Abstract
Coniosporium epidermidis sp. nov. is described from a superficial skin lesion with blackish discolouration in an 80-yr-old Chinese patient. The species produces dark, thick-walled, inflated, reluctantly liberating arthroconidia without longitudinal septa. Sequences of the ribosomal operon, as well as of the translation elongation factor 1-α support its novelty. The species is found in a lineage basal to the order Chaetothyriales, amidst relatives from rock, but also species repeatedly isolated from human skin and nails and eventually causing mild cutaneous infections. Coniosporium epidermidis is consistently found on humans, either asymptomatic or symptomatic. The species indicates a change of life style towards human pathogenicity, which is a recurrent type of ecology in derived Chaetothyriales. Superficial and cutaneous infection by melanized fungi is a new category in dermatology.
Keywords: Black yeasts, Coniosporium, superficial mycosis, taxonomy
INTRODUCTION
In recent years the clinical significance of melanized fungi involved in cutaneous infections has been underlined (Badali et al. 2008a). Several of the species concerned, although causing relatively mild infections, are regularly encountered in dermatological specimens, but usually discarded as purported contaminants. Some species, such as Phialophora europaea de Hoog et al. and Cyphellophora laciniata de Vries, however, are recurrently observed on humans, and their environmental niches thus far have remained unknown (de Hoog et al. 2000). We here report on a species that originated from the skin of an 80-yr-old male patient who manifested with a 3 yrs history of black bilateral maculae on his feet, with scales, maceration, and fissures. The infection was caused by a Coniosporium-like fungus that could not be identified with any of the known species and is therefore introduced here as a new taxon.
The genus Coniosporum is considered to comprise environmental fungi forming black spots or patches on plant leaves, bamboo surface, rotten wood, and recently particularly on rock surfaces (Hyde et al. 2002, De Leo et al. 1999, Sterflinger et al. 1997, 2001). Species have black, velvety colonies on the natural substrate, and are characterized microscopically by thick-walled, heavily pigmented arthroconidia with subsequent meristematic development. This report concerns the first human infection caused by a Coniosporium species. Coniosporium is not among the recognized human pathogens in dermatology. Several melanized fungi have been reported cause mild cutaneous infections, e.g. Cyphellophora laciniata de Vries (1962), Phialophora europaea de Hoog et al. (2000b), and Cladophialophora saturnica Badali et al. (2009). Such fungi are encountered fairly regularly in samples from human skin and nail (de Hoog et al. 2000a). A new dermatological category may be concerned, which will be introduced in this paper.
MATERIALS AND METHODS
Isolation
Clinical specimens were scraped with a scalpel from superficially sterilized blackish skin lesions. A skin biopsy was performed on the black lesion and histological specimens were stained with hematoxylin-eosin. Samples of skin flakes were plated on Sabouraud's glucose agar (SGA) with chloramphenicol and incubated at 27 °C. Strain T22 (= CBS 120353) was isolated from specimens of the first visit of the patient. Another isolate was recovered one year later from the same patient and turned out to be identical by sequence data. Studied strains of the same species included for comparison were isolates encountered during analysis of routine dermatological specimens from Denmark, and an isolate from ant garbage from Brazil. Related strains studied are listed in Table 1.
Table 1.
Isolation data of examined strains.
