Abstract
We present a phylogenetic study of black fungi in lichens, primarily focusing on saxicolous samples from seasonally arid habitats in Armenia, but also with examples from other sites. Culturable strains of lichen-associated black fungi were obtained by isolation from surface-washed lichen material. Determination is based on ITS rDNA sequence data and comparison with published sequences from other sources. The genera Capnobotryella, Cladophialophora, Coniosporium, Mycosphaerella, and Rhinocladiella were found in different lichen species, which showed no pathogenic symptoms. A clade of predominantly lichen-associated strains is present only in Rhinocladiella, whereas samples of the remaining genera were grouped more clearly in clades with species from other sources. The ecology of most-closely related strains indicates that Capnobotryella and Coniosporium, and perhaps also Rhinocladiella strains opportunistically colonise lichens. In contrast, high sequence divergence in strains assigned to Mycosphaerella could indicate the presence of several lichen-specific species with unknown range of hosts or habitats, which are distantly related to plant-inhabitants. Similar applies to Cladophialophora strains, where the closest relatives of the strains from lichens are serious human pathogens.
Keywords: Aridity, black fungi, Chaetothyriomycetidae, Dothideomycetidae, phylogeny, symbioses
INTRODUCTION
“Black fungi” is a practical term to group heterogeneous lineages of Chaetothyriomycetidae and Dothideomycetidae with melanised cell walls. It comprises fungi with diverse ecology and different growth styles, such as black yeasts or meristematic black fungi. In black yeasts, daughter cells form yeast-like multilateral or polar budding. The term meristematic was originally introduced for black fungi by de Hoog and Hermanides-Nijhof (1977) and describes non-disintegrating phenotypes which form aggregates of thick-walled, melanised cells. Meristematic growth is infrequent in the fungal kingdom, yet it was for example found in several fungi that parasitise lichens (Diederich 1990).
Black fungi can occur in extreme environments and under poor nutrient conditions, where they often grow meristematically (Sterflinger et al. 1999). In the last yr dematiaceous fungi with meristematic and yeast-like growth patterns, turned out to be, together with lichens and cyanobacteria, among the most successful inhabitants of marble, limestone, granite, and other rock types in arid and semi-arid environments (Sterflinger & Krumbein 1997, Wollenzien et al. 1997, Sterflinger 1998, Ruibal et al. 2005). They have been found in hot deserts of Arizona (U.S.A.) (Staley et al. 1982; Palmer et al. 1987), in cold Antarctic deserts (Nienow & Friedmann 1993, Selbmann et al. 2005), in Mediterranean countries as e.g. Italy, Greece, Turkey (Gorbushina et al. 2005, Ruibal et al. 2005, Sert & Sterflinger 2005), on stone monuments in Austria (Sterflinger & Prillinger 2001), and on granites of the Ivory Coast (Büdel et al. 2000). Sterflinger & Krumbein (1995) hypothesised that the ability to grow meristematically provides the colonies an optimal surface/volume ratio for enhanced stress tolerance. In particular they resist elevated temperatures, low water availability (Wollenzien et al. 1995), UV radiation (Urzì et al. 1995), high salt concentration (Zalar et al. 1999) or combinations of these factors and further stresses (Selbmann et al. 2005, Scott et al. 2007).
Due to the high selective pressure exerted by these stresses on the microbial community, black fungi are rarely found in complex microbial populations, rather they occur solitary or in communities with similarly stress resistant organisms such as lichens (Onofri et al. 2007a) and cyanobacteria (Sterflinger 2006). This is perfectly shown on rock surfaces where black fungi can form communities with epi- and endolithic lichens, cyanobacteria, chemoorganotrophic bacteria and fungi (Nienow & Friedmann 1993).
In the course of current lichenological explorations of Armenia, the first author frequently found lichen thalli with obscure discolourations. Microscopic study showed that they were caused by fungal colonisation, although not leading to sexual structures or distinctive anamorphs on lichen thalli as characteristic of most lichenicolous fungi (Lawrey & Diederich 2003). Rather, the hyphae grew isolated or as sclerotial aggregates on the thallus surface. Further diagnostic characters of the dematiaceous hyphae were missing. The phylogenetic relationships of lichen-dwelling hyphomycetes are generally unclear, due to the lack of sequence data. Despite some of the described taxa seem to be specific to their host, it is not known if other lichenicolous black fungi can colonise a wider range of hosts with similar habitat ecology.
MATERIALS AND METHODS
Sampling
Lichen material originates from Armenia. The samples were collected at different altitudes (up to ca. 2800 m), and on different substrates (basalt, calcareous and siliceous rocks, and few also bark) representing comparatively dry habitats (precipitation mostly between 400–600 mm/yr-1). Additionally, we used samples from the mountain and the Mediterranean belts of Austria, Italy and Spain to explore a wider geographic range. The selected lichen species, origins, and corresponding fungal strains isolated from the lichen thalli are listed in Table 1. Both lichen material without symptoms and with black discolourations on the thalli were selected for the isolations.
Table 1.
