Abstract
Context
Family interventions have been found to hasten episode recovery and delay recurrences among adults with bipolar disorder.
Objective
To examine the benefits of family-focused therapy for adolescents (FFT-A) and pharmacotherapy in the 2-year course of adolescent bipolar disorder.
Design and setting
Two-site outpatient randomized controlled trial with 2-year follow-up.
Patients
A referred sample of 58 adolescents (14.5 ± 1.6 yrs) with bipolar I (n = 38), II (n = 6), or not otherwise specified disorder (n = 14) with a mood episode in the prior 3 months.
Interventions
Patients were randomly assigned to FFT-A and protocol pharmacotherapy (n = 30) or enhanced care (EC) and protocol pharmacotherapy (n = 28). FFT-A consisted of 21 sessions in 9 months of psychoeducation, communication training, and problem-solving skills training. EC consisted of 3 family sessions focused on relapse prevention.
Main Outcome Measures
Independent “blind” evaluators assessed patients every 3-6 months over 2 years. Outcomes included time to recovery from the index episode, time to recurrence, weeks in episode/remission, and mood symptom severity scores.
Results
Analyses were by intent-to-treat. Rates of 2-year study completion did not differ across the FFT-A (60.0%) and EC conditions (64.3%). Although there were no group differences in rates of recovery from the index episode, patients in FFT-A recovered from their baseline depressive symptoms faster than patients in EC (HR = 1.85; 95% CI: 1.04 – 3.29; P = .037). The groups did not differ on time to recurrence of depression or mania, but patients in FFT-A spent fewer weeks in depressive episodes and had a more favorable trajectory of depression symptoms over 2 years.
Conclusions
FFT-A is effective in combination with pharmacotherapy in stabilizing bipolar depressive symptoms among adolescents. To establish full recovery, FFT-A may need to be supplemented with systematic care interventions found effective for mania symptoms.
Between 50% and 66% of bipolar patients have illness onset before age 18, and between 13% and 28% before age 13. 1-2 Early onset of the illness is associated with an unremitting course of illness, frequent switches of polarity, mixed episodes, psychosis, a high risk of suicide, and poor functioning or quality of life.3-10
The past decade has witnessed a remarkable increase in diagnoses of bipolar disorder in youth and correspondingly, drug trials for early-onset patients. 11-12 There has been comparatively little controlled examination of psychotherapy for pediatric patients. Among 35 families with bipolar children (age 8 – 11 yrs), multifamily psychoeducation groups promoted better social support and access to health care than a wait-list control.13 Two open trials found improvements in mood symptoms over 1-3 years among bipolar youth who received pharmacotherapy, family skills training, and individual therapy. 14-15
In contrast, there has been a considerable increase in the number of randomized trials examining adjunctive psychotherapies for adult bipolar patients. 16 Notably, adults given pharmacotherapy and family-focused therapy (FFT) - a 9 month, 21-session intervention consisting of psychoeducation for the family about bipolar disorder, communication training, and problem-solving skills training – had longer well intervals prior to recurrences than patients receiving medication and brief psychoeducation in one trial17 or medication and equally intensive individual psychoeducation in another trial.18 The 15-site Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that medication and up to 30 sessions of FFT, cognitive-behavior therapy (CBT), or interpersonal and social rhythm therapy were more effective than medication and minimal psychoeducation in speeding recovery from bipolar depression, maintaining wellness, and enhancing functioning and quality of life over 1 year. 19-20
In a treatment development trial (N = 20), we adapted the FFT model to adolescents (FFT-A), and showed that it was feasible, acceptable, and associated with improvements in mood symptoms over 2 years. 21 In this article, we report results from a two-site randomized trial of FFT-A and medication in comparison with enhanced care (EC; 3 sessions of family education) and medication for bipolar adolescents with a recent mood episode. We hypothesized that adolescents undergoing FFT-A would recover more rapidly from episodes of depression or mania and have longer well intervals (without recurrence) than patients in EC. Secondarily, we hypothesized that treatment with FFT-A would be associated with less time in depressed or manic moods and a more favorable trajectory of symptom severity scores over 2 years.
METHODS
PARTICIPANTS
Participants were adolescents with bipolar spectrum disorder with a minimum 1-2 week period of illness in the prior 3 months. Adolescents at the University of Colorado site were recruited through direct referral from community psychiatrists or the inpatient units of the Children’s Hospital of Denver. Patients at the University of Pittsburgh School of Medicine site were existing patients or new referrals to the Child and Adolescent Bipolar Services Clinic. Recruitment ran from October 2002 to September 2005.
ELIGIBILITY CRITERIA
Patients met the following criteria: (1) age between 12 yrs, 0 months and 17 years, 11 mos; (2) a DSM-IV22 diagnosis of bipolar I, II, or not otherwise specified (NOS) disorder based on separate Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (K-SADS-PL23-24) interviews with the child and at least one parent; and (3) concurrent physician diagnosis of bipolar I, II, or NOS based on a separate set of evaluations with the child and parent. A teen was diagnosed with bipolar NOS if he or she had had a distinct period of abnormally elevated, expansive or irritable mood plus two (three if irritable mood only) DSM-IV symptoms of mania that caused a change in functioning, lasted for at least one day, and was present for a total of at least four days in the teen’s lifetime. This operational definition of bipolar NOS was validated relative to bipolar I and II disorder and found to be associated with considerable psychosocial impairment in the Course and Outcome of Bipolar Youth (COBY) Study;9-10 (4) at least a 1-week episode of manic, mixed, or hypomanic symptoms or a 2-week episode of depressive symptoms within the past 3 months; (5) no severe psychosis lasting ≥ 3 months; (6) no evidence of mental retardation, neurological illness, or pervasive developmental disorder; (7) no substance or alcohol disorders in the prior 3 months; (8) no eating disorder or medical disorder requiring immediate hospitalization; (9) willing to proceed with pharmacotherapy from a study psychiatrist; and (10) at least one biological parent or stepparent is willing to participate in family treatment.
This study was approved by the human subject review boards of both universities. All parents, adolescent patients, and siblings gave written consent or assent following a thorough explanation of the procedures.
