Abstract
The objective of this project is to elucidate the relationship between ultrasound contrast agents (UCAs) and sonoporation. Sonoporation is an ultrasound-induced, transient cell membrane permeability change, which allows for the uptake of normally impermeable macromolecules. Specifically, this study will determine the role that inertial cavitation plays in eliciting sonoporation. The inertial cavitation thresholds of the UCA, Optison™, are directly compared to the results of sonoporation in order to determine the involvement of inertial cavitation in sonoporation. Chinese Hamster Ovary (CHO) cells were exposed as a monolayer, in a solution of Optison™, 500,000 Da Fluorescein isothiocyanate-dextran (FITC-dextran), and Phosphate Buffered Saline (PBS) to 30 seconds of pulsed ultrasound (US) at 3.15-MHz center frequency, 5-cycle pulse duration, and 10-Hz pulse repetition frequency. The peak rarefactional pressure (Pr) was varied over a range from 120 kPa to 3.5 MPa, and five independent replicates were performed at each pressure. As the Pr was increased, from 120 kPa to 3.5 MPa, the fraction of sonoporated cells among the total viable population increased from 0.63% to 10.21%, with the maximum occurring at 2.4 MPa. The inertial cavitation threshold for Optison™ at these exposure conditions has previously been shown to be in the range 0.77–0.83 MPa, at which sonoporation activity was found to be 50% of its maximum level. Furthermore, significant sonoporation activity was observed at pressure levels below the threshold for inertial cavitation of Optison™. Above 2.4 MPa, a significant drop in sonoporation activity occurred, corresponding to pressures where >95% of the Optison™ was collapsing. These results demonstrate that sonoporation is not directly due to inertial cavitation of the UCA, rather that the effect was related to linear and/or nonlinear oscillation of the UCA occurring at pressure levels below the inertial cavitation threshold.
Keywords: Chinese Hamster Ovary cells, Sonoporation, ultrasound contrast agent, inertial cavitation, thresholds
Introduction
A significant problem in cancer therapy is the compromised quality of life experienced by the patient due to the side effects of the therapeutic compounds. Delivery of molecular medicine to solid tumors is often inefficient and, as a result, the patient’s healthy cells and tissues are subject to the toxic effects of the drugs. Thus, it is important to develop approaches that deliver drugs only to the appropriate cells within the patient in a specific, efficient, and safe manner. One such method, termed sonoporation, involves the use of ultrasound (US) to enhance cell permeabilization. With this method, it is possible, using US and contrast microbubbles, to noninvasively deliver therapeutic compounds into specific target cells.
Sonoporation alters the permeability of cell membranes in a transient fashion (McNeil 1989), leaving the compounds trapped inside the cell once US exposure is complete. Small compounds (Brayman et al. 1999; Guzman et al. 2001; Keyhani et al. 2001), macromolecules (Bao et al. 1997; Greenleaf et al. 1998; Guzman et al. 2002; Miller et al. 1999; Wyber et al. 1997), and other therapeutic compounds (Harrison et al. 1996; Keyhani et al. 2001; van Wamel et al. 2004; Wu et al. 2006) have successfully been delivered into cells using US. US can also deliver protein (Mukherjee et al. 2000; Weimann & Wu 2002) and DNA (Amabile et al. 2001; Lawrie et al. 2000; Miller et al. 2003; Miller & Song 2003) into tissues. Low- and high-frequency US treatment of cells in the presence of plasmid DNA has been shown to cause mammalian cell transfection in vitro (Bao et al. 1997; Frenkel et al. 2002; Kim et al. 1996; Tata et al. 1997) and in vivo (Endoh et al. 2002; Miller et al. 1999; Miller et al. 2003; Taniyama et al. 2002). Thus, sonoporation has great possibilities in both targeted gene and drug delivery.
Little is known about the mechanism of sonoporation both physically and biologically. Tachibana et al. (1999) and Meheir-Humbert et al. (2005) have shown that large pores form in a cell membrane following US exposure. Schlicher et al (2006) provided evidence that these membrane disruptions are similar to those formed by other physical stresses and are resealed by an active process of vesicle fusion with the cell membrane. Cellular and molecular damage to human red blood cells occurs as a result of US exposure (Kawai & Iino 2003), though, the role this damage plays in pore formation is unknown. It has been shown that the enhanced membrane permeability in sonoporation is transient (Bao et al. 1997; Brayman et al. 1999; McNeil 1989; Taniyama et al. 2002) and the recovery rate does not vary significantly with US parameters or the maximum amplitude of the transmembrane current (Deng et al. 2004). Additionally, hyperpolarization of the cell membrane occurs in the presence of US and ultrasound contrast agent (UCA), most likely due to the activation of channels sensitive to mechanical stresses and nonspecific ion channels (Tran et al. 2007). However, this hyperpolarization does not explain the presence of the pores in the membrane.
The presence of a UCA is necessary to induce a significant sonoporation event (Bao et al. 1997; Greenleaf et al. 1998; Kim et al. 1996). This UCA requirement has led to the identification of inertial cavitation (IC), which is the rapid collapse of a bubble, as the probable sonoporation mechanism, theorized by several studies (Bao et al. 1997; Greenleaf et al. 1998; Hwang et al. 2005; Koch et al. 2000; Lai et al. 2006). However, the data provided in the literature are only circumstantial, not direct evidence that collapse cavitation is the sonoporation mechanism. UCAs have a complex dynamic behavior in an ultrasonic field. The major behaviors are linear oscillation, nonlinear oscillation, and IC. Determining whether oscillation or IC of UCAs is involved in producing sonoporation is essential for determining the physical phenomenon responsible for this biological effect (Fig 1).
Fig 1.
The numerous UCA responses to US and the possible bioeffects of each response, thus emphasizing the critical junction of IC versus oscillation in determining the mechanism for sonoporation.
Most UCAs are gas-filled, encapsulated microbubbles designed to increase acoustic reflectivity. As acoustic waves are incident on the UCA, it grows and shrinks due to the time-varying pressure of the wave. The behavior of the UCA is dependent on US frequency (Ammi et al. 2006b; Chen et al. 2003; Chomas et al. 2001; Giesecke & Hynynen 2003) and peak rarefactional pressure. At low-level acoustic pressure amplitudes, linear oscillation of the UCA occurs. These oscillations lead to local steady flows that are termed microstreaming. When the UCA is close to a cell, this microstreaming can lead to shearing motions on the cell membrane. Theoretical and experimental studies have shown that microstreaming near a cell boundary can adversely affect a cell membrane. The critical stress (in terms of viscous stress) for hemolysis is well defined (Rooney 1970; Williams et al. 1970). Additionally, microstreaming from a vibrating Mason horn demonstrated a threshold for enhanced membrane permeability (12±4 Pa at 21.4 kHz) (Wu et al. 2002). Also, under single-bubble controlled conditions (10 kPa at 180 kHz), Marmottant and Hilgerfeldt (2003) demonstrated experimentally that linear microbubble oscillations were sufficient to rupture lipid membranes due to large velocity gradients. Thus, microstreaming due to linear oscillation of UCAs could play a role in sonoporation.
