Abstract
Fungal strains isolated from rocks and lichens collected in the Antarctic ice-free area of the Victoria Land, one of the coldest and driest habitats on earth, were found in two phylogenetically isolated positions within the subclass Dothideomycetidae. They are here reported as new genera and species, Recurvomyces mirabilis gen. nov., sp. nov. and Elasticomyces elasticus gen. nov., sp. nov. The nearest neighbours within the clades were other rock-inhabiting fungi from dry environments, either cold or hot. Plant-associated Mycosphaerella-like species, known as invaders of leathery leaves in semi-arid climates, are also phylogenetically related with the new taxa. The clusters are also related to the halophilic species Hortaea werneckii, as well as to acidophilic fungi. One of the latter, able to grow at pH 0, is Scytalidium acidophilum, which is ascribed here to the newly validated genus Acidomyces. The ecological implications of this finding are discussed.
Keywords: Acidophilic fungi, Antarctica, black fungi, extremotolerance, halophilic fungi, ITS, lichens, phylogeny, rock-inhabiting fungi, SSU, taxonomy
INTRODUCTION
Contrary to expectations, bare rocks in arid and semi-arid climates may harbour a bewildering biodiversity of black fungi. Many species have been reported from the Mediterranean basin (Sterflinger et al. 1997, Wollenzien et al. 1997, Bogomolova & Minter 2003, De Leo et al. 1999, 2003, Bills et al. 2004, Ruibal et al. 2005, Ruibal et al. 2008). These extremotolerant fungi live and even thrive on surfaces that are too harsh to support growth of competing microorganisms; they shelter in small depressions in the marble surface, called micropits (Sterflinger 1998). Similar extremotolerant fungi were discovered in the extremely cold and ice-free McMurdo Dry Valleys, a desert area in the Antarctic (Nienow & Friedmann 1993), where temperatures are only occasionally above zero, dropping to about –50 °C in winter. Onofri et al. (1999) and Selbmann et al. (2005) even reported on the existence of possibly endemic genera, Friedmanniomyces Onofri and Cryomyces Selbmann, de Hoog, Mazzaglia, Friedmann & Onofri in these habitats, which apparently show active evolution under conditions of near-permanent frost and extreme dryness (Friedmann et al. 1987). These fungi may escape prohibitive environmental conditions by colonising air spaces in rocks, living in association with lichens and algae in cryptoendolithic communities (Friedmann & Ocampo 1976, Friedmann 1982).
In the present paper we describe three new fungal genera and species; their novelty is supported by molecular phylogeny, taking a clearly separate position within the Dothideomycetidae. One genus includes two strains isolated from rocks in the Antarctic desert, one strain from rocks collected in Monte Rosa in the Alps, Italy, and an unidentified rock fungus from Puebla de la Sierra, Spain; the other genus includes three strains isolated from different thalli of Antarctic lichens, one from cryptoendolithic Antarctic communities and one from rocks collected in Aconcagua in the Argentinian Andes. In contrast to most rock-inhabiting black fungi, which are generally scarcely differentiated, they show peculiar and distinguished morphological traits.
Fungi may also be encountered in extremely acidic environments. Some are able to grow at pH values down to pH 0 (Starkey & Waksman 1943, Harrison et al. 1966, Gould et al. 1974, Ivarsson & Morita 1982, Gimmler et al. 2001). Sigler & Carmichael (1974) compared four strains from an acidic soil (pH 1.4–3.5) with the ones previously isolated by Starkey & Waksman (1943) and Ivarsson & Morita (1982), referring them to the genus Scytalidium Pesante on the basis of scarcely differentiated brown arthroconidia. Our SSU and ITS comparison proved these fungi also to be members of a clade within the Dothideomycetidae, amidst rock-inhabiting fungi from cold and semi-arid climates.