INITIAL DIAGNOSTIC EVALUATION
Potential participants were screened during an initial telephone contact with the site’s project coordinator. Then, trained research staff members (working alone or in pairs) administered the K-SADS-PL to the adolescent and, separately, at least one parent. The interview time frame was lifetime, with current episode ratings based on the most symptomatic 1-2 week period in the prior 3 months. The mood sections of the K-SADS-PL were replaced with the K-SADS Depression and Mania Rating Scales (DRS and MRS;23-25), which offer more thorough coverage of current and past symptoms on 1-6 scales of severity and impairment. Evaluators also made 1-100 ratings of functioning on the Child’s Global Assessment Scale (CGAS26) for the prior 2 weeks, the most severe past episode, and the highest level in the past year.
Final K-SADS-PL, DRS, MRS, and CGAS scores were based on a consensus between the parents’ and adolescents’ scores. When discrepancies occurred, the adolescent and parent were consulted conjointly or information from another parent or medical records was obtained. Patients were only included if the study psychiatrist and K-SADS interviewer agreed that a bipolar I, II, or NOS disorder was present.
All K-SADS diagnosticians and independent evaluators had at least an MA or psychiatric nursing degree. Interrater reliability for K-SADS MRS and DRS scores (51 ratings) was 0.97 and 0.89 (intraclass rs), respectively, and 0.70 (kappa) for K-SADS-PL comorbid disorders. A mid-study check on rater drift between 10 Colorado raters and 4 Pittsburgh raters yielded 81% agreement on the K-SADS–DRS and 76% on the K-SADS-MRS.
PHARMACOLOGICAL TREATMENTS
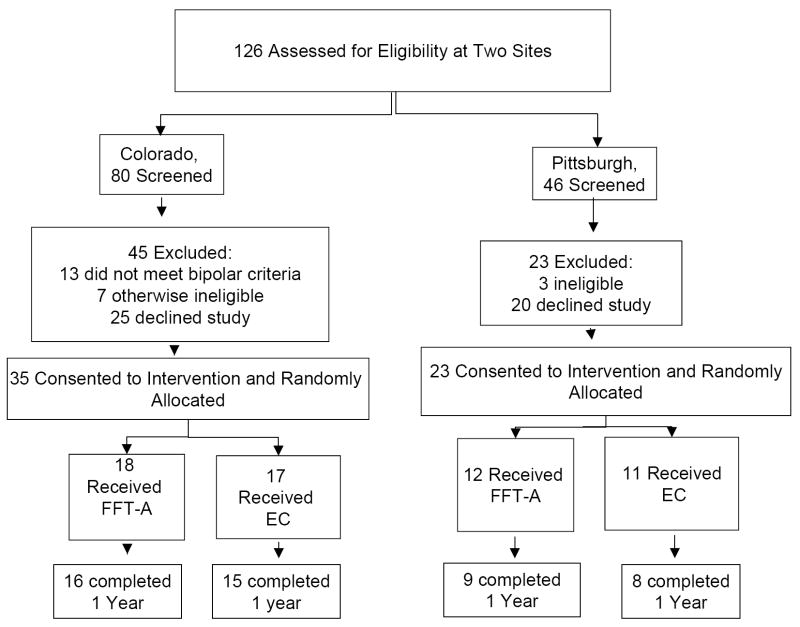
Once accepted into the trial, a data manager at the University of Pittsburgh randomly assigned subjects to pharmacotherapy and FFT-A (50%) or pharmacotherapy and Enhanced Care (EC; 50%) using a modification of Efron’s biased coin procedure. 27 Randomizations were stratified by study site (Fig 1).
Figure 1. Consort Diagram.
FFT-A indicates family-focused therapy for adolescents; EC, enhanced care.
Patients were treated by study psychiatrists for 2 years according to the best practices known at the time of treatment. Psychiatrists convened monthly teleconferences to review advances in the treatment literature and the diagnosis and management of difficult cases. Medications shown to be effective in bipolar adults and, when data were available, bipolar adolescents (i.e., lithium, anticonvulsants, second generation antipsychotics) formed the basis of pharmacotherapy plans. Adjunctive antidepressants and pharmacotherapy for comorbid ADHD or anxiety symptoms were permitted. Treatment guidelines from an expert consensus panel were integrated when they became available.12
PSYCHOSOCIAL TREATMENTS
Family-Focused Therapy for Adolescents (FFT-A)
FFT-A consisted of 21 50-minute sessions (12 weekly, 6 biweekly, and 3 monthly) over 9 months and included the patient, parents, and available siblings. The goals of the first 7-10 sessions (psychoeducation) were to (1) encourage adolescent patients and family members to develop a common understanding of the symptoms, etiology, and course of pediatric bipolar illness and the precipitants for recurrences (including life events and high family conflict21,28-30); (2) encourage ongoing adherence with pharmacotherapy; and (3) conduct a relapse prevention drill in which the family agreed on principles for early intervention when prodromal signs of recurrence appeared (e.g., arranging an emergency psychiatric evaluation). Later phases of FFT-A focused on communication enhancement training, in which participants learned, through role-playing and between-session rehearsal, skills for active listening, offering positive feedback or constructive criticism, or requesting changes in each others’ behavior; and problem-solving skills training, in which participants learned to identify, generate solutions to, and implement solutions to problems in the family’s day-to-day life.
Enhanced Care (EC)
Patients in EC received 3 weekly 50-min sessions of psychoeducation with their parents and siblings. These sessions focused on relapse prevention planning, medication adherence, and keeping the home environment low in conflict. Adolescents and family members in FFT-A and EC could receive additional family or individual crisis sessions as needed throughout the 2-year study.