List of investigated specimens with collecting sites, herbarium numbers, GenBank accessions of the ITS sequences of the black fungal strains.
|
Black fungi isolated
|
||||||
|---|---|---|---|---|---|---|
| Lichen species | Origin | Capnobotryella sp. | Cladophialophora sp. | Coniosporium sp. | Mycosphaerella sp. | Rhinocladiella sp. |
| Caloplaca erodens Tretiach, Pinna & Grube | Austria, Styria, Mt. Hochlantsch, c. 1620 m a.s.l., on limestone, 2005, Muggia (TSB 36936). | FJ265744 | ||||
| C. erythrocarpa (Pers.) Zwackh | Armenia, Syunik, Goris, c. 1550 m a.s.l., on rock, 2006, Harutyunyan (GZU 23-1006). | FJ265749 | ||||
| C. gomerana J. Steiner | Spain, Canary Islands, Tenerife, Punta Roja, 15 m a.s.l., on basalt, 2005, Muggia (TSB 36862). | FJ265765 | ||||
| C. holocarpa (Ach.) A.E. Wade | Armenia, Kotayk, Jrvej, c. 1300-1400 m a.s.l., on Prunus sp., 2005, Harutyunyan (GZU 36-105). | FJ265745 | ||||
| C. saxicola (Hoffm.) Nordin | Armenia, Kotayk, Garni gorge, c. 1180 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 05-1000). | FJ265742 | FJ265756 | FJ265760 | FJ265766 | |
| Dermatocarpon miniatum (L.) W. Mann | Austria, Styria, Grazer Bergland, Kaschlsteig, 800 m a.s.l., on limestone, 2005, Muggia & Hafellner (TSB 36921). | FJ265762 | ||||
| Fulgensia fulgida (Nyl.) Szatala | Italy, Sardegna, Nuoro, Mt. Albo Massiv, 1000 m a.s.l., on limestone, 2006, Muggia (TSB 37496). | FJ265750 | ||||
| Protoparmeliopsis muralis (Schreb.) M. Choisy | Armenia, Kotayk, Garni gorge, c. 1180 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 05-1001). | FJ265754 | FJ265770 | |||
| “ | Armenia, Kotayk, Geghardavank, c. 1600 m a.s.l., on Rhamnus catarthica, 2005, Harutyunyan (GZU 38183). | FJ265752 | ||||
| “ | Armenia, Kotayk, Tsaghkadzor, c. 1750 m a.s.l., on rock, 2005, Harutyunyan (GZU 34-206). | FJ265743 | FJ265757 | FJ265768 | ||
| “ | Armenia, Kotayk, Geghard, c. 1875 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 08-1002). | FJ265747 | ||||
| “ | Armenia, Vayots Dzor, Jermuk, c. 2050 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 12-1005). | FJ265748 | FJ265764 | |||
| “ | Armenia, Shirak, Mt. Aragats, c. 2465 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 18-1004). | FJ265771 | ||||
| “ | Armenia, Kotayk, Mt. Taghenyac, c. 2820 m a.s.l., on basalt, 2006, Harutyunyan & Mayrhofer (GZU 02-1003). | FJ265767 | ||||
| Lecidella stigmatea (Ach.) Hertel & Leuckert | Austria, Styria, Röthelstein, 1260 m a.s.l., on limestone, 2006, Muggia & Hafellner (TSB 37332). | FJ265772 | ||||
| Physcia dimidiata (Arnold) Nyl. | Armenia, Kotayk, Garni gorge, c. 1180 m a.s.l., on Salix sp., 2006, Harutyunyan & Mayrhofer (GZU 05-289). | FJ265751 | ||||
| Physconia americana Esslinger | Armenia, Syunik province, Goris, c. 1550 m a.s.l., on Quercus sp., 2006, Harutyunyan (GZU 23-727). | FJ265753 | ||||
| Tephromela atra (Huds.) Hafellner | Italy, Toscana, Mt. Labbro, 1150 m a.s.l., on silicate, 2004, Tretiach (TSB 37086). | FJ265759 | ||||
| Xanthoria elegans (Link) Th.Fr. | Armenia, Kotayk, Tsaghkadzor, c. 1750 m a.s.l., on rock, 2005, Harutyunyan (GZU 34-205). | FJ265746 | FJ265758 | FJ265763 | ||
Optical analysis
Lichens were analysed for visible colonisation with black fungi using a stereomicroscope. Hand-made transversal sections (using Gillette razor-blades) were examined in water with a Zeiss Axioscope compound microscope (Zeiss, Vienna).
Isolation of fungal strains in culture
We used young areoles or lobes, respectively, of freshly collected lichen material. The isolations were performed following the “lichen tissue culture method” as described by Yamamoto et al. (2002), with some modifications as applied in Stocker-Wörgötter (2002). To remove attached debris and substrate, small pieces of lichen thalli were cleaned mechanically using a forceps. Additionally the pieces were washed in water and in diluted Tween 80 to eliminate other organisms loosely attached to the thallus surface (Bubrick & Galun 1986). Fragments were homogenised using a mortar and pestle. The suspensions, containing small pieces of cortex, medulla and algal layer, were filtered through two sieves of 500 and 150 μm mesh size, respectively. Single fragments of about 150 μm in size were picked up with sterile bamboo sticks under a dissecting microscope and transferred on slanted agar in test tubes.