At higher pressure amplitudes, UCAs exhibit nonlinear oscillation. During these conditions, the UCA slowly expands during rarefaction and is followed by a rapid contraction, but not collapse, during compression. Nonlinear oscillation produces microstreaming, as well as the potential for liquid jets (also known as microjetting). Liquid jets are formed as a result of the asymmetric behavior of the UCA in the presence of a pressure gradient near a surface, such as a cell. These jets can then impinge on the cell membrane at high speeds. Liquid jets provide increased transport of heat and gas by streaming and have the capacity to puncture the cell membrane, producing openings that could allow for the transport of extracellular material into the cell (Prentice et al. 2005). But, the formation of jets is a bit chaotic (Prosperetti 1997).
As the pressure amplitude is increased further, the maximum-to-initial diameter ratio reaches 2, a common criterion for UCA collapse (Church 2005; Flynn 1975; Flynn & Church 1988). This collapse is termed IC because the UCA motion is dominated by the inertia of the liquid. For a shelled UCA, this violent collapse causes the shell to fragment, releasing the encapsulated gas and possibly generating daughter/free bubbles that can also oscillate. The violent collapse of the bubble during IC produces many mechanical effects and chemical agents that could cause bioeffects. Some of the consequences of collapse are mechanical shock waves, a bubble temperature that may reach thousands of degrees Kelvin (4,300–5,000 K) (Didenko et al. 1999; Suslick 2001), microjetting and free radical production (FRP). FRP is caused by the dissociation of water vapor during contraction of the UCA and can mediate chemical changes. However, it has been shown that FRP is not required for transfection (Lawrie et al. 2003).
Sonoporation is a promising drug delivery and gene therapy technique, limited chiefly by a lack of understanding regarding the biophysical mechanism that causes the cell membrane permeability change. The objective of this project is to elucidate the relationship between UCAs and sonoporation, specifically determining the role IC plays in sonoporation.
Materials and Methods
Cell Culture
Chinese Hamster Ovary (CHO) cells (American Type Culture Collection (ATCC), Manassas, VA) were cultured in F-12K Medium (ATCC, Manassas, VA) with 10% v/v fetal bovine serum (ATCC, Manassas, VA), 1% Penicillin/Streptomycin (Sigma-Aldrich, St. Louis, MO), and 0.1% Fungizone (Invitrogen, Carlsbad, CA). Nonhuman cells purchased from ATCC, cultured, and used with no animal involvement do not need IACUC approval. The cells were propagated as a monolayer in 75 cm3 tissue culture flasks at 37°C and a humidified atmosphere of 5% CO2. All work, except the US exposure, was performed in a biological safety cabinet.
Contrast Agent
Optison™ (Amersham Health Inc., Princeton, NJ) contains perflouropropane and is stabilized by a human serum albumin shell, with a mean diameter between 2 and 4.5 μm. The concentration of Optison™ is 5–8×108 mL−1 gas bodies.
Ultrasound Exposure Vessels and Cell Preparation
The sample vessel was a 96-well cell culture microplate (BD Falcon, San Jose, CA) constructed from medical-grade polystyrene. Each well is flat bottomed, holds 0.37 mL, and has a diameter of 6.4 mm, 4.25 times the −6 dB focal beamwidth of the 3.15-MHz transducer. The open face of the microplate was covered by plastic cling wrap, forming a barrier between the external water bath and internal cell solution, as well as an acoustic window for the US to pass into the well unperturbed.
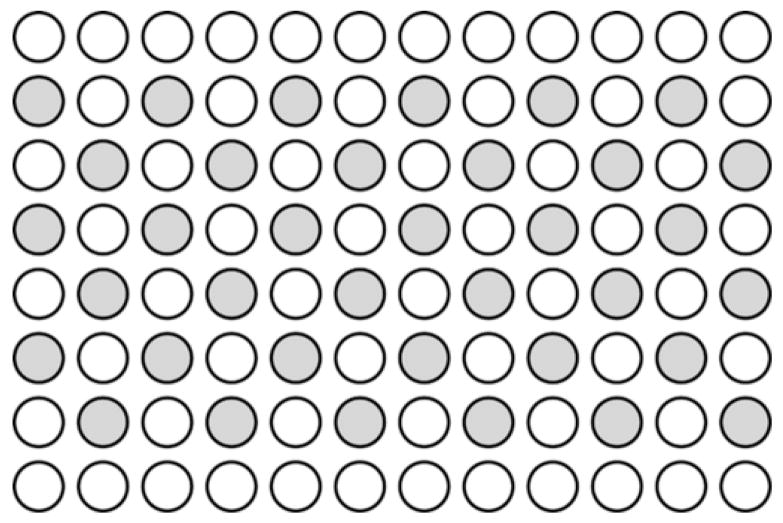
For preparation of an experiment, CHO cells were harvested with 2-mL Trypsin-EDTA (Sigma-Aldrich, St. Louis, MO), and 0.3×106 cells/mL in 0.37 mL of growth medium were added to each well of one clean, sterilized exposure vessel. Thirty-six wells were loaded with cells and the loading configuration is schematically displayed in Fig 2. Cells were loaded in every other well to prevent interaction between adjacent wells (e.g., leaking of medium from one well to the next and possible mechanical interactions). The top and bottom rows of the plate were left empty to provide a location for clamping the plate into the holder in the degassed water tank. The vessel was incubated overnight to allow the seeded cells to settle to the bottom of each well, thus forming the monolayer of greater than 90% confluence. On the day of the experiment, the growth medium was removed and the monolayer rinsed twice with Phosphate Buffered Saline (PBS) to remove any dead cells and debris.
Fig 2.
The loading pattern of the 96-well microwell plate. Note that the top and bottom rows are left empty and every other well is loaded.
The exposure medium added to each well contained Fluorescein isothiocyanate-dextran (FITC-dextran) (FD500S, Sigma-Aldrich Co., St. Louis, MO), with an average molecular weight (MW) of 500,000 Da. The FITC-dextran is normally unable to cross the cell membrane, and thus used as the marker for change in cell membrane permeability. A volume of 0.05-mL FITC-dextran solution (25 mg/mL in PBS), 8.80-μL Optison™, and 0.312-mL PBS were added to each well. The plate was then sealed with plastic cling wrap. Any wells containing air bubbles were excluded from the experiment.
