Abstract
31P-NMR spectroscopy was utilized to investigate rat and porcine pancreatic ATP:Pi ratios to assess the efficacy of existing protocols for cold preservation (CP) in maintaining organ quality. Following sacrifice, rat pancreata were immediately excised or left enclosed in the body for 15 minutes of warm ischemia (WI). After excision, rat pancreata were stored at 6°C to 8°C using histidine-tryptophan-ketoglutarate solution (HTK) presaturated with air (S1), HTK presaturated with O2 (S2), or the HTK/perfluorodecalin two-layer method (TLM) with both liquids presaturated with O2 (S3). 31P-NMR spectra were sequentially collected at 3, 6, 9, 12, and 24 hours of CP from pancreata stored with each of the three protocols examined. The ATP:Pi ratio for rat pancreata exposed to 15 minutes of WI and stored with S3 increased during the first 9 hours of CP, approaching values observed for organs procured with no WI. A marked reduction in the ATP:Pi ratio was observed beyond 12 hours of CP with S3. After 6 hours of CP, the ATP:Pi ratio was highest for S3, substantially decreased for S2, and below detection for S1. In sharp contrast to the rat model, ATP was barely detectable in porcine pancreata exposed to minimal warm ischemia (<15 minutes) stored with the TLM regardless of CP time. We conclude that 31P-NMR spectroscopy is a powerful tool that can be used to (1) noninvasively evaluate pancreata prior to islet isolation, (2) assess the efficacy of different preservation protocols, (3) precisely define the timing of reversible versus irreversible damage, and (4) assess whether intervention will extend this timing.
Islet transplantation has become a promising therapeutic alternative for the treatment of a subpopulation of patients with type 1 diabetes.1,2 Currently, clinical islet allotransplantation is limited by a shortage of suitable donor organs as well as by loss of islets during the islet manufacturing process. Islets may be predisposed to death before or during isolation due to improper handling of the organ during procurement and/or suboptimal cold preservation (CP) during transport. Significant research effort has focused on investigating the efficacy of the two-layer method (TLM), the present state of the art for pancreas preservation.3-8 In the late 1990s, many centers reported improvements in islet isolation outcome using TLM for CP. These improvements were attributed in part to increases in tissue ATP associated with enhanced tissue oxygenation with the TLM compared to the previously used CP in University of Wisconsin (UW) solution alone.3-8 However, it has recently been reported that the TLM may not be able to sufficiently oxygenate large portions of human or porcine pancreata during CP.9,10 In addition, recent retrospective studies have suggested no significant improvement in isolation outcomes for pancreata stored with the TLM when compared to pancreata stored on UW solution alone.11-13 Therefore, it is of great importance to develop tools that will aid in the assessment of the efficacy of presently utilized CP techniques (eg, TLM) as well as in the analysis of novel approaches to CP. In this study 31P-NMR spectroscopy (31P-NMR), a well-established technique for monitoring the amount of ATP present relative to inorganic phosphate (Pi) in tissues, was used to assess the efficacy of current CP methods to maintain the health of pancreata. 31P-NMR has been extensively used to study tumor biology, bioenergetics, as well as metabolism and health status of organs, such as the heart, brain, kidney, liver, and, to a limited extent, the pancreas.14-22 The noninvasive nature of 31P-NMR and its ability to provide information in real time make it an attractive tool for monitoring the health of pancreata during CP, which is the focus of this study.