THERAPIST TRAINING AND FIDELITY
All clinicians underwent standardized training in the FFT-A and EC manuals during a 2-day pre-trial seminar, and then carried at least 2 cases with weekly group supervision during a treatment development trial. 21,30 Therapist fidelity was tracked via audiotape review and ratings on the 13-item Therapist Competency and Adherence Scales (TCAS),31 which each range from 1 (poor adherence) to 7 (excellent adherence). Interrater reliability (intraclass rs) for the scales ranged from 0.74 – 0.98. During the treatment development trial, overall TCAS ratings for Pittsburgh therapists averaged 5.0 ± 1.3 (“good” adherence, based on 20 session ratings) and for Colorado therapists, 5.1 + 0.6 (27 ratings). During the randomized trial, independent fidelity judgments of 60 FFT-A or EC sessions from Colorado and Pittsburgh revealed no site differences in general therapist skills (P = .19; range 5.5 – 6.3), overall fidelity (P = .29; 5.0 – 5.9), or any other TCAS scale (all Ps > .10).
ASSESSMENT OF PRIMARY OUTCOMES
An independent evaluator interviewed the youth and at least one parent at study entry (covering the prior 3 months), every 3 months in year 1, and every 6 months in year 2 using the Adolescent Longitudinal Interval Follow-up Evaluation (A-LIFE) 10,32 and the K-SADS DRS and MRS interviews. The evaluator, who was unaware of treatment assignments, rated each week of the prior 12 weeks (year 1) or 26 weeks (year 2) on the 1 – 6 A-LIFE Psychiatric Status Rating scales (PSRs) covering the severity of mania, hypomania, and major depression symptoms. Ratings reflected a consensus between youth and parent reports. PSR ratings of 1-2 indicated asymptomatic or mildly symptomatic states; 3-4 indicated subthreshold symptoms and impairment; and 5-6, fully syndromal states with varying severity and impairment. Reliabilities for PSR ratings, established previously, were 0.74 for depressive episodes and 0.91 for manic/hypomanic episodes (8 subjects/53 ratings). 10
Clinical status designations at study entry and follow-up were based on the presence/absence of DSM-IV criteria for depression, mania, or hypomania during that 3- or 6-month interval. Recovery was assigned once all the PSR mood scales (depression and mania/hypomania) were ≤ 2 for ≥ 4 consecutive weeks. This designation was subdivided into recovery from initial depressive symptoms (PSR depression scale ≤ 2 for ≥ 4 weeks) and recovery from initial mania symptoms (PSR mania/hypomania scales ≤ 2 for ≥ 4 weeks), which could occur at different times. Recurrence was assigned if, after a 4-week or greater period of recovery, PSR scores were ≥ 5 for ≥ 1 week for mania or hypomania or ≥ 2 weeks for depression. We also calculated the number of weeks during the 2-year study that subjects spent depressed (PSR depression score ≥ 5), manic (PSR mania or hypomania scores ≥ 5), or well (free of significant depression or mania symptoms; PSR scores ≤ 2).
STATISTICAL ANALYSES
Time to recovery from the index episode was calculated as the weeks from randomization until the patient met the 4-week recovery criteria above. Separate calculations were made for time to recovery from index depression symptoms and index mania symptoms. Time to recurrence was calculated as weeks from the onset of recovery to the onset of an acute episode (PSR scores ≥ 5 for ≥ 1 week for mania/hypomania or ≥ 2 weeks for depression). Patients who did not have a recovery or recurrence or who terminated prematurely were censored at the point of their final research assessment. All randomized patients were included in the at-risk sample.
Cox proportional hazards models33 estimated the independent effect of psychosocial treatment group on time to recovery or recurrence after controlling for the effects of site, gender, initial medications, baseline illness polarity, and baseline levels of depressed or manic/hypomanic mood. Baseline mood scores were calculated as the most severe A-LIFE PSR score (calculated separately for depression and mania) recorded for a single week within a maximum of 5 weeks prior to randomization (for the 40 subjects in which pre-randomization PSR scores were available), or a maximum of 5 weeks after randomization (for the 18 subjects for which pre-randomization PSR scores were unavailable).
Our secondary hypotheses were that patients in FFT-A would spend less time in episode and have less severe mood symptoms than patients in EC over 24 months. Prior studies suggested that these treatment effects would be stronger for depression than for mania symptoms.17,20 Poisson regression models34 were used to compare the groups on the number of weeks in depressive episodes, number of weeks in manic/hypomanic episodes, the number of weeks well (all PSR mood scales ≤ 2), weeks free of depression symptoms, and weeks free of mania/hypomania symptoms, adjusted for total weeks in the study.
Group comparisons of weekly mania and depression PSR scores were undertaken using mixed effects regression (growth curve) models with random subjects effects35 using Proc Mixed in SAS.36 Covariates included site, sex, baseline mood severity, baseline polarity, bipolar subtype (I, II, or NOS), and initial medication regimens. With 29 participants in each treatment arm, mixed effects regression models had 80% power to detect a 0.75 SD group difference in means (P < .05, two-tailed).
RESULTS
STUDY SAMPLE
Participants were 58 adolescents (33 females [56.9%) and 25 males [43.1%]; 35 [60.3%] from Colorado, 23 [39.7%]) from Pittsburgh) with bipolar I (n = 38, 65.5%), bipolar II (n = 6, 10.3%), or bipolar NOS disorder (n = 14, 24.1%) with a mean age of 14.5 yrs ± 1.6 yrs (Table 1). The mean ± SD CGAS score for the 2 weeks prior to randomization was 57.8 ± 11.1 (range 35 - 95); for the most severe past episode, it was 44.9 + 12.9 (range 35 – 95). Patients who began the study in depressed, manic, mixed, or subsyndromal states did not differ in current (2-week) CGAS scores (F(3, 51) = 1.13, P = .34), nor did patients with BPI or bipolar II/NOS disorder (t (56) = 0.14, P = .89).
Table 1.
