Abstract
Melanised rock-inhabiting fungi are astonishingly resistant to environmental stresses. Also known as micro-colonial fungi (MCF), they are ubiquitous and even colonise bare rocks in deserts. To survive in nutrient poor and extremely stressful conditions, MCF have reduced morphogenetic complexity to a minimum, and rely on a broad spectrum of stress protection mechanisms. Although visual signs of carotenoid presence are masked by heavily melanised black cell-walls, we were able to isolate and characterise a variety of carotenoids (ß-carotene, ζ-carotene, phytoene, torularhodin and torulene) in the rock-inhabiting, relatively fast-growing strain A95. The desiccation/rehydration stress response was used to measure the ability of A95 to adapt to slow or fast changes in external conditions. Revival of MCF after prolonged desiccation and rehydration was documented by biochemical (analyses of lipids and protective pigments), cultivation, and microscopic methods. Survival of MCF is enhanced when desiccation is rapid and mycostasis is instant rather than following prolonged periods of low metabolic activity.
Keywords: Anhydrobiosis, carotenoids, lipids, mycostasis, protective pigments, stress responses
INTRODUCTION
Micro-colonial fungi (MCF) are the only inhabitants of varnished rock surfaces in arid regions (Staley et al. 1982) as well as ubiquitous settlers on sub-aerial rock surfaces in other climatic zones (Gorbushina et al. 1993, Urzí et al. 1995, Wollenzien et al. 1995, Sterflinger & Prillinger 2001, Ruibal 2004, Selbmann et al. 2005, Gorbushina 2007). Rock-inhabiting ascomycetes form a peculiar ecological group with simple morphology that exhibits a remarkable tolerance to stress (Palmer et al. 1987, Sterflinger & Krumbein 1995). Often stress resistance in micro-organisms is strongly correlated with an easy to simulate desiccation challenge, and here we chose desiccation / rehydration stress to investigate the capability of rock inhabiting MCF to adapt to slow or fast changes in external conditions.
Different pro- and eukaryotic organisms are able to withstand almost complete desiccation (Billi & Potts 2002). To test whether MCF are capable of surviving the removal of all but 0.1 g water / g dry weight (a condition that occurs during matric stress as well as through travel in simulated space), we took a representative strain of rock-inhabiting fungi (Sarcinomyces petricola strain A95) and measured its ability to revive. A matric stress (physical removal of water by desiccation in air) characteristic of the natural habitat of these fungi was applied for eight wks followed by sudden rehydration. Biochemical and ultra-structural changes in strain A95 were followed by analysing lipid- and pigment-composition as well as by microscopy.
MATERIAL AND METHODS
Strain
The black microcolonial fungus S. petricola strain A95 (= CBS 123872) was isolated from a marble rock surface near the Philopappos monument on Musaios Hill, Athens (Greece). This relatively fast growing strain belongs to the Chaetothyriales (Gueidan et al., 2008) and is maintained in the Geomicrobiology culture collection at the University of Oldenburg (ICBM, Oldenburg University, Germany).
Media, growth and desiccation conditions
Inocula were taken from two-wk-old pre-cultures grown on 2 % malt–extract agar (MEA) and suspended in physiological saline using a homogeniser (Ultra-Turrax T25, IKA Labortechnik, Staufen, Germany). Sterile nitrocellulose filters (Sartorius 0.22 μm, 25 mm diam) laid on MEA were inoculated with 50 μL of this suspension and fungal colonies allowed to develop in the sub-aerial environment (Fig. 1A). After eight wks of growth, the supporting filters were transferred to a desiccator. Two types of desiccation were employed: (i) fast removal of free water (to imitate environmental conditions on rock surfaces), and; (ii) slow removal of water. Phosphorus pentoxide was used as the desiccant in both cases, but for fast desiccation the nitrocellulose filters were placed in dry Petri dishes while for slow desiccation the filters were placed in Petri dishes containing a layer of an agar medium. After one wk all colonies (both desiccation types) had dried down to a constant weight (water content less then 0.1 g water / dry weight). Eight wks later, the colonies were sub-sampled for lipid and pigment analysis, as well as for ultra-structural studies by light- and transmission-electron microscopy (TEM).