MATERIALS AND METHODS
Strains
Black fungi were isolated from rock samples harbouring a cryptoendolithic lichen-dominated community and from epilithic lichens collected in different locations of Northern and Southern Victoria Land, Antarctica, in the framework of the Italian expedition 2003/2004, and from rock samples collected in Mount Aconcagua, Andes, Argentina, and Monte Rosa in the Alps, Italy, as reported in Table 1. The samples were collected using a sterile chisel, sterlilised in the field before each sampling, and preserved in sterile bags at –20 °C. To remove potential contaminants, rocks collected in other environments than Antarctica were washed 15 min in sterile physiological solution added with 0.1 % Tween 20 (Sigma - Aldrich, Munich, Germany) and rinsed 4 times with physiological solution to remove any trace of the detergent. Isolations from rocks were performed by powdering the samples and seeding fragments in triplicate Petri dishes filled with 2 % malt extract agar (MEA, AppliChem, GmbH) and dichloran-rose bengal agar (DRBC, Oxoid Ltd., Basingstoke, Hampshire, U.K.). Media were supplemented with chloramphenicol 100 ppm to prevent bacterial growth. Fungi from lichens were isolated as follows: small fragments of thalli were sterilised by washing with H2O2 (8 %) for 5 min, washed with sterile deionised water to remove any trace of H2O2 and finally seeded on MEA and DRBC. Plates were incubated at 5 and 15 °C and inspected every 15 d until no new colonies appeared. Colonies were transferred to MEA slants and incubated at 15 °C. Strains analysed for comparison are listed in Table 1; they were taken from reference collections of the Culture Collection of Fungi from Extreme Environments (CCFEE, Viterbo, Italy) and the Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands), including a large set of strains sampled by P.W. Crous from plants with leathery leaves in a semi-arid climate.
Table 1.
List of strains studied.
| Species | Strain no. | Source | Geography | ITS | Reference |
|---|---|---|---|---|---|
| Acidomyces acidophilum | CBS 335.97 | Acidophilic algae Dunaliella acidophila pH 1.0 | Germany | AJ244237 | Gimmler et al. 2001 |
| Acidomyces acidophilum (deposited as Scytalidium acidophilum) | CBS 270.74 T (ATCC 26772; UAMH 3460; IMI 183518) | Soil near acidic elemental sulphur pile pH 1.1 | Canada | — | Sigler & Carmichael 1974 |
| Acidomyces acidophilum (deposited as Botryomyces caespitosus) | CBS 899.87 | Pyrite ore acidic drainage pH 2.0 | Germany | — | — |
| dH 13081 = det 106/2003 | 2N Sulphuric acid pH 1 | Danmark (supplied by GC Frisvad) | — | Starkey & Waksman 1943 | |
| dH 11526 = det 237-1999 | Volcanic soil | Iceland (supplied by S Gross, Berlin) | — | — | |
| dH 12881 = det 142-AF1 | — | — | |||
| Acidomyces sp. | dH 13119 | Acidic industrial process water pH 1.5 | Emmen, The Netherlands | — | — |
| Batcheloromyces proteae | CBS 110696; CPC 1518 | Protea cynaroides | South Africa | — | Crous et al. 2007 |
