Abstract
Using media with low water activity, a large numbers of aureobasidium-like black yeasts were isolated from glacial and subglacial ice of three polythermal glaciers from the coastal Arctic environment of Kongsfjorden (Svalbard, Spitsbergen), as well as from adjacent sea water, sea ice and glacial meltwaters. To characterise the genetic variability of Aureobasidium pullulans strains originating from the Arctic and strains originating pan-globally, a multilocus molecular analysis was performed, through rDNA (internal transcribed spacers, partial 28 S rDNA), and partial introns and exons of genes encoding β-tubulin (TUB), translation elongation factor (EF1α) and elongase (ELO). Two globally ubiquitous varieties were distinguished: var. pullulans, occurring particularly in slightly osmotic substrates and in the phyllosphere; and var. melanogenum, mainly isolated from watery habitats. Both varieties were commonly isolated from the sampled Arctic habitats. However, some aureobasidium-like strains from subglacial ice from three different glaciers in Kongsfjorden (Svalbard, Spitsbergen), appeared to represent a new variety of A. pullulans. A strain from dolomitic marble in Namibia was found to belong to yet another variety. No molecular support has as yet been found for the previously described var. aubasidani. A partial elongase-encoding gene was successfully used as a phylogenetic marker at the (infra-)specific level.
Keywords: Arctic, Aureobasidium, black yeasts, elongase, glacier, ITS, LSU, phylogeny, polar environment, rDNA, sea ice, seawater, taxonomy, translation elongation factor, β-tubulin
INTRODUCTION
Aureobasidum pullulans (De Bary) G. Arnaud is a black yeast-like species that is particularly known for its biotechnological significance as a producer of the biodegradable extracellular polysaccharide (EPS) pullulan (poly-α-1,6-maltotriose). This component is a promising biomaterial (Rekha & Sharma 2007), and is currently used among others for the packaging of food and drugs (Singh et al. 2008). Its biotechnological potential is also seen in the production of a variety of hydrolytic enzymes (Federici 1982, Chi et al. 2006, Wang et al. 2007, Li et al. 2007, Ma et al. 2007, Zhiqiang et al. 2008).
Aureobasidum pullulans was taxonomically characterised by de Hoog & Yurlova (1994) on the basis of its morphology and nutritional physiology. These authors noted some differences in growth with galactitol, glucono-δ-lactone, creatine and creatinine, and in gelatin liquefaction. Since the species shows considerable variability in its morphological and physiological properties, three varieties have been described during the last decades, viz. Aureobasidium pullulans var. pullulans (Viala & Boyer 1891), A. pullulans var. melanogenum Hermanides-Nijhof (1977), and A. pullulans var. aubasidani Yurlova (Yurlova & de Hoog 1997). The first two of these were distinguishable by culture discolouration, while the latter is unique in its production of aubasidan-like EPS (glucans with α-1,4-D-, ß-1,6-D- and ß-1,3-D-glycosidic bonds). Diagnostically, var. aubasidani is unique due to the absence of assimilation of methyl-α-D-glucoside and lactose and by N-source assimilation for the production of EPS. In a further study using PCR ribotyping (rDNA RFLP and UP-PCR/hybridisation), Yurlova et al. (1996) divided the Aureobasidium strains into four groups, which, however, do not correlate with morphological differences. Yurlova et al. (1999) also revealed close relationships between Kabatiella lini (Laff.) Karak., the teleomorph species Discosphaerina (Columnosphaeria) fagi (H.J. Huds.) M.E. Barr and Aureobasidium pullulans.
Aureobasidum pullulans is a ubiquitous and widespread oligotrophe that can be found in environments with fluctuating water activities, such as the phyllosphere (Andrews et al. 1994), bathrooms, food and feeds (Samson et al. 2004). It can also be found in osmotically very stressed environments, such as hypersaline waters in salterns (Gunde-Cimerman et al. 2000), and rocks and monuments (Urzí et al. 1999). Due to the production of large quantities of yeast-like propagules, this fungus disperses globally, although thus far it has only rarely been reported in cold environments. This may be because most investigations on the occurrence and diversity of fungi in the cold have been limited to frozen Antarctic soils and Siberian permafrost, where basidiomycetous yeasts prevail (Abyzov 1993, Babjeva & Reshetova 1998, Deegenaars & Watson 1998, Golubev 1998, Ma et al. 1999, 2000, 2005, Margesin et al. 2002, Onofri et al. 2004, Price 2000, Vishniac 2006, Vishniac & Onofri 2003). Thus far, no investigations of mycobiota in ice had been carried out. We recently investigated ice originating from glacial and subglacial environments of three different polythermal Arctic glaciers in Svalbard (Spitsbergen, Norway) (Butinar et al. 2007, 2008, Sonjak et al. 2006). During these studies, aureobasidium-like fungi were found among the dominant ascomycetous mycota. Given the known adaptive ability of A. pullulans to low water activity (aw) and oligotrophic conditions, it appeared likely that ice from cryocarstic formations and subglacial ice in polythermal glaciers constitute a potential natural habitat. Since some of the Arctic aureobasidium-like isolates deviated phenetically from the pan-global population, a taxonomic study into the genus Aureobasidium was performed. Isolates obtained from different niches in Arctic, temperate and tropical climates were compared by multilocus analyses of rDNA internal transcribed spacers (ITS), partial large subunit of rDNA (LSU), and partial introns and exons of genes coding β-tubulin (TUB), translation elongation factor (EF1α) and elongase (ELO). The main aims of the study were to describe the total diversity of A. pullulans, to redefine its entities, to describe potentially new varieties, and to correlate these with their ecology, focusing on the Arctic sampling area investigated.
MATERIALS AND METHODS
Arctic sampling sites and sample collection
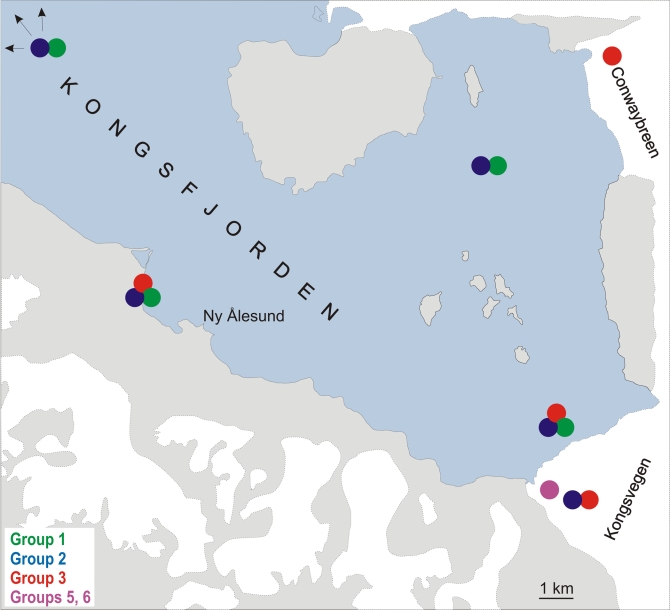
Kongsfjorden is located at 79°N 12°E, and it is one of the larger fjords along the western coast of Spitsbergen, in the Svalbard Archipelago, Norway. The greater part of this drainage basin is covered by glaciers that calve into the fjord. The annual mean temperature is around –5 °C, the mean salinity of the sea water ranges from 34.00 to 35.00 PSU. Twenty-five samples of glacial and subglacial ice were collected aseptically in 2001 and 2003, as described previously (Gunde-Cimerman et al. 2003, Butinar et al. 2007). These originated from three polythermal glaciers (Copland & Sharp 2001): Conwaybreen, Kongsvegen and austre Lovénbreen. Ice was also collected from a moulin and from a glacial cave at Kongsvegen. The subglacial samples included sediment-rich and overlying clear basal ice. Some ice samples, particularly from Kongsvegen, were rich in gypsum inclusions. During summer, subglacial meltwaters from Kongsvegen and the austre Lovénbreen glaciers were also sampled directly. The supraglacial samples comprised two samples of snow/ice mixtures from austre Lovénbreen and Kongsvegen, and eight samples of seasonal meltwaters on the glacier surfaces. During the summer season of 2001, samples of seawater and a mixture of snow and ice in the tidal area were collected from six different locations within the fjord.
Physico-chemical parameters (pH, Na+, Mg2+ and K+ concentrations, and total phosphorus content) were determined for five basal ice samples (originating from Kongsvegen), a sample of subglacial meltwater, and three samples of seawater, as described by Gunde-Cimerman et al. (2003).
Isolation and preservation
Ice samples were transported to the laboratory, where they were processed. The surface layer of ice was aseptically melted at room temperature and discarded. The remaining ice was transferred to another sterile container and melted. The resulting water, as well as directly sampled glacier meltwater and seawater, were filtered immediately (Millipore membrane filters; 0.22-μm and 0.45-μm pore sizes) in aliquots of up to 100 mL. The membrane filters were placed on general-purpose isolation media [DRBC: Dichloran (2,6-dichloro-4-nitroanilin) Rose Bengal Agar (Oxoid CM729) and Malt Extract Agar (MEA)], as well as on a medium for the detection of moderate xerophiles [18 % dichloran glycerol agar (DG18; Hocking & Pitt 1980)], and on selective media with high concentrations of salt (MEA with addition of 5 % to 15 % NaCl) or sugar (malt extract yeast extract with 20 %, 35 % and 50 % glucose). For prevention of bacterial growth, chloramphenicol (50 mg/L-1) was added to all of the media. One drop of the original water sample was applied onto a membrane and was dispersed with a Drigalski spatula. For each sample and medium, at least four and up to 10 aliquots were filtered in parallel, and average numbers of colony forming units (CFUs) were calculated (Gunde-Cimerman et al. 2000). The plates were incubated for up to 14 wk at 4, 10 and 24 °C.
