died during the observation period, compared to 80% mortality in control mice by day 5 (p=.011) (Figure 3).
Figure 2.
Mice were pretreated PGN and subsequently challenged with live S. aureus. The mice were sacrificed 6 hours later. Pretreatment with PGN was associated with significantly lower bacterial cfu’s in spleen and liver homogenates when compared to the controls (* indicates p < 0.05 vs control, N = 8 per group)
Figure 3.
Mice were pretreated with PGN prior to challenge with live S. aureus. The mice were observed for mortality for 7 days after the challenge. Mice that were pretreated had significantly better survival after the S. aureus challenge than control mice. (p < 0.05, N = 10 per group)
PGN-induced tolerance and enhancement of innate immune function was independent of signaling through the TLR4 receptor
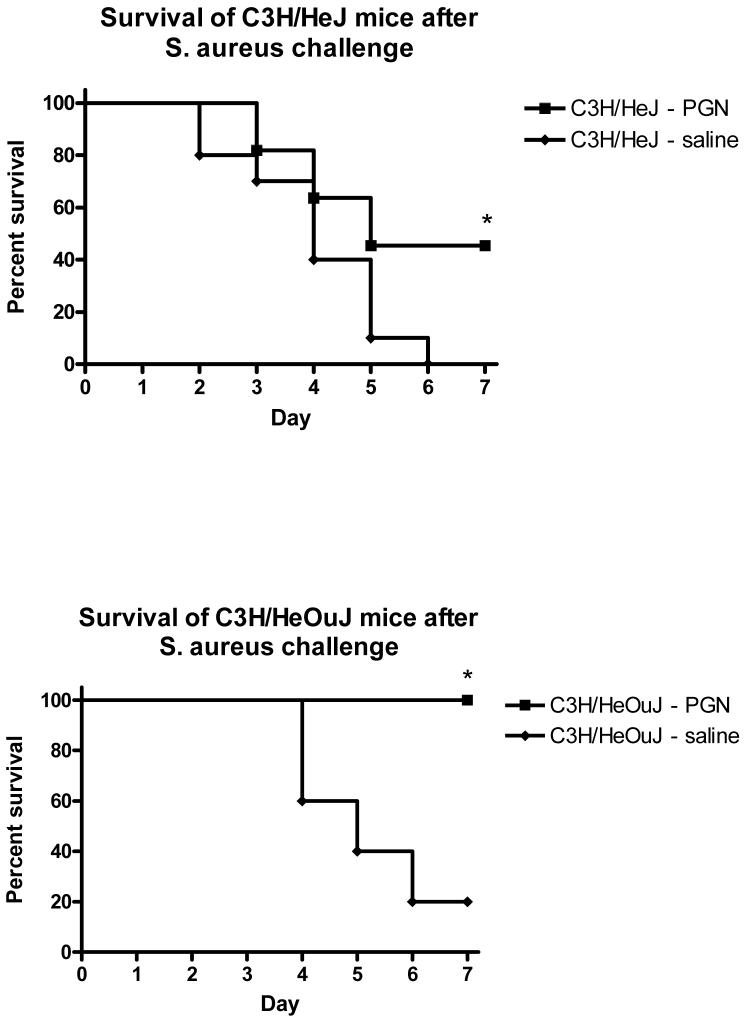
To test whether TLR4 signaling was necessary for the effects of PGN pretreatment and to verify that the effects of PGN pretreatment were not due to LPS contamination, C3H/HeJ mice were pretreated with PGN 48 hours prior to a challenge with S. aureus, and sacrificed 6 hr later for sample collection. As was demonstrated in wild-type C57BL/6 mice, pretreatment of C3H/HeJ mice with PGN was associated with diminished induction of IFNγ (p<0.01) and TNFα (p<0.001) in response to the S. aureus challenge when compared to saline-pretreated C3H/HeJ mice (Figure 4). The plasma IL-10 response to the Staphylococcus challenge was higher, but not statistically different in mice pretreated with PGN compared to saline-pretreated controls (Figure 4). Similarly, bacterial counts in spleen tissues were lower in mice pretreated with PGN when compared to saline-pretreated controls (p<0.01 in both HeJ and OuJ), although liver bacterial counts were not significantly different (Figure 5). PGN-pretreated C3H/HeJ (p=0.0327) and C3H/HeOuJ (p=.0128) mice also had a higher percentage of survival after S. aureus challenge when compared to saline-pretreated control mice (Figure 6). The effects of PGN-pretreatment on plasma cytokine profile, bacterial clearance, and mortality results were similar to those seen in the C57BL6 mice described and also similar to results observed in C3H/HeOuJ mice, which do not have the point mutation and thus have intact TLR4 signaling.