| Capnobotryella renispora | CBS 214.90 T (CBS 176.88; IAM 13014; JCM 6932) | Capnobotrys neessii | Japan | — | — |
| Catenulostroma abietis | CBS 290.90 | Man, skin lesion | The Netherland | AY128698 | Crous et al. 2007 |
| CBS 145.97 (dH 15396) | sandstone of cathedral | Zeitz, Germany | AY128699 | Butin et al. 1996 | |
| CBS 300.81 | Juniperus communis (Cupressaceae), needle | Graubünden, Grüsc, Switzerland | AJ244264 | — | |
| CBS 279.86 | Kiel, Germany | — | Butin et al. 1996; Sterflinger et al. 1999 | ||
| dH 12687 = det 396/2001 | Painted wall | Sweeden | — | — | |
| TRN 128 | Limestone | Mallorca | AY559363 | — | |
| dH 12697 = det 373/2001 RMF N113 | Desert soil | Namibia | — | — | |
| dH 13593 | See snail | Italy | — | — | |
| CBS 618.84 | Ilex sp. leaf | Germany | AY128696 | — | |
| CBS 118765 (TRN 127; dH 14531) | Limestone | Cala San Vincenc, Mallorca | AY559362 | — | |
| Catenulostroma elginense | CBS 111030 (CPC 1958) | Protea grandiceps | South Africa | AY260093 | Crous et al. 2007 |
| Catenulostroma germanicum | CBS 539.88 | Stone | Germany, former West-Germany | EU019253 | Crous et al. 2007 |
| Catenulostroma macowanii | CBS 110756 (CPC 1872) | Protea nitida | South Africa | — | Crous et al. 2007 |
| CPC 1488 | — | — | |||
| Elasticomyces elasticus | CBS 122538 (CCFEE 5313) | Lecanora fuscobrunnea | Kay Island, Northern Victoria Land, Antarctica | FJ415474 | — |
| CBS 122539 (CCFEE 5319) | Lecanora sp. | Inexpressible Island, Northern Victoria Land, Antarctica | FJ415475 | — | |
| CBS 122540 (CCFEE 5320) | Usnea antarctica | Edmondson Point, Northern Victoria Land, Antarctica | FJ415476 | — | |
| Da-004-06 | Rock | Mount Aconcagua, Andes, Argentina | — | — | |
| CCFEE 5474 (D007-06) | Sandstone | Tarn Flat, Northern Victoria Land, Antarctica | — | — | |
| Friedmanniomyces endolithicus | CBS 119423 (CCFEE 5208) | Sandstone | Northern Victoria Land, Antarctica | — | Selbmann et al. 2005 |
| CBS 119424 (CCFEE 5195) | Rock | Northern Victoria Land, Antarctica | — | Selbmann et al. 2005 | |
| CBS 119429 (CCFEE 5193) | Sandstone | Timber Peak, Northern Victoria Land, Antarctica | — | Selbmann et al. 2005 | |
| Friedmanniomyces endolithicus | CBS 119428 (CCFEE 5001) | Sandstone | Timber Peak, Northern Victoria Land, Antarctica | — | Selbmann et al. 2005 |
| Friedmanniomyces simplex | CBS 116775 T (CCFEE 5184) | Sandstone | Battleship Promontory, Southern Victoria Land, Antarctica | DQ028271 | Selbmann et al. 2005 |
| Hobsonia santessonii | Peltigera scabrosa | Sweden | — | Sikaroodi et al. 2001 | |
| Hortaea acidophila | CBS 113389 (dH 11932) | Lignite pH 1.0 | Germany | — | Hölker et al. 2004 |
| Hortaea werneckii (preserved as Pseudotaeniolina globosa) | CBS 110352 (dH 12843 = VPCI 176) | Hollow tree | Sudan | — | — |
| Hortaea werneckii | dH 12322; Poonwan 13-44-08648 | — | — | — | — |
| CBS 373.92 (dH 15813) | Ceach soil | La Palma, Spain | AJ238474 | — | |
