Abstract
In the last 5 years major advances have been made in the field of tissue engineering. However, while engineering of tissues from nearly every major system in the body have been studied and improved, little has been done with the engineering of viable lymphatic tissues. Recent advances in understanding of lymphatic biology have allowed the easy isolation of pure lymphatic cell cultures, increasing, in turn, the ability to study lymphatic biology in greater detail. This has allowed the elucidation of lymphatic properties on the structural, cellular, and molecular levels, making possible the successful development of the first lymphatic engineered tissues. Among such advances are the engineering of lymphatic capillaries, the development of a functioning bioreactor designed to culture lymph nodes in vitro, and in vivo growth of lymphatic organoids. However, there has been no research on the engineering of functional lymphangions. While the advances made in the study of lymphatic biology are encouraging, the complexities of the system make the engineering of certain functional lymphatic tissues somewhat more difficult.
Keywords: tissue engineering, lymphatics, lymphangions, vascular, endothelium, smooth muscle
Introduction
Major advances have been made in the field of tissue engineering since the topic of lymphatic engineering was taken up by this journal 5 years ago.a The relatively new discipline of tissue engineering has proven successful in using a combination of life sciences and engineering research to produce technologies that allow the assembly of multicellular, complex, and functioning tissues and organs. In the last 5 years, scientists have been able to grow and transplant human bladders, create three-dimensional (3-D) tissues using the techniques of the ink-jet printer, develop transplantable nervous tissue constructs, and advance research in cardiac, cartilage, and bone tissue engineering.1–6 In comparison, the work done in the area of lymphatic tissue engineering to this point has been more sparse. However, in the last 3 years there has been a small, yet important movement toward research geared to the engineering of lymphatic tissues.
As the critical importance of the lymphatic system in the human body becomes increasingly evident, novel research efforts will drive the need for proper models by which lymphatic biology might be studied. Tissue-engineered lymphatic organs (lymph nodes, spleens, lymphatic vessels, and lymphoid derived cells) would not only serve well as ex vivo research models, but also have the potential to be used via implantation for the alleviation of lymphatic deficiencies caused by disease or injury.
Our lab currently has been successful in the engineering of mammalian blood vessels made from cells isolated from the cardiovascular system.7 Given the similarities between the vessels in the cardiovascular and lymphatic systems, it may be useful to apply arterial tissue engineering techniques to the engineering of lymphatic vessels and organs. In this review we will concentrate on (1) current advances in lymphatic biology research and how such advances may pave the way for the initial phases of lymphatic tissue engineering; (2) differences between lymphatic and vascular vessel biology and their effects on tissue engineering strategies; and (3) recent efforts in lymphatic tissue engineering and their implications and prospects.
Relevant Advances in Lymphatic Biology
The lymphatic and blood vascular systems are interconnected—extracellular fluid flows from vascular capillaries into lymphatic microvessels and is returned to the vascular system via the thoracic duct. However, while much is known about the biology of the blood vascular system, the biology behind the development and regulation of the lymphatic system remains comparatively poorly understood.8 Until recently, the number of molecular tools that allowed us to distinguish between blood and lymphatic vessels and cells within tissues was limited. However, in recent years researchers have been able to distinguish quite definitively between the tissues of the two systems at the molecular level.9 This ability to discriminate between cell types allows for the easy isolation of pure lymphatic cell cultures, and in turn the ability to study lymphatic biology in greater detail.8–10
Blood Vessels and Lymphatic Vessels: Comparison and Contrast
A macroscopic look at the vessels of the cardiovascular and lymphatic systems makes it obvious that there are structural and organizational similarities. This is in part due to the connection between the function of the two systems. Both systems contain large networks of vessels, the vessels in both systems are composed of smooth muscle walls lined with a monolayer of endothelium, and they both range in complexity from larger multi-layered vessels to capillaries composed mostly of endothelium. However, the differences between the two also correlate with their function and are evident at the structural, cellular, and molecular levels. Such similarities and differences make an impact as to how closely the tissue engineering strategies for each system should correlate.
Structure
Current engineering of cardiovascular tissues have yielded vessels that are suitable for arterial implantation and flow.10,11 The structure of such vessels is fairly straightforward, comprising multiple layers of smooth muscle cells and interlaced extracellular matrix, lined with a monolayer of endothelium. Unlike arteries, the vessels of the venous portion of the cardiovascular system require much less mechanical strength, and many veins incorporate a valve system to prevent the backflow of blood. Lymph vessels (or lymphangions) also require less wall strength than arteries do, and utilize a two-leaflet valve system to prevent the backflow of lymph, making the structural aspects of lymph vessels more similar to venous than to arterial blood vessels. Incorporation of valvular structures into the scaffolding for engineered lymphangions increases the complexity of these structures. However, the lower mechanical requirements for function of venous and lymphangitic structures may make these “easier” to engineer in some respects (Fig. 1).