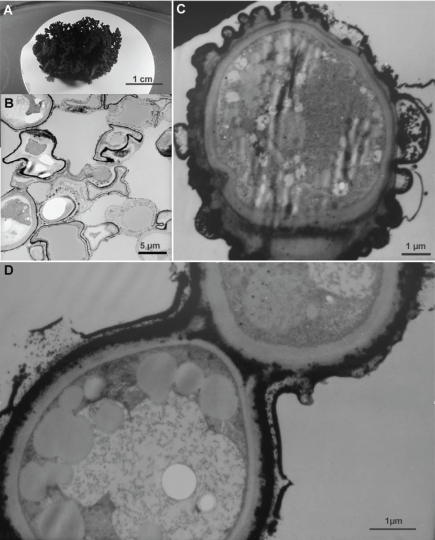
Fig. 1.
Colonies of rock-inhabiting strain A95 were grown on nitrocellulose filters (A), and subjected to fast and slow desiccation (8 wk in a desiccator containing P2O5 with and without underlying agar) and analysed microscopically. A. experimental setup, well-developed single colony just before treatment; B. TEM micrograph of a colony sectioned after 8 wk of desiccation (overview showing collapsed dehydrated cells filled with coalescent lipid inclusions). Differences were not observed between fast and slow desiccated colonies; C, D. TEM micrographs of restored cells just 24 h after rehydration.
Determination of dry weight and rate of water loss
A separate set of colonies was used to determine the rate of water loss. After transfer to a desiccator, colonies (fast- and slow-desiccated) were removed on consequent days and weighed until constant weight was achieved. These experiments were carried out in triplicate.
Cultivation studies
Discs (∼ 5 mm diameter) were cut out of desiccated colonies and placed on MEA for revival studies. These experiments were carried out in triplicate.
TEM and SEM studies
Colonies were fixed in 4 % (v/v) glutaraldehyde in 0.1 M sodium-potassium-phosphate buffer (pH 7.2) for 2 h at room temperature and post-fixed in 2 % (w/v) OsO4 overnight. An ethanol solutions series (v/v) of 30 % for 30 min, twice 50 % for 30 min, 70 % for 30 min, overnight at 80 %, 1 h at 90 % 1 h and absolute ethanol 30 min was used for dehydration. Spurr resin was used for embedding, sections were cut with an ultra-microtome. Uranyl acetate and lead citrate were used to enhance contrast (Reynolds 1963). Cryo-SEM (Hitachi S-320M, Tokyo, Japan equipped with an Oxford CT 1500 Cryostation, Oxford Instruments, U.K.) was used to examine the colonies in their native status with undisturbed extracellular matrix.
Lipid analysis
Colonies were homogenised and extracted at room temperature with chloroform / methanol (1:2) (Bligh & Dyer 1959). Lipids were fractionated on a silica-gel column eluted with chloroform, acetone, and methanol (Kates 1972). High-performance thin-layer chromatography (HPTLC) was conducted on pre-coated silica gel 60 plates (Merck, Darmstadt, Germany). Phospholipids were analysed by two-dimensional HPTLC according to the method of Vaskovsky & Terekhova (1979) using chloroform / methanol / toluene / 28 % ammonia (65:30:10:6) and chloroform / methanol / toluene / acetone / acetic acid / water (70:30:10:5:4:1) in the first and the second dimensions, respectively. Neutral lipids were separated by one-dimensional HPTLC. Toluene / hexane / formic acid (140:60:1) and hexane / diethyl ether / formic acid (60:40:1) mixtures were used sequentially as the mobile phases. The lipid spots were visualized by spraying with 5 % sulphuric acid in methanol. The contents of the individual classes of phospholipids and neutral lipids were determined by estimating phosphorus (Vaskovsky et al. 1975) and carbon (Kabara & Chen 1976), respectively. Four fungal replicates were prepared and each was sampled three times. Fatty acids were extracted using a method recommended for the Sherlock Microbial Identification System (MIDI Inc., Newark, Delaware, U.S.A.) which involves saponification of cellular lipds in hot NaOH / methanol, methylation of fatty acids with hot HCl / methanol, and extraction with hexane – methyl –tert-butyl ether. The methylated fatty acids were analysed by gas chromatography (GC 6890 Agilent Technologies, Santa Clara, CA, U.S.A.) and identified in comparison with bacterial acid methyl esters mix (Sigma-Aldrich 47080-U, St. Louis, MO, U.S.A.).