The fungal cultures were grown on MY, TM (Ahmadjian 1967), and LBM (Lilly & Barnett 1951) media for 4–5 mo at 15–20 °C, with a cycle of 14 h of light and 10 h of dark. As soon as the mycelia reached ca. 0.5 cm in diam, a small part was sub-cultured and another was used for DNA isolation. Subcultures were prepared on up to five small Petri dishes having either the same original agar media or different media. The fungus was transferred on fresh media for subcultures after 3–4 mo.
Molecular analysis: DNA-isolation, PCR-amplification and sequencing
DNA isolation was performed on a part of the mycelium grown in the slant tubes, following Cubero et al. (1999). Identity of the cultured strains were checked with sequences of the ITS regions, amplified with the primers ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990). PCR reactions were prepared for a 30 μL final volume containing 4.05 μL double-distilled water, 3 μL 10 × Taq polymerase reaction buffer (10 mM Tris pH 8.3), 1.8 μL MgCl2 (25 mM), 3 μL of 2.5 mM dNTPs, 0.15 μL Taq DNA polymerase, 1.5 μL for each of the 10 μM primers. PCR amplification were performed under the following conditions: an initial heating step of 2 min at 94 °C, 30 cycles of 1 min denaturation at 94 °C, 1 min annealing at 53 °C, and 2 min of extension at 72 °C, and one final extension step of 7 min at 72 °C, after which the samples were kept at 4 °C. PCR products were cleaned using Qiaquick spin columns (Qiagen, Vienna, Austria). Both complementary strands were sequenced with the BigDye Cycle Sequencing Ready Reaction Kit (Applera, Vienna, Austria) according to the manufacturer's instructions, and sequences were run on an ABI310 automated sequencer (Applera, Vienna, Austria).
Alignment and phylogenetic analysis
Sequences of the isolated fungal strains were subjected to BLAST search (Altschul et al. 1990, http://www.ncbi.nlm.nih.gov/BLAST/), and were assigned to genera that appeared as first matches in the GenBank. Sequences that matched closer to our data were selected from GenBank and included in the analyses.
All fungal strains corresponding to the same genus were grouped together and for each of them a small alignment was produced automatically with ClustalW (Thompson et al. 1994) as implemented in BioEdit v. 5.0.6 (Hall 1999, http://jwbrown.mbio.ncsu.edu/BioEdit/bioedit.html) and then manually adjusted. Small phylogenetic analyses were carried out to outline the genetic similarity of the isolated strains with the closest matches resulted from the BLAST search, The maximum parsimony (MP) algorithm was used in PAUP v. 4.0b10 (Swofford 2002). A heuristic search using 100 random addition replicates was conducted with tree-bisection-reconnection (TBR), branch swapping, and MulTree options. Bootstrappping was performed on 1000 pseudoreplicates (Felsenstein 1985). Ambiguously aligned positions were excluded and no root was selected. The phylogenetic trees were drawn using the program TreeView (Page 1996).
RESULTS
Several lichen species used in the present study (Caloplaca erythrocarpa, C. saxicola, some samples of Protoparmeliopsis muralis, Physconia americana and Xanthoria elegans) showed visual signs of fungal colonisation in the form of minute black hyphae or dots. Attempts to determine the colonising species using available literature that includes keys to several hyphomycetous genera occurring on lichens (e.g. Hawksworth 1979, Clauzade et al. 1989, etc.) did not give clear results. Other samples, such as C. holocarpa, some samples of P. muralis and Physcia dimidiata were free of any visual contaminants.
We obtained 70 black fungal cultures from 20 different thalli belonging to 14 lichen species, representing crustose, foliose or fruticose growth (Table 1). On the same media, black fungi grew slowly but clearly faster than lichen mycobionts (ca. 1 cm diameter black fungal colony is reached after about 3 mos, whereas most of the lichen mycobionts show only initial development of compact mycelium after this period). Sequencing of the complete ITS regions from cultures, from which DNA was successfully extracted, revealed 31 distinct strains. According to results of the BLAST searches, the obtained strains were classified as representatives of the subclasses Chaetothyriomycetidae and Dothideomycetidae. In particular, they belong to five genera: Capnobotryella, Cladophialophora, Coniosporium, Mycosphaerella and Rhinocladiella. Molecular data of 16 cultures were not subjected to phylogenetic analyses because we so far obtained incomplete sequences from these strains. According to BLASTn searches these latter were most closely assigned to uncultured soil fungi (isolated from Protoparmeliopsis muralis, Teloschistes contortuplicatus and Xanthoria. elegans), melanised limestone ascomycetes (from Caloplaca saxicola, C. holocarpa, Fulgensia fulgida, Leptogium corniculatum and P. muralis), Capronia and Exophiala (representing Chaetothyriomycetidae on Physconia americana, P. muralis and X. elegans). In addition to black fungal groups we also found a species showing sequence similarity with Nectria (representing Hypocreales) in Physcia scopulorum.
