Abstract
Covalent binding to immunogenic proteins increases the immunogenicity of the capsular polysaccharides of Haemophilus influenzae type b (Hib) and pneumococcus type 6A (Pn6A). Conjugates composed of Hib, Pn6A, or the cross-reacting Escherichia coli K100 covalently bound to tetanus toxoid (TT) were injected into young adult volunteers. Local reactions were common and were probably due to Arthus reactivity mediated by the preexisting antibodies reacting with the TT component of the conjugates. Fever occurred in about 10% of the volunteers after the first injection; no volunteers had fever after the second injection. Similar levels of Hib or Pn6A antibodies were elicited by either 50- or 100-micrograms doses or by concurrent injection of two different conjugates (Hib-TT and Pn6A-TT or Hib-TT and K100-TT). The Hib-TT elicited about a 180-fold increase in Hib antibodies, and the Pn6A-TT conjugate elicited about an 8-fold increase in Pn6A antibodies after one injection. Booster reactions were not elicited in adults; similar levels of antibodies in the five experimental groups suggested that the responses elicited by the conjugates were maximal. A one-way cross-reaction was noted as Pn6A conjugates elicited rises of Hib antibodies in 13 of 20 volunteers; only 4 of 59 volunteers immunized with Hib-TT had increases in Pn6A antibodies. The preimmunization Hib antibodies were composed of immunoglobulin M (IgM), IgA, and IgG. The postimmunization sera showed an increase in all three isotypes; the elevation of the IgG was the highest of the three isotypes. Conjugate-induced antibodies to both the polysaccharide and TT exerted biological activities that have been correlated with immunity. Adsorption of the Hib-TT onto aluminium hydroxide resulted in higher levels and an earlier Hib antibody response in infant rhesus. These results encourage the evaluation of Hib and Pn6A conjugates in human children and infants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. B., BJORNEBOE M. GAMMA GLOBULIN TURNOVER IN RABBITS BEFORE AND DURING HYPERIMMUNIZATION. J Exp Med. 1964 Apr 1;119:537–546. doi: 10.1084/jem.119.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemeier W. A., 3rd, Bellanti J. A., Buescher E. L. The IgM response of children to Salmonella typhosa vaccine. I. Measurements of anti-typhoid concentrations and their proportions of total serum IgM globulins. J Immunol. 1969 Nov;103(5):917–923. [PubMed] [Google Scholar]
- Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983 Jan;39(1):233–238. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Pichichero M., Insel R., Farsad P., Santosham M. Capsular antigens noncovalently or covalently associated with protein as vaccines to Haemophilus influenzae type b: comparison in two-year-old children. J Infect Dis. 1985 Sep;152(3):634–636. doi: 10.1093/infdis/152.3.634. [DOI] [PubMed] [Google Scholar]