Carotenoid analysis
Methanolic extracts were separated by HPLC using a mixture of acetonitrile / tetrahydrofuran / water (5:3:1, v/v/v) at a flow rate of 1 mL / min using a C18 column (Nucleosil 100 RP 18 5 μm; 4,8 × 250 mm; Varian, Palo Alto, Ca, U.S.A.) and a diode-array detector. Carotenoids were identified by comparisons of retention times and spectral characteristics to those of pure compounds and literature data. Quantitative spectrophotometric analyses were performed on methanolic extracts that were re-extracted with hexane. Phytoene concentrations were calculated by using its specific extinction coefficient E1 %1cm = 1100 (Foppen 1971). Carotenoids that absorb visible radiation (red carotenoids) were quantified by using E1 %1cm = 3240 for torulene (Foppen 1971).
RESULTS
Water loss
In colonies subjected to fast desiccation (FD), constant weight (corresponding to the complete loss of free water) was reached in 2 to 3 h. In contrast, colonies left to dry on agar (slow desiccation – SD), more than 80 h was necessary to achieve the same result. As a consequence, fungal metabolic activities were rapidly terminated in the first treatment (FD), but only slowly declined in the latter (SD). After 7 d, all colonies were completely desiccated and this status was maintained for 8 wks under both FD and SD conditions.
Morphology
Colonies of control samples consisted of mostly grossly deformed and highly-stressed cells that contained only a limited number of lipid globules. Nevertheless, the intracellular membranes were preserved and the cytoplasm remained granular. An overwhelming majority of desiccated cells had lost turgor, contained an increased number of lipid globules (Fig. 1B). Nuclei and intracellular membranous structures were not always visible, the cytoplasm was not evident and mostly replaced by lipid inclusions (abundant coalescing lipid droplets as shown in Fig. 1B). Although the shape of the cells was restored in rehydrated colonies, intracellular structures were not always re-formed and only some cells showed fully functional granular cytoplasm and intracellular membranes (Fig. 1C,D). Budding cells were observed in rehydrated colonies (Fig. 2). Nevertheless, lipid inclusions were the most obvious feature of dehydrated cells subjected to FD and SD.
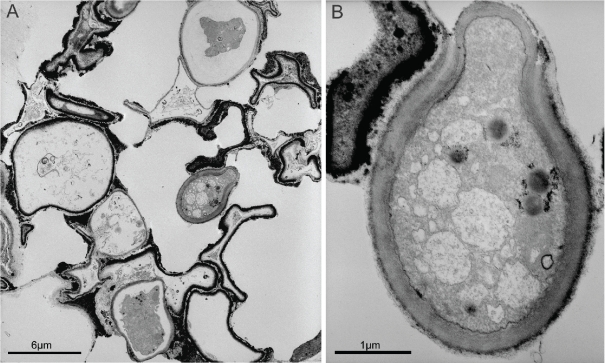
Fig. 2.
TEM micrographs of microcolonial strain A95 subjected to 8 wks of fast desiccation and rehydrated by the addition of water. A. TEM micrograph of a colony showing one surviving cell among many deformed and seemingly inactive ones. B. close-up of a budding survivor cell.
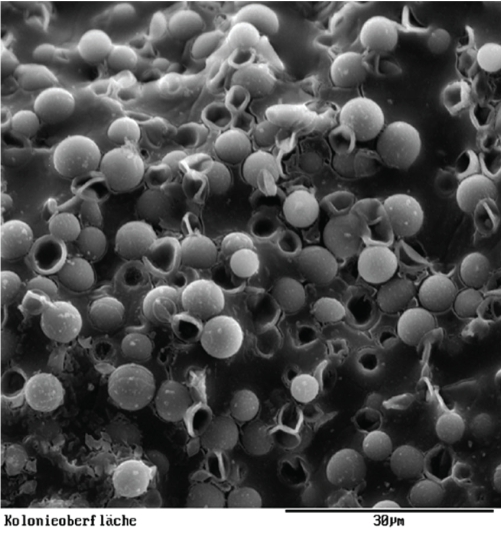
Colonies of A95 were examined by cryo-SEM to reveal extra-cellular slime (Fig. 3). Following rehydration, external layers of the cell walls were remarkably swollen (Figs 1C,D), showing that the extra-cellular matrix of A95 was capable of rapidly absorbing large amounts of water.
Fig. 3.
Cryo-SEM micrograph of microcolonial strain A95 showing abundant colony slime.
Lipid composition
The lipid fraction comprised of monoacylglycerols, 1,2- and 1,3,-diacylglycerols, triacylglycerols, phosphotidincholine, phosphatidlethanolamine, phosphatidylinositole, phosphatidic acids and sterols (both sterol esters and free fatty acids). Fatty acids were mostly unsaturated C16:0, and C18:0 although some C18:2 were present. These lipids play strikingly different physiological roles including in membranes [phosphatidylcholine (PC), phosphatidylethanolamine (PE), sterols (S)] and as reserves or neutral lipids [diacyl- and triacyl-glycerols, sterol ethers (SE) and free fatty acids (FA)].