| CBS 359.66 (dH 15803) | Can, tinea nigra palmaris | Suriname, Paramaribo | AJ244249 | — | |
| CBS 122.32 (dH 15340) | Can, tinea nigra palmaris | — | AJ238473 | — | |
| CBS 117.90 (UAMH 4978; dH 15327) | Salted fish, Osteoglossum bicirrhosum | Brazil | AJ238472 | Mok et al. 1981 | |
| CBS 116.90 (ATCC 52681; UAMH 5389; dH 15311) | Cantharus cantharus, eye infection, black sea bream in aquarium | Italy | AJ238471 | Todaro et al. 1983 | |
| CBS 115.90 (UAMH 4985; dH 15303) | Bufo granulosus, kidney | Brazil | AJ238470 | — | |
| CBS 111.31 (dH 15284) | Man, keratomycosis nigricans palmaris | Brazil | AJ238679 | — | |
| CBS 100455 (MZKI B-675) | Coral, sea water | Croatia | AY128704 | Zalar et al. 1999 | |
| CBS 107.67 (dH 15206) | Man, tinea nigra | Lisboa, Portugal | AJ238468 | McGinnis 1979 | |
| MZKI B-987 | Ipersaline water | Spain | — | — | |
| dH 13416 | Angelfish | — | — | — | |
| CBS 117931 (TRN 122, dH 14528) | Limestone | Cala San Vincenc, Mallorca | AY559357 | — | |
| BMU00057 | Patient's foot | China | — | — | |
| Mycocalicium victoriae | CBS 109863 | Soil, garden museum | Messina, Italy | AJ312123 | — |
| Mycosphaerella tasmaniensis | CBS 114556 (CMW 14663; STE-U 1556) | E. nitens | Australia | DQ267592 | — |
| CBS 111687 (CMW 14780; STE-U 1555) | E. nitens | Australia | AY667578 | — | |
| Pseudotaeniolina globosa | CBS 110353 (dH 12840; det M108/2002) | 58-yr-old man, aorta, at autopsy | Würzburg, Germany | — | Kurzai et al. 2003 |
| CBS 109889 T (MC 769) | Church roof | Sicily, Italy | AY128700 | De Leo et al. 2003 | |
| CBS 113249 (dH 13060) | — | — | — | — | |
| CBS 303.84 | Wood | Germany | AJ244268 | de Hoog et al. 1999 | |
| CBS 119923 (dH 16905) | Window | Japan | — | — | |
| Recurvomyces mirabilis | CBS 119434 (CCFEE 5264; dH 14759) | Sandstone | Battleship Promontory, Southern Victoria Land, Antarctica | FJ415477 | — |
| Recurvomyces mirabilis | CCFEE 5480 (D016-02) | Sandstone | Battleship Promontory, Southern Victoria Land, Antarctica | — | — |
| CCFEE 5391 | Rock | Mount Rosa, P.ta Indren, Alps, Italy | — | — | |
| Recurvomyces sp. | CBS 117957 (TRN 491; dH 14566) | Quarzite | Puebla de la Sierra, Madrid, Spain | AY1843175 | — |
| Teratosphaeria cryptica | TC 0.56 | E. globulus | Australia | DQ665661 | — |
| Teratosphaeria mexicana | CBS 110502 (CMW 14461) | E. globulus | Manjimup, Darling View, Plantation, Western Australia | AY725558 | Crous et al. 2007 |
| Teratosphaeria microspora | CBS 110890 (CPC 1832) | Protea leaf | South Africa | AY260097 | Taylor et al. 2003 |
| Teratosphaeria molleriana | CBS 111164 (CMW 4940; STE-U 1214) | E. globulus | Abrantes, Portugal | AF309620 | Crous et al. 2007 |
| CPC11842 | Eucalyptus sp. | Portugal | DQ302989 | Crous et al. 2006 | |
| CPC11845 | Eucalyptus sp. | Portugal | DQ302990 | Crous et al. 2006 | |
| CBS 116370 (CPC 10397) | E. globulus | Spain | AY725561 | ||
| CPC 12056 | DQ302991 | Crous et al. 2006 | |||
| Teratosphaeria nubilosa | CBS 116005 E (CMW 3282; CPC 937) | E. globulus | Austria | AY725572 | Crous et al. 2007 |