Subcultures were maintained at the Culture Collection of Extremophilic Fungi (EXF, Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia), while a selection have been deposited at the Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands). Reference strains were obtained from the CBS, and were selected either on the basis of strain history, name, or on the basis of their ITS rDNA sequence. The strains were maintained on MEA and preserved for long periods in liquid nitrogen or by lyophilisation. The strains studied are listed in Table 1. A detailed map of the sampling area, with the sites of retrieved isolates marked, is shown In Fig. 1.
Table 1.
List of analysed strains subjected to DNA sequence analyses and morphological studies.
| Taxon | Accession no. | dH number | Source | Geography | Collector | GenBank accession no. (LSU, ITS, BTUB, EF, ELO) |
|---|---|---|---|---|---|---|
| Group 1: Aureobasidium pullulans var. pullulans | ||||||
| (T, Dematoidium nigrescens) | CBS 146.30 | dH 15398 | Slime flux of Quercus sp. | Germany, Ohlsdorf near Hamburg | — | FJ150916, FJ150902, FJ157871, -, FJ039824 |
| (T, Candida malicola) | CBS 701.76 = ATCC 11942 | Malus sylvestris, fruit | — | — | FJ150951, FJ15090, FJ157865, -, FJ039834 | |
| (NT, var. pullulans) | CBS 584.75 | dH 16041 | Vitis vinifera, fruit | France, Beaujolais, Beaujeu | E.J. Hermanides-Nijhof | FJ150942, FJ150906, FJ157869, FJ157895, FJ039835 |
| CBS 109810 | dH 12237 | Chernobil radioactive wall | Ukraine, Kiev region | V.A. Zakharchenko | FJ150953, FJ150901, FJ157868, -, FJ039838 | |
| (T, var. aubasidani) | CBS 100524 | — | Betula, slime flux | Russia, Leningrad Region | J.M. Voznjakovskaja | FJ150952, FJ150905, FJ157867, FJ157900, FJ039839 |
| CBS 100280 | — | Hypersaline saltern water | Slovenia, Sečovlje salterns | P. Zalar | FJ150941, FJ150910, FJ157864, FJ157906, FJ039831 | |
| EXF-915 = CBS 122385 | dH 12636 | Glacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150947, FJ150911, FJ157870, FJ157899, FJ039830 | |
| EXF-88 | dH 16414 | Hypersaline saltern water | Slovenia, Sečovlje salterns | P. Zalar | FJ150957, -, -, FJ157904, FJ039833 | |
| MZKI B-985 | ||||||
| EXF-150 | dH 16416 | Hypersaline saltern water | Slovenia, Sečovlje salterns | P. Zalar | FJ150915, FJ150908, -, FJ157905, FJ039832 | |
| EXF-1668 | dH 13836 | Glacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150949, FJ150900, FJ157875, FJ157898, FJ039827 | |
| EXF-1702B | dH 13844 | Glacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150950, FJ150899, FJ157861, FJ157897, FJ039828 | |
| EXF-2449 | dH 13859 | Glacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150955, FJ150898, FJ157866, FJ157907, FJ039837 | |
| MZKI B-700 | dH 16413 | Hypersaline saltern water | Slovenia, Sečovlje salterns | P. Zalar | FJ150956, FJ150909, -, FJ157903, FJ039826 | |
| — | dH 13843 | Subglacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150954, FJ150904, FJ157876, FJ157896, FJ039829 | |
| — | dH 12637 | Glacial ice from sea water | Norway, Svalbard, Conwaybreen, Kongsvegen | N. Gunde-Cimerman | FJ150948, -, FJ157855, FJ157901, FJ039825 | |
| Group 2: Aureobasidium pullulans var. melanogenum | ||||||
| (T, var. melanogenum) | CBS 105.22 | dH 15197 | — | — | — | FJ150926, FJ150886, FJ157858, FJ157887, FJ039812 |
| (AUT, Torula schoenii) | CBS 123.37 | dH 15346 | — | — | — | FJ150917, FJ150881, FJ157852, -, FJ039818 |
| CBS 621.80 | dH 16090 | Deteriorated army supplies | Russia | — | FJ150921, FJ150885, FJ157859, FJ157890, FJ039813 | |
| CBS 109800 | dH 11797 | Endoperitoneal fluid | Greece, Athens | — | FJ150925, FJ150880, FJ157851, -, FJ039814 | |
| CBS 100225 | dH 15131 | Bathroom glass | Netherlands, Hilversum | G.S. de Hoog | FJ150923, FJ150890, FJ157854, -, - | |
| CBS 110373 | Soil | Thailand | M. Sudhadham | FJ150928, FJ150887, -, FJ157888, FJ039810 | ||
| CBS 110374 | Public fountain | Thailand, Bangkok | M. Sudhadham | FJ150929, FJ150888, -, FJ157886, FJ039808 | ||
| EXF-924 | dH 13831 | Ponds on sea ice | Norway, Svalbard, Kongsfjorden | N. Gunde-Cimerman | FJ150918, FJ150883, FJ157850, FJ157885, FJ039817 | |
| EXF-926 | dH 13840 =dH12625 | Surface glacier ice | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150922, FJ150884, FJ157853, FJ157894, FJ039816 | |
| EXF-3382 | 275-1-1 | Deep sea (4500 m depth) | Japan | F. Abe | FJ150930, FJ150876, -, -, FJ039807 | |
| EXF-3383 | N11 | Deep sea (4500 m depth) | Japan | F. Abe | FJ150931, FJ150877, -, FJ157884, FJ039806 | |
| EXF-3384 | 671-3-PI | Deep sea (4500 m depth) | Japan | F. Abe | FJ150932, FJ150879, -, -, FJ039820 | |
| EXF-3385 | 671-3-MI | Deep sea (4500 m depth) | Japan | F. Abe | FJ150933, FJ150878, -, -, FJ039821 | |
| — | dH 12640 | Fountain | Thailand | M. Sudhadham | FJ150919, FJ150889, FJ157857, FJ157889, FJ039809 | |
| — | dH 12643 | Air | Thailand | M. Sudhadham | FJ150924, FJ150882, FJ157856, FJ157892, FJ039815 | |
| — | dH 12676 | Soil | Thailand | M. Sudhadham | FJ150927, -, FJ157860, FJ157893, FJ039811 | |
| — | dH 12740 | — | Thailand | M. Sudhadham | FJ150920, FJ150874, FJ157862, FJ157891, FJ039819 | |
| Group 3: Aureobasidium pullulans var. subglaciale | ||||||
| EXF-2479 = CBS 123388 | dH 13860 | Glacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150935, FJ150893, FJ157877, FJ157910, FJ039846 | |
| EXF-2480 | dH 13880 | Subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150934, FJ150891, FJ157879, FJ157909, FJ039841 | |
| (T, var. subglaciale) | EXF-2481 = CBS 123387 | dH 13862 | Subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150913, FJ150895, FJ157878, FJ157911, FJ039845 |
| EXF-2491 | dH 13865 | Subglacial ice | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150936, FJ150894, FJ157880, FJ157902, FJ039844 | |
| EXF-2510 = CBS 123386 | dH 13868 | Moulin | Norway, Svalbard, Conwaybreen | N. Gunde-Cimerman | FJ150938, -, -, -, - | |
| EXF-3640 | dH 13864 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150939, FJ150896, FJ157882, FJ157913, FJ039842 | |
| — | dH 13876 | coastal ponds of melted snow and ice | Norway, Svalbard, Kongsfjorden, Ny Ălesund | N. Gunde-Cimerman | FJ150958, FJ150892, FJ157881, FJ157912, FJ039843 | |
| Group 4: Aureobasidium pullulans var. namibiae | ||||||
| (T, var. namibiae) | CBS 147.97 | — | dolomitic marble | Namibia, Namib Desert | U. Wollenzien | FJ150937, FJ150875, FJ157863, -, FJ039822 |
| Group 5, Unnamed | ||||||
| EXF-914 = CBS 122350 | dH 12626 = 13830 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150961, -, -, -, - | |
| EXF-3639 = CBS 122359 | dH 13832 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150966, -, -, -, - | |
| EXF-935 = CBS 122362 | dH 12635 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150963, -, -, -, - | |
| EXF-922 | dH 12623 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150964, -, -, -, - | |
| EXF-1934 | dH 13842 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150962, -, -, -, - | |
| EXF-1936 | dH 13839 | subglacial ice from sea water | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150965, -, -, -, - | |
| Group 6, Unnamed | ||||||
| EXF-2500 = CBS 123390 | dH 13881 | glacial ice | Norway, Svalbard, Kongsvegen | N. Gunde-Cimerman | FJ150914, -, -, -, - | |
| — | dH 12633 | N. Gunde-Cimerman | FJ150960, -, -, -, - | |||
| EXF-938 = CBS 123389 | dH 12634 | subglacial ice from sea water | Norway, Svalbard, Conwaybreen | N. Gunde-Cimerman | FJ150959, -, -, -, - | |
| Species included for comparison. | ||||||
| Dothichiza pithyophila | CBS 215.50 | — | Abies concolor, dead bark | Norway | — | FJ150968, -, FJ157883, -, - |
| Dothichiza pithyophila | — | dH 12609 | Vishniac Y-31 | — | — | FJ150969, -, -, -, - |
| Dothichiza sp. | EXF-3641 = CBS 122355 | dH 13858 | sea ice with sediment | Norway, Svalbard | N. Gunde-Cimerman | FJ150967, -, -, -, - |
| Kabatiella caulivora | CBS 242.64 | dH 15602 | Trifolium incarnatum | U.S.A., Oregon | — | FJ150944, FJ150871, -, -, FJ039836 |
| Kabatiella microsticta | CBS 114.64 = MUCL 18713 | dH 15299 | Hemerocallis sp. | Netherlands, Wageningen | — | FJ150940, FJ150873, -, FJ157914, FJ039848 |
| Kabatiella microsticta | CBS 342.66 | dH 15774 | Convallaria majalis, dying leaf | Germany | W. Gams | FJ150945, FJ150903, FJ157872, -, FJ039823 |
| Kabatiella lini | CBS 125.21T = MUCL 8712 | dH 15350 | Linum usitatissimum | U.K. | — | FJ150946, FJ150897, FJ157873, FJ157908, FJ039840 |
| Pringsheimia smilacis | CBS 873.71T | — | Smilax aspera, twig | Italy, Napoli | L. Froidevaux | FJ150970, -, -, -, - |
| Selenophoma mahoniae | CBS 388.92T | dH 15823 | Mahonia repens, leaf | U.S.A., Colorado | A.W. Ramaley | FJ150943, FJ150872, FJ157874, FJ157915, FJ039847 |
| Sydowia polyspora | CBS 750.71 | — | Pinus strobus, twig | Canada, Quebec; Lac Normand | E. Müller | FJ150912, -, -, -, - |
* Abbreviations used: ATCC = American Type Culture Collection; CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; dH = de Hoog Culture Collection, CBS, Utrecht, The Netherlands; EXF = Culture Collection of Extremophilic Fungi, Ljubljana, Slovenia; MUCL = Mycotheque de l'Universite catholique de Louvain; MZKI = Microbiological Culture Collection of the National Institute of Chemistry, Ljubljana, Slovenia; NT = ex-neotype strain; T = ex-type strain.