Demographic and Illness History Variables (N = 58)
| Variable1 | M (or N) | SD (or %) |
|---|---|---|
| Age | 14.5 | 1.6 |
| K-SADS Depression Rating Scale, Entry2 | 28.6 | 9.4 |
| K-SADS Mania Rating Scale, Entry2 | 24.3 | 9.5 |
| Child’s Global Assessment Scale | ||
| Current (last 2 weeks) | 57.8 | 11.1 |
| Most severe past episode | 44.9 | 12.9 |
| Highest in prior year | 59.7 | 11.1 |
| Female Sex | 33 | 56.9 |
| Race | ||
| African American | 1 | 1.7 |
| Native American | 1 | 1.7 |
| Asian/Pacific Islander | 1 | 1.7 |
| Biracial | 3 | 5.2 |
| Hispanic ethnicity | 3 | 5.2 |
| Live with both biological parents | 26 | 44.8 |
| Bipolar I | 38 | 65.5 |
| Bipolar II | 6 | 10.3 |
| Bipolar Not Otherwise Specified | 14 | 24.1 |
| Index Mood Episode | ||
| Depression | 18 | 31.0 |
| Mania | 12 | 20.7 |
| Mixed | 3 | 5.2 |
| Subthreshold3 | 25 | 43.1 |
| Comorbid disorders | ||
| Anxiety | 2 | 3.5 |
| ADHD | 11 | 19.0 |
| ODD | 7 | 12.1 |
| Substance abuse or dependence | 0 | 0.0 |
| Medication treatments at study entry | ||
| One mood stabilizer | 35 | 60.0 |
| Two mood stabilizers | 9 | 15.5 |
| One atypical antipsychotic | 34 | 58.6 |
| Two atypical antipsychotics | 7 | 12.1 |
| Adjunctive antidepressant | 13 | 22.4 |
| Adjunctive anxiolytic | 3 | 5.2 |
| Type of mood stabilizer at study entry | ||
| Divalproex sodium | 7 | 12.1 |
| Lithium | 21 | 36.2 |
| Lamictal | 3 | 5.2 |
| Carbamazepine | 1 | 1.7 |
| Oxcarbazepine | 13 | 22.4 |
| Topiramate | 3 | 5.17 |
| Type of antipsychotic at baseline | ||
| Aripiprazole | 10 | 17.2 |
| Risperidone | 8 | 13.8 |
| Quetiapine | 21 | 36.2 |
| Olanzapine | 4 | 6.9 |
Abbreviations: K-SADS = Kiddie Schedule for Affective Disorders and Schizophrenia; ADHD = attention deficit hyperactivity disorder; ODD = oppositional defiant disorder.
None of the above variables significantly distinguished between the psychosocial treatment groups or sites, based on Mantel-Haenszel χ2 and two-way analysis of variance tests.
Refers to scores collected at intake into the study, covering the worst 1-2 week period in the previous 3 months.
Adolescents with subthreshold index mood episodes had at least 1-2 weeks in the past 3 months with A-LIFE mania or depression scores ≥ 3 and < 5.
The 58 included youths did not differ from 68 who were excluded (Fig. 1) on age (P = .08), sex (P = .54), or racial or ethnic distribution (P = .41). Exclusions were equally common at the Colorado (45/80, or 56.3%) and Pittsburgh (23/46, or 50.0%) sites (χ2 (1) = .46, P = .50).
BASELINE COMPARISONS OF TREATMENT GROUPS AND SITES
Patients randomly assigned to FFT-A (n = 30) and EC (n = 28) did not differ on any of the demographic or illness variables listed in Table 1. The groups did not differ at randomization on the proportion treated with one versus two mood stabilizers (P = .12), atypical antipsychotics (P = .38), or adjunctive antidepressants (P = .09). There were no site differences or interactions between treatment and site on any baseline clinical, demographic, or medication variable (all Ps > .10).
STUDY ATTRITION AND TREATMENT COMPLETION
As indicated in Table 2 and Fig. 2, 48/58 (82.8%) adolescents completed one year of treatment and follow-up, and 36/58 (62.1%) completed 2 years. These proportions did not differ across the treatment groups in either year (P = .90 and P = .61, respectively) or across the sites in either year (P = .15 and P = .87). Rates of 2-year study completion were 60% in FFT-A (18/30) and 64.3% in EC (18/28) (P = .73). Subjects were followed for a mean ± SD of 83.8±31.9 wks in FFT-A and 81.8±32.1 wks in EC (P = .83). There were no significant associations between duration of participation and gender, age, race, ethnicity, bipolar subtype, or baseline mood symptoms.
Table 2.
Rates of Completed Outcome Assessments Across Treatments
| FFT-A | EC | Total (%) | |
|---|---|---|---|
| 3 month | 30 | 28 | 58 (100) |
| 6 month | 27 | 25 | 52 (89.7) |
| 9 month | 26 | 24 | 50 (86.2) |
| 12 month | 25 | 23 | 48 (82.8) |
| 18 month | 21 | 19 | 40 (70.0) |
| 24 month | 18 | 18 | 36 (62.1) |
Note: FFT-A = family-focused treatment for adolescents; EC = enhanced care.
Figure 2.
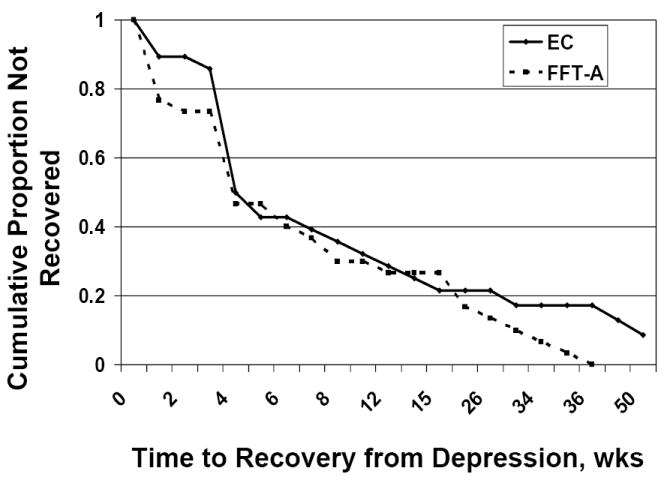
Time to recovery from depressive symptoms at study intake (N = 58; sample mean, 12.4 weeks ± 17.1). After accounting for baseline depression severity, site, and gender, a Cox proportional hazards model indicated that patients in family-focused treatment for adolescents (FFT-A, n = 30) and medication recovered from their baseline depressive symptoms faster than patients in enhanced care (EC, n = 28) and medication (χ2 (1) = 4.36, P = .037; hazard ratio = 1.85, 95% CI, 1.04 – 3.29).