Figure 4.
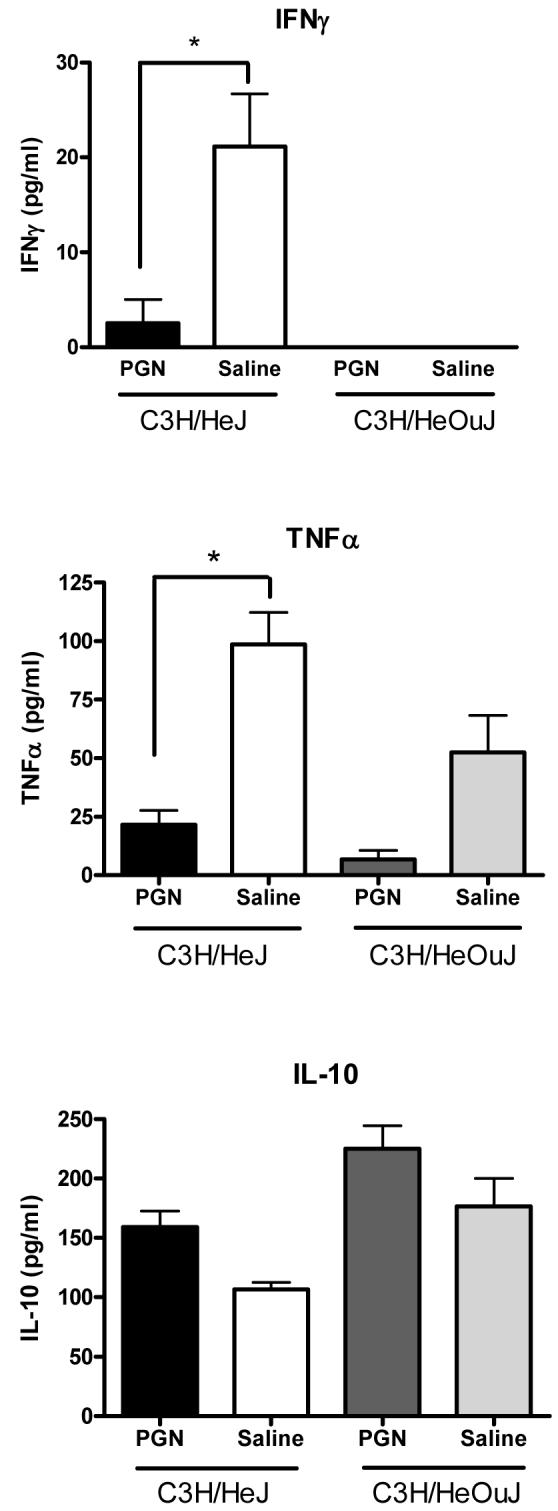
C3H/HeJ (TLR4 mutation) and C3H/HeOuJ (TLR4 intact) mice were pretreated with PGN prior to challenge with live S. aureus. Plasma samples were collected from the mice 6 hours later. Mice that were pretreated had significantly lower plasma concentrations of TNFα and IFNγ and higher plasma concentrations of IL-10 than control mice (* indicates p < 0.05, N = 5-8 per group).
Figure 5.