Fig. 1.
Detailed map of the sampling area in Svalbard, with sites of retrieved aureobasidium-like isolates marked.
Cultivation and microscopy
For growth rate determination and the phenetic description of colonies, the strains were point-inoculated onto potato-dextrose agar (PDA; Oxoid CM139), and Blakeslee's MEA (Samson et al. 2004), and then incubated at 25 °C for 7–14 d in darkness. Surface colours were rated using the colour charts of Kornerup & Wanscher (1978). For microscopic morphology, MEA blocks of about 1 × 1 cm2 were cut out aseptically, placed on sterile microscope slides, and inoculated at the upper four edges by means of a conidial suspension (Pitt 1979). Inoculated agar blocks were covered with sterile cover slips and incubated in moist chambers for 2, 4, and 7 d at 25 °C in the dark. The structure and branching pattern of the immersed hyphae were examined under magnifications of 100× and 400× in intact slide cultures under the microscope without removing the cover slips from the agar blocks. For higher magnifications (400×, 1000×) the cover slips were carefully removed and mounted in 60 % lactic acid.
DNA extraction, sequencing and analysis
For DNA isolation, the strains were grown on MEA for 7 d. Their DNA was extracted according to Gerrits van den Ende & de Hoog (1999), by mechanical lysis of approx. 1 cm2 of mycelium. A fragment of rDNA including ITS region 1, 5.8S rDNA and ITS 2 (ITS) was amplified using the ITS1 and ITS4 primers (White et al. 1990). LSU (partial 28 S rDNA) was amplified and sequenced with the NL1 and NL4 primers (Boekhout et al. 1995). For amplification and sequencing of the β-tubulin (TUB) gene, primers Bt2a and Bt2b were used (Glass & Donaldson 1995). Translation elongation factor EF-1α (EF1α) was amplified and sequenced with the primers EF1-728F and EF1-986R (Carbone & Kohn 1999). For amplification and sequencing of the partial elongase gene (ELO), the ELO2-F (5'-CAC TCT TGA CCG TCC CTT CGG-3') and ELO2-R (5'-GCG GTG ATG TAC TTC TTC CAC CAG-3') primers were used, designed for Aureobasidium pullulans. Reactions were run in a PCR Mastercycler Ep Gradient (Eppendorf) with a profile of initial denaturation of 2 min at 94 °C, followed by 6 cycles of 15 s at 94 °C, 15 s at 58 °C and of 45 s at 72 °C, and 30 cycles of 15 s at 94 °C, 15 s at 56 °C and of 45 s at 72 °C, with a final elongation of 7 min at 72 °C. BigDye terminator cycle sequencing kits were used in sequence reactions (Applied Biosystems, Foster City, CA, U.S.A.). Sequences were obtained with an ABI Prism 3700 (Applied Biosystems). They were assembled and edited using SeqMan 3.61 (DNAStar, Inc., Madison, U.S.A.). Sequences downloaded from GenBank are indicated in the gene trees by their GenBank accession numbers; newly generated sequences are indicated by their strain numbers (see also Table 1).
Phylogenetic analyses
Sequences were automatically aligned using ClustalX 1.81 (Jeanmougin et al. 1998). Alignments were adjusted manually using MEGA4 (Tamura et al. 2007). Gene trees were generated with MrBayes software, applying Bayesian inference (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003). Three parallel runs were performed for three million generations with mixed amino-acid models, the default temperature and five chains. The gene trees were sampled every 100 generations. Gene trees sampled before the analysis that reached stationarity of likelihood values, and those sampled before the mean standard deviation of the split frequencies decreased to under 0.5 % were excluded from the final analysis. The stationarity of likelihood values was checked using the Tracer software (Rambaut & Drummond: MCMC Trace Analysis Tool, version 1.4, 2003–2007). In phylogenetic analysis of LSU rDNA the LSU sequence of Elsinoe veneta (DQ678060) was selected as an outgroup, according to Schoch et al. (2006). Isolates were grouped on the basis of multilocus analyses and representative strains were selected for morphological analyses.
RESULTS
Isolates from Arctic samples
The fresh isolates from Arctic samples are listed in Table 1. Subglacial ice samples without and with gypsum inclusions, incubated on MEA with 5 % NaCl at 10 °C, contained aureobasidium-like propagules in the highest CFU range (>30 CFU/mL). Strains were also isolated from gypsum crystals collected from soil bordering subglacial ice with gypsum inclusions. Lower CFU numbers of Aureobasidium (2–3 CFU/100 mL) were detected also in other ice samples: in sea ice and moulin ice, and in specimens from an ice cave.
Phylogenetic analyses
Alignments for the phylogenetic analyses included 599 base pairs for LSU, 488 for ITS, 704 for ELO, 323 for EF1α, and 425 for TUB. Internodes were considered strongly supported if they received posterior probabilities ≥95 % (Lutzoni et al. 2004). Good convergence of the runs was reached when constructing all of the gene trees with MrBayes. The likelihood values reached plateaus after approximately 24,000 (LSU), 4,000 (ITS), 6,000 (TUB), 7,000 (EF1α) and 15,000 (ELO) generations, while the mean standard deviations of the split frequencies dropped below 1 % after 600,000 (LSU), 300,000 (ITS), 800,000 (TUB), 300,000 (EF1α) and 200,000 (ELO) generations. The first 6,000 (LSU), 3,000 (ITS), 8,000 (TUB), 3,000 (EF1α) and 2,000 (ELO) trees were discarded as burn-in.