| CPC 4661 | E. globulus | Spain | AY725570 | Crous et al. 2004 | |
| CBS 111445 (CPC 4660) | E. globulus | Spain | AY725569 | Crous et al. 2004 | |
| CPC 3722 | E. globulus | Spain | AY725568 | Crous et al. 2004 | |
| CPC 1099 | E. globulus | Tanzania | AY725567 | Crous et al. 2004 | |
| CBS 111969 (CPC 1078) | E. globulus | Kenia | AY725563 | Crous et al. 2004 | |
| CPC 4663 | E. globulus | Spain | AY725571 | Crous et al. 2004 | |
| CPC 11882 | E. globulus | Portugal | DQ302999 | Crous et al. 2006 | |
| CPC 11723 | — | ||||
| CPC 11487 | E. globulus | Spain | DQ302994 | Crous et al. 2006 | |
| CPC 11249 | E. globulus | Spain | DQ302993 | Crous et al. 2006 | |
| CPC 11246 | E. globulus | Spain | DQ302992 | Crous et al. 2006 | |
| CBS 114419 (CPC 10497) | Eucalyptus globulus (Myrtaceae) | New Zealand | AY725574 | Crous et al. 2007 | |
| TC 0.42 | E. globulus | Australia | DQ665659 | — | |
| TC 0.40 | E. globulus | Australia | DQ665657 | — | |
| TC 0.47 | E. globulus | Australia | DQ665658 | — | |
| CBS 116283 (CPC 10495) | E. globulus | New Zeland | AY725573 | Crous et al. 2004 | |
| Teratosphaeria nubilosa ( | CPC 11885 | E. globulus | Portugal | DQ303000 | Crous et al. 2006 |
| Teratosphaeria ohnowa | CPC 1005 | — | — | AF309605 | Crous et al. 2001 |
| CBS 112896* (CMW 4937; CPC 1004) | E. globulus | South Africa | AF309604 | Crous et al. 2001 | |
| — | — | AF173299 | — | ||
| CMW 9103 | — | — | AF468881 | — | |
| CBS 110949 (STE-U 1006) | E. grandis (Myrtaceae), leaves | Hazyview, South Africa | AY725575 | — | |
| Teratosphaeria toledana | CBS 113313 H (CMW 14457) | Eucalyptus sp., leaves | Toledo, Spain | AY725580 | — |
| CPC 10840 | Eucalyptus sp. | Spain | AY725581 | Crous et al. 2004 |
Abbreviations used: ATCC — American Type Culture Collection, Manassas, VA, U.S.A.; CBS — Centraalbureau voor Shimmelcultures, Utrecht, The Netherlands; dH — GS de Hoog private collection, CBS, Utrecht, The Netherlands; CCFEE — Culture Collection of Fungi From Extreme Environments, Università degli Studi della Tuscia, Viterbo, Italy; CMW — Culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; CPC — Culture collection of P Crous, housed at the CBS; IMI — International Mycological Institute, U.K.; MC — Collection of Istituto di Microbiologia di Messina, Italy; MZKI — Microbiological Culture Collection, National Institute of Chemistry, Ljubljana, Slovenia; STE-U — University of Stellenbosch fungal culture collection, Stellenbosch, South Africa; TRN — T Ruibal private collection; UAMH — The University of Alberta Microfungus Collection and Herbarium, Edmonton, AB, Canada.
Ex-type cultures.
Morphology
Hyphal maturation and conidiogenesis were studied using both light and scanning electron microscope (SEM). Slide cultures were seeded onto MEA, incubated for 10 wk and mounted in lactic acid. Samples for SEM observations were prepared according to methods described by Onofri et al. (1980).