FIGURE 1.
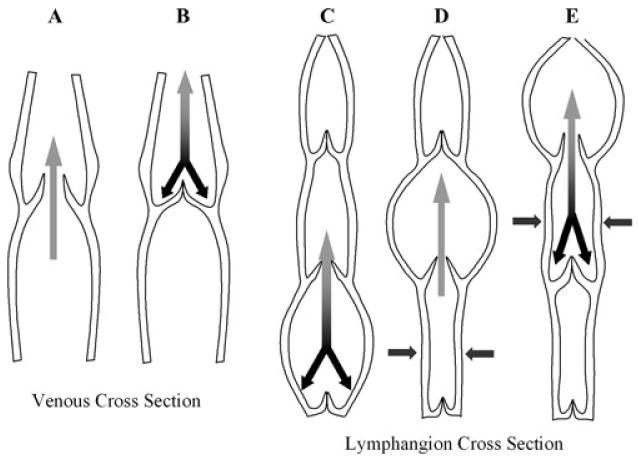
Cross sections of veins and lymphangions.
Cells
The cells that make up the vessels of the lymphatic and cardiovascular system, too, are quite similar. Noting such similarities is very important when considering cell sourcing for tissue engineering strategies. While the origin of the smooth muscle cells that make up the cardiovascular and lymphatic systems is not completely known, it is thought that smooth muscle is recruited by the endothelium that initially constitutes each system.12 Smooth muscle cells, along with the extracellular matrices that they produce, provide the mechanical strength of the vessels. However, the function of the smooth muscle cells between the two systems differs in their contractile properties. While vascular smooth muscle cells contract in a radial fashion to either increase or decrease the lumen size of the vessel, lymphatic smooth muscle contracts in a rhythmic and unidirectional manner in order to propel lymph toward the thoracic duct.12,13 It is now thought that native smooth muscle tissues contain specialized pacemaker cells that serve to regulate any spontaneous electrical activity, and that it is these cells that provide the distinct contractile properties of the tissues and not any particular differences in the smooth muscle itself.14 This implies that current strategies to obtain smooth muscle by the selective culturing of mesenchymal stem cells derived from the peripheral blood or bone marrow may be useful for both systems. However, pacemaker cells would have to be either isolated and cultured, or recruited from surrounding tissues upon engineered graft implantation, adding another consideration to the composition of a working lymphangion.
Developmentally, the lymphatic endothelium (or lymphatic endothelial cells, LECs) originates from a subpopulation of embryonic venous endothelium (or blood endothelial cells, BECs) which expresses the lymphatic-specific homeobox transcriptional factor, prox1.15 LECs bud off from the venous endothelial populations, and commit to lymphatic lineages. It is debated whether LECs originate directly from the existing venous endothelium or from lymphangioblast precursor cells, which along with angioblasts (vascular endothelial precursors) are derived from mesodermal somites.15,16 Either way, BECs and LECs are relatively close in origin, function, and structure. When considering cell source for use in tissue engineering, mixtures of BECs and LECs are readily available and easily obtained from harvest of excess dermal tissue.17 Although the cell types are similar, it is unlikely that they are completely interchangeable on account of the dissimilar sets of genes expressed by each.18
Molecular Makeup
Gene array studies have delineated about 300 genes that are differentially expressed between BECs and LECs.18 Among the genes that are differentially expressed are the known LEC-specific genes (VEGFR-3, LYVE-1, podoplanin, β-chemokine receptor D6, and prox1) as well as an array of proinflammatory cytokines, chemokines and their receptors, cadherins and integrins.19–23 Interestingly enough, induced over-expression of the PROX1 gene in BECs allows for the upregulation of many of the LEC-specific genes and suppresses expression of ∼40% of BEC-specific genes.18 This suggests that BECs may be a default lineage, with prox1 inducing lymphangiogenesis of the cells during development. Such insights may allow researchers to use endothelium obtained from less invasive means (i.e., peripheral blood–derived vascular endothelial progenitor cells) along with simple gene therapies in order to supply LEC-like cells for tissue engineering purposes.
Progress of Lymphatic Tissue Engineering
Within the last 5 years, some progress has been made in the area of lymphatic tissue engineering. While the research is still somewhat sparse, it is at the same time relatively diverse and promising. Of the lymphoid tissues, research has been published on the engineering of lymphatic capillaries, lymph nodes, and nonspecific secondary lymphoid tissue-like organoids. Interestingly enough, absent from the list is any research into the engineering of lymphangions.