Strains belonging to the genera Cladophialophora (8) and Rhinocladiella (11) were found more frequently in lichen thalli than Capnobotryella (4), Coniosporium (3), and Mycosphaerella (5). Cladophialophora and Rhinocladiella did not show any preference to elevation and were isolated from six lichen species at different altitudes. Not supporting a species-specific occurrence, the same fungal strains representing Coniosporium were isolated from co-occurring Caloplaca saxicola and Protoparmeliopsis muralis in Armenia (h11 and h6). Fungal strains identified as Capnobotryella, Cladophialophora, and Rhinocladiella did not show any specificity for the lichen growth habit. All of them were isolated from crustose, foliose and fruticose lichens, respectively. Fungal strains assigned to Coniosporium and Mycosphaerella were so far isolated only from crustose lichens. Among all the five genera only Cladophialophora was isolated also from the two epiphytic lichens included, i.e. Physcia dimidiata and Physconia americana. These strains represented distinct lineages.
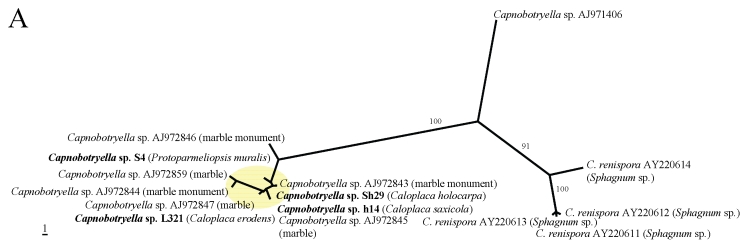
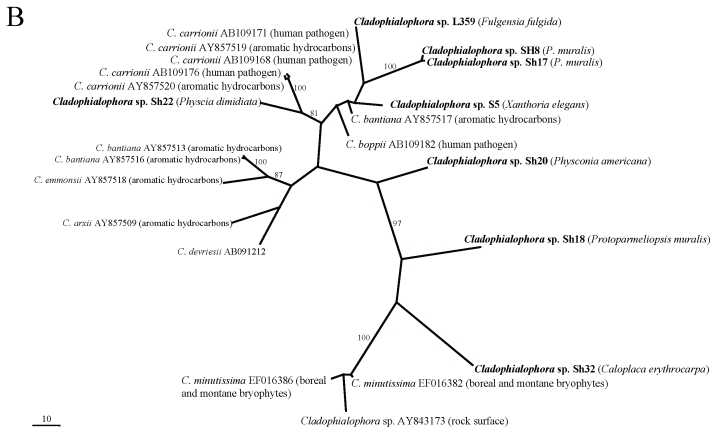
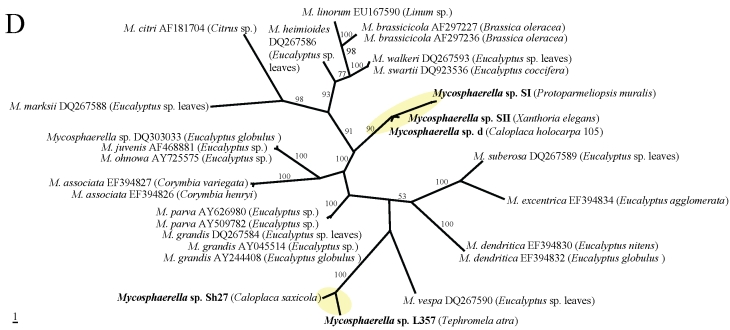
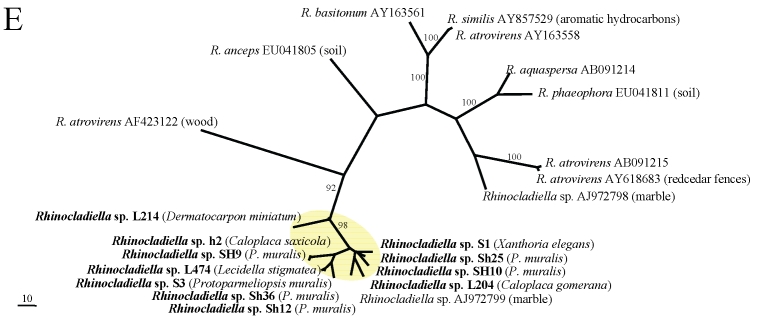
In the phylogenetic analyses (Fig. 1A–E), all fungal strains identified as Rhinocladiella, are grouped in a single clade with the exception of one rock-inhabiting strain from Turkey (AJ972799). Except for the latter, these strains are genetically rather similar although they were isolated from different lichen species. In the remaining genera, by contrast, sequences from lichens are genetically rather heterogeneous and are scattered among strains coming from other different sources. Two lineages of lichen-colonisers were so far found in Mycosphaerella and more lineages colonising lichens are present in Cladophialophora. Several distinct strains were also found in Capnobotryella.
Fig 1.
MP phylogenetic hypotheses based on ITS sequences of the black fungal genera Capnobotryella (a), Cladophialophora (b), Coniosporium (c), Mycosphaerella (d), and Rhinocladiella (e). Sequences obtained in this study are shown in bold, the isolation sources are written in parentheses, and yellow ellipses highlight the clades they form. Trees are unrooted, bootstrap cut-offs higher then 75% are reported.