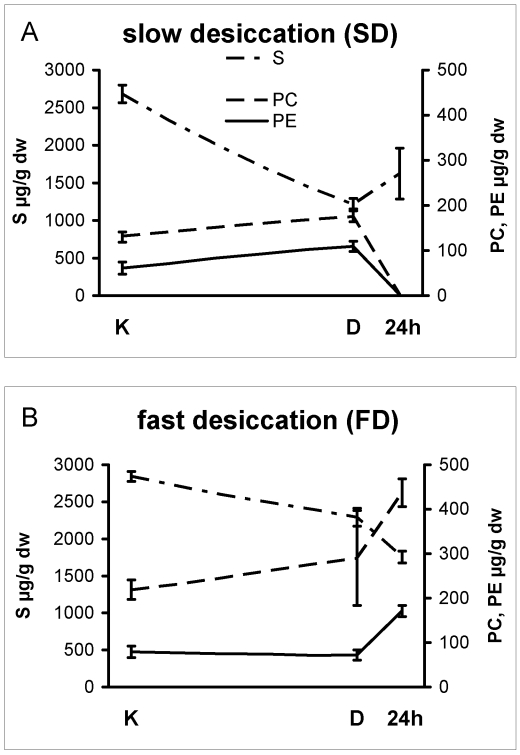
Lipid contents varied significantly between samples that were subjected to fast- or slow-desiccation (cf. Figs 4, 5). Colonies that were desiccated slowly lost the major part of their sterols during drying (Fig. 4A), whereas those that dried rapidly maintained a stable level of membrane lipids (Fig. 4B). The different rehydration regimes only magnified these differences: major membrane lipids like phosphatidylcholine and phosphatidylethanolamine were practically absent in SD colonies (Fig. 4A, 24 h), while FD colonies showed a steady increase in both phosphatidylcholine and phosphatidylethanolamine levels (Fig. 4B).
Fig. 4.
Membrane lipids in colonies of microcolonial strain A95 subjected to slow (A) and fast (B) desiccation. The time axis is not to scale, as the distance between K and D equals 8 wks of desiccation, while 24 h is 24 hs after water was added to the desiccated colonies. K = control (stationary phase colony, before being transferred to a desiccator), D = after 8 wk of desiccation; 24 h = re-hydrated colony 24 h later. S = sterols, PC = phosphatidylcholine, PE = phosphatidylethanolamine.
Fig. 5.
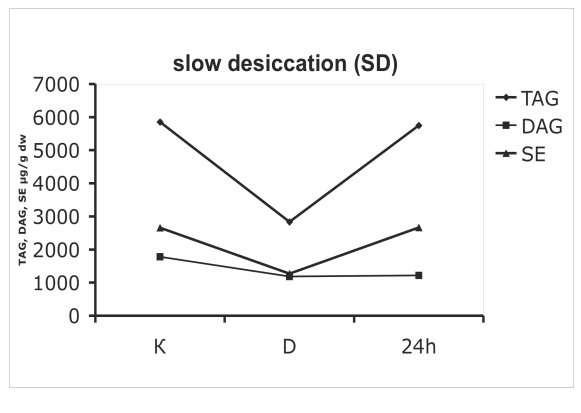
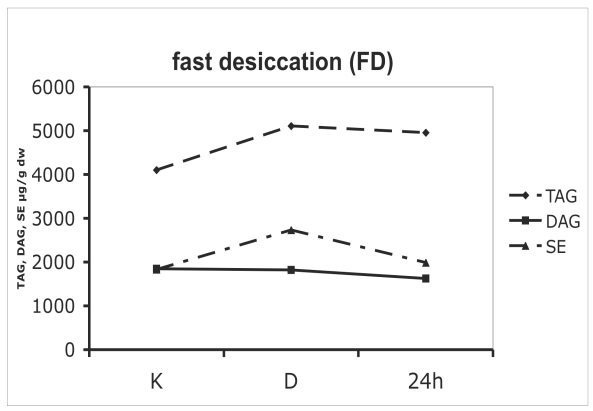
Neutral (storage) lipids in colonies o microcolonial strain A95 subjected to slow (A) and fast (B) desiccation. The time axis is not to scale - the distance between K and D equals 8 wk of desiccation, while 24 h represents 24 hs after re-hydration. K = control, D = after 8 wk of desiccation; 24 h = re-hydrated colony after 24 h. TAG = triacylglycerol; DAG = diacylglycerol, SE = sterol esters.