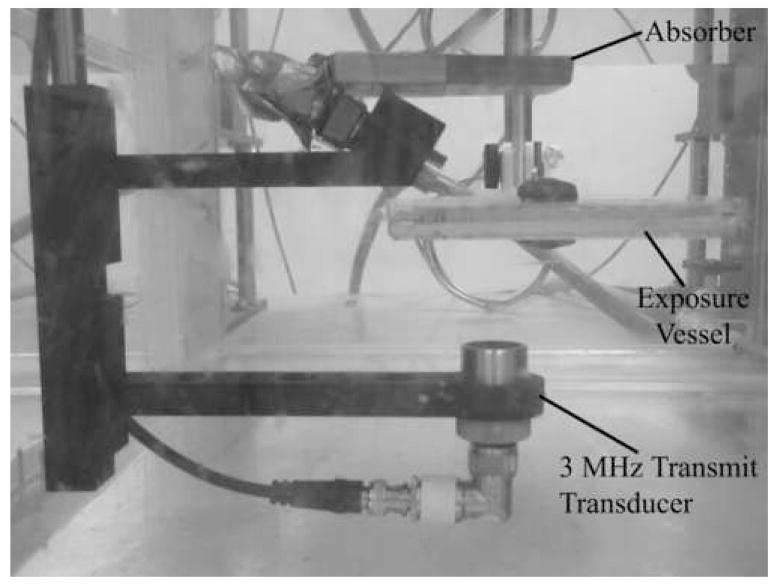
The vessel was placed in a room temperature, degassed water bath with the plastic cling wrap located near the transducer (Fig 3). Thus, the monolayer was on the back window of the chamber, allowing the UCAs to rise to the monolayer due to buoyancy and be pushed toward the monolayer by the radiation force. The vessel was located so the focus of the transducer was positioned at the bottom of the cell, where the cells were located. Also, a water level was used to align the vessel with the horizontal plane. Before each exposure, the vessel was centered with respect to the US beam, so exposure occurred at the center of the exposed well; this centering process took approximately 5 minutes. The first well was exposed approximately 30 seconds following completion of alignment. Each well was independently exposed or sham exposed (US turned off) at the predetermined conditions. The order of well exposure was varied to avoid any influences regarding the order of exposure.
Fig 3.
The experimental setup.
Ultrasound Exposure
US was produced by a 3.15-MHz f/3 19-mm-diameter single-element focused transducer (Valpey Fisher, Hopkinton, MA). The -6 dB beamwidth at the focus was 1.5 mm, and the depth of focus was 29 mm, both measured quantities (Raum & O’Brien 1997). For exposure, the transducer was mounted in a degassed water bath and aimed upward at the exposure chamber located at the focus. Sinusoidal tone bursts were generated by a pulser-receiver (Ritec RAM5000, Warwick, RI) for a pulse duration (PD) of 5 cycles, pulse repetition frequency (PRF) of 10 Hz, and exposure duration of 30 s. The peak rarefactional pressure (Pr) was varied over a range from 0.12 MPa to 3.5 MPa, and five independent replicates were performed at each Pr value (0, 0.122, 0.204, 0.436, 0.908, 1.31, 1.74, 2.40, 2.69, and 3.50 MPa). This Pr range encompassed the threshold range for Optison™ collapse, 0.77–0.83 MPa (Ammi 2006; Ammi et al. 2006b).
The calibrated pressure amplitude at the focus was varied using the pulser-receiver’s output control settings. To obtain smaller changes in the pressure amplitude, a step-variable attenuation was used. The pressure waveforms were calibrated at the field’s focus for each exposure condition. Calibrations were routinely performed according to well-established calibration techniques (Preston et al. 1983; Zachary et al. 2001), using an NPL-calibrated PVDF bilaminar shielded membrane hydrophone (diameter of the active element: 0.5 mm, Marconi 699/1/00001/100; GEC Marconi Ltd., Great Baddow UK). The hydrophone was located in the field’s focus at the same position that the exposure vessel was located during experiments.
The attenuation of the plastic cling wrap and reflection coefficient of the bottom of the vessel were found by measuring the US amplitude of the polystyrene and/or plastic cling wrap in comparison to a Plexiglas block with known reflection. The attenuation of the plastic cling wrap was negligible and the pressure reflection coefficient of the polystyrene was 0.33. This reflection was taken into account. The peak rarefactional pressure reported here included the measured pressure via calibration, without the microwell plate present, plus the pressure of the reflected wave due to the reflection that occurs from the polystyrene when the microwell is present. Additionally, an absorber was placed above the microplate to prevent reflection from the water-air interface at the top of tank from interfering with the exposure conditions (Fig 3).
Post-exposure analysis
Following exposure, the vessel was removed from the water bath. The exposure medium in each of the wells was transferred to correspondingly labeled microcentrifuge tubes and placed on ice. 0.1-mL Trypsin-EDTA was added to the monolayer in each well and after 5 minutes, the trypsinized cells were added to the same microcentrifuge tube as the exposure medium. Thus, the microcentrifuge tube contains all the cells in the monolayer and any cells that may have been dislodged from the monolayer during the procedure for a single well of the microwell plate. Each cell suspension was immediately washed twice with 1-mL cold PBS to avoid pinocytosis. Viewing of the cells with fluorescence microscopy verified that the FITC-Dextran was distributed uniformly throughout the cytoplasm and not confined to small intracellular vacuoles, as would be expected for pinocytosis or phagocytosis (Results not shown). To assess cell viability, 1-μL propidium iodide (PI) (Sigma-Aldrich Co., St. Louis, MO) was added to each sample. Samples were analyzed using flow cytometry (Beckman Coulter, Inc. Epics XL-MCL, Fullerton, CA).
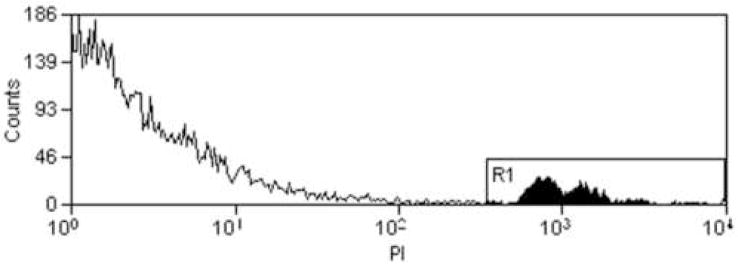
Fluorescent probes were excited at 488 nm by an argon laser and the emitted light was detected at 520 nm for FITC and 608 nm for PI. A minimum of 18,000 cells was examined in each sample. Fig 4-A shows an example of the side-scatter versus forward-scatter histogram obtained by the flow cytometer, with the whole cell and cell debris populations labeled. Results from flow cytometry are expressed in percentages of positively labeled cells, using the software program Summit v3.1 For MoFlo® Acquisition and Sort Control (Cytomation Inc., Fort Collins, CO). The percent of positive cells is relative to whole cells only, as debris from cells was ignored. Cell debris consists of parts of cell membrane, mitochondria, other organelles, dust particles, etc. It is not known the number of cells contributing to this debris, thus for this discussion of sonoporation, the debris does not provide any additional information. The data obtained provided the percent of fluorescent cells and dead cells in the entire population. Fig 4-B shows an example of the FITC histogram. The control histogram is from the sham exposed sample (US turned off) and the exposed histogram is from the sample exposed to US. To determine the sonoporated cells, the control histogram is subtracted from the exposed histogram. Both the control histogram and the exposed histogram are normalized to 18,000 counts. The number of cells in the subtraction histogram is divided by the number of cells in the exposed histogram to obtain the percentage of sonoporated cells. FITC-dextran does not bind to the cell membrane (McNeil 1989), so fluorescence of a cell indicates internalized material (i.e., sonoporation). Fig 4-C shows an example of the PI histogram. The cells in region R1 are those that had PI uptake and designated as nonviable.
