Abstract
The cell division cycle gene (CDC42) controlling cellular polarization was studied in members of Chaetothyriales. Based on ribosomal genes, ancestral members of the order exhibit meristematic growth in view of their colonization of inert surfaces such as rock, whereas in derived members of the order the gene is a putative virulence factor involved in expression of the muriform cell, the invasive phase in human chromoblastomycosis. Specific primers were developed to amplify a portion of the gene of 32 members of the order with known position according to ribosomal phylogeny. Phylogeny of CDC42 proved to be very different. In all members of Chaetohyriales the protein sequence is highly conserved. In most species, distributed all over the phylogenetic tree, introns and 3rd codon positions are also invariant. However, a number of species had paralogues with considerable deviation in non-coding exon positions, and synchronous variation in introns, although non-synonomous variation had remained very limited. In some strains both orthologues and paralogues were present. It is concluded that CDC42 does not show any orthologous evolution, and that its paralogues haves the same function but are structurally relaxed. The variation or absence thereof could not be linked to ecological changes, from rock-inhabiting to pathogenic life style. It is concluded that eventual pathogenicity in Chaetothyriales is not expressed at the DNA level in CDC42 evolution.
Keywords: Cell Division Cycle CDC42, Chaetothyriales, chromoblastomycosis, muriform cell, paralogue evolution, phylogeny, virulence factors
INTRODUCTION
Human infection by agents of chromoblastomycosis is accompanied by dramatic morphogenetic changes of fungal cells in tissue. The fungi concerned have the ability of morphogenetic switching from polarized filamentous growth to isodiametric expansion, leading to large spherical cells (Szaniszlo et al. 1983). Subdivision of the cells gives rise to muriform cells (Matsumoto et al. 1993), the invasive phase of the fungi concerned and triggering hyperphasia characteristic for the disease. Chromoblastomycosis is exclusively known to be caused by members of the ascomycete order Chaetothyriales (Haase et al. 1999, Badali et al. 2008): primarily by Cladophialophora and Fonsecaea species and occasionally by species of Exophiala, Phialophora or Rhinocladiella. The order comprises a large number of pathogens and opportunists on warm- and cold-blooded vertebrates (de Hoog et al. 2000), and hence is likely to have ancestral virulence factors.
The order Chaetothyriales is remarkable in the fungal Kingdom, for two reasons. First, a large number of the infections are observed in individuals without known immune disorder. Of the about 77 species confirmed to belong to the order by sequence data (Barr 1990, Gueidan et al. 2008), about 33 have been encountered as etiologic agents of infections in vertebrates (de Hoog et al. 2000, Zeng et al. 2006, Badali et al. 2008). This high percentage of species with an infective potential is only matched by the order Onygenales containing the dermatophytes and classical systemic fungi. Second, the diversity in clinical pictures caused by members of the Chaetothyriales is bewildering. Species of Onygenales are very consistent in their pathology, displaying a similar clinical course by members causing cutaneous or systemic infections, whereas those of Chaetothyriales encompass a wide diversity of diseases, which are nevertheless more or less characteristic within a single species (de Hoog et al. 2000). This pathogenic potential is particularly observed in the more derived parts of the order, comprising the ascomycete family Herpotrichiellaceae (Untereiner 2000). Recently it was established that members of this same group show `dual ecology', i.e., they also possess the uncommon ability to assimilate monoaromatic pollutants (Prenafeta-Boldú et al. 2006).
A second recurrent trend in the Chaetothyriales is extremotolerance, i.e. growth on exposed surfaces, having a competitive advantage at high temperature and dryness (Sterflinger et al. 1998, Ruibal 2004). Phylogenetic trees published by Lutzoni et al. (2001) and Gueidan et al. (2008) indicate that some deep branches among the pathogenic black yeasts have a shared evolution with rock-inhabiting fungi. A meristematic growth form, morphologically similar to the muriform cells described above, may be expressed under adverse environmental conditions of nutrient depletion, high temperature and dryness. This suggests a functional change in the course of evolution, from an ancestral rock-inhabiting lifestyle to a derived strategy in which ultimately pathogenicity to vertebrate hosts enhances the fitness of species.
Polarized vs. isodiametric growth in fungi is regulated by the Rho-related GTPase Cdc42p (Cdc = Cell Division Cycle), reviewed by Johnson (1999). Cdc42p is essential for the reorganization of the actin cytoskeleton during the shift from polarized to isodiametric growth. Upon activation, Cdc42p is recruited to the plasma membrane to initiate actin nucleation. Localization and activity of Cdc42p are mediated by guanine-nucleotide exchange factors (GEFs). In S. cerevisiae, the only GEF for Cdc42p is Cdc24p, controlled by cell cycle proteins (Cdc28p) and additional proteins (Cla4p and Bemp1; Bose et al. 2001). The eleven current members of the CDC42 family display between 75 and 100 % amino acid identity and are functionally as well as structurally homologous. In filamentous fungi another member of this family is present, RacAp. This is a well studied GTP-binding protein in mammalian cells and it has been shown that RacAp is required for the formation of lamellipodia in fibroblast cells. Recently, RacAp was found in basidiomycetes (Gorfer et al. 2001) as well as in ascomycetes (Hurtado et al. 2000, Boyce et al. 2001, Virag et al. 2007), but RacAp orthologus are absent in S. cerevisiae and have not been reported to be present in Chaetothyriales. Analysis of transformants overexpressing a dominant active allele of RacAp(racAG12V) displays unpolarized growth in Aspergillus niger, resembling the muriform cells, the pathogenic tissue-phase characteristic for chromoblastomycosis (A.F.J. Ram, unpublished data). This form in the agents of chromoblastomycosis, and even the agent of phaeohyphomycosis, Exophiala dermatitidis, is easily expressed in culture by a low pH of the growth medium and conditions of calcium limitation at more neutral pH (Mendoza et al. 1993, Karuppayil & Szaniszlo 1993, Szaniszlo et al. 1993, Badali et al. 2008). The Cdc42 proteins act as molecular switches by responding to exogenous and/or endogenous signals and relaying those signals to activate downstream components of a biological pathway. Ye & Szaniszlo (2000) confirmed that Cdc42p plays a unique regulatory role in the morphogenesis of the black yeast E.dermatitidis during its phenotype transition from yeast to isodiametric cells and muriform cells in vitro.. They also found that the derived Cdc42 protein is highly conserved member of the Cdc42 subfamily. These results suggest that the CDC42 gene products seem to play an important role in the regulation of stress-induced fungal cellular morphogenesis. One of the aspects of this therefore is its implication in human chromoblastomycosis.