| Name | Accession no. | Country | Source | GenBank |
|---|---|---|---|---|
| Antarctic black fungus | CCFEE 5323 | Antarctic | Thallus of Lecanora sp. | FJ392866 |
| CCFEE 5314 | Antarctic | Thallus of Xanthoria elegans | FJ392865 | |
| CCFEE 5324 | Antarctic | Thallus of Acarospora flavocordia | FJ392867 | |
| Coniosporium apollinis | CBS 100213 | Greece | Rock | AJ244271 |
| CBS 100218 | Greece | Marble | AJ244273 | |
| CBS 109867 | Greece | Marble | ||
| CBS 100216 | Spain | Rock | AJ244272 | |
| CBS 109860 | Spain | Rock | ||
| CBS 109865 | Italy | Rock | ||
| Coniosporium epidermidis | CBS 123233 | Denmark | Hand, female | |
| dH 17028 | Denmark | Axilla, male | ||
| CBS 123466 | China | Nail with onychomycosis | ||
| CBS 120353 (T) | China | Skin infection | EU730589 | |
| CBS 120388 | Denmark | Toenail, female | ||
| CBS 123279 | Denmark | Toenail, female | ||
| dH 17006 | Denmark | Toenail, female | ||
| CBS 123261 | Denmark | Toenail, male | ||
| dH 17086 | Denmark | Toenail, male | ||
| Coniosporium perforans | dH 17016 | Denmark | Axilla, female | |
| CBS 109861 | Italy | Marble | ||
| dH 16682 | Denmark | Nail, male | ||
| CBS 885.95 (T) | Greece | Marble | ||
| Coniosporium species | CBS119726 | Italy | Stone monument | |
| dH 14071 | Australia | Cattle | ||
| dH 16979 | France | Chronic nasal oedema | ||
| dH 14084 | Italy | Marble monument | ||
| dH 14085 | Italy | Rock monument | ||
| CBS 109864 | Italy | Rock | ||
| CBS 109866 | Italy | Rock | ||
| CBS 665.80 | Italy | Rock | ||
| Cryptoendolithic fungus | CCFEE 457 | Antarctic | University Valley | |
| Exophiala placitae | CPC 13707 | Australia | Eucalyptus placita | EU040215.1 |
| Exophiala species | CPC 12172 | Canada | Prunus sp. | |
| CPC 12173 | Canada | Prunus sp. | ||
| CPC 12171 | Canada | Prunus sp. | EU035420 | |
| Meristematic fungus | CBS119729 | Italy | Stone | |
| Phaeococcomyces catenatus | Det M175 | Netherlands | Nail | |
| CBS 650.76 (T) | Switzerland | Air | AF050277 | |
| dH 11392 | Unknown | |||
| dH 14721 | Austria | Bloodplasma | ||
| TRN4 | Spain | Rock surface | AY843222 | |
| Phialophora europaea | CBS 656.82 | France | Nail | FJ489612 |
| dH 12320 | Germany | Nail | ||
| CBS 101466 (T) | Netherlands | Skin scales | ||
| Sarcinomyces petricola | CBS 726.96 (T) | Guinea | Dung of cow | FJ489613 |
| CBS 600.93 | Greece | Pentelic marble | ||
| CDC 2008006858 | USA | Scalp rash, male | ||
| Uncultured ascomycete | AM901753 | Finland | Indoor dust | AM901753 |
Abbreviations used: CBS = CBS Fungal Biodiversity Centre, Utrecht, The Netherlands; CCFEE = Culture Collection of Fungi from Extreme Environments, Viterbo, Italy; CDC = Centers for Disease Control and Prevention, Atlanta, U.S.A.; CPC = culture collection of Pedro Crous, housed at CBS; dH = G.S. de Hoog working collection; TRN = Tino Ruibal working collection.
T = ex-type culture.
Morphology
Strains were transferred to malt extract agar (MEA), potato dextrose agar (PDA), cormeal agar (CMA), oatmeal agar (OA) and Czapek agar (CZA) and incubated at 25 °C and 37 °C for at least 4 wk under alternate near-ultraviolet light for growth rate determination and phenetic description of colonies. For study of microscopic morphology strains were point-inoculated on PDA. Blocks of agar of approximately 1 × 1 cm were excised aseptically on sterile microscope slides. Blocks were inoculated, covered with sterile cover slips and incubated in moist chambers for 14 d at 27 °C. Structure and branching pattern of conidiophores were observed at magnifications ×100, ×200 and ×400 in intact slide cultures under the microscope without removing the cover slips from the agar blocks. For higher magnifications, cover slips were removed and mounted in lactic acid with aniline blue.