METHODS
Male Lewis rats and female Landrace pigs were sacrificed using institutionally approved procedures. Rat pancreata were immediately excised or left enclosed in the body for 15 minutes of warm ischemia (WI). Porcine pancreata were excised as soon as possible (less than 15 minutes). After procurement, pancreata were placed directly into prechilled preservation solution (300 mL for rats or 2 L for pigs) and stored in a thermoelectrically refrigerated cooler at 6°C to 8°C using one of three CP techniques: histidine-tryptophan-ketoglutarate (HTK) + air, HTK solution presaturated with air (S1); HTK + O2, HTK solution presaturated with O2 (S2); and TLM + O2, HTK/perfluorodecalin, two-layer method with both solutions presaturated with O2 (S3). All storage solutions were either recharged with oxygen or exposed to air for 15 minutes every 3 hours and spectra were acquired after 3, 6, 9, 12, and 24 hours of CP. During data acquisition, the rat pancreata were placed into a custom-made container designed to hold 44 mL of CP solution. The container was placed inside a radiofrequency coil tuned to 86.025 MHz, then positioned in the center of the bore of a horizontal 5-T magnet maintained at 6°C. The solenoid coil had a total length of 1.8 cm and a diameter of 3.5 cm and consisted of 3 three-turn coils bridged by a 5-pF chip capacitor in the center of each coil, with each of the coils connected in parallel. The solenoid coil was attached to a balanced configuration circuit with variable 4 to 18 pF capacitors for the tuning and matching. A schematic depicting the rat pancreas container is shown in Fig 1. Porcine organs were investigated at 1.5 T using a 7-cm diameter surface coil tuned to 25.5085 MHz. 31P-NMR spectra were collected using 1024 scans with 2048 points, a dwell time of 100 μs, and a relaxation time of 1 second. All spectra were analyzed with an exponential line broadening of 25 using the ACD Labs 1-D NMR Processor software (Toronto, Ontario, Canada) by fitting a Lorentzian function to all discernible peaks. The areas of the α-, β-, and γ-ATP peaks were summed and normalized to the area of the Pi peak to obtain the ATP:Pi ratio used to compare the metabolic health of the organs.
Fig 1.
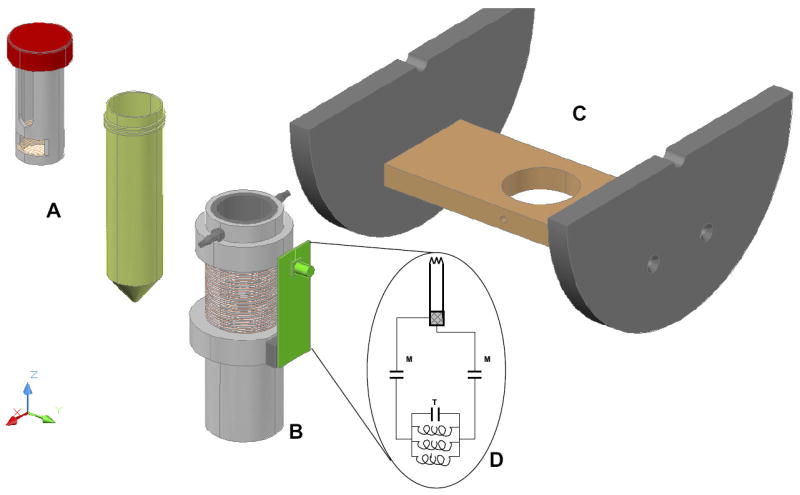
A three-dimensional rendering of the rat pancreas cold preservation container (A), solenoid coil (B), and the platform (C) used to center the coil in the bore of the magnet, used for the acquisition of the 31P spectra shown in Fig 2. Also shown is a circuit diagram (D) for the coil used in the study.
RESULTS
Spectra obtained from rat pancreata treated with each of the three methods of CP exhibited differences in the ATP:Pi ratio that were large enough to be obvious upon basic visual inspection (Fig 2). The plot of ATP:Pi for S1, S2, and S3 versus time is shown in Fig 3. For HTK + air (S1), no ATP peaks were detected throughout the preservation period. For HTK + O2 (S2), the ratio ATP:Pi trended slightly upward for the first 9 hours but leveled off and even decreased slightly at 24 hours. For TLM + O2 (S3), the ATP:Pi ratio was substantially higher than both HTK conditions and increased for the first 9 hours of CP. This increase was followed by a precipitous drop at 12 hours, and the ratio leveled out at 24 hours to values similar to those obtained with S2. TLM for CP was also investigated in the porcine model system; however, no discernible ATP peaks were detected in any of the organs examined.