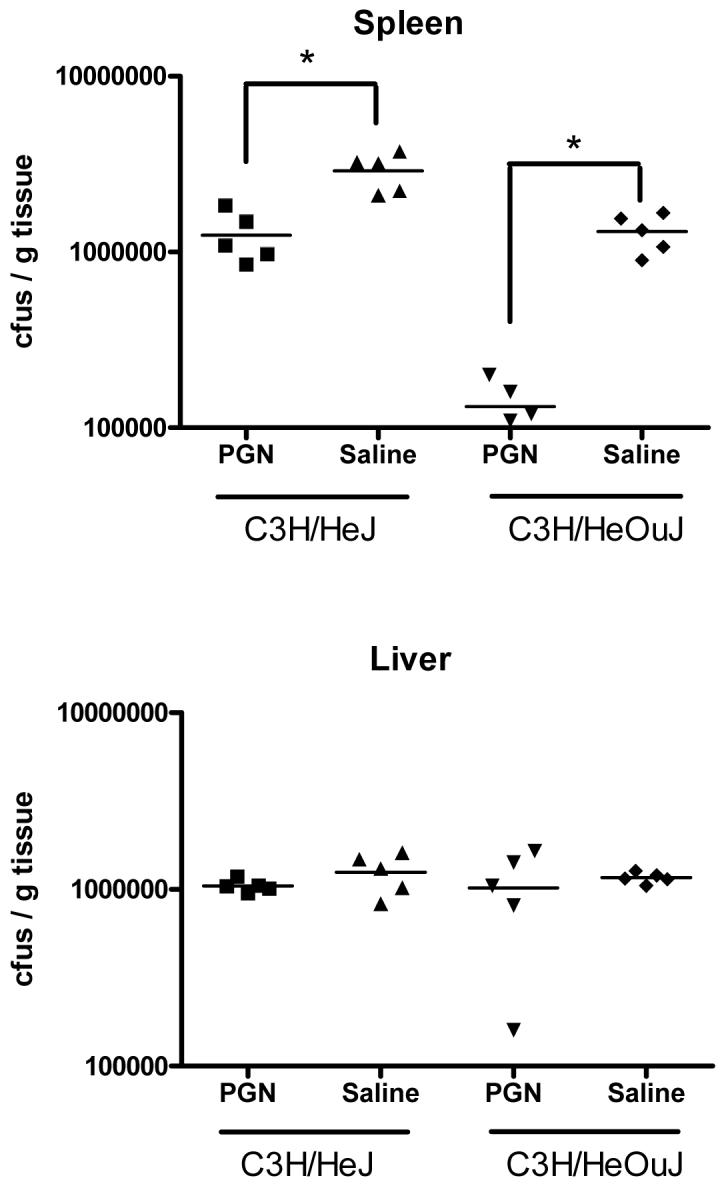
C3H/HeJ (TLR4 mutation) and C3H/HeOuJ (TLR4 intact) mice were pretreated with PGN prior to challenge with live S. aureus. The mice were sacrificed 6 hours later. Mice that were pretreated had significantly decreased numbers of bacterial-forming colonies in spleen tissue homogenates when compared to control mice (* indicates p < 0.05, N = 5 per group).
Figure 6.
C3H/HeJ (TLR4 mutation) and C3H/HeOuJ (TLR4 intact) mice were pretreated with PGN prior to challenge with live S. aureus. The mice were observed for mortality for 7 days after the challenge. Mice that were pretreated had significantly better survival after the S. aureus challenge than control mice. (p < 0.05, N = 10-11 per group)
PGN-induced tolerance and enhancement of innate immune function was independent of signaling through the TLR2 receptor
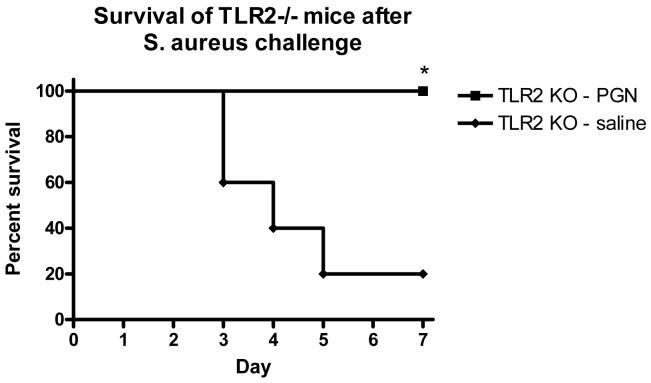
To test whether TLR2 signaling was necessary for the effects of PGN pretreatment, TLR2 knockout mice were pretreated with PGN 48 hours prior to a challenge with S. aureus, and sacrificed 6 hrs later for sample collection. As was demonstrated in wild-type mice, pretreatment of TLR2 knockout mice with PGN was associated with diminished plasma concentrations of IFNγ (p<0.05) and TNFα (p<0.001) in response to a Staphylococcus aureus challenge when compared to saline-pretreated control mice (Figure 7). The plasma IL-10 response to a Staphylcoccus challenge was higher in TLR2 KO mice pretreated with PGN when compared to saline-pretreated controls (p<0.05) (Figure 7). Similarly, bacterial counts in spleens were significantly lower in TLR2 KO mice pretreated with PGN when compared to mice pretreated with saline (p<0.001) (Figure 8). PGN-pretreated TLR2-/- mice also had a higher percentage of survival after S. aureus challenge when compared to saline-pretreated control mice (p=.0128) (Figure 9) and these results were similar to those observed in the background strain C57BL6 mice (Figure 3).
Figure 7.