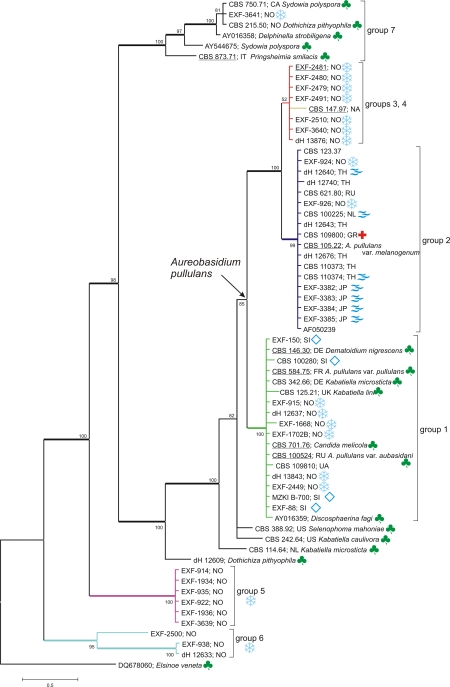
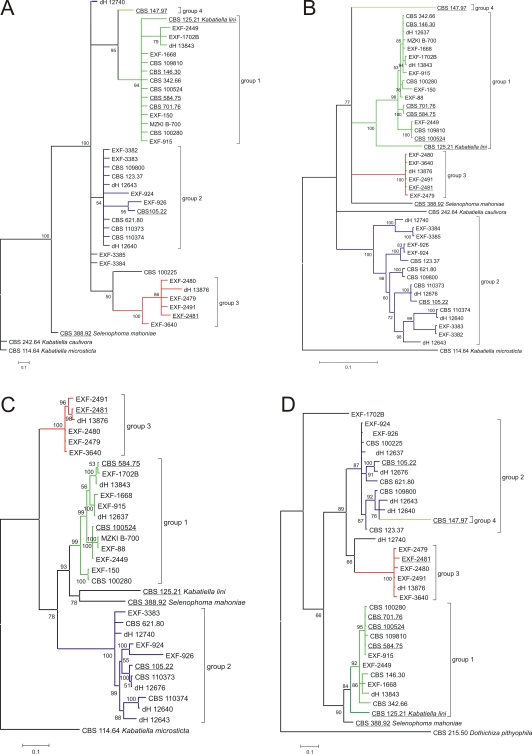
According to the LSU rDNA analysis (Fig. 2), a high level of support was evident for the clade containing A. pullulans (groups 1–4) together with Selenophoma mahoniae A.W. Ramaley (CBS 388.92), Kabatiella caulivora (Kirchn.) Karak (CBS 242.64) and Kabatiella microsticta Bubák (CBS 114.64). Group 7, consisting of Sydowia polyspora (Bref. & Tavel) E. Müll., Pringsheimia smilacis E. Müll., Delphinella strobiligena (Desm.) Sacc. ex E. Müll. & Arx and Dothichiza pithyophila (Corda) Petr., formed a well supported, but separate, clade. Separate well-supported clades (groups 5 and 6) joined arctic strains of no affinity to any of the known taxa. Clade Aureobasidium pullulans was badly supported (85 posterior probability). Groups 1 and 2 within this clade were statistically supported, while groups 3 and 4 reached a poor posterior probability value. Group 1 contained the ex-neotype strain of A. pullulans var. pullulans (CBS 584.75), its supposed teleomorph Discosphaerina (Columnosphaeria) fagi, the ex-type strain of Kabatiella lini (CBS 125.21), the ex-type strain of Dematoidium nigrescens Stautz (CBS 146.30), the ex-type strain of A. pullulans var. aubasidani (CBS 100524), and a strain of Kabatiella microsticta (CBS 342.66). Another strain of K. microsticta was placed on the basal branch as the sister taxon of K. caulivora (CBS 242.64) and Selenophoma mahoniae (CBS 388.92). Group 2 contained the ex-type strain of A. pullulans var. melanogenum. Group 3 contained exclusively Arctic strains, while group 4 consisted of one strain only (CBS 147.97). Analyses of the more variable ITS spacers (Fig. 3A), and ELO (Fig. 3B), EF1α (Fig. 3C), and TUB (Fig. 3D) introns and exons almost consistently supported the first three groups, with only a few exceptions. For example, several strains of group 2 were dispersed outside the clade of group 2 in ITS analysis, while in other analyses they formed a monophyletic group. In analyses of ITS and ELO, group 4 was supported, whereas based on TUB it was grouped together with group 2, but on a separate and long branch. The amplification of the EF1α gene failed in the only strain of group 4; therefore, its phylogenetic position concerning this gene is unknown.
Fig. 2.
Consensus phylogram (50 % majority rule) of 24000 trees resulting from a Bayesian analysis of the LSU sequence alignments using MrBayes v. 3.1.2. Bayesian posterior probabilities are indicated at the nodes, branches with posterior probabilities >95 in bold. The tree was rooted to the sequence of Elsinoe veneta (DQ678060). Ex-type and ex-neotype strains are underlined; when known origin two digit country codes are listed after strain numbers. The colour marks stand for:
 - plant associated;
- plant associated; - originating from
Arctic ice;
- originating from
Arctic ice; - originating from
hyperosmotic environment;
- originating from
hyperosmotic environment; - clinical strain.
- clinical strain. - water.
- water.
Fig. 3.
Consensus phylograms (50 % majority rule) resulting from a Bayesian analysis of: A. ITS rDNA; B. elongase gene; C. translation elongation factor EF-1α gene; D. β-tubulin gene. Bayesian posterior probabilities are indicated at the nodes. The trees are not rooted. Ex-type and ex-neotype strains are underlined.
Morphology
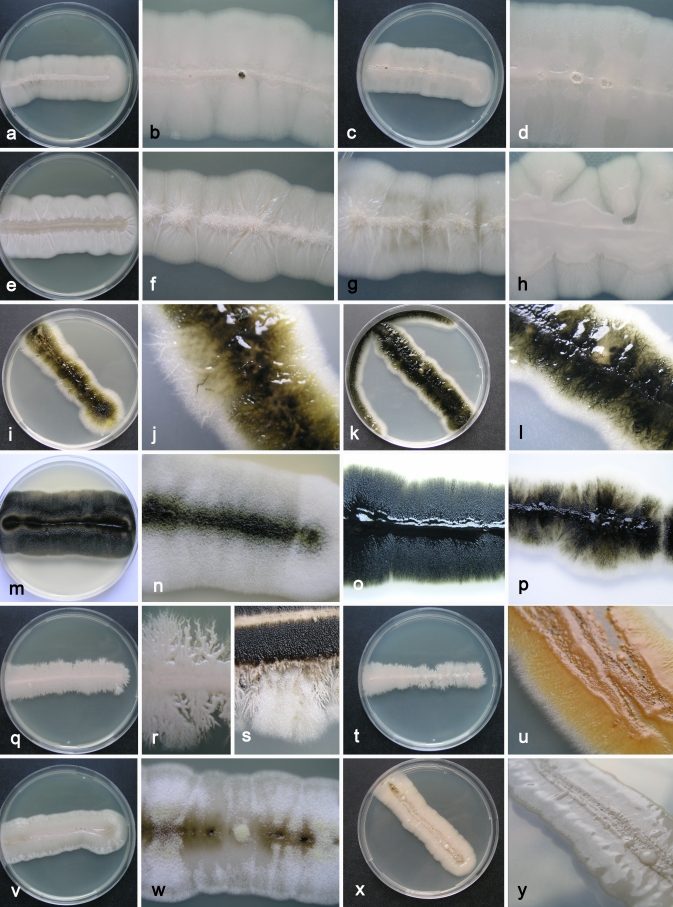
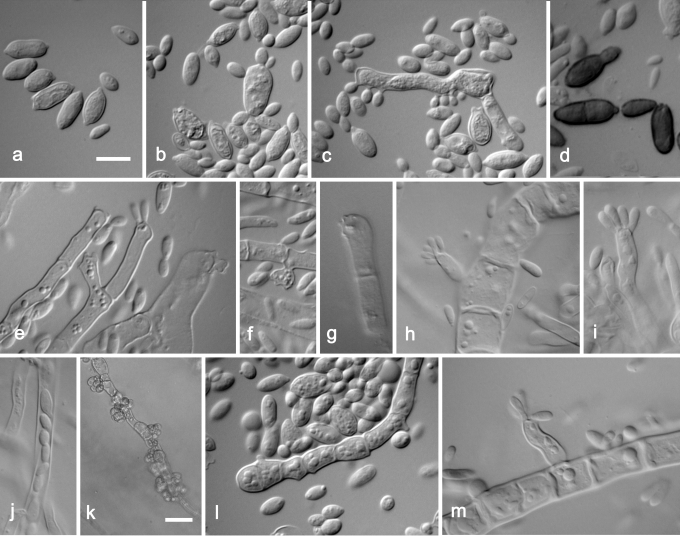
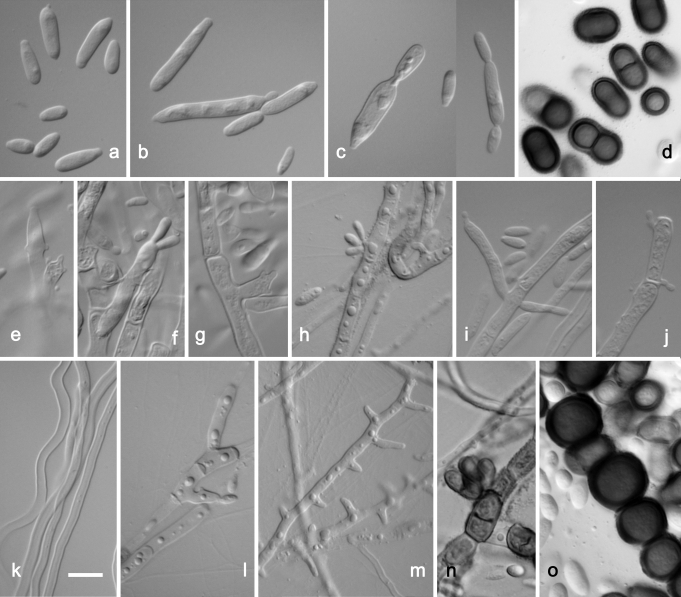
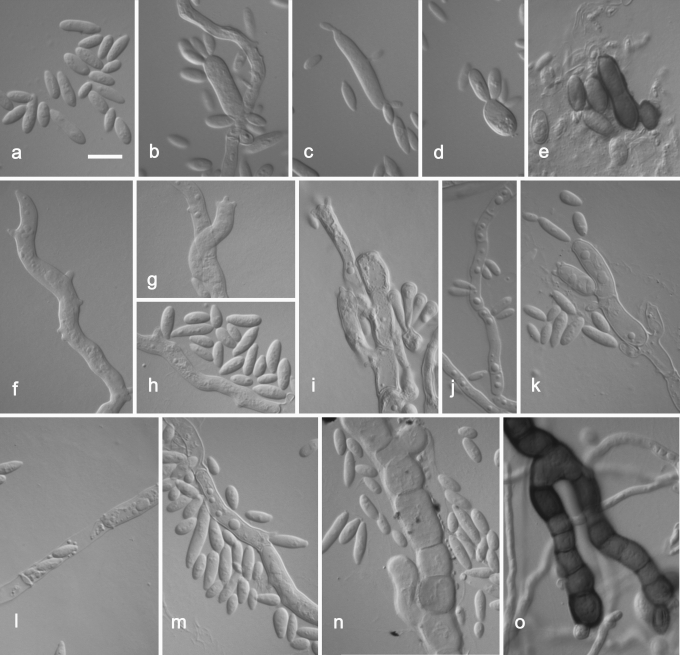
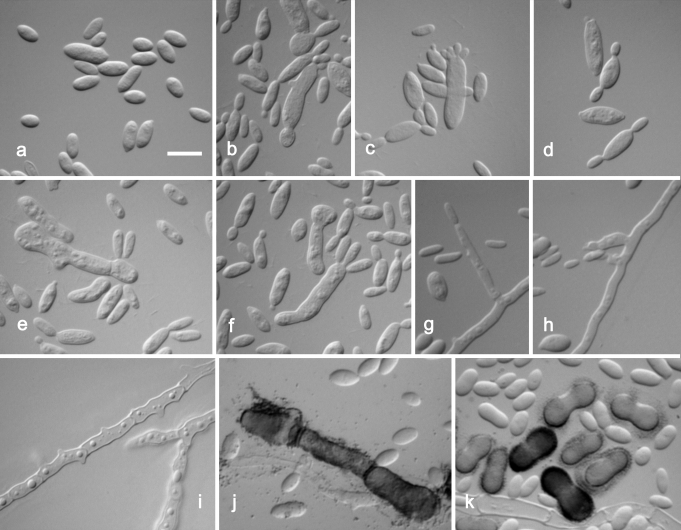
The main difference observed among isolates was pigmentation of cultures (Fig. 4). Strains belonging to groups 1 and 3 remained pinkish for at least 1 w. The majority of strains from group 1 became pigmented only after 3 wk, or even later. The only exception among this group was the ex-type strain of Dematoidium nigrescens (CBS 146.30), which was darkly pigmented already after 1 wk of incubation due to melanised septated hyphae. This was also the only strain that was exclusively filamentous and formed no conidia. Strains of group 3 became melanised only at the margin, where dark pigmented, heavily branched hyphae developed, while the colony centre remained pinkish at least for 3 wk. All strains of group 2 were green or black after 7 d of incubation, due to the production of melanised hyphae and conidia. Structures of cultures differed within different varieties, from almost entirely yeast-like to entirely filamentous, and also yeast-like with marginal or central aerial mycelium. Marginal areas of colonies were significantly different in at least two pink pigmented groups, consisting of arachnoid mycelium in group 1 and of thick and undulating hyphae in group 3. Conidiogenesis seen in the groups of A. pullulans (groups 1–4) studied was synchronous, either on rather undifferentiated short denticles, intercalary or terminally on hyaline (groups 1, 3, 4) or melanised (group 4) hyphae. This kind of conidiogenesis was also seen on enlarged globose conidiogenous areas that developed laterally on hyphae, giving rise to multiple conidia, or on enlarged yeast cells, which were synchronously budding from both poles. Synchronous conidiogenesis was sometimes difficult to observe due to heavy yeast proliferation. More complex conidiophore-like structures were noted in group 3. Another mode of conidiation seen in all varieties was percurrent conidiogenesis alongside the hyphae. Conidia formed either synchronously or percurrently, and were budding secondarily in all groups; therefore, the size and shape of conidia in Aureobasidium in general was very variable. Conidia in groups 1 and 4 were almost exclusively non-pigmented, while in group 2, as well as 1-celled non-pigmented conidia, also melanised 1–2-celled conidia were also abundant. Pigmented conidia were also seen with the strain CBS 100524, the ex-neotype strain of A. pullulans var. aubasidani. Endoconidia were seen in only some strains of groups 1 and 3.