Fig 4.
A) Example of the side-scatter (SS) versus forward scatter (FS) histogram obtained by the flow cytometer. The cells within the ellipsoid region are the whole cells, viable and nonviable, that are used in the sonoporation analysis. All points not located within the ellipse are designated as cell debris. B) Example of the FITC histogram obtained by the flow cytometer. The control histogram is from the sham exposed sample (US turned off) and the exposed histogram is from the sample exposed to US. The subtraction histogram is the result when subtracting the control histogram from the exposed histogram. The number of cells in the subtraction histogram divided by the number of cells in the exposed histogram is the percentage of sonoporated cells. C) Example of the PI histogram. The region R1 represents the cells stained with PI and designated as nonviable.
Acoustic pressure thresholds for collapse of Optison™ microbubbles
A passive cavitation detector (PCD) (Madanshetty et al. 1991) was used to determine collapse thresholds of Optison™ in degassed water (Ammi 2006; Ammi et al. 2006b). A 13-MHz measured center-frequency focused transducer (12.7-mm diameter and 15.4-mm focal length), mounted confocal and at a 115° angle to the transmit beam axis, was used to passively collect emissions from the bubbles injected into degassed water. The -6-dB field limits were determined for each transducer by measurement (Raum & O’Brien 1997). The approximate confocal volume was 0.12 mm3. The outputs from both transducers were amplified (44 dB), digitized (12-bit, 200 MHz, Strategic Test digitizing board UF 3025, Cambridge, MA) and saved to a computer. The data were processed off-line using Matlab®.
The signals detected from the PCD revealed postexcitation acoustic emissions with broadband spectral content. The observed acoustic emissions were consistent with the acoustic signature that would be anticipated from inertial collapse followed by “rebounds” when a microbubble ruptures and thus generates daughter/free bubbles that grow and collapse. Logistic regression analysis (Agresti 1996) was used to analyze the dependence of ruptured microbubble occurrence rates on Pr, and both the first IC event and the 5% occurrence rate were used to quantify the shell rupture (IC) thresholds; an automated algorithm applied to the PCD signals detected the number of IC events out of 128 data realizations at each acoustic pressure level.
Results
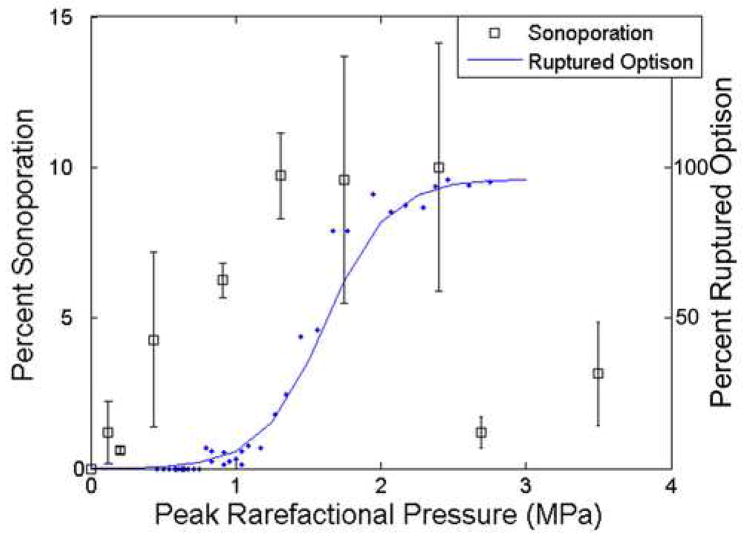
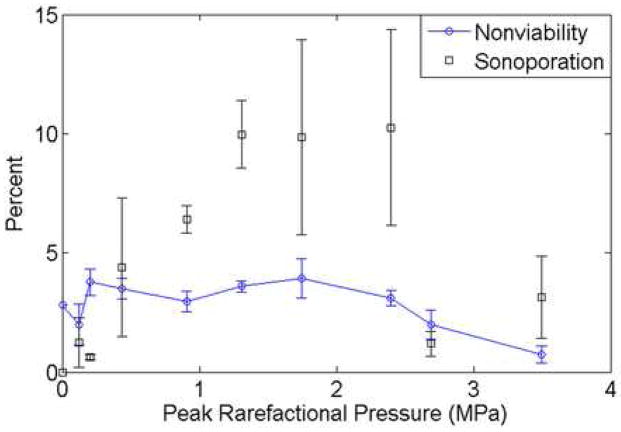
CHO cells exposed to US in the presence of the UCA, Optison™, were observed, by means of FITC-dextran internalization, to have undergone sonoporation. A threshold-type study examining sonoporation activity as a function of Pr was performed. Over the Pr range, 120 kPa to 3.5 MPa, the fraction of sonoporated cells among the total viable population varied from 0.63% to 10.2%, with the sonoporation activity increasing as Pr increased up to a maximum occurring at 2.4 MPa (Fig 5). The error was calculated using standard error of measurement for the 5 independent replicate samples at each Pr. It is important to note that only 5.5% of the cells in each well were exposed within the -6 dB beamwidth at the 3.15-MHz transducer focus (1.5 mm); however the entire well was sampled for sonoporation.
Fig 5.
Sonoporation of CHO cells exposed at 3.15 MHz, 5 cycles, 10 Hz and for 30 s compared to the occurrence of ruptured Optison™. The collapse threshold for Optison™ occurs at 0.83 MPa.
The 2.8-MHz 5-cycle collapse data of Optison™ are also plotted on Fig 5 (data obtained from Fig V.6 in (Ammi 2006); see also (Ammi et al. 2006a)) to show the relationship between sonoporation and IC. The percentage of observed bubbles that underwent collapse at each Pr is displayed. Two 2.8-MHz 5-cycle thresholds were determined for Optison™: first IC event and 5% occurrence rate using logistic regression analysis. The first IC event was at 0.77 MPa and the 5% occurrence rate was at 0.83 MPa (Ammi 2006). First IC event thresholds at 0.9, 2.8 and 4.6 MHz for 3, 5 and 7 cycles are graphically represented in Ammi et al. (2006) and show that the first IC event thresholds increase as frequency increases; this frequency trend suggests that at 3.15 MHz, the collapse thresholds would be slightly greater than those at 2.8 MHz. Around this threshold pressure (0.77–0.83 MPa), sonoporation had already reached over 50% relative to the maximum sonoporation activity, indicating that significant sonoporation is taking place at Pr levels where IC of Optison™ was not occurring.