DNA extraction and sequencing
DNA was extracted from mycelial fragments taken from 6-mos-old MEA slants grown at 10 °C, using Nucleospin Plant kit (Macherey-Nagel, Düren, Germany) following the protocol optimised for fungi. PCR reactions were performed using BioMix (BioLine GmbH, Luckenwalde, Germany). In each 25 μL reaction tube 5 pmol of each primer and 40 ng on template DNA were added. The amplification was carried out using MiniCycler™ (MJ Research, Waltham, Massachusetts, U.S.A.) equipped with a heated lid. The first denaturation step at 95 °C for 3 min was followed by: denaturation at 95 °C for 2 s, annealing at 55 °C for 30 s, extension at 72 °C for 30 s. The last three steps were repeated 35 times, with a last extension 72 °C for 5 min. The products were purified using Nucleospin Extract kit (Macherey-Nagel, Düren, Germany). Primers NS1, NS2, NS3, NS4, NS5, NS8, ITS1, ITS4 (White et al. 1990), SR10R (Bruns et al. 1992), ITS5, and ITS4a (Larena et al. 1999) were employed to amplify SSU and ITS rDNA portions. Sequencing reactions were performed according to the dideoxynucleotide method (Sanger et al. 1977) using the TF Big Dye Terminator 1,1 RR kit (Applied Biosystems). Fragments were analysed using an ABI 310 Genetic Analyser (Applied Biosystems). Sequence assembly was done using the software Chromas (v. 1.45 1996–1998, Conor McCarthy School of Health Science, Griffith University, Southport, Queensland, Australia).
Alignment and tree reconstruction
SSU sequences were aligned with ARB beta-package (v. 22-08-2003, Ludwig et al. 2004; www.mikro.biologie.tu-muenchen.de/pub/ARB). The SSU alignment spanned positions 141–2512, which corresponds to 1515 bp with reference to Saccharomyces cerevisiae. Trees based on SSU sequences were reconstructed with neighbour-joining in ARB.
ITS sequences were aligned iteratively with Ward's averaging (Van Ooyen 2002) in a research data base of black yeasts present at CBS using the BioNumerics package (Applied Maths, Kortrijk, Belgium). Due to gaps necessary for alignment, the ITS1 domain spanned 187 positions (real lengths 147–154 bp), the 5.8S gene 156 positions and the ITS2 domain 184 positions (real lengths 143–155 bp). The alignments were based on the positions 28–481, the initial and the final parts were cut off to compare fragments with the same length. Alignments were exported and the best-fit substitution model was determined using Modeltest MrAic.pl 1.4.3 (Nylander 2004, program distributed by the author) estimated using Phyml (Guindon & Gascuel 2003) through hierarchical likelihood ratio tests. MrAic calculates the Akaike Information Criterion (AIC), corrected Akaike Information Criterion (AICc) and Bayesian Information Criterion (BIC); Akaike weights for nucleotide substitution model and model uncertainty. All 56 models implemented in Modeltest were evaluated. Phylogenetic trees were reconstructed by Maximum Likelihood, using Treefinder (Jobb et al. 2004) and the resulting tree was displayed using Treeview v. 1.6.6 (Page 1996). The robustness of the phylogenetic inference was estimated using the bootstrap method (Felsenstein 1985) with 100 pseudoreplicates generated and analysed with Treefinder.
As alignment over the entire complex was highly ambiguous, an algorithm for tree reconstruction without alignment, the DNA-walk Divergence method (DNAWD, Licinio & Caligiorne 2004; Caligiorne et al. 2005) was used, involving the entire spacer region. DNA-walks are defined by incrementing walk steps for each nucleotide in the sequence (for example a positive step for purines, and negative for pyrimidines). It makes simultaneous comparisons of the three-dimensional walks (representing three composition skews): AG-TC, AC-TG, and AT-CG for each pair of sequences. One sequence slides against the other until the minimum squared walk difference is found, corresponding to a global alignment. This is then taken as a measure of their distance since statistically independent mutations and indels increase the mean square walk differences linearly. The resulting distance matrices are then fed into the Kitsch program of the Phylip package (v. 3.572c, Felsenstein 1996).
Cultural preferences
Cultural characteristics and growth rates were recorded on Potato-Dextrose Agar (PDA), MEA, Czapek Dox Agar (CzA) and Oatmeal Agar (OA). Strain CBS 119434 was incubated at 10 °C, strains CBS 122538, 122539 and 122540 at 20 °C and strains CBS 899.87, CBS 335.97, dH 12881, dH 11526 and dH 13081 at 25 °C. The diameter of the colonies was recorded monthly. Tests were performed in triplicate.