Sequencing
Approximately 0.1 g of fungal material was transferred to a 2-mL Eppendorf tube containing a 2:1 (w/w) mixture of silica gel and Celite (silica gel H, Merck 7736/Kieselguhr, Celite 545, Machery, Merck, Amsterdam, The Netherlands); DNA was extracted according to methods described previously (Li et al. 2008). Amplifications were done with primers ITS1 and ITS4 (for rDNA Internal Transcribed Spacer ITS), NS1, BF83, OLI1, BF963, BF1438 and NS24 (for rDNA Small Subunit nucSSU), D1/D2 (for rDNA Large SubUnit nucLSU), and EF1-728F and EF1-986R (for Translation Elongation Factor 1-α EF1α). PCR was performed in 50 μL volumes of a reaction mixture containing 10 mM Tris HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2·6H2O, 0.01 % gelatin, 200 mM of each deoxynucleotide triphosphate, 25 pmol of each primer, 10–100 ng rDNA, and 0.5 U Taq DNA polymerase (Bioline, GC Biotech, Alphen a/d Rijn, The Netherlands), as follows: 95 °C for 4 min, followed by 35 cycles consisting of 94 °C for 45 s, 52 °C for 30 s, and 72 °C for 2 min. Amplicons were cleaned with GFX columns (GE Healthcare, Sweden). Sequence PCR was performed as follows: 95 °C for one min, followed by 30 cycles consisting of 95 °C for 10 s, 50 °C for five s, and 60 °C for two min. DNA was purified with Sephadex G-50 Superfine. Purified amplicons were then sequenced on both strands using the same primers described above. BigDye terminator cycle sequencing Ready Reaction kits (Perkin Elmer Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands) were used according to the manufacturer's instructions and DNA was sequenced using a DYE-ET terminator.
Sequence analysis and taxonomy
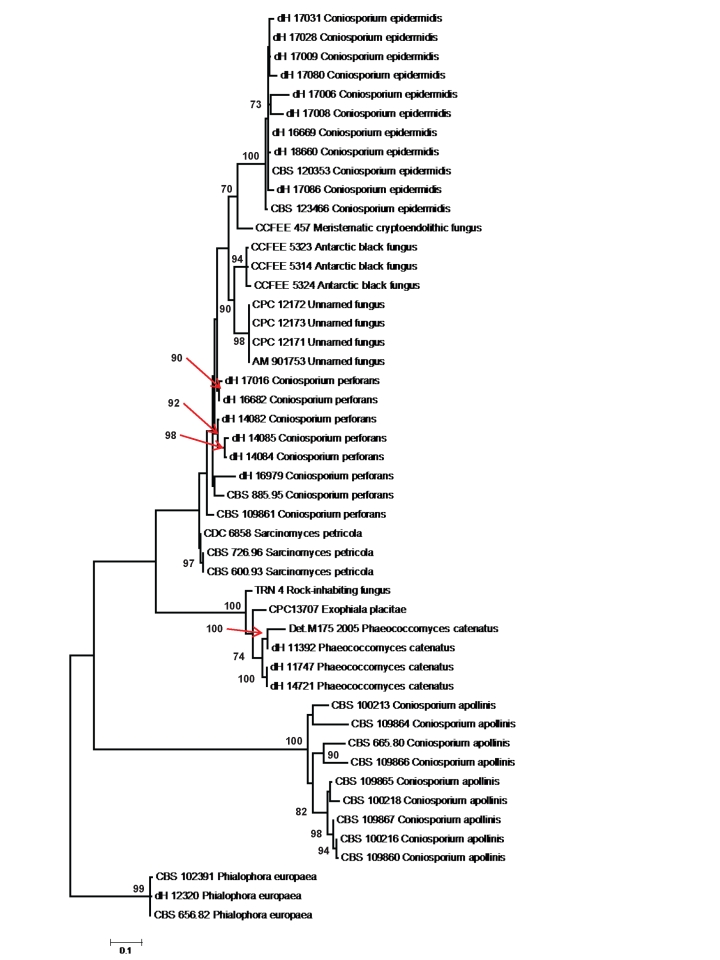
Sequences were compared in GenBank and using a research database available at the Centraalbureau voor Schimmelcultures Biodiversity Centre (CBS), Utrecht, The Netherlands. Alignment was done in a database using BioNumerics software v. 4.61 (Applied Maths, Kortrijk, Belgium). SSU sequences were aligned with the ARB beta-package (v. 22-08-2003) developed by Ludwig et al. (2004). A distance tree of Coniosporium epidermidis and allied black fungi based on the completed ITS 1-2 domain including the 5.8S rDNA gene were reconstructed using neighbor-joining algorithm with Kimura 2 correction with 100 bootstrap replications in Treefinder.