When the functional change of Cdc42p, from extremotolerance to pathogenicity, concerns an evolutionary adaptation, we might expect the transition to be reflected at the DNA level. Comparing CDC42 DNA gene sequences with the phylogenetic scaffold based on ribosomal genes as the gold standard, might thus provide additional insights about whether structural and functional changes in the gene concern adaptive changes in ecological strategies. In the present paper, tools are developed for the detection of the CDC42 gene in Chaetothyriales in view of a phylogenetic study of the CDC42 gene, in parallel to genes with known phylogenetic content.
MATERIAL AND METHODS
Strains and culture conditions
The isolates studied are listed in Table 1. A total of 32 members of Chaetothyriales including basal lineages were obtained from the reference collection of the Centraalbureau voor Schimmelcultures Fungal Biodiversity Centre and grown on Malt Extract Agar (MEA) at 25 °C.
Table 1.
Strains used in this study
| Name | CBS no. | Status | Ortho-/para- | Other reference | Source | Origin |
|---|---|---|---|---|---|---|
| Exophiala dermatitidis | CBS 525.76 | T | +/+ | ATCC 34100; NIH 8656 | Human sputum | Japan |
| CBS 292.49 | +/- | DH 15696 | Faeces | U.S.A. | ||
| CBS 207.35 | T | +/+ | ATCC 28869; UAMH 3967 | Man, facial chromoblastomycosis | Japan; Osaka University | |
| Exophiala heteromorpha | CBS 232.33 | T | -/+ | MUCL 9894; NCMH 17 | Wood pulp | Sweden |
| Capronia mansonii | CBS 101.67 | T | +/- | ATCC 18659; IMI 134456 | Populus tremula | Sweden |
| Capronia munkii | CBS 615.96 | T | +/- | DH 16078 | Populus tremuloides, wood | Canada, Alberta; south of Hinton |
| Capronia epimyces | CBS 606.96 | +/+ | DH 16065 | Nectria, fruit bodies, on Pinus wood | Canada; Ontario | |
| Rhinocladiella mackenziei | CBS 650.93 | T | +/- | MUCL 40057 | Human, cerebral phaeohyphomycosis | Saudi Arabia |
| Capronia acutiseta | CBS 618.96 | T | -/+ | ATCC 56428;ATCC 76482 | Dacrydium cupressinum, wood | New Zealand; Saltwater State, Westland County |
| Capronia parasitica | CBS 123.88 | +/- | DH 15347 | Hypoxylon cohaerens var. microsporum, on Quercus sp. | France; Bois de Lourdes | |
| Rhinocladiella anceps | CBS 181.65 | NT | +/- | ATCC 18655; IMI 134453; MUCL 8233 | Soil under Thuja plicata | Canada, Ontario; Campbellville |
| Capronia villosa | CBS 616.96 | +/- | ATCC 56206 | Decorticated wood | New Zealand | |
| Fonsecaea monophora | CBS 269.37 | +/- | DH 12659 | Human, chromoblastomycosis | South America | |
| Phialophora verrucosa | CBS 286.47 | -/+ | ATCC 9541; MUCL 9768 | Mycetoma hand, human | Brazil | |
| Phialophora americana | CBS 840.69 | -/+ | MUCL 15537 | Decaying timber | Finland; Helsinki | |
| Cladophialophora carrionii | CBS 260.83 | -/+ | ATCC 44535 | Human, chromoblastomycosis | Venezuela, Falcon State, Uganda | |
| Cladophialophora boppii | CBS 126.86 | T | +/+ | DH 15357 | Human, skin lesion, on limb | Brazil |
| Exophiala castellanii | CBS 158.58 | NT | +/- | ATCC 18657; IFM 4702; MUCL 10097 | Human | Sri Lanka |
| Exophiala nigra | CBS 546.82 | +/- | DH 15993 | Soil under ice | Russia | |
| Exophiala bergeri | CBS 353.52 | T | +/- | DH 15792 | Human, chromoblastomycosis | Canada |
| Exophiala spinifera | CBS 899.68 | T | +/- | ATCC 18218; IHM 1767; NCMH 152 | Human, nasal granuloma | U.S.A. |
| Exophiala jeanselmei | CBS 507.90 | T | +/- | ATCC 34123; CBS 664.76; IHM 283; NCMH 123 | Human | Uruguay |
| Exophiala oligosperma | CBS 725.88 | T | +/- | DH 16212 | Human, tumour of sphenoidal cavity | Germany; Würzburg |
| Phialophora europaea | CBS 129.96 | T | -/+ | DH 10389 | Human, chromoblastomycosis of toe | Germany; Giessen |
| Fonsecaea pedrosoi | CBS 271.37 | NT | +/- | ATCC 18658;IMI 134458 | Human, chromoblastomycosis | Argentina |
| Coniosporium perforans | CBS 885.95 | T | +/- | DH 16308 | Marble | Greece |
| Phaeoannellomyces elegans | CBS 122.95 | +/- | DH 15343; NCMH 1286 | Human, skin infection of toe nail | Canada; Toronto | |
| Exophiala pisciphila | CBS 661.76 | +/- | DH 16145 | Heterodera schachtii egg from cyst, recovered from soil | Germany; Elsdorf | |
| Phialophora reptans | CBS113.85 | T | +/- | DH 5543 | Food | Sweden |
| Exophiala mesophila | CBS 402.95 | T | +/- | DH 15838 | Silicone, shower cabinet, | Germany, Hamburg |
| Cladophialophora modesta | CBS 985.96 | T | +/- | NCMH 108; UAMH 4004 | Human, brain | U.S.A.; Chapel Hill |
| Exophiala salmonis | CBS 157.67 | T | +/- | Brain, Salmo clakii | Canada; Galgary |
DNA extraction
Approximately 1 cm2 mycelium of 30-d-old cultures was transferred to a 2 mL Eppendorf tube containing 300 μL TES-buffer (Tris 1.2 % w/v, Na-EDTA 0.38% w/v, SDS 2 % w/v, pH 8.0) and about 80 mg of a silica mixture (Silica gel H, Merck 7736, Darmstadt, Germany / Kieselguhr Celite 545, Machery, Düren, Germany, 2 : 1, w/w). Cells were disrupted mechanically in a tight-fitting sterile pestle for approximately 1 min. Subsequently 200 μL TES-buffer was added, the mixture was vortexed, 10 μL proteinase K was added and incubated for 10 min at 65 °C. After addition of 140 μL of 5 M NaCl and 1/10 vol CTAB 10 % (cetyltrimethylammoniumbromide) solution, the material was incubated for 30 min at 65 °C. Subsequently 700 μL SEVAG (24 : 1, chloroform: isoamylalcohol) was added to the solution and shortly mixed by shaking, incubated for 30 min on ice water and centrifuged for 10 min at 1125 × g. The supernatant was transferred to a new tube with 225 μL 5 M NH4-acetate, incubated on ice water for 30 min. and centrifuged again for 10 min at 1125 × g. The supernatant was transferred to another Eppendorf tube with 0.55 vol isopropanol mixed carefully by flipping and spin for 5 min at 1125 × g. Subsequently, the pellet was washed with ice cold 70 % ethanol. After drying at room temperature it was re-suspended in 48.5 μL TE buffer (Tris 0.12 % w/v, a-EDTA 0.04 % w/v) plus 1.5 μL RNAse 20 U/mL and incubated for 15–30 min at 37 °C. DNAs were purified with GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences) as recommended by the manufacturer.