Temperature preferences
Temperature preferences for the strains CBS 119434, 122538, 122539 and 122540 were tested by incubating them on MEA, in Petri dishes at 0–35 °C (in 5° intervals) ± 1 °C. The diameter of the colonies was recorded monthly. Tests were performed in triplicate. Optimum temperatures for growth and development of the strains CBS 899.87, CBS 335.97, dH12881, dH11526 and dH13081, were determined by seeding 25 mL flasks containing 2 % Malt Extract Broth (MEB) with 0.25 mL of a 105 cells/mL suspension and incubating in shaken culture at 70 r.p.m. After 30 d of incubation at temperatures of 4, 10, 18, 25, 30, 37 °C, cultures were filtered and the biomass dry-weighed. The test was performed in duplicate.
Growth at different salt concentrations
The ability to grow at different salinities was tested in duplicate on plates of MEA amended with 1.2, 1.5, 3, 5, 7, 10 or 12 % NaCl. Strains were inoculated in three spots on each plate and incubated at 25 °C for one mo, when the colony diameter was recorded. Colonies with a diameter >2 mm were considered positive (Kane & Summerbell 1987).
Growth at different pH
The ability to grow at different pH values for the strains CBS 899.87, CBS 335.97, dH12881, dH11526 and dH13081 was tested in duplicate using MEB2 % medium at pH 1, 3, 5, 7 and 9. Values of pH 5 were obtained by the addition of 1N HCl; remaining pH values were obtained according to Küster & Thiel (1990) as follows: McIlvaine solution for pH 2–7, Clark & Lubs solution for pH 8–9; buffer HCl/KCl for pH 1. Strains were incubated at 25 °C in shaken culture at 70 r.p.m. for one mo, cultures were filtered and the biomass dry-weighed.
RESULTS
Physiology
Temperature relations and cultural features of the strains CBS 119434, 122538, 122539 and 122540 are shown in Table 2. Physiological data for the strains CBS 899.87, CBS 335.97, dH12881, dH11526 and dH13081 are reported in Tables 3 and 4. All fungi tested were able to grow on natural media showing clearly visible growth on MEA as well as on PDA and OA, whilst there was a marked reduction of ultimate colony diameter when seeded on CzA. CBS 119434 was able to grow in the range 0–20 °C, with an optimum at 15 °C, whereas strains CBS 122538, 122539 and 122540 grew in a wider range of temperatures between 0 and 25 °C, with an optimum at 15–20 °C. Strains CBS 899.87, CBS 335.97, dH12881, dH11526 and dH13081 grew best in the range of 18–30 °C, with the optimum temperature at 18 °C for the strains CBS 335.97 and dH 12881, 25 °C for the strains CBS 899.87 and dH 13081, and 30 °C for the strain dH 11526. Since almost all strains (except CBS 899.87) were still able to reproduce at 4 °C, they can be referred as mesophilic-psycrotolerant (Zucconi et al. 1996). None of them grew at 37 °C. Furthermore, they were able to grow in very acidic conditions (pH 1) and most of them grew best at pH 5 or below. In particular the optimum was pH 5 for the strain CBS 899.87, around pH 3–5 for the strains CBS 335.97, dH11526 and dH 13081 and pH 3 for the strain dH 12881. All the strains studied showed a decreasing growth at pH 7 and stopped to grow at pH 9, with the exception of strain CBS 899.87 which was able to grow in a very wide range of pH values showing a weak growth even at pH 9. The strains demonstrated also to be moderately halophilic being able to grow in up to 7 % NaCl (strains CBS 335.97, dH11526, dH 13081, dH 12881) and in up to 10 % for the strain CBS 899.87.
Table 2.