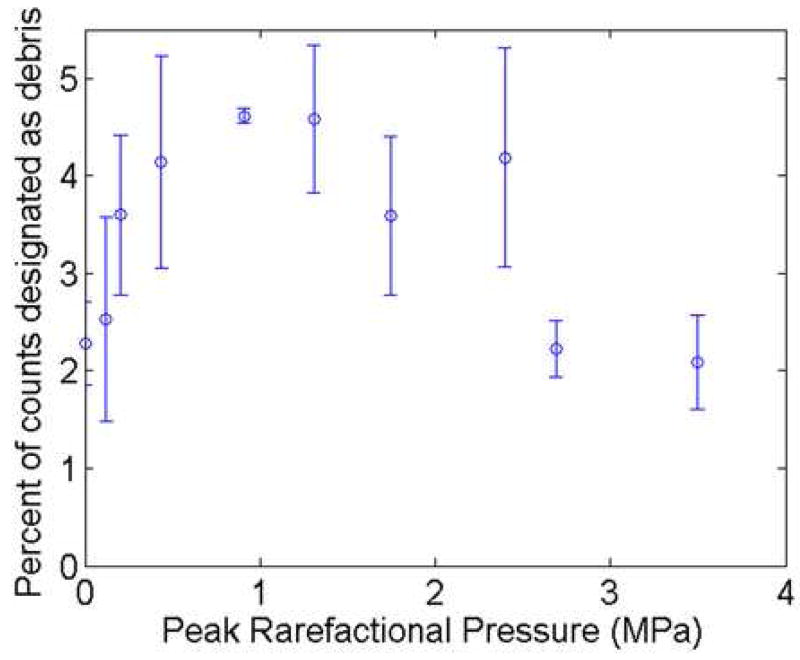
At Pr>2.4 MPa a significant drop in sonoporation activity was observed. This decrease corresponds to the pressure where greater than 95% of the Optison™ was collapsing (Fig 5). The percentage of nonviable cells at each Pr is plotted in Fig 6. For the range of Pr examined, 120 kPa to 3.5 MPa, the nonviable cells varied between 0.74% and 3.9%, with no distinct pattern emerging with respect to Pr. This emphasizes that sonoporation is not immediately lethal to the cells and that cell death is not related to the activity of the UCA, nor is cell death a contributor for the drop in sonoporation seen above 2.4 MPa. Additionally, the percentage of total items counted by the flow cytometer that were classified as cell debris is presented in Fig 7. At the higher pressure settings, where a drop in sonoporation activity occurred, there is no increased percentage of cell debris. Thus, the drop in sonoporation at these higher pressures is also not due to increased destruction of cells resulting in increased cell debris.
Fig 6.
Percentage of nonviable cells exposed at 3.15 MHz, 5 cycles, 10 Hz and for 30 s in the presence of Optison™ compared to the sonoporation activity for the same exposure conditions.
Fig 7.
Percentage of items counted by flow cytometer that were designated as cell debris from samples exposed at 3.15 MHz, 5 cycles, 10 Hz and for 30 s in the presence of Optison™.
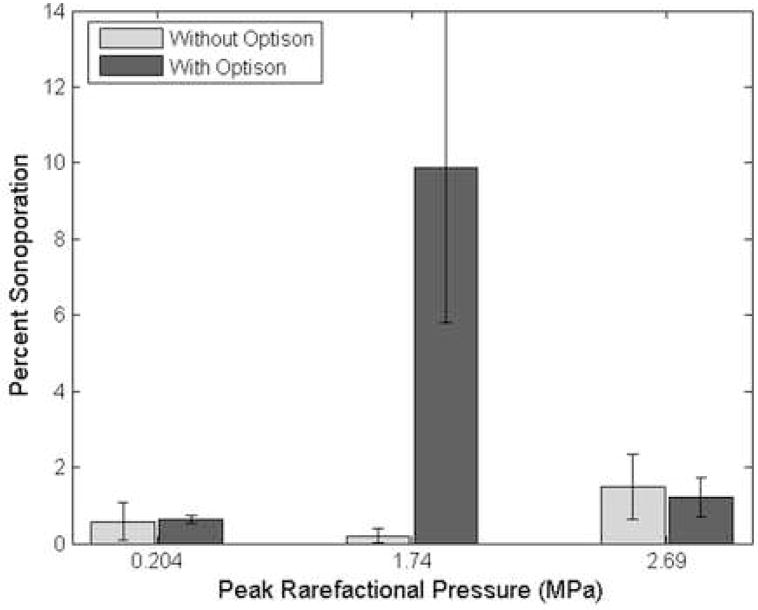
The CHO cells were exposed to US without Optison™ added to the solution. Fig 8 presents the percentage of sonoporated cells without Optison™ compared to the percentage of sonoporated cells with Optison™ for 3 Pr levels (0.204, 1.74, and 2.69 MPa). The percentage of sonoporated cells without Optison™ is not significantly different from the control sample (US off). However, the percentage of sonoporated cells with Optison™ differs from the samples without Optison™ and the control samples for a Pr of 1.74 MPa.
Fig 8.
Percentage of sonoporated cells exposed at 3.15 MHz, 5 cycles, 10 Hz and for 30 s without Optison™ compared to the percentage of sonoporated cells with Optison™ for Pr of 0.204, 1.74, and 2.69 MPa.
Discussion
The role of IC as the sonoporation mechanism has previously not been elucidated with direct evidence. Hallow et al. (2006) did show that acoustic cavitation correlates well with sonoporation activity through a simultaneous monitoring of inertial cavitation dose and cell exposure. However, an integration of the average broadband noise was used as the inertial cavitation dose; further this method is unable to quantify the amount of IC and UCA oscillation. This quantification becomes important when looking at exposure conditions around the threshold for collapse. The experimental observations reported herein provide evidence that sonoporation is not directly due to IC of UCAs, using characteristic rebounds that occur when a microbubble ruptures as the criteria for the IC event.. This is most apparent in comparing the collapse threshold of Optison™ to the sonoporation response to Pr, as in Fig 5. The threshold for Optison™ collapse is around a Pr of 0.77–0.83 MPa, hence below this pressure range, few, if any, microbubbles undergo collapse. However, below this collapse threshold significant sonoporation is occurring. Furthermore, at the threshold for Optison™ collapse, sonoporation activity is half of the maximum observed activity. These results demonstrate that sonoporation is occurring while Optison™ is intact, thus IC is not the mechanism responsible for sonoporation.
To verify that sonoporation was a UCA-mediated event, samples were exposed to US without Optison™ present. Streaming within the well due to US could potentially impact the cell membrane. Additionally, cells that have been dislodged from the bottom of the well from the US could have a membrane permeability change due to this dislodging process (McNeil 1989). If either of these mechanisms were responsible for the uptake of FITC-Dextran, then samples exposed to US without UCA would show uptake. However, as seen in Fig 8, without Optison™ present sonoporation activity is not observed. Therefore, the sonoporation observed in these studies was mediated by UCA activity.