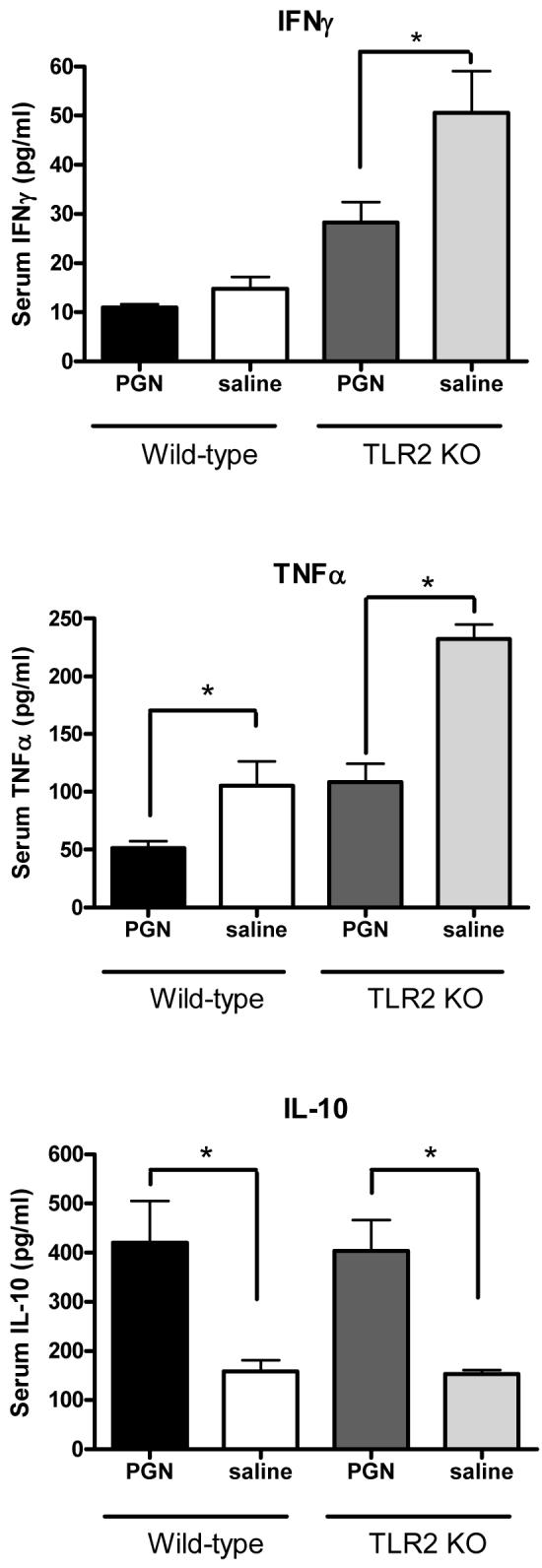
TLR2 Knockout and wild-type mice were pretreated with PGN prior to challenge with live S. aureus. Plasma samples were collected from the mice 6 hours later. Mice that were pretreated had lower plasma concentrations of IFNγ and TNFα and higher plasma concentrations of IL-10 than control mice (*indicates p < 0.05, N = 5-8 per group for IFNγ and TNFα; 3-5 per group for IL-10).
Figure 8.