RESULTS
Mycology
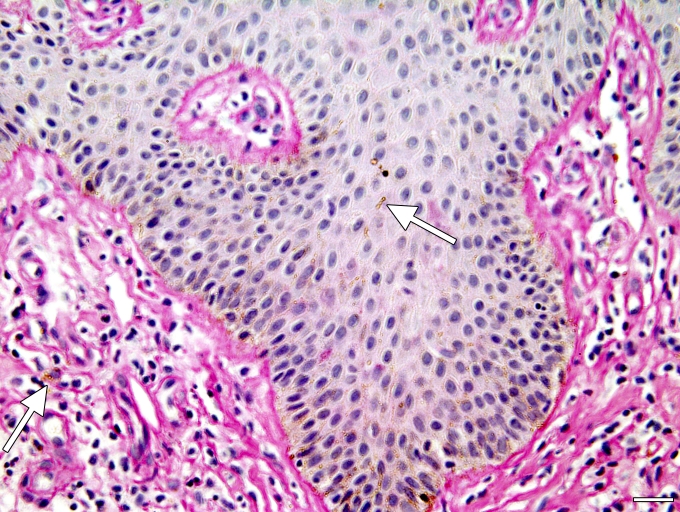
A skin biopsy performed on the black lesion (Fig. 1) and histological specimens stained with hematoxylin-eosin (Fig. 2) showed hyperkeratosis and acanthosis. Numerous hyphae and swollen cells were observed in the stratum corneum (Fig. 2). Pigmented hyphae and loose cells were displayed in the entire layers of epidermis, predominated among the low layers. Cells also penetrated the basal membrane to the dermis. Direct examination of skin scrapings with KOH was positive for pigmented arthroconidia and dark-walled hyphae. The disease was considered to be an infection.
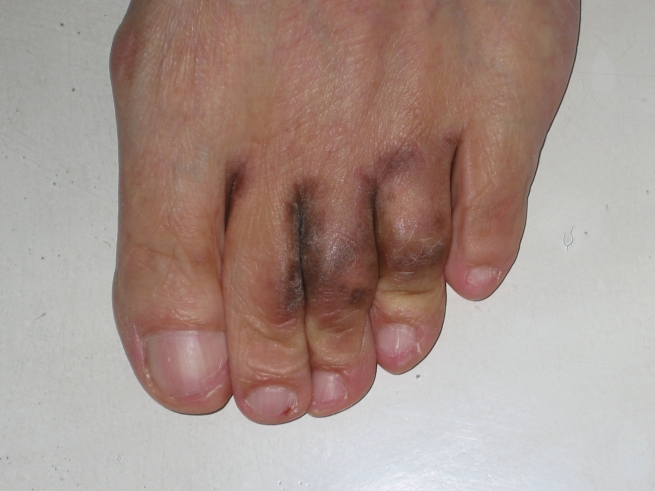
Fig. 1.
Blackish discoloured skin of toes and toe webs with scaling.
Fig. 2.
Skin biopsy stained with HAE; some fungal elements are visible (arrows). Size bar = 5μm.