- Avery O. T., Goebel W. F. CHEMO-IMMUNOLOGICAL STUDIES ON CONJUGATED CARBOHYDRATE-PROTEINS : II. IMMUNOLOGICAL SPECIFICITY OF SYNTHETIC SUGAR-PROTEIN ANTIGENS. J Exp Med. 1929 Sep 30;50(4):533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Hardegree M. C., Pittman M. Immunization against neonatal tetanus in New Guinea. 3. The toxin-neutralization test and the response of guinea-pigs to the toxoids as used in the immunization schedules in New Guinea. Bull World Health Organ. 1970;43(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Bell C. G. Fine-specificity of the lambda and chi L chains associated with antibodies directed to alpha (1 leads to 3) glucosyls in dextran. Scand J Immunol. 1983 Dec;18(6):473–484. doi: 10.1111/j.1365-3083.1983.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Bell C. V and C genes in creating antibodies directed to alpha (1 replaced by 3)-linked D-glucosyls in dextran. III. Frequency of alpha (1 replaced by 3)-responsive, primary B precursors in a1 high- and b low-receptor allogroups. Scand J Immunol. 1982 Jan;15(1):71–80. doi: 10.1111/j.1365-3083.1982.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
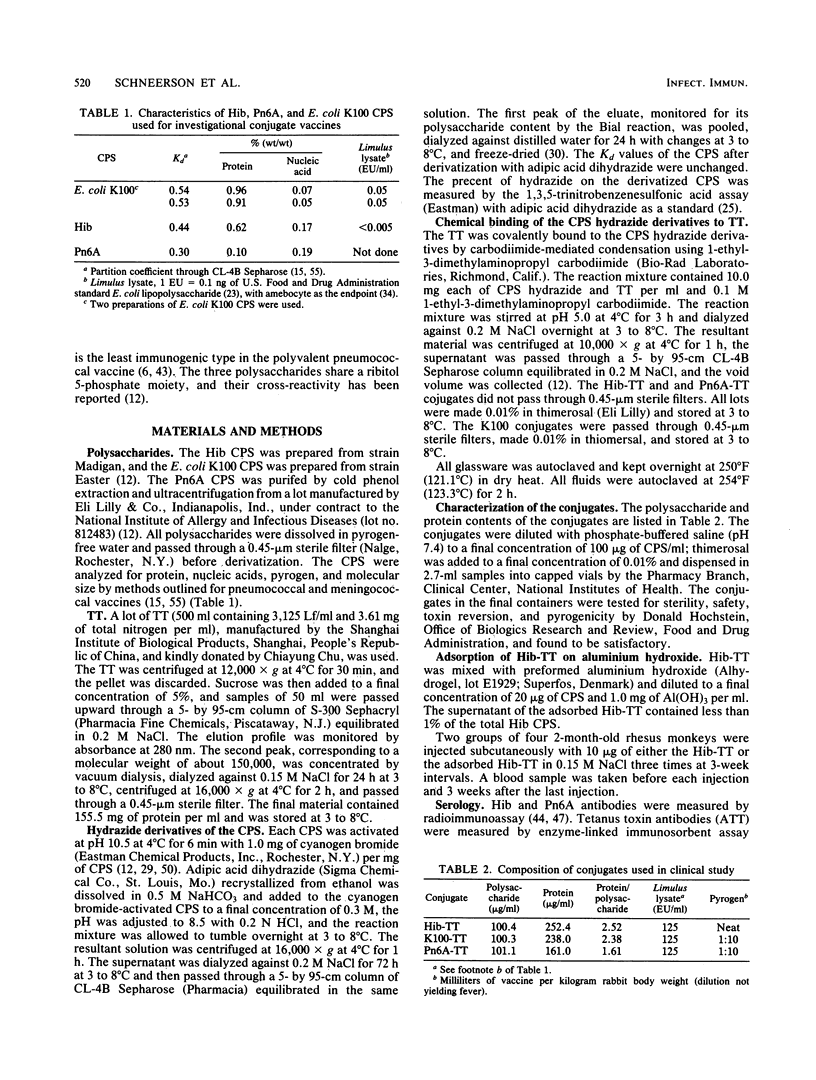
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Boies E. G., Munson R. S., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide--diphtheria toxoid conjugate vaccine in adults. J Pediatr. 1984 Jul;105(1):22–27. doi: 10.1016/s0022-3476(84)80350-9. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- Hochstein H. D., Mills D. F., Outschoorn A. S., Rastogi S. C. The processing and collaborative assay of a reference endotoxin. J Biol Stand. 1983 Oct;11(4):251–260. doi: 10.1016/s0092-1157(83)80013-4. [DOI] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W., Jr Cross-reactivity with Escherichia coli K100 in the human serum anticapsular antibody response to Haemophilus influenzae type B. J Immunol. 1982 Mar;128(3):1267–1270. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost R., Miron T., Wilchek M. The mode of adsorption of proteins to aliphatic and aromatic amines coupled to cyanogen bromide-activated agarose. Biochim Biophys Acta. 1974 Aug 7;362(1):75–82. doi: 10.1016/0304-4165(74)90028-2. [DOI] [PubMed] [Google Scholar]
- Kuronen T., Peltola H., Nors T., Haque N., Mäkelä P. H. Adverse reactions and endotoxin content of polysaccharide vaccines. Dev Biol Stand. 1977;34:117–125. [PubMed] [Google Scholar]
- Käyhty H., Jousimies-Somer H., Peltola H., Mäketä P. H. Antibody response to capsular polysaccharides of groups A and C neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J Infect Dis. 1981 Jan;143(1):32–41. doi: 10.1093/infdis/143.1.32. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Mäkelä P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984 Nov;74(5):857–865. [PubMed] [Google Scholar]