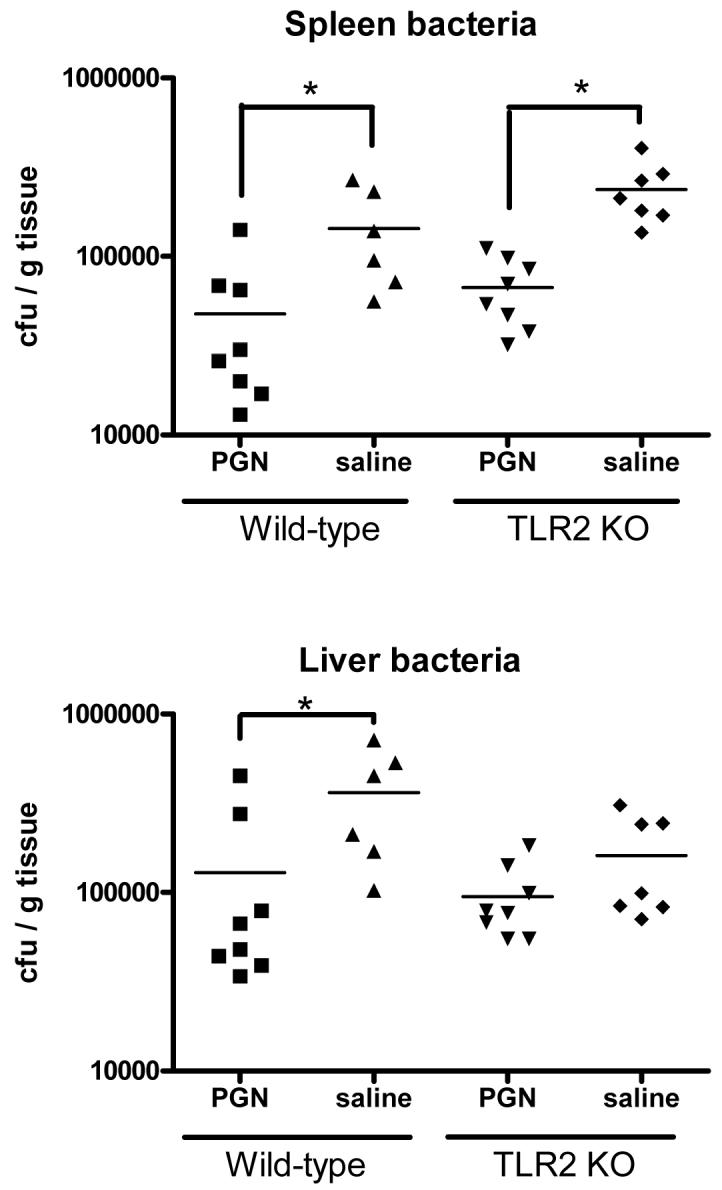
TLR2 Knockout and wild-type mice were pretreated with PGN prior to challenge with live S. aureus. The mice were sacrificed 6 hours later. Mice that were pretreated with PGN had decreased numbers of bacteria in spleen homogenates and diminished bacterial colonies in liver homogenates (* indicates p < 0.05, N = 8 per group).
Figure 9.
TLR2 Knockout mice were pretreated with PGN prior to challenge with live S. aureus. The mice were observed for mortality for 7 days after the challenge. Mice that were pretreated had significantly better survival after the S. aureus challenge than control mice. (p < 0.05, N = 5 per group)
Discussion
These studies demonstrate that, similar to LPS from Gram-negative bacteria, peptidoglycan from the cell wall of Gram-positive bacteria can reduce inflammation and enhance innate immune function in mice in response to a subsequent bacterial challenge. In the present study, tolerance was manifested by a diminished proinflammatory cytokine (TNFα) response; diminished Th1 cytokine response (IFNγ); and an increased Th2 cytokine response (IL-10). Despite the predominant Th2 response to the Staphylococcus aureus challenge, tolerant animals demonstrated enhanced bacterial clearance and greatly attenuated mortality after the S. aureus challenge. Neither TLR2 nor TLR4 signaling were necessary for these effects and the results in LPS-resistant C3H/HeJ mice suggested that the tolerance induction was not a result of LPS contamination of the peptidoglycan (PGN).
LPS tolerance was first described in 1947 by Beeson who noted that consecutive administrations of fractionated bacterial suspensions to rabbits were associated with progressively diminished physiologic responses (15). Since these observations, LPS has been reported to induce tolerance to a variety of other stimuli and injuries, including tolerance to CpG DNA (16), TNFα and IL-1β (17), ethanol intoxication (18) and an attenuation of inflammation and injury after a variety of ischemic insults (2-7). Similarly, tolerance to LPS has been described to occur after a variety of other pretreatments, including cytokines (17,19), heat shock proteins (20,21), and other bacterial peptides including lipoproteins and lipoteichoic acid (22-24). In the last few decades, a number of studies of tolerance have been conducted with isolated cells. In vivo study of tolerance induction offers an advantage over in vitro or ex vivo studies in that a functional immune endpoint (bacterial clearance) can be studied directly. Further, different cell types and origins have had conflicting results in cell culture under the same experimental conditions (25).
Tolerance to components of gram-positive bacterial cell walls has not been studied as extensively as tolerance induced by LPS. In cell culture, exposure of macrophages to either lipoteichoic acid (LTA) or PGN results in suppression of cytokine release after restimulation with the same molecule (24,26). In the case of LTA, tolerance induction was dependent upon TLR2 (24). In animal models, pretreatment with LTA reduced cardiac injury induced by ischemia/reperfusion similar to the protection provided by pretreatment with LPS (27). There are few studies of gram-positive tolerance induction and subsequent effects on innate immune function. Ausobsky et al tested muramyl dipeptide, a component of peptidoglycan, in an in vivo model and reported an attenuation of mortality after challenge with Klebsiella (28). O’Brien et al demonstrated tolerance and enhanced clearance of a Gram-negative bacterial challenge in mice pretreated with bacterial lipoprotein, a molecule that can be derived from both Gram-positive and Gram-negative bacteria (29).