CDC42 primer design
Primers specific for CDC42 amplifications were selected using a complete alignment of the amino acid sequences of species listed in Table 2. Two highly conserved areas were detected for CDC42 that were absent from the Rac and Rho gene families. Degenerated forward (CDC42-F1, CDC42-F2) and reverse (CDC42-R1, CDC42-R2) primers were designed matching the target regions. In addition, primers (Table 4) were synthesized in the same positions but that were specific for the published sequence of Exophiala dermatitidis, CBS 525.76 (NIH 8656 = AF162788) as control (Ye & Szaniszlo 2000). The resulting specific primers CDC42-F1s, CDC42-F2s, CDC42-R1s and CDC42-R2s (Table 4) were subsequently tested with the aim to establish amplification conditions. Different combinations of specific primers were then tested for the 32 strains listed in Table 1.
Table 2.
CDC42 reference sequences taken from GenBank.
Table 4.
Degenerate and specific primers designed in this study.
| Degenerate primer: | ||||
|---|---|---|---|---|
| Gene: | Amino acid sequence: | Nucleotide sequence: | ||
| CDC42-F1: | M V V A T I | 5′-ATG GTI GTI GCI ACI ATH | ||
| CDC42-F2: | I G D E PYT | 5′-ATH GGI GAY GAR CCI TAY AC | ||
| CDC42-R1: | R M A K EL G | 5′-CCI ARY ICY TTI GCC ATI CK | ||
| CDC42-R2: | Y K L K D VF | 5′-RAA IAC RTC YTT IAR YTT RTA | ||
| Specific primers for CDC42 orthologue: | ||||
| CDC42-F1s: 5′-ATG GTT GTC GCA ACG ATC | ||||
| CDC42- F2s: 5′-GGA TTA CGA CCG GCT TCG | ||||
| CDC42-R1s: 5′-CCA ACT CCT TGG CCA TTC | ||||
| CDC42-R2s: 5′-AAA GAC GTC TTT GAG TTT GTA | ||||
| Primer combination | ||||
| CDC42 F1s--R2s 698 bp | CDC42 F1s--R1s 639 bp | CDC42 F2s--R2s 372 bp | CDC42 F2s--R1s 315 bp | |
| Six specific backward primers for CDC42 paralogue: | ||||
| CDC42-F1s: | 5′-ATG GTT GTC GCA ACG ATC | |||
| CDC42- R1d: | 5′-GGA CTT GTG GGT CGT CA | |||
| CDC42-R2d: | 5′-TCA GCG ACG GAT GGG T | |||
| CDC42-R3d (CBS 232.33): | 5′-GTT CCA ACA ATC AGA CA | |||
| CDC42-R4d (CBS 606.96): | 5′-TCC CAA CAA TCA GAC AT | |||
| CDC42-R5d (CBS 616.96): | 5′-CTT GCG GGT AGT CAC GAA | |||
| CDC42-R6d (CBS 618.96): | 5′-AAC AAT CAA ACA AGG CAC T | |||
Amplification of CDC42 orthologue
Approximately 100 ng of genomic DNA was used as a template in semi-nested PCR with primer pair CDC42-F1s-R2s and followed by a second PCR using the amplicon of the first PCR with primer CDC42-F1s-R1s. Both PCR were performed in 25 μL PCR-mix consisting of GoTaq green master mix 7 μL (Promega, Leiden), MQ 13 μL, DMSO 1 μL, primers 1 μL each, DNA 2 μL, using a Biosystems 2720 thermal cycler with an initial cycle of 1 min at 98 °C, subsequently 30 cycles of 30 s at 98 °C, 30 s at 54 °C, and 1 min at 72 °C, and a final extension of 7 min at 72 °C.
Specific primers for CDC42 paralogue
Backward primers were designed (Table 4) specific for each deviating motif using CDC42-F1s as forward primer. With both PCR amplifications, approximately 100 ng of genomic DNA was used as a template in first PCR with the forward primer combined with the backward primer in six separate reactions, and nested with the same primer pair. PCR was performed in a 25 μL PCR-mix consisting of GoTaq green master mix 7 μL (Promega, Leiden), MQ 13 μL, DMSO 1 μL, primers 1 μL each, DNA 2 μL using a Biosystems 2720 thermal cycler with an initial cycle of 1 min at 98 °C. Program was as follows: 30 cycles of 30 s at 98 °C, 30 s at 54 °C and 1 min at 72 °C, and a final extension of 7 min at 72 °C. The sond PCRs used amplicons from the first PCR and a touch-down program with an initial cycle of 1 min at 95 °C, subsequently 10 cycles of 30 s at 95 °C, 30 s at 62 °C/-1°C/cycle and 1 min at 72 °C, followed by 20 cycles of 30 s at 95 °C, 30 s at 52 °C, and 1 min at 72 °C, and a final extension of 7 min at 72 °C in sond PCR.