Multiple strains of black fungi were isolated from the same thallus of four crustose lichen species (Caloplaca holocarpa, C. saxicola, Protoparmeliopsis muralis and Xanthoria elegans) (Table 1). The most diverse of these was C. saxicola, yielding four isolates representing the genera Capnobotryella, Coniosporium, Mycosphaerella and Rhinocladiella. P. muralis contained Capnobotryella, Mycosphaerella and Rhinocladiella, and X. elegans hosted Cladophialophora, Mycosphaerella and Rhinocladiella sp. occurring simultaneously. The thallus of C. holocarpa was a substrate for Capnobotryella and Mycosphaerella. Two separate thalli of P. muralis hosted Coniosporium with Rhinocladiella and Cladophialophora with Rhinocladiella respectively. Only one culturable fungal strain from the above-mentioned genera of black fungi was present in the lichen samples of Caloplaca erodens, Dermatocarpon miniatum, Fulgensia fulgida, Physcia dimidiata, Physconia americana, and Tephromela atra according to the sequencing results with several isolates retrieved from these lichens (Table 1).
DISCUSSION
High diversity of life-styles characterises the Chaetothyriomycetidae and Dothideomycetidae (Ruibal et al. 2008). In these two subclasses, genera can comprise animal and human pathogens, endophytes or epiphytes of living plants and fungi (de Hoog 1994, Geiser et al. 2006, Schoch et al. 2006). On the other hand, phylogenetic studies indicate a rather scattered distribution of lichenised fungi in Dothideomycetidae (Del Prado et al. 2006, Muggia et al. 2007), whereas in Chaetothyriomycetidae, lichenised forms belong to the large monophyletic orders Pyrenulales and Verrucariales (Geiser et al. 2006). Repeated loss of lichenisation and lichenicolous habit occurred in Verrucariales (Navarro-Rosinés et al. 2007). However, black fungi detected by us do not belong to any of the lineages of lichenised fungi in Chaetothyriomycetidae or Dothideomycetidae. The BLAST analysis revealed indeed high sequence similarity primarily with the genera Capnobotryella, Cladophialophora, Coniosporium, Mycosphaerella, and Rhinocladiella. Within these genera genetic similarities were found with undetermined fungal strains isolated directly from rock surfaces, or with plant and human pathogenic species.
Lichens can host a wide range of associated fungi with rather varied ecologies, specificities, and biological behaviours (Lawrey & Diederich 2003). Some fast-growing lichenicolous species (e.g. Athelia, Marchandiomyces) with often low host specificity can rapidly eradicate lichen vegetation, whereas many others grow slowly without expressing any or showing only local pathogenic symptoms on their specific hosts. Pathogenic and commensalic interactions with their hosts appear to be corners of an ecological continuum, yet, known lichenicolous fungi have clear affinity to lichens as hosts and are not found without their hosts.
In this publication we find indirect evidence that some lineages of black fungi can opportunistically grow on lichens. Generally lichen-colonising forms did not form monophyletic groups, although in Rhinocladiella (Arnanlou et al. 2007), only one published marble-colonising strain was found among a genetically homogeneous group of lichen inhabitants. This pattern might nevertheless be incomplete because only few strains from rock are published so far. We suspect that Rhinocladiella is only a facultative lichen coloniser (Fig. 2), also because the same strains can be found in different co-occurring lichen species. More evidence for this hypothesis is found in Coniosporium and Capnobotryella, where lichen-inhabiting strains are scattered among groups of rock-inhabitants. This clearly contrasts with the ecological relationships found in Mycosphaerella and Cladophialophora. Mycosphaerella strains from lichens form two clades which are related to plant-associated fungi. Sequence divergence suggests that the lichen associates could represent distinct species with so far undetermined host specificity. The lichenicolous genus Stigmidium is phenotypically recognised in the mycological literature by the similar phenotypic features as the phytopathogenic Mycosphaerella (Mycophaerella Johanson being a younger name than Stigmidium Trevis.). The understanding of relationships between these two genera will clarify whether some our Mycosphaerella-like strains could in fact represent Stigmidium species. If this is the case, it will be interesting to assess whether they represent new species or individuals growing cryptically in a suboptimal host. Stigmidium species are regarded as highly specialised for their lichen host species but they are only recognised by their fertile structures. Cladophialophora isolates from lichens are represented in several distinct lineages. Some of them are related to the human pathogens C. bantiana, C. boppi and C. carrionii, while others are related to the moss-inhabitant C. minutissima (and a strain isolated from rocks).
Fig 2.
A. Co-culture on LB medium of Rhinocladiella sp. and Trebouxia sp. both isolated from Lecanora muralis, Bar = 1 mm; B. Thallus of Lecanora muralis showing symptoms of black fungal infection, Bar = 1 mm.