Fig 2.
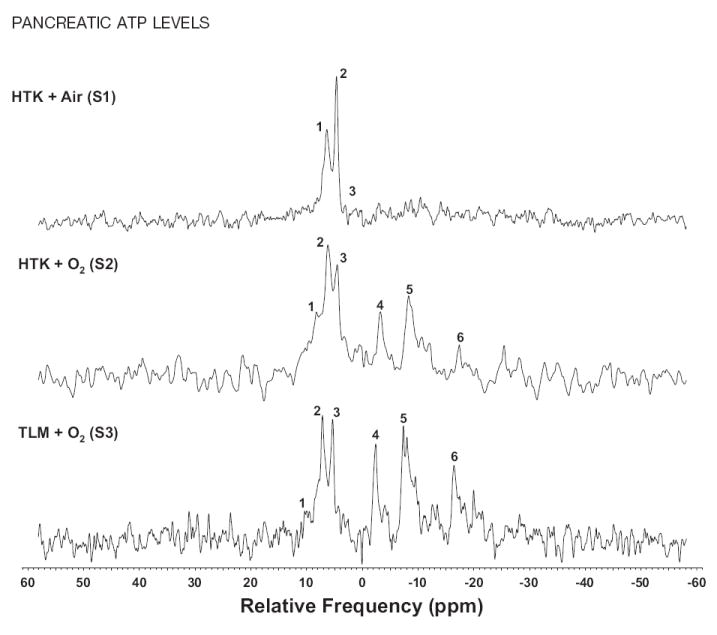
31P-NMR spectra acquired from rat organs exposed to 15 minutes of WI following 9 hours of cold preservation. Method of cold preservation: HTK + air (S1); HTK + O2 (S2); TLM + O2 (S3). Peak numbering corresponds to: (1) phosphomonoester, (2) inorganic phosphate, (3) phosphodiester, (4) γ-ATP, (5) α-ATP, and (6) β-ATP.
Fig 3.
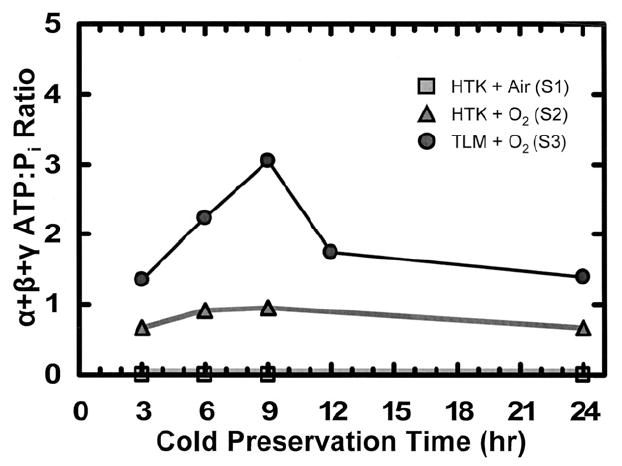
Plot of α + β + γ ATP:Pi ratios versus time of cold preservation. Rat pancreata were placed into a custom-made container with 44 mL of the appropriate cold preservation solution(s). The container was placed into a coil, which was tuned to 86.025 MHz and centered in a 5-T magnet maintained at 6°C. Three separate CP methods were compared: HTK + air (S1); HTK + O2 (S2); TLM + O2 (S3). All storage solutions were either recharged with O2 or exposed to air for 15 minutes every 3 hours and spectra were acquired after 3, 6, 9, 12, and 24 hours of cold preservation. During data acquisition, the 31P-NMR spectra were collected for 1024 scans with 2048 points, a dwell time of 100 μs, and a relaxation time of 1 second. Spectra were analyzed with an exponential line broadening of 25 by fitting a Lorentzian function to all discernible peaks. The areas of the α-, β-, and γ-ATP peaks were summed and normalized to the area of the Pi peak to obtain the ratios shown.