Recognition of microbial organisms is the first step in host defense. The role of the innate immune system is to recognize a vast number of different pathogens and to distinguish them from self. Lipoteichoic acid, peptidoglycan, and other bacterial molecules such as LPS of gram-negative bacteria are highly conserved across broad classes of bacteria and, as such, provide the innate immune system with an efficient method for detection of a diverse multitude of microbial organisms. A consequence of the interaction of these pathogen-associated molecular patterns (PAMPs) with Toll-like receptors on macrophages, monocytes, and neutrophils is up-regulation of inflammatory gene expression and subsequent release of inflammatory cytokines and chemokines. Both TLR2 and the alternative innate immune receptor NOD2 have been described as required for a full response to Staphylococcus aureus (30,31), although our study seems to demonstrate that neither TLR2 knockout nor TLR4 deficient (HeJ) control mice cleared the bacterial challenge as efficiently as the wild-type control mice. Further, both TLR2 knockout and TLR4 deficient mice had a more pronounced TNFα and IFNγ cytokine response when compared to their respective wild-type groups suggesting that TLR2 and TLR4 signaling may play a role in the regulation of those cytokines.
While it is not surprising that a defect in TLR2 signaling would be associated with increased susceptibility to a Gram positive bacterial infection (32), it is not clear why the C3H/HeJ mice had worse bacterial clearance and mortality compared to the OuJ control mice groups. The point mutation involving the TLR4 receptor renders mice more susceptible to Gram negative bacterial infections (33) but previous investigations in C3H/HeJ mice have not reported an increased susceptibility to Staphylococcus aureus compared to C3H strains with intact TLR4 signaling. During the course of these studies, we attempted to minimize variability by purchasing both strains at the same time from the same vendor; housing under the same conditions, and infecting and sampling the mice at the same time. It is possible that additional genetic drift has occurred between these 2 C3H strains since their separation in the 1960’s that either renders the HeJ mice more susceptible or the OuJ mice less susceptible to Staphylococcus. Nevertheless, PGN administration effectively enhanced innate immune function in both TLR2-/- and HeJ mice when compared to saline-treated TLR2-/- or HeJ mice, suggesting that the enhancement of immune function occurred through mechanisms other than TLR2 and TLR4 signaling. This may have involved activity downstream of NOD-2 receptor activation. Peptidoglycan, consisting of glycan strands cross-linked by peptides, is the major component of the cell wall of gram-positive bacteria. Detection and activation of immune cells in response to PGN is not only through TLR2 but also through alternate pattern recognition receptors including NOD1 and NOD2, CD14, and a family of peptidoglycan recognition proteins (PGRP’s) (34). Other investigators have demonstrated that additional receptors, such as C5aR, contribute significantly to the immune response of TLR2 knockout mice to S. aureus (35) and it remains possible that the mechanism of PGN-induced tolerance worked through these as well.
Other components of gram-positive bacteria include lipoteichoic acid which is a polymer anchored in the outer cytoplasmic membrane by a lipid moiety and, as such, is the Gram-positive bacterial equivalent to LPS from Gram-negative bacteria. LPS-binding protein and CD14 facilitate recognition of lipoteichoic acid, although cell stimulation occurs through TLR2 rather than TLR4 (36,37). We have tested lipoteichoic acid in experiments similar to those in this report and found that tolerance induction did occur although not as strongly compared to that induced by PGN (unpublished observations).
It was interesting to note that although lack of TLR2 or TLR4 signaling appeared to be associated with diminished ability to clear bacteria in untreated mice, neither was necessary for the induction of tolerance and enhancement of bacterial clearance. How tolerance induction exerts a positive effect on bacterial clearance is not entirely clear. The number of phagocytic cells is increased after tolerance induction, and surface expression of complement receptor 3 and FcγIII/IIR have been shown to be increased on cells from tolerant animals (23). Both of those molecules have roles in phagocytosis, so tolerance enhancement of bacterial clearance may be due to increased numbers and activity of phagocytic cells. Recent work confirms that phagocytosis is significantly improved after induction of tolerance by LPS (38).