The mean ± SD number of psychotherapy sessions (including non-protocol crisis contacts) for patients in FFT-A was 19.3 ± 7.8 (median = 21), and in EC, 4.96 ± 3.68 (median = 3). Patients in EC obtained 0.88 ± 3-4). There was no effect 1.86 extra family crisis sessions and 0.96 ± 2.27 individual crisis sessions; the corresponding numbers for FFT-A were 0.25 ± 0.61 and 0.79 ± 2.32, respectively. There was no difference between the sites (P = .09) and no treatment by site interaction for number of psychotherapy sessions (P = .23). The frequency of pharmacotherapy visits over 2 years (mean 13.4 ± 9.4, range 1 – 48) did not differ across the FFT-A and EC conditions (P = .89), the sites (P = .29), or the psychosocial conditions stratified by site (P = .42).
PSYCHOSOCIAL TREATMENT AND RECOVERY
Of the 58 patients, 53 (91.4%) had full recovery from the index mood episode (all PSR mood scales ≤ 2 for 4 or more weeks) during the 2-year study. The average time to recovery was 19.8 weeks (SE = 3.28). Patients in FFT-A and EC did not differ in time to full recovery (χ2 (1) = 0.95, P = .33; HR = 1.33; for FFT, mean 17.4 wks, SE = 3.74; for EC, mean = 22.3 wks, SE = 5.40).
The rate of recovery from index episode depression symptoms was high in both the FFT-A (30/30 or 100.0%) and EC (25/28 or 89.3%) conditions. However, patients in FFT-A experienced more favorable and rapid recovery from index depressive symptoms than patients in EC (χ2 (1) = 4.36, P = .037; hazard ratio (HR) = 1.85, 95% CI, 1.04 – 3.29) (Fig 2). The mean time to depression recovery in FFT-A was 10.2 weeks (SE = 2.1; 25% median = 2 wks, 95% CI, 1 – 4) and in EC, 14.1 weeks (SE = 3.34; 25% median = 4 wks; 95% CI, 3 - 4). There was no effect of site (P = .81) and no interaction between treatment and site (P = .55) on recovery from depressive symptoms.
There were no interactions between treatment group and baseline PSR depression scores (P = .67) or treatment group and baseline illness polarity (P = .83) on time to recovery from depression. In the subsample of 18 patients with a major depressive episode prior to randomization, the mean time to recovery from depression was 15.3 wks (SE = 4.47) in FFT and 22.5 wks (SE = 8.01) in EC (HR = 4.87, 95% CI, 1.67 to 14.18). Within the subgroup whose index episode was subsyndromal, the mean time to depression recovery was 5.7 wks (SE = 1.68) in FFT and 9.2 wks (SE = 2.86) in EC (HR = 2.18, 95% CI, 0.75 to 6.27).
There was no interaction between treatment group and bipolar subtype (I versus II/NOS) on time to recovery from index depression symptoms (χ2 (1) = 1.60, P= .21). The mean time to recovery within the bipolar I subgroup (N = 38) was 10.6 wks (SE = 2.71; 25% median = 2.5, 95% CI, 1 - 4) for FFT and 13.4 wks (SE = 4.08; 25% median = 4, 95% CI, 1 - 4) for EC (HR = 1.48, 95% CI, 0.7 to 3.0). Within the bipolar II and NOS patients (n = 20), the mean times to recovery were 9.4 wks (SE = 3.41; 25% median = 2, 95% CI, 0 – 6) for FFT and 8.1 wks (SE = 1.62; 25% median = 4, 95% CI, 4 – 7) for EC (HR = 2.83, 95% CI 0.9 to 8.5).
By one year, 29/30 (96.7%) of the patients in FFT-A and 28/28 (100%) of the patients in EC met the 4-week recovery criteria for mania/hypomania. The group difference in time to recovery from mania, although favoring FFT-A (mean 7.6 weeks [SE = 1.37]); for EC, 13.79 weeks [SE = 3.54]), did not reach significance (χ2 (1) = 2.53, P = .11; HR = 1.58; 95% CI, 0.9 to 2.8). There were no interactions between treatment and index polarity (P = .48) or treatment and bipolar subtype (P = .52) on time to recovery from mania symptoms.
PSYCHOSOCIAL TREATMENT AND RECURRENCE
Of the 53 adolescents who achieved 4 weeks of full recovery, 26 (49.1%) had at least one recurrence. Of these, 20 (37.7%) had a recurrence of depression and 15 (28.3%) had a recurrence of mania (n = 12) or hypomania (n = 3). The average time between onset of recovery and recurrence was 62.4 weeks (SE = 7.43). There were no effect of psychosocial treatment on time to any mood disorder recurrence (χ2 (1) = .50, P = .48, HR = 0.74), depression recurrence (χ2 (1) = .11, P = .74, HR = 0.85) or mania/hypomania recurrence (χ2 (1) = 0.34, P = .56; HR = 1.36).
Consistent with our hypotheses, patients in FFT-A spent less time in acute states of depression (estimate 3.3 weeks, 95% CI, 2.7 -4.0) during the 2-year study than patients in EC (5.0 weeks, 95% CI, 4.2-5.9; χ2 (1) = 13.03, P = .0003). Patients in FFT-A and EC did not differ on the number of weeks free from all mood disorder symptoms (χ2 (1) = 1.30, P = .25). Patients in FFT-A, however, spent more time (average 70.3% of total weeks) without symptoms of depression (estimate 52.6 wks, 95% CI, 50.0 - 55.4) than patients in EC (average 66.3% of total weeks; estimate 48.3 wks, 95% CI, 45.7 – 51.0; χ2 (1) = 5.93, P = .015).
There were no interactions between treatment group and polarity of the index episode (P = .77) or treatment group and bipolar subtype (P = .68) on number of depression-free weeks. Notably, patients in FFT-A who began the study in a syndromally depressed state had more depression-free weeks (mean 53.8 wks ± 27.9) at follow-up than patients in EC who began in a depressed state (mean 44.7 wks ± 35.2). Comparable effects of FFT-A were not observed for time spent in manic episodes (χ2 (1) = .01, P = .91) or time spent remitted from manic episodes (χ2 (1) = 1.99, P = .16).