Microscopic observations of the interactions between black fungi and their host lichens are difficult. Some black fungi, including the ones found by us, are well pigmented only at the surface of the thalli. Their hyphal walls can lose their pigmentation when they stretch downwards through lichen cortex plectenchyma into the thallus, and then become unapparent. At present we cannot assess how extensively hyphae can invade the thallus and how they interact with the lichen symbionts. Only Intralichen (Hawksworth & Cole 2002) species form mycelia that also visibly extend deep into the thallus. Clear mycoparasitic or algal-parasitic behaviour is not evident and the hyphae of Intralichen grow well between fungal plectenchyma, which might be evidence for an affinity to the fungal partner in lichens. However, this is perhaps not a general feature of lichen-colonising black fungi. A direct involvement of black fungi in fungal-algal interactions was earlier described for Coniosporium aeroalgicolum, which seems to establish a balanced stage of algal parasitism (Turian 1977). Also, co-culture of various rock-inhabiting microcolonial fungi with lichen algae can develop into lichenoid structures after 2–12 mo, with the fungi contacting algae by haustoria- and appressoria-like structures (Gorbushina et al. 2005). Finally, Brunauer et al. (2007) showed that a lichen-associated black fungus (with unclear position at the basis of Chaetothyriomycetidae) discovered on Lecanora rupicola forms lichenoid structures with a range of coccal green algae in vitro.
As some Cladophialophora strains are involved in degradation of aromatic hydrocarbons (Prenafeta-Boldú et al. 2006), it might be possible that black fungi can take benefits from the numerous aromatic polyketide secondary metabolites found in lichens, but there is so far no clear evidence for this from our observations. Parts of the lichens colonised by black fungi are not necessarily bleaching or devoid of secondary compounds. Moreover, aromatic hydrocarbons can also originate from other sources. Turian (1975) noticed that Coniosporium aeroalgicolum was tolerant to high levels of air pollution, and was abundant at urban places where hydrocarbons originate from traffic exhausts. High incidence of black fungi is also noticed on roadside trees in Armenia (de Hoog, unpubl. data).
In extremely hostile places on Earth, meristematic fungi are frequently associated with lichens (Onofri et al. 2007b), where they can directly or indirectly benefit from primary production of the algae at very low temperatures and limiting water conditions. Lichens from increasingly arid habitats are often more pronouncedly colonised by dark pigmented fungi, not only at the edges of the thallus areoles and squamules, but also on the more central surfaces. The isolation of black fungi from several visually uninfected thalli suggests that black fungi are ubiquitous in lichens and wait for chances to grow out, e.g. when parts of the host become senescent (Aptroot & Alstrup 1999). Microscopic studies also confirm the frequent presence of dark-walled hyphal fragments in otherwise healthy parts of lichens. We hypothesise that black fungi readily colonise most, if not all, lichen thalli from dry sites. Moreover, we have shown here that several distinct strains can be present on a single thallus at the same time. Preliminary results using single strand conformation polymorphisms (SSCP, L. Muggia, prelim. data), indicate that up to 8 fungi can be present in a single lichen thallus (incl. the mycobiont). It is possible that we might have been able to isolate additional black fungal strains from the same samples with specific media for xerophilic fungi. However, black discolourations, especially at the thallus or areole margins do not always indicate the presence of black fungi. They may rather represent thallospores or prothalline edges formed by the lichen itself. These dark coloured mitospores can be found in a various crustose lichens, and are particularly common in arid habitats (Poelt & Obermayer 1990).
We have no evidence that associated black fungi overgrow the host when it is in a wealthy ecological state, but they may become more prominent when the lichen hosts are somewhat affected by sustained aridity. We observed that the upper cortex becomes more brittle in arid habitats than found in samples of the same species collected from more humid situations. Water dropped on such brittle surfaces is usually taken up very quickly. The thalli also do not keep the water for extended time in a gelatinous intercellular matrix, which is often better developed in the samples from more humid locations (unpubl. observation). Some of the lichen-associated meristematic black fungi may be rock-inhabitants, which appear as opportunists on lichens under certain circumstances. We observed more commonly meristematic forms on the brittle surfaces, whereas gelatinous surfaces might more often host filamentous forms of hyphomycetes. Adjacent lichens, which slightly differ in their cortical structures, can contain black fungi that were different in appearance and abundance, but further studies are required to see whether these represent different co-inhabiting species or just represent growth modifications of the lichen-colonisers. We also observed that the algal layers beneath the colonised parts of the host lichens can look rather wealthy, indicating that the algae seem to proliferate under a cover of black fungi rather than being negatively affected. It is still unclear whether black fungi could influence hydration of the lichens or even dissipate excessive sunlight to protect the algae.
However, when black fungal hyphae become abundant on the surface, the thallus structure is severely impaired. Reinfection of Lecanora rupicola with a concentrated inoculum of a black fungus isolated from the same lichen actually led to necrotic symptoms (Brunauer et al. 2007). Moreover, there are also examples for negative interactions and rather aggressive dematiaceous hyphomycetes on lichens. Black fungi apparently interact in various ways with lichens and they likely have different degrees of specificity. Future studies will show which black fungi are facultative opportunists on lichens, and which ones represent obligate and specialised lichen inhabitants. Such studies will also elucidate relationships with described lichenicolous fungi. Growing on their hosts, some of our fungi have at least some similarities with poorly understood lichenicolous species assigned to, e.g., the genera Taeniolella or Torula. Some of these described species are regarded as highly host-specific (e.g. Etayo & Calatayud 2005). This may perhaps be questioned, if future sequencing data may reveal that growth and behaviour of these fungi can be modified in other potential lichen hosts. Future studies may also resolve the relationships with the fertile lichenicolous genus Lichenostigma and poorly known species assigned to the lichenised genus Lichenothelia, which are capable of meristematic growth.