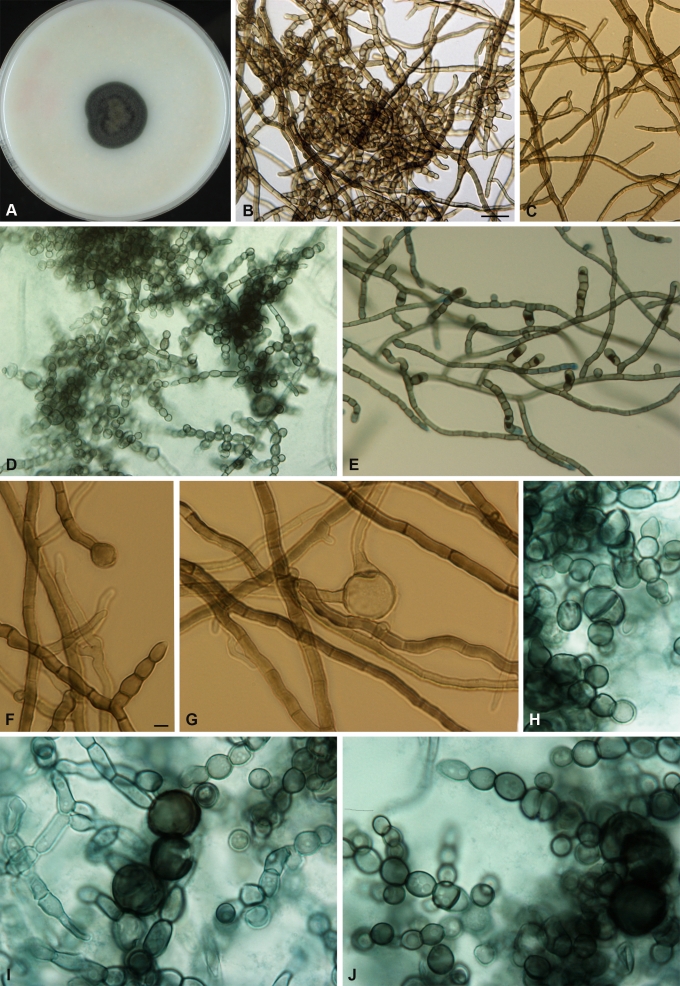
At primary isolation, isolate CBS 120353 grew slowly with 8 mm/wk. Colonies on SGA were convex with papillate surface. Obverse and reverse were black, while colonies on MEA, OA, CMA, CZA and PDA were velvety with dark brown to olive obverse. Growth was stimulated under near-UV light; 37 °C was tolerated. Subcultures initially were cream-coloured, smooth and turned black within a wk; this phenomenon disappeared after several transfers. Hyphae were septate, olivaceous-black, forming reluctantly disarticulating arthroconidia with transverse but without longitudinal septa (Fig. 3). Cells were pigmented, thick-walled, and maturated meristematically, the mother cell wall frequently rupturing in an irregular fashion. With time, monilioid conidia (Fig. 3h, i) were predominant and occasional chlamydospores occurred.
Fig. 3.
Coniosporium epidermidis, CBS 120353. A. Colony on OA (3 wks). B, C. Elongated and monilioid hyphae, anthroconidia; D, E. Conidial chains; F–J. Mature conidial chains and large conidia with transverse septa. Scale bars 5 μm (B–E); 1 μm (F–J).
Molecular data
1743 bp of the rDNA SSU gene were sequenced of strain CBS 120353 (data not shown). Phylogenetic analysis of aligned sequences revealed close relationship with species in Cladophialophora, Exophiala, Phialophora, Rhinocladiella, Fonsecaea and Capronia, which all are members of the order Chaetothyriales. However, nearest neighbours were Coniosporium perforans Sterflinger (CBS 665.80), and C. apollinis Sterflinger (CBS 352.97). Of the LSU domain, 616 bp were sequenced. Nearest neighbour at 97 % similarity was a species published by Crous et al. (2007) in a tree as `Exophiala sp. 3', CPC 12173 = EU035422.
Length of ITS domain of CBS 120353 was 541 bp. ITS rDNA sequences compared in a dedicated black yeast data base maintained at CBS and containing about 11,000 entries revealed no close match with any known species. Alignment was only partially confident. Among the nearest neighbours were the known rock-inhabiting species Coniosporium perforans and C. apollinis, as well as a number of undescribed rock-inhabiting species prevalently from the Mediterranean and from the Antarctic (Table 1). `Exophiala sp. 3' of Crous et al. (2007) was also close; the ITS of this species proved to be identical to a hyperparasitic `Coniosporium sp.', AM901753 published by Harutyunyan et al. (2008). In addition, strains from human skin and nail samples were involved (Table 1). Identity was found with a group of strains from dermatological skin and nail samples from Denmark, as well as with an environmental sample from Brazil; all these strains either were morphologically identical to CBS 120353, or consisted of sterile melanized hyphae. These strains were therefore regarded to represent a hitherto undescribed taxon, which is introduced below.
Coniosporium epidermidis D.M. Li, de Hoog, Saunte & X.R. Chen, sp. nov. – MycoBank MB512506, Figs 3, 5.