Fig. 4.
Macromorphology of different Aureobasidium pullulans varieties incubated for 7 d at 25 °C in the dark on MEA (left 2 columns) and on PDA (right two columns). a–h. A. pullulans var. pullulans: a, b. CBS 584.75 (MEA); c, d. CBS 584.75 (PDA); e. CBS 109810 (MEA); f, g. CBS 701.76 (MEA, PDA); h. MZKI B-700 (PDA). i–p. A. pullulans var. melanogenum: i, j. CBS 105.22 (MEA); k, l. CBS 105.22 (PDA); m. EXF-3382 (MEA); n. CBS 621.80 (MEA); o. EXF-924 (PDA); p. CBS 100225 (PDA). q–u. A. pullulans var. subglaciale: q, r. EXF-2481 (MEA); s. EXF-2481 after 14 d incubation (MEA); t. EXF-2481 (PDA); u. EXF-2479 (PDA). v–y. A. pullulans var. namibiae. v, w. CBS 147.97 (MEA); x, y. CBS 147.97 (PDA).
TAXONOMY
Aureobasidium pullulans (de Bary) G. Arnaud var. pullulans – Annales École Nat. Agric. Montpellier 16: 39, 1918. MycoBank MB101771). Fig. 5.
Fig. 5.
Aureobasidium pullulans var. pullulans. a. Liberated conidia transforming to budding cells. b. Synchronous production of conidia on a transformed conidium – yeast cell. c. Short hypha synchronously producing conidia. d. Dark brown conidia. e–i, m. Poorly differentiated, terminal and intercalar conidiophors performing synchronous conidiation. k. Immersed hypha with lateral accumulation of conidia. l. Hypha with lateral scars – conidiogenous loci. j. Endoconidia. a–c, e–g, k–m. CBS 584.75 (ex-neotype strain); d. CBS 100524; h–i, j, m. EXF-1702B. Scale bars: a–j, l–m= 10 μm; k= 20 μm.
Synonyms: Dematium pullulans de Bary 1884 (MB 219317; NT = CBS 584.75)
Aureobasidium pullulans (de Bary) Arn. var. aubasidani Yurlova in Yurlova & de Hoog 1997 (MB 442903; T = CBS 100524)
Candida malicola D.S. Clark & R.H. Wallace 1955 (MB 294033; T = CBS 701.76)
Dematoidium nigrescens Stautz 1931 (MB 272259; T = CBS 146.30)
Cultural characteristics: Colonies on MEA/PDA at 25 °C attaining about 40/30 mm diam after 7 d, appearing smooth and slimy due to abundant sporulation, pinkish (pinkish white, 7A2) to yellowish (light yellow, 3A4), reverse yellowish (pale yellow (4A3) to light yellow (4A4)). Black sectors composed of dark pigmented hyphae or conidia develop in some isolates after 14 d. Margin composed of arachnoid mycelium, sometimes in sectors. No aerial mycelium. Deviations: White aerial mycelium at the edge of cultures present in some strains (CBS 109800, EXF-915), some strains entirely filamentous (dH 12637), some develop white, setae-like mycelial formations in colony centre and marginal leathery mycelium (CBS 701.76). Strain CBS 146.30 was black and filamentous already after 1 wk of incubation.
Microscopy: Vegetative hyphae hyaline, smooth, thin-walled, 4–12 μm wide, transversely septate, in older cultures sometimes locally converted to dark-brown hyphae. Conidiogenous cells undifferentiated, intercalary or terminal on hyaline hyphae. Conidia produced synchronously in dense groups from small denticles, and also formed percurrently on short lateral denticles. Conidia hyaline to dark brown. Hyaline conidia one-celled, smooth, ellipsoidal, very variable in shape and size, 7.5–16 × 3.5–7 μm, often with an indistinct hilum. Dark brown conidia (measured in strain CBS 100524, developed after 2 wk) 1–2 celled, one celled 10–17 × 5–7 μm, two celled slightly constricted at septum, 14–25 × 5–11 μm. Budding of hyaline and dark brown conidia frequently seen, with the secondary conidia being smaller than the primary ones. Conidia in old cultures transfer to globose, brownish structures of 10–15 μm diam. Endoconidia, about 6 × 3 μm occasionally seen in intercalary cells.
Maximum tolerated salt concentration: 15 % NaCl.
Cardinal temperatures: Minimum at 4 °C, optimum at 25 °C, maximum at 30 °C.
Specimens examined: France, fruit of Vitis vinifera, 1974, coll. and isol. E.J. Hermanides-Nijhof, ex-neotype culture CBS 584.75; for additional specimens, see Table 1.
Aureobasidium pullulans (de Bary) G. Arnaud var. melanogenum Hermanides-Nijhof – Stud. Mycol. 15: 161, 1977. MycoBank MB352628. Fig. 6.
Fig. 6.
Aureobasidium pullulans var. melanogenum. a–c. Liberated conidia transforming to budding cells. d. Dark brown conidia. e–h. Poorly differentiated, terminal and intercalar conidiophors performing synchronous conidiation. i, l. Hypha with long lateral conidiogenous cells. j. Hypha with prolonged lateral pegs. k. Vegetative hyphae. m. Immersed hyphae with multiple lateral pegs. n. Melanized hyphae with intercalar synchronous conidiogenesis. o. Melanized hyphae / chlamydospores. a–c, e–n. CBS 105.22 (ex-type strain); d, o. EXF-926. Scale bar: as marked on k (a–o) = 10 μm.