Desiccated cells contained high amounts of neutral lipids like triacylglycerol (TAG) and diacylglycerol (DAG). Storage lipids also displayed significantly diverse dynamics between slow- and fast-dehydrated mycelia. Neutral lipids decreased during the dehydration when the process was slow (Fig. 5A), while a slight increase occurred when desiccation was fast. TAG levels of SD cells were reduced by 50 % as compared to controls (Fig 5A), while FD cells contained 1,4-fold higher amounts of TAG (Fig. 5B). After rehydration, the constant levels of neutral lipids like triacylglycerols and sterol ethers suggest a stable metabolic state that was not significantly influenced by desiccation stress (Fig. 5B). The proportion of rapidly and slowly oxidised lipids in cell membranes and intracellular inclusions was checked in A95 following the desiccation challenge. The proportion of unsaturated fatty acids was always relatively high. In control cells, 81 % of all fatty acids were unsaturated. After 8 wk of anhydrobiosis the ratio of unsaturated fatty acids in SD and FD cells equalled respectively 80 and 67 % of the total fatty acids. In desiccated cells after 24 h of rehydration the values reached 86 % in SD cells, while rehydrated FD cells had equal proportions of saturated and unsaturated fatty acids (50:50 %).
As shown by biochemical methods, intra-cellular structures in fast desiccated cells were largely preserved and their biochemical status (synthesis of carotenoids and phospholipids) supported these observations (Figs 4B, 5B, 6B). In contrast, SD colonies (Figs 4A, 5A, 6A), were less viable although some cells in a colony were still capable of re-growth. Decreased levels of triglycerols and sterol esters suggested lower levels of storage lipids in SD cells. Only trace amounts of phospholipids were found following rehydration, confirming the complete loss of membrane structure in SD colonies. Under TEM, these cells looked like vials filled with lipid droplets, confirming the decay of intracellular membrane structures.
Fig. 6.
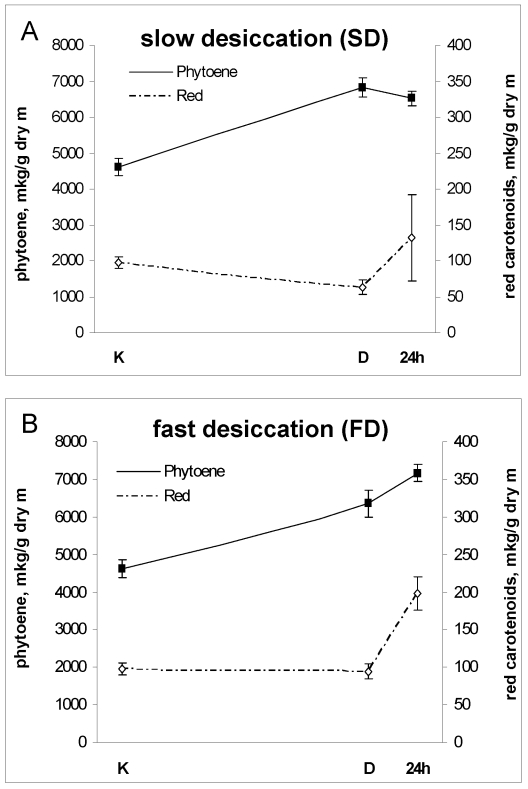
Pigment composition in colonies of microcolonial strain A95 subjected to slow (A) and fast (B) desiccation. For further details see Figs 3 and 4.
Pigment analysis
As expected (Gorbushina et al. 1993, Butler & Day 1997) melanin was obvious under TEM (Figs 1, 2) and no further characterisation of this pigment group was carried out. Pigments found in A95 absorbed radiation in UV- and visible-wavelengths. Both red carotenoids and colourless UV-absorbing carotenoids as well as mycosporines were present. Five different carotenoid pigments were identified in A95: ß-carotene, ζ-carotene, phytoene, torulene and torularhodin. Mycosporines included mycosporine-glutaminol, mycosporine-glutaminol-glucoside, mycosporine-glutamicol, and mycosporine-glutamicol-glucoside (Volkmann & Gorbushina 2006). Carotenoid contents also varied during desiccation / rehydration stress (Fig. 6). Levels of phytoene, a colourless precursor of carotenoid synthesis were particularly sensitive to stresses, but the amounts of red carotenoids decreased under SD (Fig. 6A) or remained unchanged in conditions of FD (Fig. 6B).