The exposure-dependent sonoporation activity between 120 kPa and 2.4 MPa, a Pr range that transitions the UCAs’ response from linear to nonlinear to inertial collapse (IC), suggests a mechanism that is also exposure-dependent throughout this range of rarefactional pressures. It is likely that microstreaming is exposure-dependent between 120 kPa and 2.4 MPa, and supported by the observation of Marmottant and Hilgenfeldt (2003). The shear stress due to microstreaming from microbubble oscillation is
| (1) |
where δ is the boundary layer thickness, A is the initial bubble radius, η is the shear viscosity of the medium, f is the frequency, and εo is the radial oscillation amplitude (Rooney 1970). The εo is dependent on Pr (Emmer et al. 2007; Marmottant et al. 2005), with larger microbubble oscillation amplitudes occurring as Pr is increased. Marmottant et al. (2005) presented experimental results of a 0.8 μm radius BR14 UCA oscillating in a single 5-cycle pulse of 2 MHz US. At Pr=200 kPa, εo was approximately 0.15 μm, at Pr=250 kPa, εo was approximately 0.2 μm, and at Pr=300 kPa, εo was approximately 0.35 μm. Emmer et al. (2007) presented similar results using a 3.5 μs pulse at 1.7 MHz. For BR14 UCA of 3 μm, at Pr=100 kPa, εo was approximately 0.08 μm, at Pr=200 kPa, εo ranged from 0.23 to 0.6. μm, and at Pr=250 kPa, εo ranged from 0.38 to 0.78 μm. Hence, as Pr increases, εo increases; resulting in an increased S. Therefore, the shear stress due to microstreaming is dependent on the applied Pr, with increased microstreaming occurring with increased Pr.
Using this definition of shear stress it is possible to demonstrate that microstreaming around UCAs has the potential induce sonoporation. Several studies have presented observations of streamlines that develop around a vibrating bubble (Marmottant & Hilgenfeldt 2003; Rooney 1970). Through visual observation, the smallest streamline traced was approximately 1 and 2 times the circumference of the bubble, respectively. Let us assume that one circuit of the streamline is sufficient for a fully developed streaming flow to develop around a bubble that is initially at rest and then excited by US. If we use the more recent value, then the distance the fluid must travel is 2πA. The limiting tangential fluid velocity at the surface of the bubble is given by
| (2) |
(Coakley & Nyborg 1978). Thus, the time to complete one circuit of the smallest streamline is
| (3) |
If we assume the radial oscillation amplitude is where the UCA undergoes inertial cavitation (2 times the initial radius), ξo is A. Thus, τ becomes
| (4) |
The frequency used in this study was 3.15 MHz, thus the time for microstreaming to develop around a bubble is 0.32 μs. The time duration for a single pulse in this study was 1.67 μs, thus microstreaming would develop within a single pulse of US.
The shear stress, S, can be calculated for this study. The boundary layer thickness, δ, is defined as
| (5) |
where ρ is the density of the medium (Coakley & Nyborg 1978). The exposure medium used in this study has a concentration of 0.30% Dextran with a 500 kDa molecular weight, which results in a shear viscosity around 0.002–0.003 Pa-s (Nyborg 1975). If we assume a shear viscosity of 0.002 Pa-s, δ is 0.45 μm. If the initial radius of the UCA is 2 μm, S is calculated to be 1.76×105 dyn-cm−2 using Equation 1. Rooney found that at the threshold stress for hemoglobin release of red blood cells the duration of the applied stress was 25 μs (Rooney 1972). In this study, the time of the applied stress of a single pulse was 1.67 μs, however as the relaxation time of biological materials is long under these conditions, it can be assumed that successive pulses would have a cumulative effect on streaming. Thus, 15 pulses would be required to achieve a 25 μs applied stress, which is approximately 1.5 seconds of exposure. The ED for this study was 30 seconds, so it is reasonable that a biologically active microstreaming flow pattern is developed during the exposure. Ammi et al. (2006b) presented evidence that Optison™ microbubbles collapse within a single pulse, thus when a UCA undergoes collapse, there is not sufficient time for microstreaming to impact the cells and thus no sonoporation occurs.
At Pr>2.4 MPa, a drop in sonoporation activity occurs. At these Pr values (2.7 and 3.5 MPa), greater than 95% of the bubbles are collapsing quite rapidly. Due to their rapid collapse within a single pulse, the UCAs are not present to oscillate and contribute to microstreaming. Thus, at higher Pr microstreaming will be minimized. A similar drop in sonoporation activity was seen in Hallow et al (2006) using 1.7 vol% Optison™, 1.1 MHz, and 3-s exposure duration. The percentage of viable cells increased as the pressure was increased from 0.5 MPa to 1.7 MPa. From 1.7 MPa to 2.0 MPa the percentage sonoporated cells decreased from 20% to around 7%. Furthermore, it has been shown that as the percentage of Optison™ bubbles destroyed increases, the molecular uptake of macromolecules by cells in suspension was found to decrease (Kamaev et al. 2004).
The drop in sonoporation activity seen in this study does not have a corresponding increase in the percentage of nonviable cells, nor an increase in lysed cells and debris. The results presented here suggest that increased cell death is not a cause of this decreased sonoporation. However, several studies have shown that at higher acoustic pressures cell viability does decrease (Bao et al. 1997; Hallow et al. 2006). The major difference between those studies and this one is the configuration of the cells. This study was performed as a monolayer, whereas the cited studies were suspension cells.
Published reports that have rigorously examined the behavior of liquid jets are scarce and none replicate the situation in this study. However, results suggest that liquid jets are a less likely explanation for the sonoporation results presented in this paper. Kodama and Takayama (1998) investigated the interaction of shock waves with bubbles attached to rat livers. They observed that liquid jets due to oscillating bubbles are capable of penetrating into rat livers and the penetration depth of the liquid jet rapidly decreased with decreasing equilibrium radius of the bubble. The smallest bubble examined had a radius of 100 μm, while Optison™ has a mean radius of 2 to 4.5 μm. Additionally, jet formation shows some irregularities near the threshold. Due to this irregularity, we do not anticipate jet formation could be responsible for the trends in sonoporation results observed here. Prentice et al. (2005) provided evidence that microjets from shelled microbubbles can produce pits on the surface of a cell membrane. However, they do not show that macromolecules are able to pass into the cell through those pits. Additionally, they concluded that the extent of those microjet-induced pits would suggest that lysis of the cell was inevitable and the exposed cells in this study do not undergo increased lysis. Literature has shown that microjets do have the potential to contribute to sonoporation, but in this study are unlikely to be the dominant mechanism.