Synonyms:Torula schoenii Roukhelman 1937 (MB 445735; AUT = CBS 123.37) (Invalid; Art. 37 ICBN)
Pullularia fermentans Wynne & Gott var. schoenii (Roukhelman) Wynne & Gott 1956 (MB 352450)
Aureobasidium pullulans (de Bary) G. Arnaud var. melanogenum Hermanides-Nijhof 1977 (MB 352628; T = CBS 105.22)
Cultural characteristics: Colonies on MEA/PDA at 25 °C attaining 25 mm diam after 7 d, appearing smooth and slimy due to abundant sporulation and EPS formation, olive brown (4F3-4F8) to black in centre, towards margin mustard yellow (3B6), margin yellowish white (3A2); reverse olive-grey (3E2) at the centre, towards margin dull yellow (3B4), at the margin yellowish white (3A2). Margin composed of arachnoid to thick undulating hyphae growing into the agar, sometimes sectorial. After 14 d the entire colonies are green to black. Aerial mycelium develops in some parts of the colonies. Deviations: White aerial mycelium present in strain CBS 621.80.
Microscopy: Vegetative hyphae in the central part of colonies, dark brown, smooth to slightly roughened, thick walled, 6–12 μm wide, transversely septate, constricted at septa, embedded in EPS, disarticulating to 1–2-celled, dark brown chlamydospores, one celled 13–16 × 8–12 μm, two celled 17–24 × 10–12 μm. Vegetative hyphae at colony edge hyaline, smooth, thin-walled, 2–10 μm wide, transversely septate, getting thicker and darker with age. Immersed hyphae with multiple lateral pegs. Conidiogenous cells undifferentiated, intercalary or terminal on hyaline hyphae, sometimes grown in the form of an outgrowth with three denticles. Conidia produced synchronously in dense groups from small denticles (1.0–2.5 μm long), and also formed percurrently alongside hyphae and on short lateral branches. Conidia hyaline and dark brown. Hyaline conidia one-celled, smooth, ellipsoidal, very variable in shape and size, 8–30 × 3.5–5 μm, often with an indistinct hilum. Dark brown conidia 1–2-celled, smooth, ellipsoidal when one celled, 7 × 6 μm, slightly constricted at septa when two celled, 12–20 × 4–12 μm. Unilateral and bilateral budding of hyaline conidia frequently seen, with the secondary conidia being smaller than the primary ones. Endoconidia not seen.
Maximum tolerated salt concentration: 10 % NaCl.
Cardinal temperatures: Minimum at 10 °C, optimum at 30 °C, maximum at 35 °C.
Specimens examined: Unknown, culture ex-type CBS 105.22 = ATCC 12536 = CECT 2658 = IMI 062460 = NRRL Y-7469, isolated by M. Church; additional specimens see Table 1.
Aureobasidium pullulans (de Bary) G. Arnaud var. subglaciale Zalar, de Hoog & Gunde-Cimerman, var. nov. MycoBank MB512380. Fig. 7.
Fig. 7.
Aureobasidium pullulans var. subglaciale. a. Conidia. b–d. Budding conidia. e. Dark brown conidia. f, g. Hyphae with multiple lateral pegs, which develop into synchronous conidiation aparatus. h, m. Hyphae with lateral pegs. i, k. Conidiophore-like structure synchronously producing conidia. l. Endoconidia. n. Hyaline vegetative hyphae. o. Melanized hyphae. a–o. EXF–2481 (ex-type strain). Scale bar: as marked on a (a–o) = 10 μm.
Coloniae in agaro MEA vel PDA 25 °C ad 20 mm diam post 7 dies, leves, haud lucidae, copiose sporulantes, roseae, reverso dilute aurantiaco; post 15 dies in medio roseae, marginem versus obscure brunneae; hyphae marginales superficiales latae, undulantes, nonnumquam sectores formantes; hyphae aeriae absentes. Hyphae vegetativae hyalinae, leves, tenuitunicatae, 2–10 μm latae, in coloniis vetustis nonnumquam hyphae fuscae, crassitunicatae, 5–9 μm latae. Conidia hyalina vel fusca, hyalina unicellularia, levia, ellipsoidea, forma magnitudineque variabilissima, 5.5–28 × 2–6.5 μm, fusca 1- vel bicellularia, 8–16(–25) × 5–9 μm. Conidia saepe gemmantia, secundaria primariis minora; endoconidia circa 8 × 3 μm nonnumque in cellulis intercalaribus formata. Temperatura optima et maxima crescentiae 25 °C.
Holotype: CBS H-20186
Cultural characteristics: Colonies on MEA/PDA at 25 °C attaining 20 mm (10–35 mm) diam after 7 d, appearing smooth and matt due to abundant sporulation, pinkish (pinkish white, 7A2), reverse pale orange (5A3). After 14 d central areas of colonies remain pinkish, towards the margin becoming dark-brown (greyish brown, 5F3). Margin composed of thick undulating superficial and immersed branched hyphae, sometimes with sectors. Aerial mycelium absent.
Deviations: Culture EXF-2479 develops more intensively pigmented colonies than others, pink in centre and yellowish orange towards the colony margin on MEA, and golden-yellow on PDA.
Microscopy: Vegetative hyphae hyaline, smooth, thin-walled, 2–10 μm wide, transversely septate, in older cultures locally converted to dark brown, thick-walled hyphae of 5–9 μm diam. Conidiogenous cells mostly undifferentiated, intercalary or terminal on hyaline hyphae, sometimes developed in clusters as conidiophore-like structure. Conidia produced synchronously in dense groups from small denticles, and also percurrently on short lateral branches. Conidia hyaline to dark brown. Hyaline conidia one-celled, smooth, ellipsoidal, very variable in shape and size, 5.5–28 × 2–6.5 μm, often with an indistinct hilum. Dark conidia 1–2-celled, one celled 8–16 ×5–9 μm, two celled 9–25 × 5.5–7.5 μm. Budding frequently seen, with secondary conidia being smaller than the primary ones. Endoconidia, about 8 × 3 μm, sometimes present in intercalary cells.
Maximum tolerated salt concentration: 10 % NaCl.
Cardinal temperatures: Minimum at 4 °C, optimum and maximum at 25 °C.
Specimen examined: Norway, Spitsbergen, subglacial ice from sea water, 2003, coll. and isol. N. Gunde-Cimerman, Holotype CBS H-20186, culture ex-neotype EXF-2481 = CBS 123387; additional specimens see Table 1.
Aureobasidium pullulans (de Bary) Arnaud var. namibiae Zalar, de Hoog & Gunde-Cimerman, var. nov. MycoBank MB512381. Fig. 8.
Fig. 8.
Aureobasidium pullulans var. namibiae. a. Conidia. b–f. Liberated conidia transforming to budding cells. g–h. Hypha with long lateral conidiogenous cells. i. Immersed hyphae with multiple lateral pegs. j. Melanized hypha surrounded by EPS. k. Hyaline and dark brown conidia. a–k. CBS 147.97 (ex-type strain). Scale bar: as marked on a (a–k) = 10 μm.
Coloniae in agaro MEA 25 °C ad 25 mm diam post 7 dies, leves, lucidae textura coriacea, roseae, in medio brunneae, margine hyphis aereis albae, reverso griseoluteo; coloniae in agaro PDA post 7 dies ad 20 mm diam, leves, sporulatione copiosa lucidae, in medio aurantio-albae olivascentes, nonnumquam hyphales et setosae, reverso armeniaco. Hyphae aeriae absentes. Hyphae vegetativae hyalinae, leves, tenuitunicatae, 2–13 μm latae, in coloniis vetustis nonnumquam hyphae fuscae. Conidia hyalina vel fusca, hyalina unicellularia, levia, ellipsoidea, forma magnitudineque variabilissima, 7–17 × 3.5–7 μm, fusca 1- vel bicellularia, 8–13(–24) × 5–9(–10) μm, saepe granulis ectoplasmaticis circumdata. Conidia saepe gemmantia, secundaria primariis minora; endoconidia haud visa. Temperatura optima crescentiae 25 °C, et maxima 30 °C.
Holotype: CBS H-20184
Cultural characteristics: Colonies on MEA at 25 °C attaining 25 mm diam after 7 d, appearing smooth and shiny due to the leathery structure of colonies, pinkish (pinkish white, 7A2) with brownish (greyish brown, 5E3) central part, margin white (5A1), reverse yellowish (greyish yellow, 4B4); margin composed of superficial aerial mycelium. Colonies on PDA at 25 °C attaining 20 mm diam after 7 d, appearing smooth and shiny due to abundant sporulation, orange-white (5A2) with olive-brown (4F3) centre, sometimes hyphal and with setae, reverse apricot (orange white, 5A2). No aerial mycelium.
Microscopy: Vegetative hyphae hyaline, smooth, thin-walled, 2–13 μm wide, transversely septate, locally converted to dark brown, thick-walled hyphae. Conidiogenous cells undifferentiated, intercalary or terminal on hyaline hyphae and on larger transformed conidia. Conidia produced synchronously in dense groups from small denticles, later formed percurrently on short lateral branches. Conidia hyaline and dark brown. Hyaline conidia one celled, smooth, ellipsoidal, very variable in shape and size, 7–17 × 3.5–7.0 μm, often with an indistinct hilum. Dark brown conidia 1-2-celled, one celled 8–13 × 5–9 μm, two celled 8–24 × 2–10 μm, surrounded by granular EP; if two-celled, constricted at the septum. Budding frequently seen, with secondary conidia smaller than the primary ones. Endoconidia not seen.
Maximum tolerated salt concentration: 10 % NaCl.