Lymphatic Capillaries
In the engineering of 3-D tissues, one of the greatest challenges is getting nutrition to all of the cells within a complex structure. For this reason, in correlation with the engineering of larger, more complex tissues, much work is being done in the area of tissue engineering microvasculature. With the ability to reliably differentiate between vascular and lymphatic endothelial cells, some labs are including LECs in their microvasculature research.24,25 This has led to the development of lymphatic endothelium–lined microstructured extracellular matrices and has allowed investigators to study the function of the lymphatic endothelium in a 3-D in vitro system, as opposed to typical 2-D flow systems. In such systems, the endothelium of both the lymphatic and cardiovascular systems can be studied and compared in relation to their response to different stresses and conditions. It is essential to note that research on the microvasculature of lymphatic and blood capillaries should differ in that their functions differ (i.e., exchange of gasses and nutrients with continual flow versus uptake of excessive interstitial fluid).
Researchers have shown that extracellular matrix composition differentially influences the organization of lymphatic and vascular endothelium, with lymphatic endothelium showing the most extensive organization in fibrin-only matrix and vascular endothelium preferring a matrix containing collagen.24 It is also observed that the types of organizational structures produced are distinct between the two types of endothelium. Lymphatic endothelium tends to produce slender, overlapping networks with fine lumina, while vascular endothelium produces thick, branched networks containing wide lumina.24 Such differences may be observed in vivo in the several days' time lapse that occurs between angiogenesis and lymphangiogenesis during dermal wound healing.26 It is hypothesized that the change in matrix that occurs during the process of wound-healing produces the optimal environment for the transition from angiogenesis to lymphangiogenesis.
Other research has studied the effects of interstitial and laminar flow on lymphatic endothelium in both 3-D and 2-D environments, respectively.27 Here we observe that under interstitial flow, lymphatic endothelium tends to form large vacuoles and long extensions. This is in contrast to the multicellular branched lumen-containing networks formed by vascular endothelium under the same conditions. Under interstitial flow, lymphatic endothelium in a 2-D confluent monolayer tends to decrease its cell–cell adhesions in comparison to vascular endothelium. These observations make sense, as they agree with the native function of lymphatic capillaries which provide for looser junctions to allow for the uptake of excess interstitial fluids. This shows that it is not only the matrix that may influence LEC development during tissue culture, but also the mechanical stress that is imparted on the cells. Such observations may further elucidate differences between vascular and lymphatic endothelium in vivo, and address key concerns as to what specific cell source, matrix, and environmental stresses are required for lymphatic endothelialization in engineered tissues and creation of lymphatic capillary networks.
Human Lymph Nodes In Vitro
Secondary lymphoid organs are tissues that allow for the development of the immune system within the mammalian body. Some success has been obtained in the development of a bioreactor made for the engineering of a human lymph node in vitro.28 This system was developed to culture human dendritic cells generated from peripheral blood mononuclear cells using an 11-day protocol involving the timed addition and removal of cytokines and growth factors (GM-CSF, IL-4, and TNF-alpha). Such cells were tested for maturation by selecting for a panel of CD-markers and for phagocytic properties.
A small, disposable bioreactor was fashioned that allowed perfusion of cells and medium from a 4-mL outer culture space into a 1-mL central culture space (Fig. 2). The central culture space contained a matrix composed of sheets of agarose and nonwoven polyamide fibers, which were chosen on the basis of their flexibility, cell adhesion properties, porosity, and ease of manipulation. Primed dendritic cells were placed onto two sheets of matrix stabilized by a macro-porous membrane and enclosed in the central space, which is supported by a set of microporous hollow fibers that allow medium and gas supply. The porosity of the system would allow any suspended cells within the outer space to freely pass into the central space and have contact with any matrix-bound cells, as well as allow continual medium exchange. After 2 weeks of bioreactor culture, both the nonwoven polyamide sheets and agarose matrix sheets showed evidence of sustainable lymphocyte clusters containing antigen-specific leukocytes. Responses of the cells to IL-2 and LPS showed evidence of early T cell activation and long-term viability.
FIGURE 2.

Bioreactor incorporates two external fluidic systems, O-ring sealed caps, and a sample reservoir.28 Used with permission from ProBioGen AG.
This bioreactor system allowed for the construction of an environment similar to that of the lymph node, leading to T cell and B cell swarming and T cell clustering. This type of system could be useful for mimicking the effects of drugs and cell therapies within physiologic environments. However, it is not evident that such a system is useful for the construction of implantable tissues.
There has also been success in the area of growing lymphoid tissues in vivo. Transplantation of biocompatible “sponge-like” collagenous scaffolds that are seeded with thymus stromal cells (TEL-2-LTα) into mice have yielded “lymphoid tissue-like organoids” that have similar structure to secondary lymphoid organs and are able to produce antibodies in the host.29 Addition of activated dendritic cells to the stromal cell–containing matrix resulted in the promotion of T cell and B cell cluster formation more than two times as much as without dendritic cells. However, in these experiments it was essential that the TEL-2-LTα stromal cells be present, suggesting that they play a major role in cluster formation. Another intriguing point is that sections of explanted organoids showed evidence of blood vessel and high endothelial venule development, which are typical of secondary lymphoid tissues.