LONGITUDINAL TRAJECTORY OF MOOD SYMPTOMS
Patients in FFT-A had greater overall reductions in mood severity scores (the worst score recorded for the mania/hypomania or depression PSR scales for each week of follow-up) than patients in EC over 2 years (F(1, 5017) = 7.49, P = .006). This treatment by time interaction was independent of the main effects of baseline depression (F(1, 51) = 12.90, P = .0007) or baseline mania or hypomania symptoms (F(1,51) = 3.68, P = .06) on mood severity scores at follow-up. The treatment by time interaction was equally robust (F(1, 5017) = 7.58, P = .006) when baseline illness polarity was included in the model. No treatment by polarity interaction was observed (F(3, 48) = 1.20, P = .31). There was no main effect of site (P = .27) or bipolar I versus II/NOS subtype (P = .28) on the trajectory of mood symptom scores.
Decomposing the overall mood symptom score revealed that FFT-A was associated with a more favorable trajectory of PSR depression scores even when baseline depression scores were covaried (treatment by time interaction: linear effect, F(1, 5014) = 9.23, P = .002; quadratic effect, F(1, 5014) = 4.70, P = .03; difference in −2 log-likelihood = 14.0 (df = 2), P <.001; Fig 3). The groups did not differ in the trajectory of mania/hypomania symptoms over 24 months (F(1, 5044) =0.003, P = .96).
Figure 3.
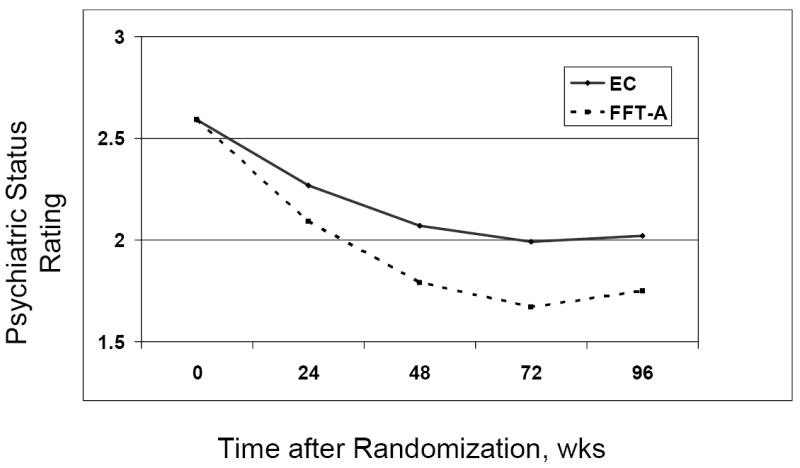
Longitudinal trajectory of Psychiatric Status Ratings (PSR) from the Adolescent Longitudinal Interval Follow-up Interview (N = 58). Family-focused treatment for adolescents (FFT-A) was associated with a more favorable trajectory of PSR depression scores than enhanced care (EC) (treatment by time interaction, linear effect, P = .002; quadratic effect, P = .03; difference in −2 log-likelihood = 14.0 (df = 2), P < .001).
EFFECTS OF MEDICATION REGIMENS
At the 12-month follow-up, patients in FFT and EC did not differ on the likelihood of treatment with one versus two mood stabilizers (P = .99), antipsychotics (P = .89), antidepressants (P = .99), or anxiolytics (P = .49). The effects of FFT-A on speed of recovery from depression did not change when antidepressant exposure at baseline (present/absent) and initial PSR depression scores were covaried (χ2 (1) = 3.87, P = .049; HR = 1.84). Moreover, FFT-A remained associated with more weeks free of depressive symptoms (52.7 ± 3.9) than EC (48.2 ± 3.8) (χ2 (1) = 5.88, P = .015) and fewer weeks in depressive states (3.12 ±.93) than EC (5.3 ± 1.5) (χ2 (1) = 18.48, P < .0001) after including these covariates in Poisson models.
A mixed effects regression model, including baseline PSR depression scores, site, bipolar I or II/NOS subtype, number of mood stabilizers per patient, atypical antipsychotics, and presence/absence of antidepressant exposure as covariates revealed a strong psychosocial group by time interaction on PSR depression scores over 2 years (linear trend: F(1, 5014) = 9.08, P = .003; quadratic trend: F(1, 5014) = 4.62, P = .032; difference in -2 log likelihood ratio test for treatment effects = 13.8, P < .01). These interactions indicate greater stabilization in the FFT-A than the EC condition.
COMMENT
To our knowledge, this is the first randomized controlled trial of a psychosocial intervention for adolescent bipolar disorder. We examined the efficacy of medication and FFT-A, a psychosocial intervention originally developed for adults, in 58 bipolar adolescents followed for 2 years after an illness episode. The FFT-A was administered with high levels of therapist fidelity across two sites. In comparison with teens treated with medication and 3 sessions of family psychoeducation (EC), patients in FFT-A had shorter times to recovery from depression, less time in depressive episodes, and lower depression severity scores over 2 years. Naturalistic studies find that 70% - 100% of bipolar children and adolescents recover from acute episodes within 1-5 years.5,8,10 However, the speed and quality of recovery may be enhanced by involving the family in psychoeducational treatment.
The results are similar to those of recent trials comparing intensive psychotherapy to brief psychoeducation or routine care for bipolar adults. 16-20,37 In the multisite STEP-BD study, patients (N = 293) who began in a bipolar depressive episode and who received medication and up to 30 sessions of psychotherapy (FFT, interpersonal therapy, or CBT) had more rapid recoveries from depressive episodes than patients who received medication and 3 psychoeducational sessions. 19-20 Much like STEP-BD, the present study found effects for FFT-A on depression symptoms, but not mania symptoms.