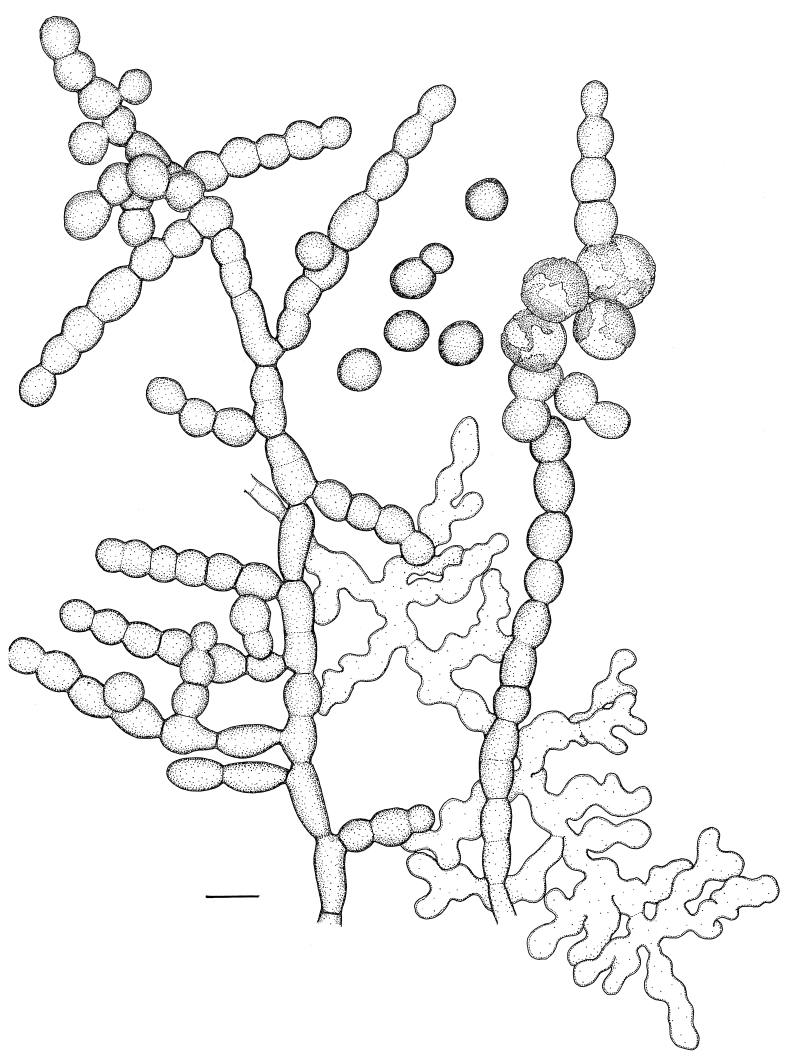
Fig. 5.
Microscopic morphology of Coniosporium epidermidis, CBS 120353; slide culture on SGA, 28 d.
Coloniae primum fuscae, effusae, deinde elevatae, velutinae vel cottoneae, griseo-olivaceae; reversum olivaceo-nigrum. Coloniae in agaroso maltoso, agaro PDA, vel agaro farina avenae confecto (OSD) dicto 25 °C 10–15 mm diam post 28 dies. Mycelium immersum vel superficiale, ex hyphis ramosis, septatis compositum, olivaceo-nigrum vel brunneum. Conidiophora vix distinguenda, ramose vel simplicia, terminalia vel intercalaria. Conidia singula, primaria in apice conidiophori, ellipsoidea vel subglobosa, levia, 2-3 μm diam. Mycelium torulosum praesens. Teleomorphosis ignota.
Holotype: dried culture in CBS herbarium (CBS-H-20167); ex-type strain CBS 120353 = T22, isolated from nigramacula, superficial infection of the feet of a 80-yr-old male patient, China, D.M. Li. Additional strains listed in Table 1.
The following description is of CBS 120353 on PDA after 28 ds incubation at 25 °C.