The evidence provided suggests that the sonoporation effect was caused by linear or nonlinear oscillation of the UCA. These responses occur at lower pressure amplitudes and could thus explain the presence of sonoporation at the lower pressure levels. Therefore, we conclude that IC is not the responsible mechanism for sonoporation and hypothesize that microstreaming due to microbubble oscillations is principally responsible.
Acknowledgments
This work was supported in part by an Illinois Distinguished Fellowship for predoctoral students, by NIH Grant F31EB06634, and by NIH Grant R37EB02641. Cell culture facilities were provided by the Imaging Technology Group of the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A. An Introduction for Categorical Analysis. New York: Wiley; 1996. [Google Scholar]
- Amabile PG, Lewis JM, Lewis TN. High-efficiency endovascular gene delivery via therapeutic ultrasound. Journal of the American College of Cardiology. 2001;37:1975–80. doi: 10.1016/s0735-1097(01)01253-0. [DOI] [PubMed] [Google Scholar]
- Ammi AY. Doctoral Thesis. University of Paris VI: 2006. Detection et caracterisation de la destruction des microbulles de produit de contrast e ultrasonore (Detection and characterization of the destruction of ultrasound contrast agents) In English. [Google Scholar]
- Ammi AY, Bridal SL, Mamou J, Wang GI, O’Brien WD., Jr Automatic Detection of Ultrasound Contrast Microbubble Shell Rupture. Proceedings of the 2006 IEEE Ultrasonics Symposium; 2006a. pp. 297–300. [Google Scholar]
- Ammi AY, Cleveland RO, Mamou J, Wang GI, Bridal SL, O’Brien WD., Jr Ultrasonic contrast agent shell rupture detected by inertial cavitation and rebound signals. IEEE transactions on Ultrasonics, Ferroelectrics, and Frequency control. 2006b;53:126–36. doi: 10.1109/tuffc.2006.1588398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound in Medicine and Biology. 1997;23:953–9. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Coppage ML, Vaidya S, Miller MW. Transient poration and cell surface receptor removal from human lymphocytes in vitro by 1-MHz ultrasound. Ultrasound in Medicine and Biology. 1999;25:999–1008. doi: 10.1016/s0301-5629(99)00039-3. [DOI] [PubMed] [Google Scholar]
- Chen WS, Brayman AA, Matula TJ, Crum LA, Miller MW. The pulse length-dependence of inertial cavitation dose and hemolysis. Ultrasound in Medicine and Biology. 2003;29:739–48. doi: 10.1016/s0301-5629(03)00029-2. [DOI] [PubMed] [Google Scholar]
- Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. Journal of Biomedical Optics. 2001;6:141–50. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- Church CC. Frequency, pulse length, and the mechanical index. Acoustics Research Letters Online. 2005;6:162–8. [Google Scholar]
- Coakley WT, Nyborg WL. Ultrasound: Its Applications in Medicine and Biology. Amsterdam, The Netherlands: Elsevier Scientific Publishing Comany; 1978. Chapter 2: Cavitation; Dynamics of Gas Bubbles; Applications; pp. 77–159. [Google Scholar]
- Deng CX, Sieling F, Pan H, Cui J. Ultasound-induced cell membrane porosity. Ultrasound in Medicine and Biology. 2004;30:519–26. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Didenko YT, McNamera WB, III, Suslick KS. Hot spot conditions during cavitation in water. Journal of the American Chemical Society. 1999;24:5817–8. [Google Scholar]
- Emmer M, van Wamel A, Goertz D, de Jong N. The onset of microbubble vibration. Ultrasound in Medicine and Biology. 2007;33:941–9. doi: 10.1016/j.ultrasmedbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Endoh M, Koibucki N, Sato M. Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound. Molecular Therapy. 2002;5:501–8. doi: 10.1006/mthe.2002.0577. [DOI] [PubMed] [Google Scholar]
- Flynn HG. Cavitation dynamics. II. Free pulsations and models for cavitation bubbles. Journal of the Acoustical Society of America. 1975;58:1160–70. [Google Scholar]
- Flynn HG, Church CC. Transient pulsations of small gas bubble in water. Journal of the Acoustical Society of America. 1988;84:985–98. doi: 10.1121/1.397253. [DOI] [PubMed] [Google Scholar]
- Frenkel PA, Chen S, Thai T, Shohet RV, Grayburn PA. DNA-loaded albumin microbubbles enhance ultrasound-mediated transfection in vitro. Ultrasound in Medicine and Biology. 2002;28:817–22. doi: 10.1016/s0301-5629(02)00518-5. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Hynynen K. Ultrasound-mediated cavitation thresholds of liquid perfluorocarbon droplets in vitro. Ultrasound in Medicine and Biology. 2003;29:1359–65. doi: 10.1016/s0301-5629(03)00980-3. [DOI] [PubMed] [Google Scholar]
- Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound in Medicine and Biology. 1998;24:587–95. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Guzman HR, Nguyen DX, McNamera A, Prausnitz MR. Equilibrium loading of cells with macromolecules by ultrasound: Effects of molecular size and acoustic energy. Journal of Pharmacological Sciences. 2002;91:1693–701. doi: 10.1002/jps.10156. [DOI] [PubMed] [Google Scholar]
- Guzman HR, Nguyen DX, Sohail K, Prausnitz MR. Ultrasound-mediated disruption of cell membranes I. Quantification of molecular uptake and cell viability. Journal of the Acoustical Society of America. 2001;110:588–96. doi: 10.1121/1.1376131. [DOI] [PubMed] [Google Scholar]
- Hallow DM, Mahajan AD, McCutchen TE, Prausnitz MR. Measurement and Correlation of Acoustic Cavitation with Cellular Bioeffects. Ultrasound in Medicine and Biology. 2006;32:1111–22. doi: 10.1016/j.ultrasmedbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Harrison GH, Balcer-Kubiczek E, Gutierrez PL. In vitro mechanisms of chemopotentiation by tone-burst ultrasound. Ultrasound in Medicine and Biology. 1996;22:355–62. doi: 10.1016/0301-5629(95)02053-5. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound in Medicine and Biology. 2005;31:553–64. doi: 10.1016/j.ultrasmedbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kamaev PP, Hutcheson JD, Wilson ML, Prausnitz MR. Quantification of Optison bubble size and lifetime during sonication dominant role of secondary cavitation bubbles causing acoustic bioeffects. Journal of the Acoustical Society of America. 2004;115:1818–25. doi: 10.1121/1.1624073. [DOI] [PubMed] [Google Scholar]
- Kawai N, Iino M. Molecular damage to membrane proteins induced by ultrasound. Ultrasound in Medicine and Biology. 2003;29:609–14. doi: 10.1016/s0301-5629(02)00786-x. [DOI] [PubMed] [Google Scholar]