To enhance full symptomatic and functional recovery among adolescents, FFT-A may need to be supplemented with collaborative care interventions found effective in mania stabilization. Notably, two trials of systematic care management for adults with bipolar I disorder found that the combination of group psychoeducation, prodromal symptom monitoring, and systematic application of pharmacotherapy guidelines was more effective than usual care in reducing the severity and duration of manic symptoms. 38-39 In contrast, the emphasis in FFT on reducing conflict in family relationships, enhancing social supports, and teaching interpersonal skills may underlie its stronger effects on bipolar depression.40-41
We did not examine whether the effects of family intervention on time to recovery or symptom severity translated into differences in functioning or quality of life among teens, as we had observed among bipolar adults.19 DelBello et al. 42 observed that 86% of bipolar adolescents recovered syndromally in the year after an acute episode, but only 41% achieved functional recovery. Future studies should consider the course of adolescent functioning, notably during the transition to adulthood, in patients treated with FFT-A or other psychosocial interventions.
The conclusions from this study are tempered by several limitations. First, there was considerable variability in the clinical status of patients at entry: 43% (n = 25) had subsyndromal episodes, 31% (n = 18) had depressive episodes, and 12 (21%) had manic episodes; 38/58 (65.5%) had bipolar I disorder. This heterogeneity is not atypical in samples of bipolar youth.10,12 We saw no evidence that pretreatment clinical status moderated the effects of FFT-A on symptom outcomes. However, our design was underpowered to detect treatment by baseline polarity (or treatment by bipolar subtype) interactions which, for a moderate effect size (Cohen’s d = 0.5, P < .05), would have required 64 subjects in each of 4 cells, or 256 participants.
By design, this study did not equate the treatment conditions on number of therapy contacts. Thus, we were unable to determine whether the effects of FFT-A were due its specific content, nonspecific factors such as the therapist/patient working alliance,43-45 or simply its greater number of contact hours (21 hrs versus 3 hrs in EC) and by extension, greater opportunities for therapists to communicate changes in patients’ mood states to the treating psychiatrists. Brief interventions such as EC are typically associated with only a 20% improvement rate in childhood mood disorders.10, 46-47 Possibly, a more intensive EC treatment with the same amount of therapist contact and prodromal symptom monitoring as FFT-A, but without the additional skills training components, would have performed just as well in reducing time to recovery and episode length.
The issue of therapy specificity is particularly important in light of Goodyer et al.’s46 finding that a 9-session nonspecific care intervention in combination with an SSRI performed just as well as a 19-session CBT plus an SSRI in stabilizing major depression among adolescents. In contrast, a randomized trial in bipolar, manic adults found that 21 sessions of FFT and medication were more effective than a time-matched, 21-session individual psychoeducational treatment and medication in preventing recurrences and rehospitalizations over 2-3 years.18 Comparisons of active and control treatments of identical duration and frequency, and systematic examination of treatment mediators (which in prior FFT studies have included improvements in family communication and medication adherence16-17) would help to disentangle the contributions of treatment content versus frequency to the outcomes of youth with bipolar disorder.
Although pharmacotherapy was administered by study psychiatrists who followed best-practice guidelines, regimens could be modified at any point during the study based on clinical need. Patients in FFT-A and EC did not differ at time of randomization or at a 12-month follow-up in medication regimens, nor did baseline regimens account for the effectiveness of FFT-A. Nonetheless, group differences in drug dosages or adherence might have emerged at any point during the 2-year follow-up. Future research should examine whether intensive psychosocial intervention in the interval following an acute episode enhances pharmacological adherence or reduces the later need for complex pharmacotherapy among pediatric bipolar patients.
Acknowledgments
We thank Adrine Biuckians MA, Tina Goldstein PhD, Eunice Kim, PhD, Kimberley Mullen MA, Amy Schlonski LCSW, and Tim Winbush, LCSW for serving as study therapists; Susan Wassick RN, Amy Mechels MA, Chad Morris PhD, Victoria Cosgrove MA, and Laura Wagenknecht MA for serving as independent evaluators; Mary Beth Hickey for serving as the study’s data manager; and Joel Sherrill Ph.D. for input regarding study design and methods.
Dr. Miklowitz verifies that he had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Dickinson was the project statistician.
Funding/Support: This study was funded by National Institute of Mental Health (NIMH) grants R21-MH62555 and R01-MH073871, a Distinguished Investigator Award from the National Alliance for Research on Schizophrenia and Depression, and a Faculty Fellowship from the University of Colorado’s Council on Research and Creative Work (Dr Miklowitz); and NIMH Center Grant No. MH066371 (Dr Brent).
Footnotes
The results were presented in part at the annual meeting of the American Association of Child and Adolescent Psychiatry, Boston MA, Oct 24, 2007.
Financial Disclosure: Dr Miklowitz has had funding from the NIMH, the National Association for Research on Schizophrenia and Depression, the Robert Sutherland Foundation, and the Danny Alberts Foundation. He receives book royalties from Guilford Press and John Wiley & Sons. Dr Birmaher has received honoraria from Solvay Pharmacueticals and Abcomm, Inc, and book royalties from Random House, Inc. Dr Craighead has received honoraria from Forest Laboratories, Eli Lilly Co., and Novadel; and book royalties from John Wiley and Sons.