DISCUSSION
Cultivation, biochemical- and ultra-structural analyses have shown that MCF strain A95 is capable of surviving desiccation / rehydration stress and sudden changes in the availability of water. Desiccated cells maintained their typical MCF spore-like ultra-structure (Sterflinger 1998, Gorbushina 2003, Gorbushina et al. 2003), and all colonies were able to re-grow after long-term desiccation, as well as following the rehydration challenge. Large inocula (discs of 5 mm) aided survival as parts of colonies always contained viable cells that gave rise to new colonies. Even when viable cells were few, some survivor cells in the middle of a colony were protected by the surrounding cells (Fig. 2) and were abundant enough to permit re-growth.
The decrease in the proportion of unsaturated fatty acids in both SD and FD colonies with and without dehydration indicates significant stress. Unsaturated fatty acids in membranes of A95 remained high (50 to 80 %) in all cases. Rehydrated SD colonies managed to keep the ratio of unsaturated fatty acids at the level of unstressed (unchallenged) controls, while in FD cells this parameter decreased to slightly more than 50 %. This value is, however, still high and shows an intrinsic ability of A95 to adapt to desiccation / rehydration stress.
Desiccated A95 cells contained high amounts of neutral lipids that served as a reserve for the synthesis of phospholipid-membrane following stress relief. Rapidly desiccated cells largely retained their normal physiological status. After 8 wk of matric stress and one day of rehydration, phospholipid synthesis was restored to levels that permitted correct functioning of membranes (Fig. 4B). TEM showed that budding cells were indeed present after 24 h of rehydration, confirming that the colonies remained viable (Fig. 2).
In contrast, slow desiccation (complete water loss over a period of several days, which kept metabolism active for approx. 80 h) followed by rehydration, drastically reduced phospholipid contents (Fig. 4A), and resulted in complete degradation of membrane systems. This could have been caused by the exhaustion of internal lipid resources as TAG levels of SD cells were reduced by 50 % compared to control values (Fig. 5A).
Typical fungal carotenoids include molecules with 13 conjugated double bonds that are important antioxidants (e.g. torulene and torularhodin), which help to stabilise membranes under unfavourable conditions All carotenoids found in A95 have been previously observed in different asco- and basidiomycetous yeasts and thus are typical fungal pigments (Davoli & Weber 2002, Weber & Davoli 2002, Davoli et al. 2004). As A95 was grown in the dark, the carotenoids described here belong to the natural metabolites of MCF. Constitutive levels of carotenoids in a majority of MCF strains (data not shown) suggest a readiness to counteract stress. Fungal carotenoids are reported to occur predominantly in cytoplasmic lipid bodies, the endoplasmatic reticulum, cell walls and EPS layers (Rikkinen 1995). Carotenoids are abundant in lipid inclusions of fungi, but are also major components of the cell wall and the cell membrane (Rikkinen 1995).
Phytoene is generally accepted as a precursor of less-saturated C40 carotenoids, which are synthesised from phytoene through a series of desaturation reactions (Simpson 1972). All types of environmental stresses promote accumulation of this colourless precursor (Fig. 6). The amount of red carotenoids either remained unchanged under conditions of fast desiccation or UV-radiation or was reduced when the cells were slowly desiccated. Fast desiccation stimulated both phytoene and red carotenoid synthesis (Fig. 6B) while preventing degradation of protective pigments.
During slow desiccation, colonies are forced to subsist with ever lower levels of available water and as a result retain synthetic activities until their internal resources (especially reserve lipids) are exhausted. In contrast, fast drying forces vegetative MCF to shift rapidly to a dormant state in which levels of reserve storage compounds, protective carotenoid precursors and constitutive antioxidants like melanins and mycosporines are maintained.
In many rock surface environments, access to sufficient sources of energy, nutrients and water rarely coincides. For this reason, micro-organisms that dwell on and in rocks need to be able to maintain biomass during extended periods of dormancy. We have shown here that MCF are capable of fast recovery after prolonged desiccation, proving that the cellular machinery remains in a state of suspended animation. This immediate revival from an anhydrobiotic state clearly demonstrates the ability of MCF to recover from water deficits that might be lethal to many prokaryotes.