Acknowledgments
SH acknowledges support by the Austrian Exchange Service (ÖAD). Helmut Mayrhofer (Graz) is thanked for continuous discussion and logistic support. We are grateful to Sigrun Kraker and Theodora Kopun (both Graz) for technical help.
References
- Ahmadjian V (1967). The lichen symbiosis. Blaisdell Publishing Company, Massachusetts.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Aptroot A, Alstrup V (1999). Three hyphomycetes isolated from the necrotic lichen Cladonia rangiformis. Cryptogamie, Mycologie 20: 189–191. [Google Scholar]
- Büdel B, Becker U, Follmann G, Sterflinger K (2000). Algae, fungi and lichens on inselbergs. In: Inselbergs (Porembski S, Barthlott W, eds.). Ecological Studies 146: 69–90. [Google Scholar]
- Bubrick P, Galun M (1986). Spore to spore resynthesis of Xanthoria parietina. The Lichenologist 18: 47–49. [Google Scholar]
- Brunauer G, Blaha J, Hager A, Türk R, Stocker-Wörgötter E, Grube M (2007). Lichenoid structures in vitro of a cultured lichenicolous fungus. Symbiosis 44: 127–136. [Google Scholar]
- Clauzade G, Diederich P, Roux C (1989). Nelikenigintaj fungoj likenlogaj. Ilustrita determinlibro. Bulletin de la Societé Linnéenne de Provence, Numero Spécial 1: 1–142. [Google Scholar]
- Cubero OF, Crespo A, Fatehi J, Bridge PD (1999). DNA extraction and PCR amplification method suitable for fresh, herbarium stored and lichenised fungi. Plant Systematics and Evolution 217: 243–249. [Google Scholar]
- Del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch T (2006). Molecular data place Trypetheliaceae in Dothideomycetes. Mycological Research 110: 511–520. [DOI] [PubMed] [Google Scholar]
- Diederich P (1990). New or interesting lichenicolous fungi. 1. Species from Luxembourg. Mycotaxon 37: 297–330. [Google Scholar]
- Etayo J, Calatayud V (2005). Taeniolella diederichiana, a new lichenicolous hyphomycete of Placopsis. Lichenologist 37: 303–305. [Google Scholar]
- Felsenstein J (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD (1993). ITS primers with enhanced specificity for basidiomycetes. Application for the identification of mycorrhizae and rust. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, Hofstetter V, Schoch CL, Tibell L, Untereiner WA, Aptroot A (2006). Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1053–1064. [DOI] [PubMed] [Google Scholar]
- Gorbushina AA, Beck A, Schulte A (2005). Microcolonial rock inhabiting fungi and lichen photobionts: evidence for mutualistic interactions. Mycological Research 109: 1288–1296. [DOI] [PubMed] [Google Scholar]
- Hall TA (1999). BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symposium Series 41: 95–98. [Google Scholar]
- Hawksworth DL (1979). The lichenicolous hyphomycetes. Bulletin of the British Museum (Natural History), Botany Series 6: 183–300. [Google Scholar]
- Hawksworth DL, Cole MS (2002). Intralichen, a new genus for lichenicolous `Bispora' and `Trimmatostroma' species. Fungal Diversity 11: 87–97. [Google Scholar]
- Hoog GS de (1994). Ecology and pathogenicity of black yeasts. Annual Report, Research Institute of Pathogemic Fungi, Chiba Japan, pp 50–62.
- Hoog GS de, Hermanides-Nijhof EJ (1977). The black yeast and allied hyphomycetes. Studies in Mycology 15: 1–222. [Google Scholar]
- Lawrey JD, Diederich P (2003). Lichenicolous fungi: interactions, evolution and biodiversity. The Bryologist 106: 80–120. [Google Scholar]
- Lilly VG, Barnett HL (1951). Physiology of fungi. McGraw-Hill, New York.
- Muggia L, Hafellner, J, Wirtz N, Hawksworth DL, Grube M (2007). The sterile microfilamentous lichenised fungi Cystocoleus ebeneus and Racodium rupestre are relatives of plant pathogens and clinically important dothidealean fungi. Mycological Research 112: 50–56. [DOI] [PubMed] [Google Scholar]
- Navarro-Rosinés P, Roux C, Gueidan C (2007). La genroj Verrucula kaj Verruculopsis (Verrucariaceae, Verrucariales). Bulletin de la Societé Linnéenne de Provence 58: 133–180. [Google Scholar]
- Nienow JA, Friedman EI (1993). Terrestrial lithophytic (rock) communities. In: Antartic Microbiology (Friedmann EI, ed). Wiley-Liss, New York: 343–412.
- Onofri S, Selbmann L, Zucconi L, Hoog GS de, de los Rios A, Ruisi S, Grube M (2007a). Fungal Associations at the Cold Edge of Life. In: Algae and Cyanobacteria in Extreme Environments (Seckbach J, ed). Springer, Netherlands: 735–757.