- Lepow M. L., Samuelson J. S., Gordon L. K. Safety and immunogenicity of Haemophilus influenzae type B polysaccharide-diphtheria toxoid conjugate vaccine in adults. J Infect Dis. 1984 Sep;150(3):402–406. doi: 10.1093/infdis/150.3.402. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Péterfy F., Outschoorn I. G., Richter A. W., Seppälä I. Immunogenic properties of alpha (1----6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand J Immunol. 1984 Jun;19(6):541–550. doi: 10.1111/j.1365-3083.1984.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Parke J. C., Jr, Schneerson R., Robbins J. B., Schlesselman J. J. Interim report of a controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mecklenburg county, North Carolina (March 1974-March 1976). J Infect Dis. 1977 Aug;136 (Suppl):S51–S56. doi: 10.1093/infdis/136.supplement.s51. [DOI] [PubMed] [Google Scholar]
- Pedersen F. K., Nielsen J. L., Ellegaard J. Immunoglobulin classes and persistence of anti-pneumococcal antibodies in splenectomized adults and adolescents after pneumococcal vaccination. Acta Pathol Microbiol Immunol Scand C. 1983 Aug;91(4):245–249. [PubMed] [Google Scholar]
- Peebles T. C., Levine L., Eldred M. C., Edsall G. Tetanus-toxoid emergency boosters: a reappraisal. N Engl J Med. 1969 Mar 13;280(11):575–581. doi: 10.1056/NEJM196903132801102. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Kuronen T., Haque N., Sarna S., Mäkelä P. H. Meningococcus group A vaccine in children three months to five years of age. Adverse reactions and immunogenicity related to endotoxin content and molecular weight of the polysaccharide. J Pediatr. 1978 May;92(5):818–822. doi: 10.1016/s0022-3476(78)80165-6. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Porro M., Costantino P., Viti S., Vannozzi F., Naggi A., Torri G. Specific antibodies to diphtheria toxin and type 6A pneumococcal capsular polysaccharide induced by a model of semi-synthetic glycoconjugate antigen. Mol Immunol. 1985 Aug;22(8):907–919. doi: 10.1016/0161-5890(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Chu C., Sutton A., Vann W., Vickers J. C., London W. T., Curfman B., Hardegree M. C., Shiloach J. Serum antibody responses of juvenile and infant rhesus monkeys injected with Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-protein conjugates. Infect Immun. 1984 Sep;45(3):582–591. doi: 10.1128/iai.45.3.582-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975 May 22;292(21):1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Ambrosino D. M., McIver J., Ervin T. J., Schiffman G., Sallan S., Grady G. F. Preparation of human hyperimmune globulin to Haemophilus influenzae b, Streptococcus pneumoniae, and Neisseria meningitidis. Infect Immun. 1984 Jul;45(1):248–254. doi: 10.1128/iai.45.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Trinca J. C. Antibody response to successive booster doses of tetanus toxoid in adults. Infect Immun. 1974 Jul;10(1):1–5. doi: 10.1128/iai.10.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Barrera O., Sutton A., May J., Hochstein D. H., Robbins J. D., Robbins J. B., Parkman P. D., Seligmann E. B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5(3):197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]