Further research with these “artificial lymph nodes” has yielded promising results in immunodeficient SCID mice. It has been found that implantation of the artificial lymph nodes in the SCID mice led to strong antigen-specific antibody responses, migration of lymphoid cells to the spleen and bone marrow, and maintenance of secondary immune responses over time.30 Such technology could lead to the successful treatment of immunodeficiency diseases in the future, and allow for the synthesis of engineered lymphoid tissues that can be used for both in vitro therapeutic analysis as well as in vivo implantation.
Prospects for Lymphatic Tissue Engineering
While the advances made in the study of lymphatic biology are encouraging, the complexities of the system that have been elucidated make the engineering of certain functional lymphatic tissues somewhat more daunting than previously thought. Specifically, such difficulties are apparent in the engineering of functional lymphangions, which may affect how we translate techniques used in engineering blood vessels to engineering lymphatic vessels. Contrary to what has been proposed in the past, it may not be sufficient to simply design a conduit for the passive movement of lymph. A lymphatic vessel must be functional: the vessel wall must be able to respond to lymph flow, leakage of lymph backwards in the system must be prevented, and there must be active contraction to move lymph forward. While the mechanical requirements that must be met for cardiovascular vessel engineering are much higher, the anatomic design of an arterial blood vessel seems comparatively simpler. Such differences may be one reason why current research in lymphatic tissue engineering has not yet broached the engineering of lymphangions.
When we look at the progress made in the engineering of secondary lymphoid tissues and the prospects of furthering such research, it is easy to become optimistic. Such findings may lead the way to therapies aimed at diseases, such as HIV and leukemia. As with all fields of tissue engineering, there is a long way to go before engineered lymphatic tissues can be implemented as a clinical regimen. However, looking at the progress made in the last several years, we see that it is more a question of timing than plausibility.
Acknowledgments
Work supported by ROI HL076485 and RZI HL081560 (both LEN).
Footnotes
See The Lymphatic Continuum, Volume 979 of the Annals of the New York Academy of Sciences (2002), edited by Stanley G. Rockson and Reparative Medicine: Growing Tissues and Organs, volume 961 of the Annals of the New York Academy of Sciences
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Atala A, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Mironov V, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 3.Pfister BJ, et al. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J Neurosci Meth. 2006;153:95–103. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Risbud MV, Sittinger M. Tissue engineering: advances in in vitro cartilage generation. Trends Biotechnol. 2002;20:351–356. doi: 10.1016/s0167-7799(02)02016-4. [DOI] [PubMed] [Google Scholar]
- 5.Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148–159. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 6.Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Curr Opin Biotechnol. 2004;15:430–434. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Poh M, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 9.Ezaki T, et al. Production of two novel monoclonal antibodies that distinguish mouse lymphatic and block vascular endothelial cells. Anat Embryol. 2006;211:379–393. doi: 10.1007/s00429-006-0091-3. [DOI] [PubMed] [Google Scholar]
- 10.Niklason LE, et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 11.Niklason LE, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 12.von der Weid PY, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Gashev AA. Physiologic aspects of lymphatic contractile function. Ann NY Acad Sci. 2002;979:178–187. doi: 10.1111/j.1749-6632.2002.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 14.McHale M, et al. Origin of spontaneous rythmicity in smooth muscle. J Physiol. 2006;570:23–28. doi: 10.1113/jphysiol.2005.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kono T, et al. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler Thromb Vasc Biol. 2006;26:2070–2076. doi: 10.1161/01.ATV.0000225770.57219.b0. [DOI] [PubMed] [Google Scholar]
- 16.Wilting J, Becker J. Two endothelial cell lines derived from the somite. Anat Embryol. 2006;211:S57–S63. doi: 10.1007/s00429-006-0120-2. [DOI] [PubMed] [Google Scholar]
- 17.Hirakawa S, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrova TV, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the PRox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale NW, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman J, et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1–10. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 21.Nibbs RJB, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schacht V, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigle JT, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helm ELE, Zisch A, Swartz MA. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol Bioeng. 2006;96:167–176. doi: 10.1002/bit.21185. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CM, Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518–523. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291:H1402–H1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng CP, Helm CE, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Giese C, et al. A human lymph node in vitro: challenges and progress. Artif Organs. 2006;30:803–808. doi: 10.1111/j.1525-1594.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 29.Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol. 2004;22:1539–1545. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto M, et al. Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. J Clin Invest. 2007;117:997–1007. doi: 10.1172/JCI30379. [DOI] [PMC free article] [PubMed] [Google Scholar]


