Abstract
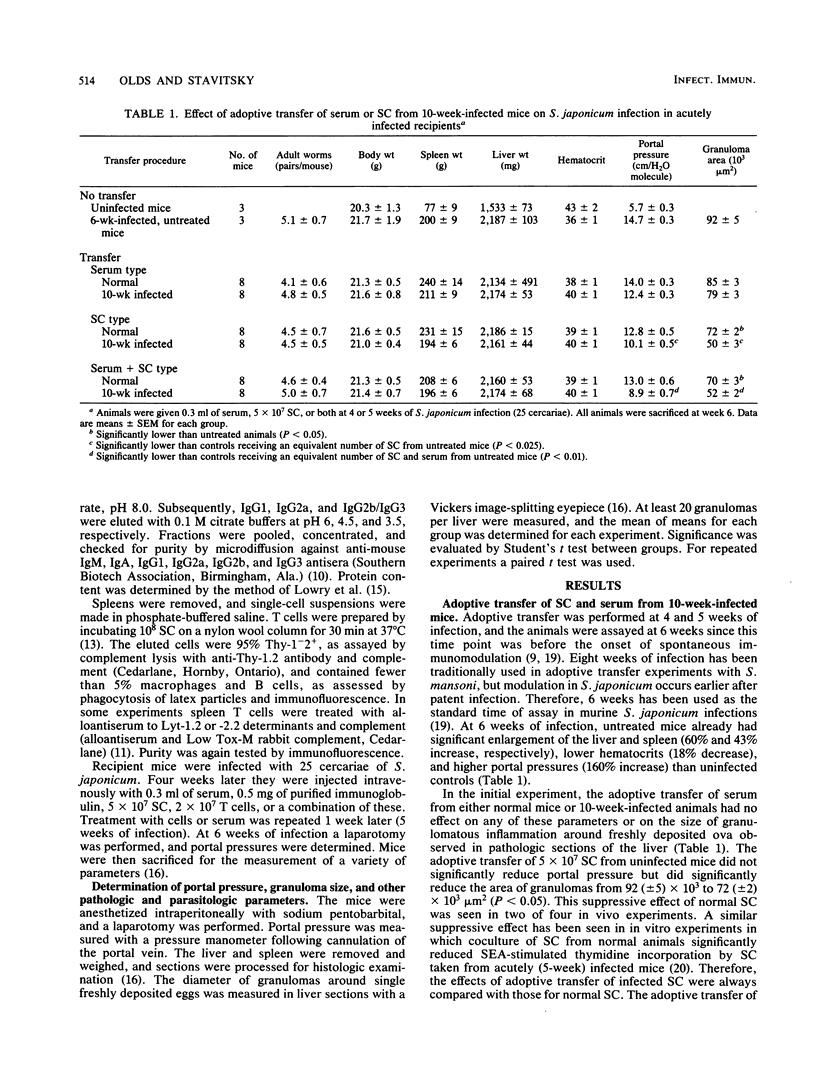
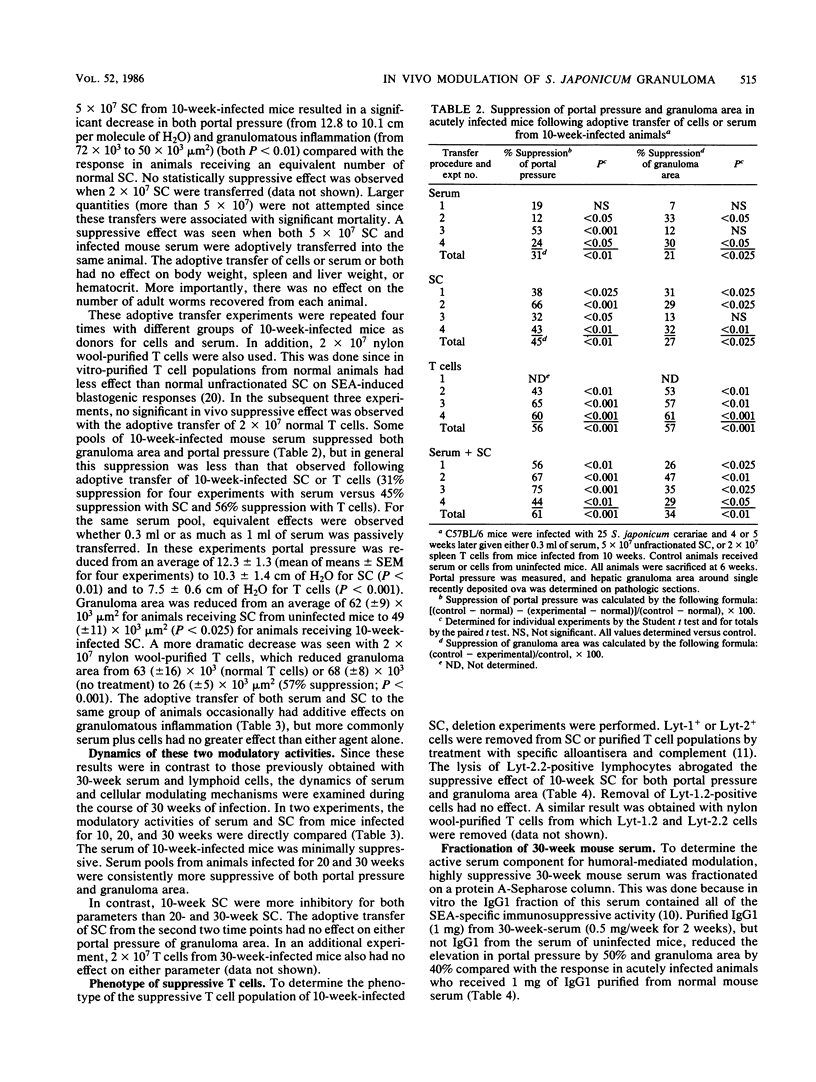
In schistosomiasis japonicum, the major pathologic lesion is the granulomatous inflammation that occurs around parasite eggs trapped in the liver. The size of these granulomas and their major sequela, a rise in portal pressure, both peak between 8 and 10 weeks after infection and then spontaneously decrease. We have shown that the adoptive transfer of the serum, but not lymphoid cells, of 30-week-infected mice caused decreases in both the size of the hepatic granulomas and the portal pressure of acutely infected recipient mice. The present study examines the role of both humoral (serum) and cellular immune mechanisms of modulation throughout the course of murine infection. Pools of serum (0.3 ml), spleen cells (5 X 10(7)), or splenic T cells (2 X 10(7)) from mice infected for 10, 20, and 30 weeks were adoptively transferred into mice at 4 and 5 weeks of infection. One week later (6 weeks postinfection), the portal pressure and size of hepatic granulomas in all recipient mice were determined. The 10-week-infected mouse serum occasionally lowered these values, but serum from 20- and 30-week-infected animals was consistently suppressive. The active component of 30-week-infected mouse serum coeluted with immunoglobulin G1. In contrast, 10-week-infected spleen cells or T cells consistently lowered portal pressure and granulomatous inflammation, but 20- and 30-week spleen cells did not. The phenotype of these suppressive T cells was Lyt-1-2+. These in vivo observations confirm earlier in vitro studies on cellular and humoral immune modulation of egg antigen-induced spleen cell blastogenesis. The current study demonstrates that both cellular and humoral regulation of granulomatous inflammation occur in murine schistosomiasis japonicum but with different kinetics: cellular mechanisms are maximal early (10 weeks) while humoral mechanisms predominate late during the chronic stage of infection (20 and 30 weeks).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Stone S. H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: description of the phenomenon and the effect of splenectomy. Immunology. 1965 Sep;9(3):205–217. [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V. The effect of previous injections of tuberculoprotein on the development of tuberculin sensitivity following B.C.G. vaccination in guinea-pigs. Br J Exp Pathol. 