Cloning of the CDC42 paralogue
CDC42 paralogues were cloned using a cloning kit (pGEM-T vector; Promega, Madison, WI, U.S.A.), according to the manufacturer's instructions. We picked up one white colony to do direct PCR with primer M13 fw [5′-GTA AAA CGA CGG CCA GT-3′], M13 rv [5′-GGA AAC AGC TAT GAC CAT G-3]. PCR was performed in a 25 μL PCR-mix consisting of PCR buffer 10x 2.5 μL, MQ 15 μL, dNTP mix (1 mM) 2.5 μL, Taq polymerase (1 U/μL) 1 μL, primers 1 μL each and one white colony. amplifications were with a Biosystems 2720 thermal cycler using an initial cycle of 3 min at 94 °C, 28 subsequent cycles of 1 min at 93 °C, 1 min at 52 °C, and 2 min at 72 °C, and a final extension of 3 min at 72 °C. The resulting CDC42 paralogue sequences were compared to the previously obtained orthologous CDC42 gene sequences using BioNumerics software v. 4.61 (Applied Maths, Kortrijk, Belgium)
SSU and LSU amplification and sequencing
Primers (http://www.biology.duke.edu/fungi/mycolab/primers.htm) shown in Table 3 were used to amplify part of the nuclear rDNA operon spanning the 3' end of the 18S r RNA gene (SSU), then first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region and 5' end of the 28S rRNA gene (LSU). The PCR conditions followed the methods of Crous et al. (2006b). PCR amplifications were performed as follows: 95 °C for 1 min, followed by 30 cycles consisting of 95 °C for 10 s, 50 °C for 5 s and 60 °C for 4 min. Reaction products were then purified with Sephadex G-50 fine (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and sequencing was done on an ABI 3730XL automatic sequencer (Applied Biosystems, Foster City, CA, U.S.A.). Sequence data were adjusted using the SeqMan of Lasergene software (DNAStar Inc., Madison, Wisconsin, U.S.A.).
Table 3.
Primer sequences for PCR amplification and sequencing of LSU and SSU.
Alignment and phylogenetic reconstruction
Phylogenetic analyses were carried out on data with a taxon sampling representative of the order Chaetothyriales and including the three genes nucLSU, nucSSU and CDC42. This dataset was used in order to assess the phylogenetic evolution of CDC42 gene on diverse species within Chaetothyriales. SSU, LSU and CDC42 data sets were analyzed separately. Alignments were adjusted manually. Ambiguous regions were excluded from the alignments. Concatenated sequences of LSU and SSU were submitted to the Cipres Portal v.1.14 (http://www.phylo.org/portal/Home.do) using the RAxML web server. Maximum likelihood searches for the best-scoring tree were made after the bootstrap estimate proportion of invariable sites automatically determined the number of bootstrapping runs: if checked, RAxML will automatically determine the point at which enough bootstrapping replicates have been produced (Stamatakis et al. 2008). CDC42 data were analyzed in the same way. Bootstrap values equal to or greater than 80 % were considered significant (Hillis & Bull 1993).
RESULTS
Evaluation of designed primers for CDC42
The degenerate primers designed proved to be insufficiently specific to amplify the CDC42 gene. Four oligonucleotide primer sets, i.e. CDC42-F1s-R2s, CDC42-F1s-R1s, CDC42-F2s-R2s and CDC42-F2s-R1s were synthesized and tested for amplification with genomic DNA from 32 selected strains in the CBS reference collection representing the order Chaetothyriales. Primer pairs CDC42-F2s-R2s and CDC42-F2s-R1s provided poor results and were excluded from the analysis. Primer sets CDC42-F1s-R2s yielded a PCR product of 690 bp, whereas primer pair CDC42-F1s-R1s produced an amplicon of 630 bp in length, spanning two introns. The primer sets were found to be largely specific for their target groups. Semi Nested PCRs were successfully employed, with the first PCR giving only very weak bands but clear bands being visible with second PCR. With the second amplification the ratio of concentration of inhibitors vs. template DNA allowed amplification of the product. In general it was difficult to amplify the desired CDC42 gene fragments from strains of Chaetothyriales, because of the following: (1) PCRs had frequently to be repeated using the first amplicon, because the product from the first PCR was mostly not visible on the gel; (2) Successful PCRs were only obtained with GoTaq and DMSO, not with BioTaq; (3) Although the PCR programs were optimized for amplification of Chaetothyriales, some strains had to be done with touch-down programs in order to avoid generation of unspecific bands; (4) Frequent heavy backgrounds in electropherograms suggested contaminated PCR products. BLAST searches in GenBank showed that the sequences determined in this study deviated maximally 7 % from the published CDC42 sequence of CBS 525.76, E. dermatitidis (AF162788) (Ye & Szaniszlo 2000).