- Onofri S, Selbmann L, Hoog GS de, Grube M, Barreca D, Ruisi S, Zucconi L (2007b). Evolution and adaptation of fungi at boundaries of life. Advances in Space Research 40: 1657–1664. [Google Scholar]
- Page RDM (1996). TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Palmer FE, Emery DR, Stemmler J, Staley JT (1987). Survival and growth of microcolonial rock fungi as affected by temperature and humidity. New Phytologist 107: 155–162. [Google Scholar]
- Poelt J, Obermayer W (1990). Über Thallosporen bei einigen Krustenflechten. Herzogia 8: 273–288. [Google Scholar]
- Prenafeta-Boldú FX, Summerbell R, Hoog GS de (2006). Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiology Reviews 30: 109–130. [DOI] [PubMed] [Google Scholar]
- Ruibal C, Platas G, Bills GF (2008). High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Platas G, Bills GF (2005). Isolation and characterization of melanized fungi from limestone in Mallorca. Mycological Progress 4: 23–38. [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Scott JA, Untereiner WA, Ewaze JO, Wong B, Doyle D (2007). Baudoinia, a new genus to accommodate Torula compniacensis. Mycologia 99: 592–601. [DOI] [PubMed] [Google Scholar]
- Selbmann L, Hoog GS de, Mazzaglia A, Friedmann EI, Onofri S (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic deserts. Studies in Mycology 51: 1–32. [Google Scholar]
- Sert H, Sterflinger K (2005). Biodiversity of black microcolonial fungi isolated from Antalya/Turkey (Side, Perge, Termessos) and their biodeterioration-potential. In: XVII International Botanical Congress – Abstracts, Vienna, Austria: 486.
- Staley JT, Palmer FE, Adams JB (1982). Microcolonial fungi: common inhabitants on desert rocks? Science 215: 1093–1095. [DOI] [PubMed] [Google Scholar]
- Sterflinger K (1998). Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie van Leeuwenhoek 74: 271–281. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, Hoog GS de, Haase G (1999). Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology 43: 5–22. [Google Scholar]
- Sterflinger K, Krumbein WE (1995). Multiple stress factors affecting growth of rock inhabiting black fungi. Botanica Acta 108: 490–496. [Google Scholar]
- Sterflinger K, Krumbein WE (1997). Dematiaceous fungi as a major agent for biopitting on Mediterrenean marbles and limestones. Geomicrobiological Journal 14: 219–230. [Google Scholar]
- Sterflinger K, Prillinger H (2001). Molecular taxonomy and biodiversity of rock fungal communities in an urban environment. Antonie van Leeuwenhoek 80: 275–286. [DOI] [PubMed] [Google Scholar]
- Sterflinger K (2006). Black yeast and meristematic fungi: ecology, diversity and identification. In: The Yeast Handbook. Biodiversity and Ecophysiology of Yeasts (Péter G, Rosa C, eds). Springer, Berlin, Heidelberg: 501–514.
- Stocker-Wörgötter E (2002). Investigating the production of secondary compounds in cultured lichen mycobiont. In: Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring (Kranner I, Beckett RP, Varma AK, eds). Springer-Verlag, Berlin, Heidelberg: 296–306.
- Swofford DL (2002). PAUP*, Phylogenetic Analysis Using Parsimony (and other methods), Version 4.10. Illinois Natural History Survey, Champaign, Illinois.
- Thompson JD, Higgins DG, Gibson TJ (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turian G (1975). Maxi-tolérance d'une moissisure-dématiée algicorticole du genre Coniosporium. Berichte der Schweizer Botanischen Gesellschaft 85: 204–209. [Google Scholar]
- Urzì C, Wollenzien U, Criseo G, Krumbein WE (1995). Biodiversity of the rock-inhabiting microflora with special reference to black fungi and black yeast. In: Microbial Diversity and Ecosystem Function (Allosopp D, Colwell RR, Hawksworth DL, eds). CAB International, Wallington, U.K.: 289–301.
- White TJ, Bruns TD, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal DNA genes for phylogenies. In: PCR protocols, a guide to methods and applications (Innis MA, Gelfand DH, Snisky JJ, White TJ, eds). Academic Press, San Diego: 315–322.
- Wollenzien U, Hoog GS de, Krumbein WE, Urzì C (1995). On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Science of the Total Environment 167: 287–294. [Google Scholar]
- Wollenzien U, Hoog GS de, Krumbein WE, Uijthof JMJ (1997). Sarcinomyces petricola, a new microcolonial fungus from marble in the Mediterranean basin. Antonie van Leeuwenhoek 71: 281–288. [DOI] [PubMed] [Google Scholar]
- Zalar P, Hoog GS de, Gunde-Cimerman N (1999). Ecology of halotolerant dothideaceous black yeast. Studies in Mycology 43: 38–48. [Google Scholar]
- Yamamoto Y, Kinoshita Y, Yoshimura I (2002). Culture of thallus fragments and redifferentiation of lichens. In: Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring (Kranner I, Beckett RP, Varma AK, eds). Springer-Verlag, Berlin, Heidelberg: 34–46.