Colonies effuse, becoming raised, attaining 10-15 mm diam, velvety to fluffy, black, blackish-brown to greyish olivaceous; reverse olivaceous-black. Mycelium superficial, regularly and densely septate, profusely branched at nearly right angles, olivaceous-black or dark brown, smooth-, or occasionally rough-walled. Cells gradually swelling at maturation up to 3-8 μm diam; cell walls very thick at maturity. Conidia formed by liberation of arthric cells, swelling to become ellipsoidal or nearly spherical, smooth-walled, mostly 2-3 μm diam, up to 8 μm wide, then frequently the mother cell wall remaining visible on the daughter cell. Truly muriform cells absent. Teleomorph unknown. Cardinal temperatures: optimum 27 °C, maximum 37 °C.
DISCUSSION
Coniosporium classically includes black, slow-growing anamorphs, most of which are plant colonizers (Hyde et al. 2002). Species are characterized by pigmented anthroconidia, and tend to develop meristematically into chains (Ellis 1971). The mother cell wall may eventually rupture during development, leading to irregular cell wall ornamentation (Ellis 1976).
Coniosporium epidermidis was isolated from a tinea nigra-like skin infection on the foot. Tinea nigra, characterized by brown to black superficial macules, is a strictly asymptomatic colonization of dead epidermis (Schwartz 2004, Bonifaz et al. 2008) caused by a halophilic member of the order Capnodiales, Hortaea werneckii (Zalar et al. 1999). In contrast, pathological slides of C. epidermidis clearly showed that the fungus grew into all layers of the epidermis, and some cells penetrated down to the basal membrane reaching the superficial dermis. Thus the present species causes a real disease process, as was also observed with the tinea nigra-like infection caused by Cladophialophora saturnica Badali et al. (2008a), another member of Chaetothyriales. The optimal growth temperature of C. epidermidis at 27 °C (maximum 37 °C) is comparable to that of true pathogens of the skin, the dermatophytes, ranging between 25 and 35 °C (Weitzman & Summerbell 1999). We thus conclude that ordinal relationships predict opportunistic potential. In the course of the present study Coniosporium epidermidis was repeatedly isolated from routine dermatological samples in Denmark. It is supposed that the fungus may be common in cutaneous samples, but is generally discarded as a contaminant. We therefore recommend to pay more attention to melanized fungi occurring on skin and nails, and to establish their precise role in pathology.
With SSU rDNA, the black fungus recovered from affected human skin appeared to be an undescribed species, located within a group of species causing mild cutaneous infections, such as Phialophora europaea and Cyphellophora laciniata. The group was basal to the order Chaetothyriales, an order having Capronia teleomorphs and being notorious for containing numerous opportunists (Badali et al. 2008b). In the corresponding ITS tree (Fig. 4) the group showed considerable diversification among species. The similarity of C. epidermidis to the nearest described species, Coniosporium perforans, was less than 91 %. This species is, however, a colonizer of rock and monuments in the Mediterranean Basin (Sterflinger et al. 1997), as is the case in the majority of taxa in this group. The rock-inhabiting species were attributed to the genus Coniosporium, although the generic type species, C. olivaceum Link (Ellis 1971), has as yet not been redefined according to modern standards.
Fig. 4.
Phylogenetic tree of Coniosporium epidermidis and allied black fungi based on the completed ITS 1–2 domain including the 5.8S rDNA gene, generated with the TREEFINDER package using the Neighbor-joining algorithm and Kimura correction. The tree was subjected to 100 bootstrap replications; Phialophora europaea, CBS 656.82 was selected as outgroup.
Sterflinger et al. (1997) supposed that the Coniosporium-clade of Chaetothyriales was entirely rock-associated. However, with recent additions to this group we notice that it contains several undescribed taxonomic species from human skin and nail samples. In addition, many of the species described as having a rock-inhabiting life-style, such as Sarcinomyces petricola Wollenzien & de Hoog, Coniosporium perforans and Phaeococcomyces catenatus (de Hoog & Hermanides-Nijhof) de Hoog, can also be found on human skin (Table 1). A similar dual ecology was found earlier in the unrelated meristematic fungus Catenulostroma abietis (Butin & Pehl) Crous et al. (Butin et al. 1996). We suppose that these predominantly meristematic skin colonizers are taken up from the environment where they live as oligotrophs on rock, leathery plant leaves and other relatively inert surfaces. They are likely to display a similar oligotrophic character on human skin, and thus probably behave as commensals rather than pathogens. Nevertheless occasional mild infections may occur, such as the ones observed in Cladophialophora saturnica (Badali et al. 2008a) and the present fungus.