References
- 1.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. STEP-BD Investigators: Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Brent DA, Perper JA, Goldstein CE, Kolko DJ, Allan MJ, Allman CJ, Zelenak JP. Risk factors for adolescent suicide: a comparison of adolescent suicide victims with suicidal inpatients. Arch Gen Psychiatry. 1988;45:581–588. doi: 10.1001/archpsyc.1988.01800300079011. [DOI] [PubMed] [Google Scholar]
- 4.Geller B, Bolhofner K, Craney JL, Williams M, Delbello MP, Gunderson K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adol Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- 6.Coryell W, Solomon D, Turvey C, Keller M, Leon AC, Endicott J, Schettler P, Judd L, Mueller T. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60:914–920. doi: 10.1001/archpsyc.60.9.914. [DOI] [PubMed] [Google Scholar]
- 7.Schneck CD, Miklowitz DJ, Calabrese JR, Allen MH, Thomas MR, Wisniewski SR, Miyahara S, Shelton MD, Ketter TA, Goldberg JF, Bowden CL, Sachs GS. Phenomenology of rapid cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder. Am J Psychiatry. 2004;161:1902–1908. doi: 10.1176/ajp.161.10.1902. [DOI] [PubMed] [Google Scholar]
- 8.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adol Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 9.Axelson DA, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 10.Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 12.Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M Child Psychiatric Workgroup on Bipolar Disorder. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adol Psychiatry. 2005;44(3):213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Fristad MA, Gavazzi SM, Mackinaw-Koons B. Family psychoeducation: an adjunctive intervention for children with bipolar disorder. Biol Psychiatry. 2003;53:1000–1009. doi: 10.1016/s0006-3223(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 14.West AE, Henry DB, Pavuluri MN. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: A pilot feasibility study. J Am Acad Child Adol Psychiatry. 2007;46:205–212. doi: 10.1097/01.chi.0000246068.85577.d7. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein TR, Axelson DA, Birmaher B, Brent DA. Dialectical behavior therapy for adolescents with bipolar disorder: a 1-year open trial. J Am Acad Child Adol Psychiatry. 2007;46:820–830. doi: 10.1097/chi.0b013e31805c1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miklowitz DJ. A review of evidence-based psychosocial interventions for bipolar disorder. J Clin Psychiatry. 2006;67(suppl 11):28–33. [PubMed] [Google Scholar]
- 17.Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003;60:904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- 18.Rea MM, Tompson M, Miklowitz DJ, Goldstein MJ, Hwang S, Mintz J. Family focused treatment vs. individual treatment for bipolar disorder: results of a randomized clinical trial. J Cons Clin Psychol. 2003;71:482–492. doi: 10.1037/0022-006x.71.3.482. [DOI] [PubMed] [Google Scholar]
- 19.Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, Thase ME, Calabrese JR, Marangell LB, Ostacher MJ, Patel J, Thomas MR, A M, Gonzalez JM, Wisniewski SR. Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial. Am J Psychiatry. 2007;164:1–8. doi: 10.1176/appi.ajp.2007.07020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Nierenberg AA, Calabrese JR, Marangell LB, Gyulai L, Araga M, Gonzalez JM, Shirley ER, Thase ME, Sachs GS. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419–427. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miklowitz DJ, Biuckians A, Richards JA. Early-onset bipolar disorder: a family treatment perspective. Dev Psychopathol. 2006;18(4):1247–1265. doi: 10.1017/S0954579406060603. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (Text Revision) (DSM-IV-TR) Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 23.Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semi-structured interview: test-retest reliability. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Ch Adol Psychiatry. 1997;36:98–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale for children and adolescents. J Child Adol Psychopharmacol. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird HA. Children’s Global Assessment Scale (C-GAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 27.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36:81–90. [PubMed] [Google Scholar]
- 28.Johnson SL. Life events in bipolar disorder: towards more specific models. Clinical Psychol Rev. 2005;25:1008–1027. doi: 10.1016/j.cpr.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, Mintz J. Family factors and the course of bipolar affective disorder. Arch Gen Psychiatry. 1988;45:225–231. doi: 10.1001/archpsyc.1988.01800270033004. [DOI] [PubMed] [Google Scholar]
- 30.Miklowitz DJ, George EL, Axelson DA, Kim EY, Birmaher B, Schneck C, Beresford C, Craighead WE, Brent DA. Family-focused treatment for adolescents with bipolar disorder. J Affect Disord. 2004;82(Suppl 1):113–128. doi: 10.1016/j.jad.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisman AG, Okazaki S, Gregory J, Goldstein MJ, Tompson MC, Rea M, Miklowitz DJ. Evaluating therapist competency and adherence to behavioral family management with bipolar patients. Fam Process. 1998;37:107–121. doi: 10.1111/j.1545-5300.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 32.Keller MB, Lavori PW, Friedman B, et al. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 33.Cox DR. Regression models and life tables. J R Stat Soc [Ser B] 1972;34:187–220. [Google Scholar]
- 34.Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118:392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 35.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 36.Ger D, Everitt BS. Handbook of Statistical Analyses Using SAS. 2. London: CRC Press; 2001. [Google Scholar]
- 37.Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H. Cognitive behaviour therapy for severe and recurrent bipolar disorders: a randomised controlled trial. Br J Psychiatry. 2006;188:313–320. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- 38.Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, Beresford T, Kilbourne AM, Sajatovic M Cooperative Studies Program 430. Collaborative care for bipolar disorder: Part II. Impact on clinical outcome, function, and costs. Psychiatr Serv. 2006;57:937–945. doi: 10.1176/ps.2006.57.7.937. [DOI] [PubMed] [Google Scholar]
- 39.Simon GE, Ludman EJ, Bauer MS, Unutzer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry. 2006;63:500–508. doi: 10.1001/archpsyc.63.5.500. [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, Miklowitz DJ. Expressed emotion as a predictor of outcome among bipolar patients undergoing family therapy. J Affect Disord. 2004;82:343–352. doi: 10.1016/j.jad.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, Sachs-Ericsson N, Suddath R. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol Psychiatry. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. [DOI] [PubMed] [Google Scholar]
- 42.DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve month outcome of adolescents with bipolar disorder following first-hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 43.Luborsky L, McLellan AT, Woody GE, O’Brien CP, Auerbach A. Therapist success and its determinants. Arch Gen Psychiatry. 1985;42:602–611. doi: 10.1001/archpsyc.1985.01790290084010. [DOI] [PubMed] [Google Scholar]
- 44.Startup M, Wilding N, Startup S. Patient treatment adherence in cognitive behaviour therapy for acute psychosis: the role of recovery style and working alliance. Behav Cog Psychoth. 2006;34:191–199. [Google Scholar]
- 45.Castonguay LG, Goldfried MR, Wiser S, Raue PJ, Hayes AM. Predicting the effect of cognitive therapy for depression: a study of unique and common factors. J Consult Clin Psychol. 1996;64:497–504. [PubMed] [Google Scholar]
- 46.Goodyer I, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, Breen S, Ford C, Barrett B, Leech A, Rothwell J, White L, Harrington R. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomised controlled trial. BMJ. 2007;335(7611):142–149. doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington RC, Whittaker J, Shoebridge P, Campbell F. Systematic review of efficacy of cognitive behavioural therapies in childhood and adolescent depressive disorder. BMJ. 1998;316:1559–1563. doi: 10.1136/bmj.316.7144.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]