Cardinal temperatures: Minimum at 10 °C, optimum at 25 °C and maximum at 30 °C.
Specimen examined: Namibia, dolomitic marble in Namib Desert, 1997, coll. and isol. U. Wollenzien, holotype CBS H-20184, culture ex-type CBS 147.97.
DISCUSSION
The elongase-encoding gene (ELO) was used as phylogenetic marker for the first time. Southern blotting of A. pullulans genomic DNA did not suggest the existence of more than one copy of the elongase gene in the genome of A. pullulans, while this is the case in other fungi (Gostinčar et al. 2008); this would have diminished its value for routine studies. The gene provided excellent resolution of the Aureobasidium complex and thus could reliably be used for tree reconstruction.
The anamorph genus Aureobasidium phylogenetically belongs to Ascomycota, order Dothideales, family Dothideaceae (Schoch et al. 2006). The fungi have been known since the late 19th century, when Viala & Boyer (1891) described A. vitis as a common coloniser of the sugary surface of grapes (Vitis vinifera). Type material is not known to be preserved. In her revision of the genus, Hermanides-Nijhof (1977) neotypified Dematium pullulans De Bary (1884) with CBS 584.75, thus establishing A. pullulans as the oldest name for the type species of Aureobasidium. The genus was circumscribed using criteria of conidiogenesis, i.e., synchronous holoblastic conidium production. This feature is also known in sporodochial Kabatiella species forming defined leaf spots on specific host plants. When these fungi are cultured, the sporodochia fall apart, and the micromorphology becomes very similar to that of Aureobasidium pullulans. For this reason, Hermanides-Nijhof (1977) classified all Kabatiella species in Aureobasidium, even though most Kabatiella species have not been cultured and are only known from the sporodochial anamorph on the host plant. LSU sequences of the few species thus far available for study indeed show affinity to A. pullulans.
Kabatiella zeae Narita & Y. Hirats. (Hermanides-Nijhof 1977) is found in isolated positions away from other aureobasidia (de Hoog et al. 1999, Yurlova et al. 1999). Synchronous conidiation is thus polyphyletic. However, in addition to molecular differences for some species, morphological distinctions may also be possible, since most Kabatiella species have sickle-shaped conidia, such as K. caulivora, K. harpospora (Bres. & Sacc.) Arx, K. phoradendri (Darling) Harvey f. umbellulariae Harvey, and K. zeae. In Kabatiella lini, a species clustering within A. pullulans (Fig. 2), the conidia have similar shape, but are slightly larger than in the var. pullulans. Kabatiella microsticta, K. caulivora and another, related pycnidial fungus, Selenophoma mahoniae deviate in all of the genes studied at the variety level. Thus, they might be regarded as separate varieties of A. pullulans, but the possibility cannot be excluded that unintentially the ubiquitous phyllosphere fungus A. pullulans was isolated instead of the pathogen. It appears likely that the plant-invading, host-specific pathogens are consistently different from A. pullulans, which on host plants colonises surfaces only, but unambiguously identified strains are needed to prove this.
Our multilocus analysis shows that Aureobasidium pullulans consists of three robust main groups, two of which have high statistic support in LSU and show the same topology with all of the genes sequenced. The ex-neotype of the species, CBS 584.75, is in group 1, A. pullulans var. pullulans. This group also contains CBS 146.30, the ex-type strain of Dematoideum nigrescens Stautz, CBS 701.76, the ex-type strain of Candida malicola D.S. Clark & R.H. Wallace, and CBS 100524, the ex-type strain of A. pullulans var. aubasidani Yurlova, which should thus be regarded as synonyms. The production of aubasidan rather than pullulan as the main extracellular exopolysaccharide (Yurlova & de Hoog 1997) is apparently strain dependent. Although the production of EPS and other previously described diagnostic characters for this variety were not evaluated in this study, we believe that used multilocus approach as molecular diagnostic tool would show the difference of var. aubasidani to other varieties. The ex-type strain of Dematoidium nigrescens (CBS 146.30) was the only initially darkly pigmented strain in group 1, which is probably due to its degeneration. The var. pullulans, which is newly defined, is phenetically characterised by rapidly expanding, pinkish cultures that can develop radial dark brown sectors due to the local presence of thick-walled, melanised hyphae. Most isolates attributed to this variety originate from sugary or osmotically fluctuating habitats, such as saline water in the salterns, tree slime flux, fruit surfaces and phyllosphere (Table 1). This well supported variety was obtained pan-globally from temperate to tropical habitats, and was also found trapped in Spitsbergen glaciers and in ice released from these glaciers into the sea water. Its distribution is wide, ranging from the Arctic to the Mediterranean coast. Given the small degree of diversity with TUB, ELO and EF1α, the taxon can be regarded as being relatively recent.
Group 2, A. pullulans var. melanogenum contains CBS 105.22, the ex-type strain of this variety, and an authentic strain, CBS 123.37 with the invalid description of Torula schoenii Roukhelman. Earlier data (Yurlova et al. 1995) suggested that this taxon cannot be distinguished from var. pullulans, but current sequence data show that the groups are strictly concordant. Cultures are characteristically black from the beginning. They produce an abundance of dark, ellipsoidal conidia, which can either originate from disarticulating hyphae (arthroconidia) or transfer from hyaline conidia. The hyaline conidia are ellipsoidal and emerge from inconspicuous scars alongside undifferentiated hyphae; the process of conidiogenesis is synchronous in addition to percurrent, the latter being identical to that in the anamorph genus Hormonema. The sources of isolation of the strains of this variety, as far as is known, are low-nutrient, mostly low-strength environments, such as moist metal and glass surfaces, showers, fountains, as well as ocean water. Only one strain of this variety was retrieved from a human patient, but it is also possible that this was a culture contaminant, since it is often reported in air, especially in warmer climates (Punnapayak et al. 2003). Strains of this variety have a world-wide distribution, from the Arctic to the tropics. Given its marked diversity with TUB, ELO and EF1α, this may be an ancestral taxon, the introns having accumulated more mutations than var. pullulans.
Group 3, A. pullulans var. subglaciale Zalar et al. is exclusively known from Kongfjorden glacial and subglacial ice and sea water. Its psychrotolerant nature is in line with its active metabolism under conditions of permanently cold in Arctic glaciers.
Group 4 consists of a single isolate, CBS 147.97, the ex-type of the monotypic variety A. pullulans var. namibiae, isolated from marble in Namibia, Africa. The strain takes an isolated position with all sequenced genes, but has not drifted far away from the ancestral variety.
Other related groups are 5 and 6, which are aureobasidium-like but consistently different. Strains of these groups thus far have only been recovered from glacial ice in Spitsbergen. The species occurred with very high densities in subglacial ice in microchannels, and in gypsum-rich ice at high pH. During their travel through the glacier, these cells have been subjected to extreme variations in aw due to ice freezing and thawing. These conditions are highly selective, for which reason this entity is likely to be restricted to small endemic areas, such as Kongsfjorden (Skidmore et al. 2005). The description as novel species will be the subject of a later paper.
The overall phylogenetic structure of A. pullulans suggests that the species is strictly clonal. A possible teleomorph, Discosphaerina fagi, has been suggested on the basis of ITS sequence similarity (de Hoog et al. 2000), but this finding awaits confirmation with multilocus analysis and re-isolation from single ascospores.
The varieties of Aureobasidium pullulans are markedly different for melanin production. This can be of biotechnological interest, since the organism is highly significant for its pullulan and aubasidan production (Yurlova & de Hoog 1997). Melanin contamination leads to low pullulan quality. Attempts have been made to grow non-pigmented yeast cells, e.g. by culturing A. pullulans in a two-stage fermentation process in media with a special nutrient combination (Shabtai & Mukmenev 1995), or with melanin-deficient mutants (Gniewosz & Duszkiewicz-Reinhard 2008). From the present study, it is apparent that the use of strains of the variety pullulans is recommended.
Acknowledgments
The authors would like to thank to Nick Cox (NERC Station) for his logistic support in our Arctic excursions. We are indebted to Walter Gams, who provided Latin diagnoses. We also thank Fumiyoshi Abe, who donated valuable deep-sea isolates. The work in Kongsfjorden was funded by EU Large Scale Facility Fund. The work was supported by the Slovenian Ministry of Education, Science and Sport.
Taxonomic novelties: Aureobasidium pullulans var. subglaciale Zalar, de Hoog & Gunde-Cimerman, var. nov.; Aureobasidium pullulans var. namibiae Zalar, de Hoog & Gunde-Cimerman, var. nov.
References
- Abyzov SS (1993). Microorganisms in the Antarctic ice. In: Friedmann EI (ed.): Antarctic Microbiology, Wiley-Liss, New York: 265–297.
- Andrews JH, Harris RF, Speaer RN, Lau GW, Nordheim EV (1994). Morphogenesis and adhesion of Aureobasidium pullulans. Canadian Journal of Microbiology 40: 6–17. [DOI] [PubMed] [Google Scholar]
- Babjeva I, Reshetova I (1998). Yeast resources in natural habitats at polar circle latitude. Food Technology and Biotechnology 36: 1–5. [Google Scholar]
- Bary A de (1884). Vergleichende Morphologie und Biologie der Pilze Mycetozoen und Bacterien: 182.