DISCUSSION
In this article, we utilized 31P-NMR spectroscopy to noninvasively assess the efficacy of different preservation protocols in maintaining ATP levels (as indicated by the ATP:Pi ratio, a measure of organ health and viability) in rat and porcine pancreata. Results demonstrated that in the rat pancreas model, CP with TLM results in dramatically improved ATP:Pi ratio levels when compared to CP in HTK solution exposed to air alone, the previous state of the art. Furthermore, preservation of rat pancreata in HTK solution saturated with O2 demonstrated a minor improvement in ATP:Pi ratios but to a smaller extent compared to the improvement observed with TLM (Fig 3). These results demonstrate that TLM is indeed effective in improving oxygenation and elevating ATP levels in the rat pancreas model. However, when porcine pancreata stored with TLM were investigated, no discernible ATP peaks were observed, indicating that TLM may be less effective in this model. These data may help explain why the success of TLM with the rat and canine pancreas models has not been fully translated to the preservation of clinical organs11-13 and are consistent with previously published studies,9,10 which suggest that TLM cannot fully oxygenate the larger porcine and human pancreata. The increase in the ATP:Pi ratio with time over the first 9 hours of CP with the TLM may reflect the dynamics of reoxygenation and ATP regeneration following ischemia during procurement. This increase is consistent with the observation in the rat model by Tanaka and coworkers that pancreata exposed to moderate periods of WI yielded significantly more islets when isolation was performed after 3 to 6 hours of CP with the TLM as opposed to shortly after procurement.23 Furthermore, the marked reduction in the ATP:Pi ratio after 12 hours of CP that we observed is also consistent with the observation that isolation yields generally decrease after 8 to 9 hours of CP24 and may be indicative of the presence of additional and important factors limiting CP. Based on our results, we conclude that the noninvasive nature and the ability of 31P-NMR to provide information in real time make it an effective and powerful tool for identifying the factors currently limiting CP and for assessing and establishing new and improved methods for organ preservation.
Acknowledgments
We would like to thank Dr A.N. Balamurugan, Dr Kristen Maynard, Thomas M. Suszynski, and Bradley P. Weegman from the Diabetes Institute for Immunology and Transplantation for helpful discussions and manuscript review. We would also like to thank Denice Dudero, Laurie Macleod, Heather Nelson, and Christine Vincent from the Diabetes Institute for Immunology and Transplantation at the UMN for administrative support.
Research funding provided by grants from the National Center for Research Resources (U42 RR016598), National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (R43 DK070400), NIH, the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:289. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362:1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 3.Hiraoka K, Kuroda Y, Suzuki Y, et al. Outcomes in clinical pancreas transplantation with the two-layer cold storage method versus simple storage in University of Wisconsin solution. Transplant Proc. 2002;34:2688. doi: 10.1016/s0041-1345(02)03376-6. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimura T, Kuroda Y, Avila JG, et al. Influence of pancreas preservation on human islet isolation outcomes: impact of the two-layer method. Transplantation. 2004;78:96. doi: 10.1097/01.tp.0000133515.37892.d5. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Zhang G, Qualley S, et al. The effect of two-layer (University of Wisconsin solution/perfluorochemical) preservation method on clinical grade pancreata prior to islet isolation and transplantation. Transplant Proc. 2004;36:1037. doi: 10.1016/j.transproceed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimura T, Kuroda Y, Avila JG, et al. Resuscitation of the ischemically damaged human pancreas by the two-layer method prior to islet isolation. Transplant Proc. 2003;35:2461. doi: 10.1016/j.transproceed.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Kuroda Y. Perfluorocarbon for organ preservation before transplantation. Transplantation. 2002;74:1804. doi: 10.1097/00007890-200212270-00030. [DOI] [PubMed] [Google Scholar]