Acknowledgments
This work was supported partly by grants from the National Natural Science Foundation of China (C30570003), the China Exchange Programme of the Netherlands Academy of Sciences. D. Attili (Rio Claro, Brazil) is acknowledged for donating a strain from an ant hill. We thank Xin Lian for assistance in sequencing.
Taxonomic novelties: Coniosporium epidermidis D.M. Li, de Hoog, Saunte & X.R. Chen, sp. nov.
References
- Badali H, Carvalho VO, Vicente V, Attili-Angelis D, Kwiatkowski IB, Gerrits van den Ende AHG, Hoog GS de (2009). Cladophialophora saturnica sp. nov., a new opportunistic species of Chaetothyriales revealed using molecular data. Medical Mycology 47: 1–12. [DOI] [PubMed] [Google Scholar]
- Badali H, Gueidan C, Najafzadeh MJ, Bonifaz A, Gerrits van den Ende AHG, Hoog GS de (2008b). Biodiversity of the genus Cladophialophora. Studies in Mycology 61: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz A, Badali H, Hoog GS de, Cruz M, Araiza J, Cruz M, Fierro L, Ponce RM (2008). Tinea nigra by Hortaea werneckii, a report of 22 cases from Mexico. Studies in Mycology 61: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin H, Pehl L, Hoog GS de, Wollenzien U (1996). Trimmatostroma abietis sp. nov. (Hyphomycetes) and related species. Antonie van Leeuwenhoek 69: 203–209. [DOI] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ (2007). Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo F, Urzi C, Hoog GS de (1999). Two Coniosporium species from rock surfaces Studies in Mycology 43: 70–79. [Google Scholar]
- Ellis MB (1971). Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, UK.
- Ellis MB (1976). More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, UK.
- Harutyunyan S, Muggia L, Grube M (2008). Black fungi in lichens from seasonally arid habitats. Studies in Mycology 61 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000a). Atlas of Clinical Fungi, ed. 2. Centraalbureau voor Schimmelcultures / Universitat Rovira i Virgili, Utrecht / Reus.
- Hoog GS de, Mayser P, Haase G, Horré R, Horrevorts AM (2000b). A new species, Phialophora europaea, causing superficial infections in humans. Mycoses 43: 409–416. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Zeng JS, Harrak MJ, Sutton DA (2006). Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie van Leeuwenhoek 90: 257–268. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Zhou DQ, Dalisay T (2002). Bambusicolous fungi: A review. Fungal Diversity 9: 1–14. [Google Scholar]
- Li DM, Xiu DR, Li RY, Samson RA, Hoog GS de, Wang DL (2008). Aspergillus flavus myositis in a patient after liver transplantation. Clinical Transplant 22: 508–511. [DOI] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. (2004). ARB: a software environment for sequence data. Nucleic Acids Research 32: 1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RA (2004). Superficial fungal infections. Lancet 364: 1173–1182. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, De Baere R, Hoog GS de, De Wachter R, Krumbein WE, Haase G (1997). Coniosporium perforans and C. apollinis, two new rock–inhabiting fungi isolated from marble in the Sanctuary of Delos (Cyclades, Greece). Antonie van Leeuwenhoek 72: 349–353. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, Prillinger H (2001). Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria). Antonie van Leeuwenhoek 80: 275–286. [DOI] [PubMed] [Google Scholar]
- Tseng SS, Whittier S, Miller SR, Zalar GL (1999). Bilateral tinea nigra plantaris and tinea nigra plantaris mimicking melanoma. Cutis 64: 265–268. [PubMed] [Google Scholar]
- Vries GS de (1962). Cyphellophora laciniata nov. gen., nov. sp. and Dactylium fusarioides Fragoso et Ciferri. Mycopathologia et Mycologia Applicata 16: 147-54. [Google Scholar]
- Weitzman I, Summerbell RC (1999). The dermatophytes. Clinical Microbiology Reviews 8: 240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar P, Hoog GS de, Gunde-Cimerman N (1999). Ecology of halotolerant dothideaceous black yeasts. Studies in Mycology 43: 38–48. [Google Scholar]