- Boekhout T, Fell JW, O`Donnell K (1995). Molecular systematics of some yeast-like anamorphs belonging to the Ustilaginales and Tilletiales. Studies in Mycology 38: 175–183. [Google Scholar]
- Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007). Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie van Leeuwenhoek 91: 277–289. [DOI] [PubMed] [Google Scholar]
- Butinar L, Strmole T, Spencer-Martins I, Gunde-Cimerman N (2008). Relative incidence of ascomycetous yeasts in Arctic coastal environments. Microbial Ecology in press. [DOI] [PubMed]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Chi Z, Liu Z, Gao L, Gong F, Ma C, Wang X, Li H (2006). Marine yeasts and their applications in mariculture. Journal of Ocean University of China 5: 251–256. [Google Scholar]
- Copland L, Sharp M (2001). Mapping thermal and hydrological conditions beneath a polythermal glacier with radio-echo sounding. Journal of Glaciology 47: 232–242. [Google Scholar]
- Deegenaars ML, Watson K (1998). Heat shock response in psychrophilic and psychrotrophic yeast from Antarctica. Extremophiles 2: 41–49. [DOI] [PubMed] [Google Scholar]
- Federici F (1982). Extracellular enzymatic activities in Aureobasidium pullulans. Mycologia 74: 738–743. [Google Scholar]
- Gerrits van den Ende AHG, Hoog GS de (1999). Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Studies in Mycology 43: 151–162. [Google Scholar]
- Glass NL, Donaldson GC (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubev WI (1998). New species of basidiomycetous yeasts, Rhodotorula creatinovora and R. yakutica, isolated from permafrost soils of Eastern-Siberian Arctic. Mykologiya i Phytopathologiya 32: 8–13. [Google Scholar]
- Gostinčar C, Turk M, Trbuha T, Vaupotič T, Plemenitaš A, Gunde-Cimerman N (2008). Expression of fatty-acid-modifying enzymes in the halotolerant black yeast Aureobasidium pullulans (de Bary) G. Arnaud under salt stress. Studies in Mycology 61: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniewosz M & Duszkiewicz-Reinhard W (2008). Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant Aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydrate Polymers 72: 431–438. [Google Scholar]
- Gunde-Cimerman N, Zalar P, Hoog GS de, Plemenitaš A (2000). Hypersaline water in salterns – natural ecological niches for halophilic black yeasts. FEMS Microbiology Ecology 32: 235–240. [DOI] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Sonjak S, Zalar P, Frisvad JC, Diderichsen B, Plemenitaš A (2003). Extremophilic fungi in arctic ice: a relationship between adaptation to low temperature and water activity. Physics and Chemistry of the Earth 28: 1273–1278. [Google Scholar]
- Hermanides-Nijhof EJ (1977). Aureobasidium and allied genera. Studies in Mycology 15: 141–222. [Google Scholar]
- Hocking AD, Pitt JI (1980). Dichloran-glycerol medium for enumeration of xerophilic fungi from low-moisture foods. Applied and Environmental Microbiology 39: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000). Atlas of Clinical Fungi, 2nd ed. Centraalbureau voor Schimmelcultures / Universitat Rovira i Virgili, Utrecht / Reus, 1126 pp.
- Hoog GS de, Zalar P, Urzì C, de Leo F, Yurlova NA, Sterflinger K (1999). Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8S and ITS2 rDNA sequence comparison. Studies in Mycology 43: 31–37. [Google Scholar]
- Hoog GS, Yurlova NA (1994). Conidiogenesis, nutritional physiology and taxonomy of Aureobasidium and Hormonema. Antonie van Leeuwenhoek 65: 41–54. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998). Multiple sequence alignment with ClustalX. Trends in Biochemical Sciences 23: 403–405. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH (1978). Methuen handbook of colour, 3rd ed. London: Eyre Methuen. 243 pp.
- Li H, Chi Z, Wang X, Duan XH, Ma LY, Gao LM (2007). Purification and characterisation of extracellular amylase from the marine yeast Aureobasidium pullulans N13d and its raw potato starch digestion. Enzyme and Microbial Technology 40: 1006–1012. [Google Scholar]
- Lutzoni F, Kauff F, Cox C, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James T, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O`Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004). Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Catranis CM, Starmer WT, Rogers SO (1999). Revival and characterization of fungi from ancient polar ice. Mycologist 13: 70–73. [Google Scholar]
- Ma LJ, Catranis CM, Starmer WT, Rogers SO (2005). The significance and implications of the discovery of filamentous fungi in glacial ice. In: Castello JD, Rogers SO (eds): Life in Ancient Ice, Princeton University Press, Princeton and Oxford.
- Ma LJ, Rogers SO, Catranis CM, Starmer WT (2000). Detection and characterization of ancient fungi entrapped in glacial ice. Mycologia 92: 286–295. [Google Scholar]
- Ma C, Ni X, Chi Z, Ma LY, Gao LM (2007). Purification and characterization of an alkaline protease from the marine yeast Aureobasidium pullulans for bioactive peptide production from different sources. Marine Biotechnology 9: 343–351. [DOI] [PubMed] [Google Scholar]
- Margesin R, Zacke G, Schinner F (2002). Characterization of heterotrophic microorganisms in Alpine glacier cryoconite. Arctic, Onofri S, Selbmann L, Zucconi L, Pagano S (2004). Antarctic microfungi as models for exobiology. Planetary and Space Science 52: 229–237. [Google Scholar]
- Punnapayak H, Sudhadham M, Prasongsuk S, Pichayangkura S (2003). Characterization of Aureobasidium pullulans isolated from airborne spores in Thailand. Journal of Industrial Microbiology and Biotechnology 30: 89–94. [DOI] [PubMed] [Google Scholar]
- Pitt JI (1979). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London.
- Price PB (2000). A habitat for psychrophiles in deep Antarctic ice. Proceedings of the National Academy of Sciences of the U.S.A. 97: 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha MR, Sharma CP (2007). Pullulan as a promising biomaterial for biomedical applications: A perspective. Trends in Biomaterials and Artificial Organs 20: 116–121. [Google Scholar]
- Ronquist F, Huelsenbeck JP (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC (2004). Introduction to Food- and Airborne Fungi, 7th ed. Centraalbureau voor Schimmelcultures, Utrecht.
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Shabtai Y, Mukmenev I (1995). Enhanced production of pigment-free pullulan by a morphogenetically arrested Aureobasidium pullulans (ATCC 42023) in a two-stage fermentation with shift from soy bean oil to sucrose. Applied Microbiology and Biotechnology 43: 595–603. [Google Scholar]
- Singh RS, Saini GK, Kennedy JF (2008). Pullulan: microbial sources, production and applications. Carbohydrate Polymers 73: 515–531. [DOI] [PubMed] [Google Scholar]
- Skidmore M, Anderson SP, Sharp M, Foght J, Lanoil BD (2005). Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Applied and Environmental Microbiology 71: 6986–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonjak S, Frisvad JC, Gunde-Cimerman N (2006). Penicillium mycobiota in Arctic subglacial ice. Microbial Ecology 52: 207–216. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007). Mega4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596-1599. [DOI] [PubMed] [Google Scholar]
- Urzì C, De Leo F, Lo Passo C, Criseo G (1999). Intra-specific diversity of Aureobasidium pullulans strains isolated from rocks and other habitats assessed by physiological methods and by random amplified polymorphic DNA (RAPD). Journal of Microbiological Methods 36: 95–105. [DOI] [PubMed] [Google Scholar]
- Viala P, Boyer G (1891). Sur un Basidiomycète inferérieur, parasite des grains de raisins. Comptes Rendues Hebdomaires des Séances de l'Académie de Sciences, Paris 112: 1148–1150. [Google Scholar]
- Vishniac HS (2006). A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microbial Ecology 52: 90–103. [DOI] [PubMed] [Google Scholar]
- Vishniac HS, Onofri S (2003). Cryptococcus antarcticus var. circumpolaris var. nov., a basidiomycetous yeast from Antarctica. Antonie van Leeuwenhoek 83: 231–233. [DOI] [PubMed] [Google Scholar]
- Wang L, Chi Z, Wang X, Liu Z, Li J (2007). Diversity of lipase-producing yeasts from marine environments and oil hydrolysis by their crude enzymes. Annals of Microbiology 57: 34–40. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA et al. (eds): PCR Protocols: Academic Press, San Diego 315–322.
- Yurlova NA, Mokrousov IV, Hoog GS de (1995). Intraspecific variability and exopolysaccharide production in Aureobasidium pullulans. Antonie van Leeuwenhoek 68: 57–63. [DOI] [PubMed] [Google Scholar]
- Yurlova NA, Uijthof JMJ, Hoog GS de (1996). Distinction of species in Aureobasidium and related genera by PCR-ribotyping. Antonie van Leeuwenhoek 69: 323–329. [DOI] [PubMed] [Google Scholar]
- Yurlova NA, Hoog GS de (1997). A new variety of Aureobasidium pullulans characterized by exopolysaccharide structure, nutritional physiology and molecular features. Antonie van Leeuwenhoek 72: 141–147. [DOI] [PubMed] [Google Scholar]
- Yurlova NA, Hoog GS de, Gerrits van den Ende AHG (1999). Taxonomy of Aureobasidium and allied genera. Studies in Mycology 43: 63–69. [Google Scholar]
- Zhiqiang L, Xiaoyu L, Zhenming C, Lin W, Jing L, Xianghong W (2008). Cloning, characterization and expression of the extracellular lipase gene from Aureobasidium pullulans HN2-3 isolated from sea saltern. Antonie van Leeuwenhoek 94: 245–255. [DOI] [PubMed] [Google Scholar]