- 9.Avgoustiniatos ES, Hering BJ, Papas KK. The rat pancreas is not an appropriate model for testing the preservation of the human pancreas with the two-layer method. Transplantation. 2006;81:1471. doi: 10.1097/01.tp.0000215389.64186.3f. [DOI] [PubMed] [Google Scholar]
- 10.Papas KK, Hering BJ, Guenther L, et al. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;38:3501. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 11.Kin T, Mirbolooki M, Salehi P, et al. Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation. 2006;82:1286. doi: 10.1097/01.tp.0000244347.61060.af. [DOI] [PubMed] [Google Scholar]
- 12.Caballero-Corbalán J, Eich T, Foss A, et al. No beneficial effect of the two layer method (PFCUW) compared with transportation in UW alone on the outcome of human islet isolation and transplantation: a report on 214 human pancreases. Am J Transplant. 2006;6(suppl 2):340. [Google Scholar]
- 13.Caballero-Corbalán J, Eich T, Lundgren T, et al. No beneficial effect of two-layer storage compared with UW-storage on human islet isolation and transplantation. Transplantation. 2007;84:864. doi: 10.1097/01.tp.0000284584.60600.ab. [DOI] [PubMed] [Google Scholar]
- 14.Arias-Mendoza F, Brown TR. In vivo measurement of phosphorous markers of disease. 2004;19:49. doi: 10.1155/2004/419095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbins RL, Malloy CR. Measuring in-vivo metabolism using nuclear magnetic resonance. Curr Opin Clin Nutr Metab Care. 2003;6:501. doi: 10.1097/00075197-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Horn M. Cardiac magnetic resonance spectroscopy: a window for studying physiology. Methods Mol Med. 2006;124:225. [PubMed] [Google Scholar]
- 17.Barnard ML, Changani KK, Taylor-Robinson SD. The role of magnetic resonance spectroscopy in the assessment of kidney viability. Scand J Urol Nephrol. 1997;31:487. doi: 10.3109/00365599709030648. [DOI] [PubMed] [Google Scholar]
- 18.Davidson BR, Barnard ML, Changani KK, et al. Liver transplantation: current and potential applications of magnetic resonance spectroscopy. Liver Transpl Surg. 1997;3:481. doi: 10.1002/lt.500030502. [DOI] [PubMed] [Google Scholar]
- 19.Khan SA, Cox IJ, Hamilton G, et al. In vivo and in vitro nuclear magnetic resonance spectroscopy as a tool for investigating hepatobiliary disease: a review of H and P MRS applications. Liver Int. 2005;25:273. doi: 10.1111/j.1478-3231.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 20.Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287. doi: 10.1146/annurev.bioeng.7.060804.100411. [DOI] [PubMed] [Google Scholar]
- 21.Ishii S, Sato Y, Terashima M, et al. A novel method for determination of ATP, ADP, and AMP contents of a single pancreatic islet before transplantation. Transplant Proc. 2004;36:1191. doi: 10.1016/j.transproceed.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Siech M, Sotak CH, Letko G, et al. A method for in vivo assessment of reversible rat pancreatic ischemia using 31P NMR spectroscopy at 2 tesla. Magn Reson Imaging. 1995;13:463. doi: 10.1016/0730-725x(94)00127-o. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Suzuki Y, Tanioka Y, et al. Possibility of islet transplantation from a nonheartbeating donor pancreas resuscitated by the two-layer method. Transplantation. 2005;80:738. doi: 10.1097/01.tp.0000174136.70282.5a. [DOI] [PubMed] [Google Scholar]
- 24.Paget M, Murray H, Bailey CJ, et al. Human islet isolation: semi-automated and manual methods. Diab Vasc Dis Res. 2007;4:7. doi: 10.3132/dvdr.2007.010. [DOI] [PubMed] [Google Scholar]