Phylogenetic analysis
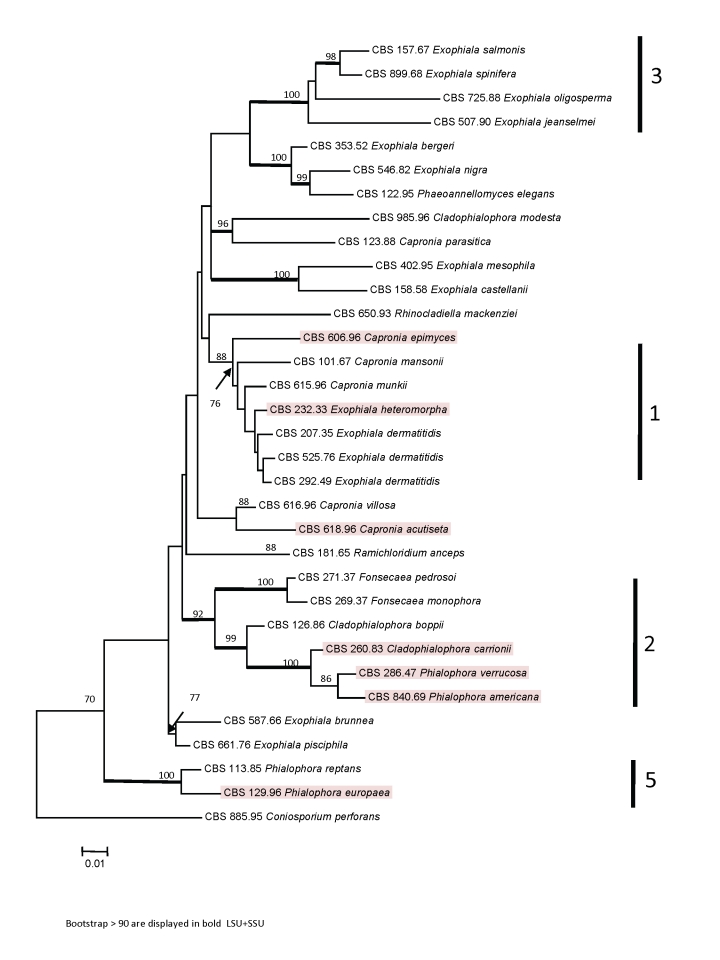
A phylogenetic tree was constructed for 32 members of Chaetothyriales using concatenated SSU and LSU ribosomal genes, with Conisporium perforans as an outgroup. This species was shown previously to be basal to the Herpotrichiellaceae by Badali et al. (2008). Four of the five clades recognized previously (e.g., Haase et al. 1999) were separated with high bootstrap support (Fig. 2): Exophiala dermatitidis clade (1), Fonsecaea pedrosoi clade (2) and Exophiala spinifera clade (3), while Haase's clade 5 is now known to represent the ancestral group (lineage 2) close to Ceramothyrium (Badali et al. 2008) and members of this clade 4 were not included in the present analysis.
Fig. 2.
Tree constructed for 32 members of Chaetothyriales obtained from a ML analysis of two combined loci (SSU and LSU) using RAxML. Bootstrap support values were estimated based on 500 replicates and are shown above the branches (thick branch for values ≥ 90 %). The tree was rooted using Coniosporium perforans, CBS 885.95.
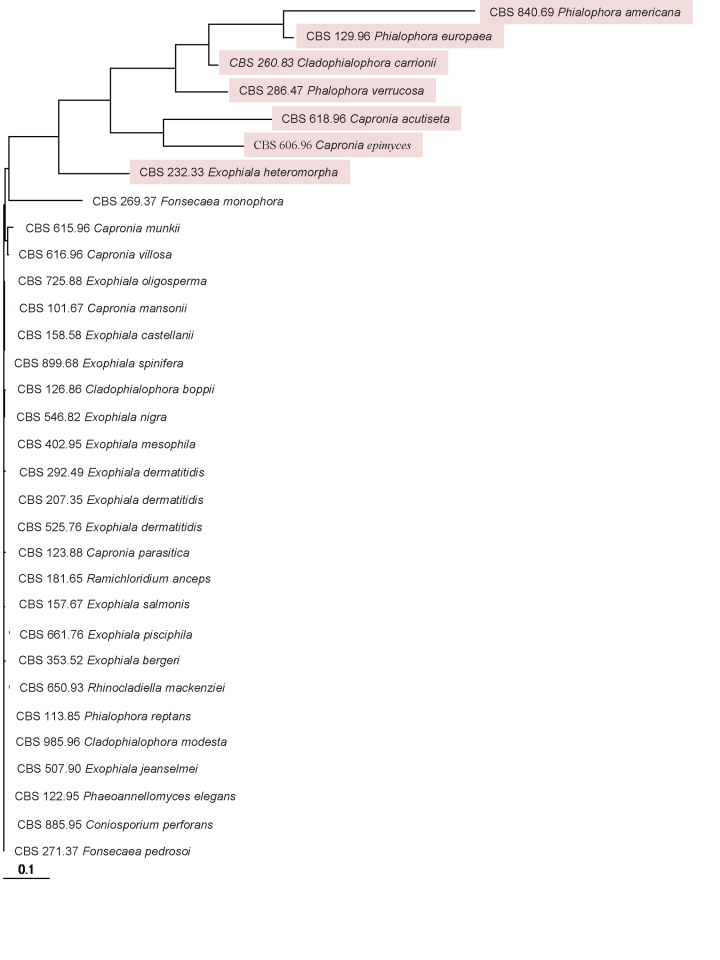
DNA sequences of the partial of CDC42 were strictly identical for 25 strains (Fig 1). In these strains also the intron and the 3rd codon positions of the exons were identical. The strains were distributed randomly over the ribosomal tree (Fig. 2). No difference was detected between Coniosporium perforans CBS 885.96, selected as the ribosomal outgroup, and the most derived groups containing e.g. Exophiala oligosperma CBS 725.88. In a number of strains significant deviations were found (Fig. 1), which were limited to the third codon positions and to the introns (Table 5). The strains clustered in four ribosomal clades (1, 2, 3, 5; Fig. 2). Nearest neighbours in the CDC42 trees were found to belong to the same ribosomal clades.
Fig. 1.
Initial tree constructed for 32 members of Chaetothyriales based on partial CDC42 sequences.
Table 5.