1957 Dec;38(6):611–617. [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., Hieny S., von Lichtenberg F., Lunde M. N., Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985 Jul;7(4):399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., von Lichtenberg F. Immunopathology of Schistosoma japonicum infection in athymic mice. Parasite Immunol. 1985 Jul;7(4):387–398. doi: 10.1111/j.1365-3024.1985.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Todd C. W. Adoptive suppression of granuloma formation by T lymphocytes and by lymphoid cells sensitive to cyclophosphamide. Cell Immunol. 1979 Aug;46(1):192–200. doi: 10.1016/0008-8749(79)90258-2. [DOI] [PubMed] [Google Scholar]
- Ferguson R. M., Anderson S. M., Simmons R. L. In vitro generation of specific and nonspecific suppression of cell-mediated cytotoxicity. Transplant Proc. 1977 Mar;9(1):919–921. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Sharrow S. O., Simpson E. T-cell populations with different functions. Nature. 1975 Feb 13;253(5492):544–546. doi: 10.1038/253544a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Little J. V., 3rd, Carter C. E., Colley D. G. Serologic responses to Schistosoma japonicum: evaluation of total and parasite-specific immunoglobulins during the course of murine infection. J Parasitol. 1982 Aug;68(4):519–528. [PubMed] [Google Scholar]
- Mahmoud A. A., Mandel A., Warren K., Webster L. T., Jr Niridazole. II. A potent long-acting suppressant of cellular hypersensitivity. J Immunol. 1975 Jan;114(1 Pt 2):279–283. [PubMed] [Google Scholar]
- Olds G. R., Kresina T. F. Network interactions in Schistosoma japonicum infection. Identification and characterization of a serologically distinct immunoregulatory auto-antiidiotypic antibody population. J Clin Invest. 1985 Dec;76(6):2338–2347. doi: 10.1172/JCI112245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981 May 15;60(2):251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Stavitsky A. B., Olds G. R., Peterson L. B. Regulation of egg antigen-induced in vitro proliferative response by splenic suppressor T cells in murine Schistosoma japonicum infection. Infect Immun. 1985 Sep;49(3):635–640. doi: 10.1128/iai.49.3.635-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