The clinical relevance of tolerance induction is unclear. Septic patients often have anti-inflammatory cytokine profiles, and ex-vivo stimulation of leukocytes from septic patients has demonstrated suppression of the inflammatory response. Sepsis due to Gram-positive infections has been associated with a lesser degree of macrophage hyporesponsiveness to inflammatory stimuli than that seen in patients with Gram-negative sepsis (39). Our previous work combined with the present study demonstrates that pretreatment of mice with either Gram-negative or Gram-positive molecules results in a diminished proinflammatory response to a subsequent bacterial challenge. At the same time, however, we observed enhanced innate immune function as manifested by accelerated bacterial clearance and decreased mortality and thus induced tolerance differs significantly from clinical cases of sepsis. Whether this occurs as a natural phenomenon, ie, whether there are groups of patients that suffer minor injuries or infections that are followed by a period of enhanced bacterial resistance is unknown. Regardless, from a clinical standpoint understanding the mechanism of LPS tolerance is of obvious interest because reduction of the proinflammatory response may have important benefits, especially if it can be achieved while maintaining or even enhancing the innate immune clearance of pathogenic organisms.
Conclusions
Exposure to peptidoglycan derived from the cell wall of gram-positive bacteria diminished the proinflammatory response but enhanced the innate immune response of mice to subsequent live bacterial challenges.
Acknowledgments
The authors would like to acknowledge the technical expertise of Kimberly Palkowetz who performed the flow cytometry analyses and Dr. Daniel Freeman who reviewed the statistical analyses in this paper. This work was supported by grants from the NIH-NIGMS and Shriners of North America.
This work was supported by grants from the NIH-NIGMS and Shriners of North America
Footnotes
The authors have not disclosed any potential conflicts of interest.
Reference List
- 1.Greisman SE, Young EJ, Carozza FA., Jr. Mechanisms of endotoxin tolerance. V. Specificity of the early and late phases of pyrogenic tolerance. J. Immunol. 1969;103:1223–36. [PubMed] [Google Scholar]
- 2.Frassdorf J, Weber NC, Obal D, Toma O, Mullenheim J, Kojda G, Preckel B, Schlack W. Morphine induces late cardioprotection in rat hearts in vivo: the involvement of opioid receptors and nuclear transcription factor kappaB. Anesth. Analg. 2005;101:934–41. doi: 10.1213/01.ane.0000172130.70274.84. [DOI] [PubMed] [Google Scholar]
- 3.Meng X, Brown JM, Ao L, Shames BD, Banerjee A, Harken AH. Reduction of infarct size in the rat heart by LPS preconditioning is associated with expression of angiogenic growth factors and increased capillary density. Shock. 1999;12:25–31. doi: 10.1097/00024382-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lastres-Becker I, Cartmell T, Molina-Holgado F. Endotoxin preconditioning protects neurones from in vitro ischemia: role of endogenous IL-1beta and TNF-alpha. J. Neuroimmunol. 2006;173:108–116. doi: 10.1016/j.jneuroim.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J. Neurosurg. 2005;103:715–23. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- 6.Gomes AS, Lima LM, Santos CL, Cunha FQ, Ribeiro RA, Souza MH. LPS from Escherichia coli protects against indomethacin-induced gastropathy in rats--role of ATP-sensitive potassium channels. Eur. J. Pharmacol. 2006;547:136–42. doi: 10.1016/j.ejphar.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Konturek PC, Brzozowski T, Konturek SJ, Taut A, Kwiecien S, Pajdo R, Sliwowski Z, Hahn EG. Bacterial lipopolysaccharide protects gastric mucosa against acute injury in rats by activation of genes for cyclooxygenases and endogenous prostaglandins. Digestion. 1998;59:284–97. doi: 10.1159/000007505. [DOI] [PubMed] [Google Scholar]
- 8.Lehner MD, Ittner J, Bundschuh DS, van RN, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect. Immun. 2001;69:463–71. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock. 2007;27:289–95. doi: 10.1097/01.shk.0000245024.93740.28. [DOI] [PubMed] [Google Scholar]
- 10.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, Toliver-Kinsky T, Sherwood ER. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect. Immun. 2005;73:7340–47. doi: 10.1128/IAI.73.11.7340-7347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphey ED, Fang G, Sherwood ER. Endotoxin pretreatment improves bacterial clearance and decreases mortality in mice challenged with Staphylococcus aureus. Shock. 2008:512–18. doi: 10.1097/shk.0b013e318150776f. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, Formal SB. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 1980;124:20–24. [PubMed] [Google Scholar]