Number of mutations in 89 codons of partial CDC42 coding region of ten strains which had paralogues. Amino acid changes from orthologue are listed
| Accession No. | Species name | Total codon | 1st base | 2nd base | 3rd base | Amino acid change |
|---|---|---|---|---|---|---|
| CBS 618.96 | Capronia acutiseta | 89 | 2 | 1 | 38 | CAA → TCA/Q → S |
| CBS 232.33 | Exophiala heteromorpha | 89 | 1 | - | 33 | - |
| CBS 260.83 | Cladophialophora carrionii | 89 | 2 | 1 | 39 | CAA → TCG/Q → S |
| CBS 126.86 | Cladophialophora boppii | 89 | 2 | 1 | 39 | CAA → TCG/Q → S |
| CBS 286.47 | Phialophora verrucosa | 89 | 3 | 1 | 46 | CAA → TCC/Q → S |
| CBS 606.96 | Capronia epimyces | 89 | - | - | 35 | - |
| CBS 207.35 | Exophiala dermatitidis | 89 | 2 | - | 33 | - |
| CBS 525.76 | Exophiala dermatitidis | 89 | 1 | - | 23 | - |
| CBS 616.96 | Capronia villosa | 89 | 2 | 1 | 5 | GAC → TAC/D → Y |
| CBS 615.96 | Capronia munkii | 89 | - | - | 5 | - |
Q glutamin; S serin; D asparaginic acid; Y tyrosin
In order to verify the different motifs obtained, specific primers were developed for each sequence type of 32 strains. Amplification of the invariant type was relatively straightforward: the same sequence was obtained consistently. However, specific amplifications of deviating types were mostly unsuccessful. Strains CBS 232.33 and CBS 618.96 consistently provided a deviating sequence. Two strains of Exophiala dermatitidis (CBS 525.76 and CBS 207.37), Capronia epimyces (CBS 606.96) and Cladophialophora boppii (CBS 126.86) revealed two deviating sequences, one identical to the core sequence of 25 strains, another clearly deviating in intron and 3rd codon sequences. Cloning results proved that the deviating genotypes were common in many strains. In some strains the invariant type could not be amplified (Table 1)
DISCUSSION
CDC42 is an essential GTPase that is ubiquitously expressed in eukaryotes, where it participates in the regulation of the cytoskeleton and a wide range of cellular processes, including cytokinesis, gene expression, cell cycle progression, apoptosis, and tumorogenesis. It transduces signals to the actin cytoskeleton to initiate and maintain polarized growth and to promote mitogen-activated protein morphogenesis. In filamentous fungi the CDC42 gene product is involved in the transition of hyphae to isodiametrically growing cells. This transition becomes apparent in the formation of meristematic cells under conditions of environmental stress, such as with growth on rock, but also concerns muriform cells, the invasive form in human chromoblastomycosis.
In the evolution of Chaetothyriales, we witness a functional change from a rock-inhabiting life style prevalent in Coniosporium and relatives (Fig. 2; Sterflinger et al. 1998) to an increased ability to infect humans and other vertebrates, e.g. in cases of chromoblastomycosis. Both life styles are characterized by isodiametric growth at least during part of the life cycle. Thus a major ecological and functional transition has taken place, the same characteristics of isodiametric expansion becoming applied in an entirely different setting. Exposure on rock is an ancestral condition in Coniosporium (Lutzoni et al. 2001, Gueidan et al. 2008), while the pathogenic role in agents of chromoblastomycosis in Cladophialophora, Fonsecaea and Phialophora is a derived condition (Badali et al. 2008). In recent publications the ribosomal tree based on SSU and LSU exhibits a basal lineage that has become individualized more clearly and referred to as the separate family Chaetothyriaceae within the Chaetothyriales (Badali et al. 2008). It contains primarily species from rock, but also those occasionally colonizing human skin or causing mild cutaneous infections (Li et al. 2008). The majority of species reported from human chromoblastomycosis (Phialophora verrucosa and Cladophialophora carrionii) are united in a separate clade which can be attributed to the family Herpotrichiellaceae (Untereiner 2000). An eventual directional evolution of CDC should reflect both ecologies including rock-inhabiting and pathogenic life styles.
Our research was initiated with the development of primer sets to detect CDC42 in Chaetothyriales. We constructed four primer sets from protein coding regions in which the PCR product would span at least one intron. Four degenerate primer sets were tested for their ability to amplify CDC42 in members of Chaetothyriales. Specific primer sets were subsequently synthesized. Only two sets worked well such that they amplified PCR products matching the published CDC42 sequence of CBS 525.76, Exophiala dermatitidis (AF162788) and that were clearly different from genes that encode functionally related proteins such as Rac and Rho. Further amplification in related species was done with these primer sets (CDC42-F1s-R2s and CDC42-F1s-R1s).
The majority of the strains, irrespective of their phylogenetic position based on rDNA, were invariable in CDC42. Part of the strains, however, showed considerable deviation in non-coding positions and introns (Table 5). Since the relationship with the ribosomal tree of the combined dataset was not directly obvious, we were uncertain whether the deviating sequences were homologous with CDC42, despite consistent highest scores in GenBank. For most CDC42 amplifications, PCRs were difficult and had to be repeated several times due to frequent heavy background in the electropherogram suggesting contaminated PCR products. In order to verify the different motifs obtained, we developed specific primers for each sequence type. PCR efficiency and sequencing was particularly difficult in the deviating sequences, even when specific backward primers were used. Amplification of the invariant type was relatively straightforward: the same sequence was obtained consistently, with CBS 618.96, CBS 232.33 as the only exceptions (Table 1). However, specific amplifications of deviating haplotypes were mostly unsuccessful. In two strains the deviating sequence was obtained in addition to the invariant type. This strongly suggests that the deviating type was a paralogue resulting from gene duplication. When the fragments were cloned, ortho- and paralogues were obtained repeatedly, the orthologue remaining absent from CBS 232.33.
The CDC42 orthologue evolution shows an unexpected topology. The analyzed exons showed very limited non-synonymous change throughout the entire phylogenetic history of Chaetothyriales, even between such remote species as Coniosporium perforans and Exophiala dermatitidis. A very strong constraint is observed on the gene, the exon having remained identical and the protein structure having remained unchanged. Remarkably, also intron and 3rd codon sequences had remained identical (Fig. 1). The gene was subject of strong functional and structural constraints.