- Keyhani K, Guzman HR, Parsons A, Lewis TN, Prausnitz MR. Intracellular drug delivery using low-frequency ultrasound: Quantification of molecular uptake and cell viability. Pharmaceutical Research. 2001;18:1514. doi: 10.1023/a:1013066027759. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Human Gene Therapy. 1996;7:1339–46. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- Koch S, Pohl P, Cobet W, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound in Medicine and Biology. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Kodoma T, Takayama T. Dynamic behavior of bubbles during extracorporeal shock-wave lithotripsy. Ultrasound in Medicine and Biology. 1998;24:723–38. doi: 10.1016/s0301-5629(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Lai C, Wu C, Chen C, Li P. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound in Medicine and Biology. 2006;12:1931–41. doi: 10.1016/j.ultrasmedbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Therapy. 2000;7:2023–7. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, Wyllie D, Kiss-Toth E, Qwarnstrom EE, Dower SK, Crossman DC, Newman CM. Ultrasound-Enhanced Transgene Expression in Vascular Cells is Not Dependent Upon Cavitation-Induced Free Radicals. Ultrasound in Medicine and Biology. 2003;29:1453–61. doi: 10.1016/s0301-5629(03)01032-9. [DOI] [PubMed] [Google Scholar]
- Madanshetty SI, Roy RA, Apfel RE. Acoustic microcavitation: Its active and passive acoustic detection. Journal of the Acoustical Society of America. 1991;90:1515–26. doi: 10.1121/1.401891. [DOI] [PubMed] [Google Scholar]
- Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423:153–6. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- Marmottant P, van der Meer S, Emmer M, Versluis M, de Jong N, Hilgenfeldt S, Lohse D. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. Journal of the Acoustical Society of America. 2005;118:3499–505. [Google Scholar]
- McNeil PL. Incorporation of macromolecules into living cells. Methods in Cell Biology. 1989;29:153–73. doi: 10.1016/s0091-679x(08)60193-4. [DOI] [PubMed] [Google Scholar]
- Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. Journal of Controlled Release. 2005;104:213–22. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Miller DL, Bao S, Gies RA, Thrall BD. Ultrasonic enhancement of gene transfection in murine melanoma tumors. Ultrasound in Medicine and Biology. 1999;25:1425–30. doi: 10.1016/s0301-5629(99)00105-2. [DOI] [PubMed] [Google Scholar]
- Miller DL, Dou C, Song J. DNA transfer and cell killing in epidermoid cells by diagnostic ultrasound activation of contrast agent gas bodies in vitro. Ultrasound in Medicine and Biology. 2003;29:601–7. doi: 10.1016/s0301-5629(02)00783-4. [DOI] [PubMed] [Google Scholar]
- Miller DL, Song J. Tumor growth reduction and DNA transfer by cavitation-enhanced high-intensity focused ultrasound in vivo. Ultrasound in Medicine and Biology. 2003;29:887–93. doi: 10.1016/s0301-5629(03)00031-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, Thomas J. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. Journal of the American College of Cardiology. 2000;35:1678–86. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- Nyborg WL. Intermediate Biophysical Mechanisms. Menlo Park, CA: Cummings Publishing Co; 1975. [Google Scholar]
- Prentice P, Cuschieri A, Dholakia K, Prausnitz MR, Campbell P. Membrane disruption by optically controlled microbubble cavitation. Nature Physics. 2005;1:107–10. [Google Scholar]
- Preston RC, Bacon DR, Livett AJ, Rajendran K. PVDF membrane hydrophone performance properties and their relevance to the measurement of the acoustic output of medical ultrasound equipment. Journal of Physics E: Scientific Instruments. 1983;16:786–96. [Google Scholar]
- Prosperetti A. A new mechanism for sonoluminescence. Journal of the Acoustical society of America. 1997;101:2003–7. [Google Scholar]
- Raum K, O’Brien WD. Pulse-echo field distribution measurements technique for high-frequency ultrasound sources. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 1997;44:810–5. [Google Scholar]
- Rooney JA. Hemolysis Near and Ultrasonically Pulsating Gas Bubble. Science. 1970;169:869–71. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- Rooney JA. Shear as a Mechanism for Sonically Induced Biological Effects. Journal of the acoustical society of America. 1972;52:1718–24. doi: 10.1121/1.1913306. [DOI] [PubMed] [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Vladimir Z, Prausnitz MR. Mechanism of Intracellular Delivery by Acoustic Cavitation. Ultrasound in Medicine and Biology. 2006;32:915–24. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Suslick KS. Encyclopedia of Physical Science and Technology. 3. Vol. 17. San Diego: Academic Press; 2001. Sonochemistry and Sonoluminescence; pp. 363–76. [Google Scholar]
- Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell membrane porosity by ultrasound. The Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Tachibana K, Hiraoka L. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Therapy. 2002;9:372–80. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- Tata DB, Dunn F, Tindall DJ. Selective clinical ultrasound signals mediate differential gene transfer and expression in two human prostate cancer cell lines: LnCap and PC-3. Biochemical and Biophysical Research Communications. 1997;234:64–7. doi: 10.1006/bbrc.1997.6578. [DOI] [PubMed] [Google Scholar]
- Tran TA, Roger S, Le Geunnec JY, Tranquart F, Bouakaz A. Effect of ultrasound-activated microbubbles on the cell electrophysiological properties. Ultrasound in Medicine and Biology. 2007;33:158–63. doi: 10.1016/j.ultrasmedbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- van Wamel A, Bouakaz A, Bernard B, ten Cate F, de Jong N. Radionuclide tumour therapy with ultrasound contrast microbubbles. Ultrasonics. 2004;42:903–6. doi: 10.1016/j.ultras.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Weimann LJ, Wu J. Transdermal Delivery of Poly-l-lysine by Sonomacroporation. Ultrasound in Medicine and Biology. 2002;28:1173–80. doi: 10.1016/s0301-5629(02)00571-9. [DOI] [PubMed] [Google Scholar]
- Williams AR, Hughes DE, Nyborg WL. Hemolysis Near a Transversely Oscillating Wire. Science. 1970;169:871–3. doi: 10.1126/science.169.3948.871. [DOI] [PubMed] [Google Scholar]
- Wu J, Pepe J, Rincón M. Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics. 2006;44:e21–e5. doi: 10.1016/j.ultras.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Wu J, Ross JP, Chiu J. Reparable sonoporation generated by microstreaming. Journal of the Acoustical Society of America. 2002;111:1460–4. doi: 10.1121/1.1420389. [DOI] [PubMed] [Google Scholar]
- Wyber JA, Andrews J, D’Emanuele A. The use of sonication for the efficient delivery of plasmic DNA into cells. Pharmacological Research. 1997;14:750–6. doi: 10.1023/a:1012198321879. [DOI] [PubMed] [Google Scholar]
- Zachary JF, Sepsrott JM, Frizzell LA, Simpson DG, O’Brien WD., Jr Superthreshold behavior and threshold estimation of ultrasound-induced lung hemorrhage in adult mice and rats. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2001;34:581–92. doi: 10.1109/58.911741. [DOI] [PubMed] [Google Scholar]