- 13.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–88. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Jones RN, Doern GV, Kugler K. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob. Agents Chemother. 1998;42:1762–70. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeson PB. Tolerance to bacterial pyrogens: I. Factors influencing its development. J Exp Med. 1947;86:29–38. doi: 10.1084/jem.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur. J. Immunol. 2006;36:2483–93. doi: 10.1002/eji.200535767. [DOI] [PubMed] [Google Scholar]
- 17.Ferlito M, Romanenko OG, Ashton S, Squadrito F, Halushka PV, Cook JA. Effect of cross-tolerance between endotoxin and TNF-alpha or IL-1beta on cellular signaling and mediator production. J. Leukoc. Biol. 2001;70:821–29. [PubMed] [Google Scholar]
- 18.Bautista AP, Spitzer JJ. Cross-tolerance between acute alcohol intoxication and endotoxemia. Alcohol Clin. Exp. Res. 1996;20:1395–1400. doi: 10.1111/j.1530-0277.1996.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphey ED, Traber DL. Protective effect of tumor necrosis factor-alpha against subsequent endotoxemia in mice is mediated, in part, by interleukin-10. Critical Care Medicine. 2001;29:1761–66. doi: 10.1097/00003246-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Kilmartin B, Reen DJ. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur. J. Immunol. 2004;34:2041–51. doi: 10.1002/eji.200425108. [DOI] [PubMed] [Google Scholar]
- 21.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J. Immunol. 2006;177:7184–92. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2-and TLR4-mediated signaling pathways. J. Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 23.Wang JH, Doyle M, Manning BJ, Blankson S, Wu QD, Power C, Cahill R, Redmond HP. Cutting edge: bacterial lipoprotein induces endotoxin-independent tolerance to septic shock. J. Immunol. 2003;170:14–18. doi: 10.4049/jimmunol.170.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 2001;166:5161–67. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 25.Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J. Infect. Dis. 2004;189:1295–303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama K, Okugawa S, Yanagimoto S, Kitazawa T, Tsukada K, Kawada M, Kimura S, Hirai K, Takagaki Y, Ota Y. Involvement of IRAKM in peptidoglycan-induced tolerance in macrophages. J. Biol. Chem. 2004;279:6629–34. doi: 10.1074/jbc.M308620200. [DOI] [PubMed] [Google Scholar]
- 27.Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzoorea S, Hafner C, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart - A comparison with endotoxin. Arteriosclerosis Thrombosis and Vascular Biology. 2000;20:1521–28. doi: 10.1161/01.atv.20.6.1521. [DOI] [PubMed] [Google Scholar]
- 28.Ausobsky JR, Cheadle WG, Brosky BG, Polk HC., Jr. Muramyl dipeptide increases tolerance to shock and bacterial challenge in mice. Br. J. Surg. 1984;71:151–53. doi: 10.1002/bjs.1800710225. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien GC, Wang JH, Redmond HP. Bacterial lipoprotein induces resistance to Gram-negative sepsis in TLR4-deficient mice via enhanced bacterial clearance. J. Immunol. 2005;174:1020–26. doi: 10.4049/jimmunol.174.2.1020. [DOI] [PubMed] [Google Scholar]
- 30.Kapetanovic R, Nahori MA, Balloy V, Fitting C, Philpott DJ, Cavaillon JM, dib-Conquy M. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect. Immun. 2007;75:830–37. doi: 10.1128/IAI.01199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165:5392–96. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi O, Hoshino K, Akira S. TLR2 knockout mice are highly susceptible to Staphylococcus aureus. J Immunol. 2000;165:5392–96. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 33.Totemyer S, Foster N, Kaiser P, Maskell DJ, Bryant CE. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica Serovar Typhimurium infection. Infect Immun. 2003;71:6653–57. doi: 10.1128/IAI.71.11.6653-6657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dziarski R, Gupta D. Peptidoglycan recognition in innate immunity. J Endotoxin Res. 2005;11:304–10. doi: 10.1179/096805105X67256. [DOI] [PubMed] [Google Scholar]
- 35.Mullaly SC, Kubes P. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J Immunol. 2006;77:8154–63. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- 36.Han SH, Kim JH, Martin M, Michalek SM, Nahm MH. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 2003;71:5541–48. doi: 10.1128/IAI.71.10.5541-5548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 2003;278:15587–94. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler DS, Lahni PM, Deneberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B.Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis SHOCK 2008, epub Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. Journal of Clinical Investigation. 1991;88:1747–54. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]