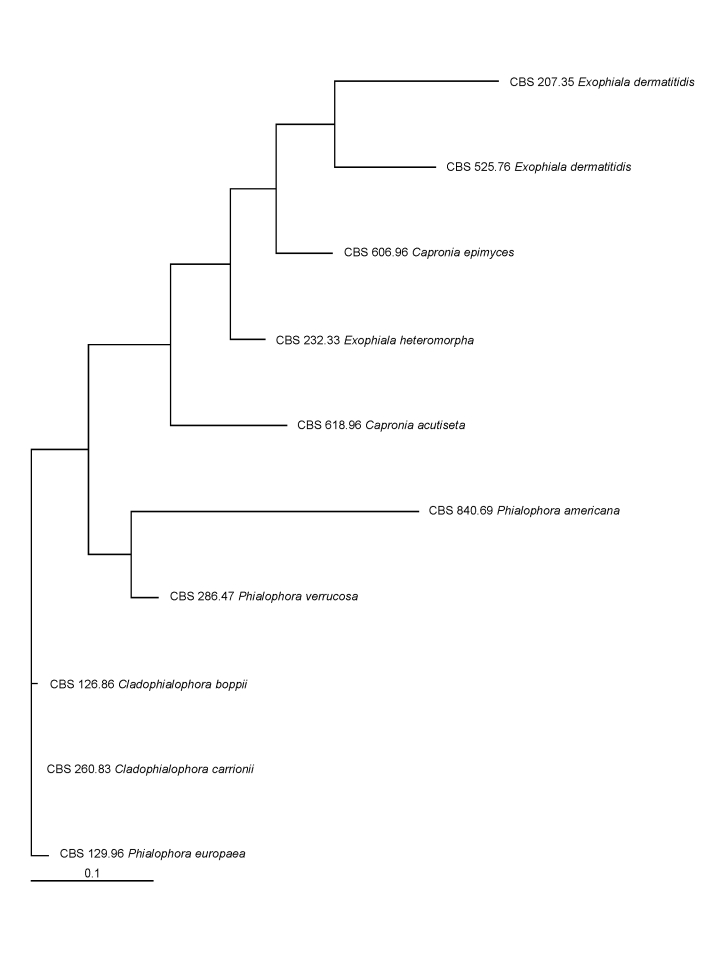
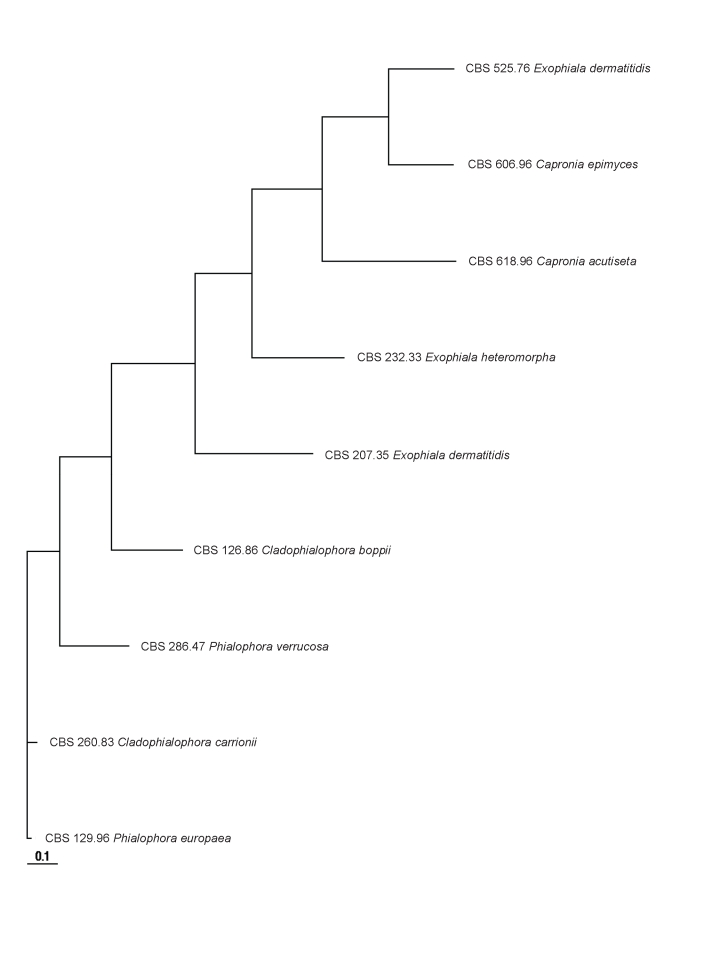
In main traits the evolution of the CDC42 paralogue was comparable to that of the scaffold of the multilocus phylogeny of the Chaetothyriales (Badali et al. 2008). SSU, LSU and CDC42 were analysed using the same set of strains (Figs 1, 2, 3, 4). SSU and LSU gene evolution is likely to reflect the phylogenetic history of Chaetothyriales. The paralogue showed considerable evolution in intron and 3rd codon positions, the tertiary structure being relaxed, allowing considerable mutation. The translated protein sequence, however, showed only limited non-synonymous change (Table 5 Figs 3, 4). Non-synonymous change is observed in Phialophora and Cladophialophora agents of chromoblastomycosis, but not in Fonsecaea even though all three cause the same disease. Hence these mutations cannot be linked to selection of an adapted genotype.
Fig. 3.
Tree constructed for 10 strains based on the partial exon of the CDC42 paralogue.
Fig. 4.
Tree constructed for 9 strains based on the sequenced intron of the CDC42 paralogue.
We hypothesise that both types are expressed with identical proteins, with gene duplication as an ancient event, and that subsequent structural evolution has taken place in the paralogue without loss of function. In the majority of the species investigated only one of the types was detected, suggesting that ortho- or paralogues may be lost. When non-synonymous vs. synonymous nucleotide divergence is high between species, functional divergence is assumed to be high, due to positive selection and/or relaxed selective constraint (e.g. Friedman & Hughes 2004). When this ratio is low, the functional properties of the gene products involved are thought to be conserved, because selective constraint is high and there is little or no positive selection (e.g. Sehgal & Lovette 2003). In CDC42, both ortho- and paralogues show a very low ratio, indicating high functional and structural selective constraint.