The most important findings of this study are: A the fact that MCF responses to stresses are unspecific and thus can be employed against various environmental challenges (in this sense, tolerance to desiccation is perhaps part of a broader range of adaptations to other stresses; Mattimore & Battista 1996), and B that rapid as opposed to slow desiccation best preserves the viability of MCF. This preference reflects an inherent capacity of MCF to respond to drastic changes in the environmental conditions typical of subaerial rock surfaces (Gorbushina 2003). Enhanced survival of MCF when subjected to rapid changes in the environment provides strong experimental support for the poikilotolerance hypothesis proposed for rock inhabiting organisms (Gorbushina & Krumbein 2000).
CONCLUSIONS
During desiccation-induced mycostasis (dormancy), MCF employ a broad range of unspecific stress response mechanisms including:
cell wall re-encrustation with irradiation-protective and antioxidant melanins (Gorbushina et al. 1993, 2003);
layers of EPS containing lipids, polysaccharides, and proteins act as an additional irradiation filter and may have properties similar to those described for glass-forming polymers (Hill et al. 1997);
presence of diverse carotenoids that are known to stabilise cell membranes;
presence of intracellular mycosporines (typical of fungal survival structures such as spores, fruit bodies and sclerotia) that act as UV-filters, antioxidants and minor osmolytes (Gorbushina et al. 2003, Volkmann et al. 2003, Kogej et al. 2006, Kogej et al. 2007), and;
as witnessed by a high proportion of unsaturated fatty acids and normal levels of membrane lipids (phosphatidylcholine, phosphatidylethanolamine), membranes in dormant desiccated MCF are maintained in a stable physiological state.
Acknowledgments
This work was financially supported by a grant of DFG (Go 897/2) as well as by the Ministry of Science and Culture of Lower Saxony (Dorothea-Erxleben-Programm). Technical help of Anette Schulte, Daria Morozova and Renate Kort is deeply appreciated. William J. Broughton is gratefully acknowledged for his valued help in editing our English text.
References
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW (2007). Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi D, Potts M (2002). Life and death of dried prokaryotes. Research in Microbiology 153: 7–12. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911–915. [DOI] [PubMed] [Google Scholar]
- Britton G (1995). UV/visible spectroscopy. Carotenoids 1B: Spectroscopy, Basel: Birkhauser, 13–62.
- Butler MJ, Day AW (1998). Fungal melanins: a review. Canadian Journal of Microbiology 44: 1115–1136. [Google Scholar]
- Davoli P, Mierau V, Weber RWS (2004). Carotenoids and fatty acids in red yeasts Sporobolomyces roseus and Rhodotorula glutinis. Applied Biochemistry and Microbiology 40: 392–397. [PubMed] [Google Scholar]
- Davoli P, Weber RWS (2002). Identification and quantification of carotenoid pigments in aeciospores of the daisy rust fungus, Puccinia distincta. Phytochemistry 60: 309–313. [DOI] [PubMed] [Google Scholar]
- Gorbushina AA, Krumbein, WE, Hamann, CH, Panina, LK, Soukharzhevski, S, Wollenzien, U. (1993). Role of black fungi in color-change and biodeterioration of antique marbles. Geomicrobiological Journal 11: 205–221. [Google Scholar]
- Gorbushina AA (2003). Microcolonial fungi: survival potential of terrestrial vegetative structures. Astrobiology 3: 543–554. [DOI] [PubMed] [Google Scholar]
- Gorbushina AA (2007). Life on the Rocks. Environmental Microbiology 9: 1613–1631. [DOI] [PubMed] [Google Scholar]
- Gorbushina AA, Krumbein WE (2000). Subaerial microbial mats and their effects on soil and rock. In: Microbial Sediments (Riding R & Awramik S, eds). Springer. Berlin: 161–170
- Gorbushina AA, Whitehead K, Dornieden Th, Niesse A, Schulte A, Hedges JI (2003). Black fungal colonies as units of survival: hyphal mycosporines synthesised by rock dwelling microcolonial fungi. Canadian Journal of Botany 81: 131–138. [Google Scholar]
- Hill DR, Keenan TW, Helm RF, Potts M, Crowe LM, Crowe JH (1997). Extracellular polysaccharide of Nostoc commune (Cyanobacteria) inhibits fusion of membrane vesicles during desiccation. Journal of Applied Phycology 9: 237–248. [Google Scholar]
- Kabara JJ, Chen JS (1976). Microdetermination of lipid classes of thin-layer chromatography. Analytical Chemistry 48: 814–817. [DOI] [PubMed] [Google Scholar]
- Kates M (1972). Techniques of lipidology. Elsevier, Amsterdam.
- Kogej T, Gostincar C, Volkmann M, Gorbushina AA, Gunde-Cimerman N (2006). Screening of halophilic and psychrophilic fungi for mycosporines – could they act as compatible solutes in fungi? Environmental Chemistry 3: 105–110. [Google Scholar]
- Kogej T, Stein M, Volkmann M, Gorbushina AA, Galinski EA, Gunde-Cimerman N (2007). Osmotic adaptation of halophilic fungus Hortaea werneckii: role of osmolytes and melanization. Microbiology (U.K.) 153: 4261–4273. [DOI] [PubMed] [Google Scholar]
- Kotlova ER, Gorbushina AA (2003). Adaptive response of rock inhabiting microcolonial ascomycetes to de- and rehydration. Joint MSA/BMS Meeting, Assilomar, July 26–31, 2003.
- Mattimore V, Battista JR (1996). Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. Journal of Bacteriology 178: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer FE, Emery J, Staley JT (1987). Survival and growth of microcolonial fungi as affected by temperature and humidity. New Phytologist 107: 155–162. [Google Scholar]
- Rikkinen J (1995). What's behind the pretty colours? A study on the photobiology of lichens. Bryobrothera 4: 1–239. [Google Scholar]
- Ruibal CV (2004). Isolation and characterization of melanized, slow-growing fungi from semiarid rock surfaces of central Spain and Mallorca. Ph.D. dissertation, Universidad Autónoma de Madrid Merck, Sharp & Dohme de España, Madrid.
- Ruibal C, Platas G, Bills GF (2008). High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbmann L, Hoog GS de, Mazzaglia A, Friedmann EI, Onofri S (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Studies in Mycology 51: 1–32. [Google Scholar]
- Simpson KL (1972). The biosynthesis of yeast carotenoids. In: The chemistry of plant pigments (Chichester CO, eds). Academic Press, New York and London.
- Simpson KL, Nakayama TOM, Chichester CO (1964). Biosynthesis of yeast carotenoids. Journal of Bacteriology 88: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JT, Palmer F, Adams JB (1982). Microcolonial fungi: common inhabitants on desert rocks? Science 215: 1093–1095. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, Prillinger H (2001). Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria). Antonie van Leeuwenhoek 80: 275–286. [DOI] [PubMed] [Google Scholar]
- Sterflinger K (1998). Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie van Leeuwenhoek 74: 271–281. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, Krumbein WE (1995). Multiple stress factors affecting growth of rock-inhabiting black fungi. Botanica Acta 108: 490–496. [Google Scholar]
- Urzi C, Wollenzien U, Criseo G, Krumbein WE (1995) Biodiversity of the rock inhabiting microbiota with special reference to black fungi and black yeasts. In Microbial diversity and ecosystem function (Allsopp D, Colwell RR, Hawksworth DL, eds). CAB International, Wallingford: 289–302.
- Vaskovsky VE, Kostetsky EY, Vasendin IM (1975). A universal reagent for phospholipid analysis. Journal of Chromatography 114: 129–141. [DOI] [PubMed] [Google Scholar]
- Vaskovsky VE, Terekhova TA (1979). HPTLC of phospholipid mixtures containing phosphatidylglycerol. Journal of High Resolution Chromatography and Chromatography Communications 2: 671–672. [Google Scholar]
- Volkmann M, Gorbushina AA (2006). A broadly applicable method for extraction and characterisation of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiology Letters 255: 286–295. [DOI] [PubMed] [Google Scholar]
- Volkmann M, Whitehead K, Rütters H, Rullkötter J, Gorbushina AA (2003). Mycosporine-glutamicol-glucoside: a native UV-absorbing secondary metabolite of rock inhabiting microcolonial fungi (MCF). Rapid Communications in Mass Spectroscopy 17: 897–902. [DOI] [PubMed] [Google Scholar]
- Weber RWS, Davoli P (2002). Autophagocytosis of carotenoid-rich lipid droplets into vacuoles during aeciospore ageing in Puccinia distincta. New Phytologist 154: 471–479. [DOI] [PubMed] [Google Scholar]
- Wollenzien U, Hoog GS de, Krumbein WE, Uijthof JMJ (1997) Sarcinomyces petricola, a new microcolonial fungus from marble in the Mediterranean basin. Antonie van Leeuwenhoek 71: 281–288. [DOI] [PubMed] [Google Scholar]