In summary, we hypothesise that members of Chaetothyriales may have two duplicate CDC42 genes, producing exactly the same protein, but having a conserved versus relaxed tertiary structure, which involves 3rd codon positions as well as introns. The evolution that takes place in the paralogue follows the main traits of evolution seen in ribosomal genes, but the genes are considerably more variable and difficult to align. The possibility is not excluded that in the course of evolution one of the duplicate genes – either ortho- or paralogue – was lost in individual species. In that case CDC42 would be a poor phylogenetic marker, as trees will consist of non-homologous genes. From the fact that both orthogue and paralogue have a considerable constraint at the protein level, it can be concluded that any eventual change of function of CDC42 in the course of evolution of Chaetothyriales (using CDC42 for rock-inhabiting or pathogenic life styles, respectively) is not reflected at the DNA level. The limited number of non-synonymous changes in the paralogue do not coincide with evolution in species causing chromoblastomycosis, and thus do not suggest any positive selection of a functional change from rock-inhabiting life styles to pathogenicity. Any role of CDC42 as a virulence factor has thus not been proven.
Acknowledgments
We are indebted to K. Voigt, P.J. Szaniszlo and G. Walther for useful comments on the manuscript.
References
- Badali H, Gueidan C, Najafzadeh MJ, Bonifaz A, Gerrits van den Ende AHG, Hoog GS de (2008). Biodiversity of the genus Cladophialophora. Studies in Mycology 61: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME (1990). Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39: 43–184. [Google Scholar]
- Bose I, Irazoqui J, Moskow JJ, Bardes ESG, Zyla TR, Lew DJ (2001). Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle regulated phosphorylation of Cdc24p. Journal Biological Chemistry 276: 7176–7186. [DOI] [PubMed] [Google Scholar]
- Boyce KJ, Hynes MJ, Andrianopoulos A (2001). The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. Journal of Bacteriology 183: 3447–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R & Hughes AL (2004). Two patterns of genome organization in mammals: the chromosomal distribution of duplicate genes in human and mouse. Molecular Biology and Evolution 21: 1008–1013. [DOI] [PubMed] [Google Scholar]
- Gargas A, Taylor JW (1992). Polymerase chain reaction (PCR) primers for amplifying and sequencing 18s rDNA from lichenised fungi. Mycologia 84: 589–592. [Google Scholar]
- Gorfer M, Tarkka MT, Hanif M, Pardo AG, Laitiainen E, Raudaskoski M (2001). Characterization of small GTPases Cdc42 and Rac and the relationship between Cdc42 and actin cytoskeleton in vegetative and ectomycorrhizal hyphae of Suillus bovinus. Molecular Plant Microbe Interactions 14: 135–144. [DOI] [PubMed] [Google Scholar]
- Gueidan C, Ruibal Villaseñor C, Hoog GS de, Gorbushina AA, Untereiner WA, Lutzoni F (2008). An extremotolerant rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Studies in Mycology 61: 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase G, Sonntag L, Melzer-Krick B, Hoog GS de (1999). Phylogenetic inference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Studies in Mycology 43 80–97. [Google Scholar]
- Hillis DM, Bull JJ (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192. [Google Scholar]
- Hoog GS de, Göttlich E, Platas G, Genilloud O, Leotta G, Brummelen J van (2005). Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Studies in Mycology 51: 33–76. [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000). Atlas of Clinical Fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands and Universitat Rovira i Virgili, Reus, Spain.
- Hughes AL, Friedman R (2004). Recent mammalian gene duplications:robust search for functionally divergent gene pairs. Journal of Molecular Evolution 59: 114–120. [DOI] [PubMed] [Google Scholar]
- Hurtado CAR, Beckerich JM, Gaillardin C, Rachubinski RA (2000). A Rac homolog is required for induction of hyphal growth in the dimorphic yeast Yarrowia lipolytica. Journal of Bacteriology 182: 2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI (1999). Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiology and Molecular Biology Reviews 63: 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppayil SM, Szaniszlo PJ (1997). Importance of calcium to the regulation of polymorphism in Wangiella dermatitidis. Journal of Medical and Veterinary Mycology 35: 379–388. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Pagel M, Reeb V (2001). Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411: 937–940. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Matsuda T, McGinnis MR, Ajello L (1993). Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36: 145–155. [DOI] [PubMed] [Google Scholar]
- Mendoza L, Karuppayil SM, Szaniszlo PJ (1993). Calcium regulates in vitro dimorphism in chromoblastomycotic fungi. Mycoses 36: 157–164. [DOI] [PubMed] [Google Scholar]
- Prenafeta-Boldú FX, Summerbell RC, Hoog GS de (2006). Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard. FEMS Microbiological Reviews 30: 109–130. [DOI] [PubMed] [Google Scholar]
- Ruibal C (2004). Isolation and characterization of melanized, slow-growing fungi from semiarid rock surfaces of central Spain and Mallorca. Ph.D. dissertation, Universidad Autónoma de Madrid.
- Sehgal RN, Lovette IJ (2003). Molecular evolution of three avian neurotrophin genes: implications for proregion functional constraints. Journal of Molecular Evolution 57: 335–342. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J (2008). A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758–771. [DOI] [PubMed] [Google Scholar]
- Sterflinger K (1998). Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie van Leeuwenhoek 74: 271–281. [DOI] [PubMed] [Google Scholar]
- Szaniszlo PJ, Geis PA, Jacobs CW, Cooper CR Jr, Harris JL (1983). Cell wall changes associated with yeast to multi-cellular form conversion in Wangiella dermatitidis In: D. Schlessinger (ed.) Microbiology 83. American Society for Microbiology, Washington, D.C. 239–244.
- Szaniszlo PJ, Karuppayil SM, Mendoza L, Rennard RJ (1993). Cell cycle regulation of polymorphism in Wangiella dermatitidis. Archives of Medical Research 24: 251–261. [PubMed] [Google Scholar]
- Untereiner WA (2000). Capronia and its anamorphs: exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Studies in Mycology 45: 141–149 [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag A, Lee MP, Si H, Harris SD (2007). Regulation of hyphal morphogenesis by CDC42 and RAC1 homologues in Aspergillus nidulans. Molecular Microbiology 66: 1579–1596. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322.
- Ye X, Szaniszlo PJ (2000). Expression of a constitutively active CDC42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. Journal of Bacteriology 182: 4941–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, Hoog GS de (2007). Spectrum of clinically relevant Exophiala species in the U.S.A. Journal of Clinical Microbiology 45: 3713–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]