Physiological profiles of Antarctic strains.
| Species | Strain no. | Cultural preferences | Thermal preferences (° C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDA | MEA | CzA | OA | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | ||
| Recurvomyces mirabilis | CBS 119434 | 1.5±0.14 | 1.8±0.14 | 0.5±0.14 | 1.65±0.07 | 0.45±0.07 | 0.8±0.2 | 1.5±0.26 | 1.9±0.26 | 0.78±0.2 | - | - | - |
| Elasticomyces elasticus | CBS 122538 | 1.7±0.2 | 1.5±0.14 | 0.5±0.14 | 1.5±0.14 | 0.82±0.064 | 0.64±0.079 | 1.2±0.2 | 1.6±0.26 | 1.54±0.09 | 0.95 ±0.15 | - | - |
| CBS 122539 | 1.5±0.1 | 1.43±0.15 | 0.3±0.02 | 1.23±0.2 | 0.77±0.1 | 0.6±0.2 | 1.1±0 | 1.5±0.14 | 1.2±0.2 | 0.6±0.2 | - | - | |
| CBS 122540 | 1.0±0.2 | 1.1±0.1 | 0.2±0.02 | 1.0±0.2 | 0.72±0.1 | 0.5±0.14 | 1.13±0.2 | 1.4±0.17 | 1.2±0.2 | 0.8±0.08 | - | - | |
Cultural and thermal preferences reported as diameter of the colonies (cm), after two mos of incubation. The values represent the average of three different tests. Plates for cultural preferences were incubated at 10 °C for strain CBS 119434 and at 20 °C for strains CBS 122538, 122539 and 122540; plates for temperature preferences were seeded on MEA; - = no growth.
Table 3.
Cultural preferences and salt tolerance of acidophilic strains.
| Species | Strain no. | Cultural preferences | NaCl % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDA | MEA | CzA | OA | 1.2 | 1.5 | 3 | 5 | 7 | 10 | 12 | ||
| Acidomyces acidophilum | CBS 899.87 | 1.17±0.1 | 1.33±0.15 | 0.3±0.02 | 1.17±0.1 | 1.3±0.02 | 0.7±0.02 | 0.6±0.03 | 0.6±0 | 0.5±0 | 0.2±0.02 | - |
| CBS 335.97 | 1.08±0.1 | 1.9±0.14 | 0.6±0.2 | 1.0±0.2 | 1.5±0.02 | 0.5±0.02 | 0.4±0.04 | 0.3±0.02 | 0.2±0.02 | - | - | |
| dH 12881 | 2.0±0.14 | 1.5±0.14 | 1.2±0.2 | 1.5±0.1 | 1.5±0.02 | 1.5±0.02 | 1.5±0.02 | 0.7±0.03 | 0.5±0.04 | - | - | |
| dH 11526 | 2.5±0.2 | 3.0±0.2 | 1.5±0.14 | 1.2±0.2 | 2.5±0.02 | 2.0±0.04 | 2±0.04 | 0.8±0.03 | 0.6±0.008 | - | - | |
| dH 13081 | 1.8±0.14 | 2.2±0.2 | 1.1±0.1 | 1.0±0.2 | 1.0±0.04 | 0.8±0.02 | 0.8±0.02 | 0.6±0.02 | 0.3±0.02 | - | - | |
Growth on different media and salt concentration expressed as diameters of the colonies (cm); - = no growth.
Table 4.
Thermal and pH preferences of acidophilic strains.
| Species | Strain no. | Thermal preferences (° C) | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 10 | 18 | 25 | 30 | 37 | 1 | 3 | 5 | 7 | 9 | ||
| Acidomyces acidophilum | CBS 899.87 | - | - | + | ++ | + | - | + | + | ++ | + | ± |
| CBS 335.97 | + | + | ++ | + | + | - | + | ++ | ++ | + | - | |
| dH 12881 | + | + | ++ | ++ | + | - | + | ++ | + | ± | - | |
| dH 11526 | + | + | + | + | ++ | - | + | ++ | ++ | + | - | |
| dH 13081 | + | + | + | ++ | + | - | ± | ++ | ++ | + | - | |
++ = maximum growth recorded; + = growth; ± = weak growth; - = no growth.
Phylogeny
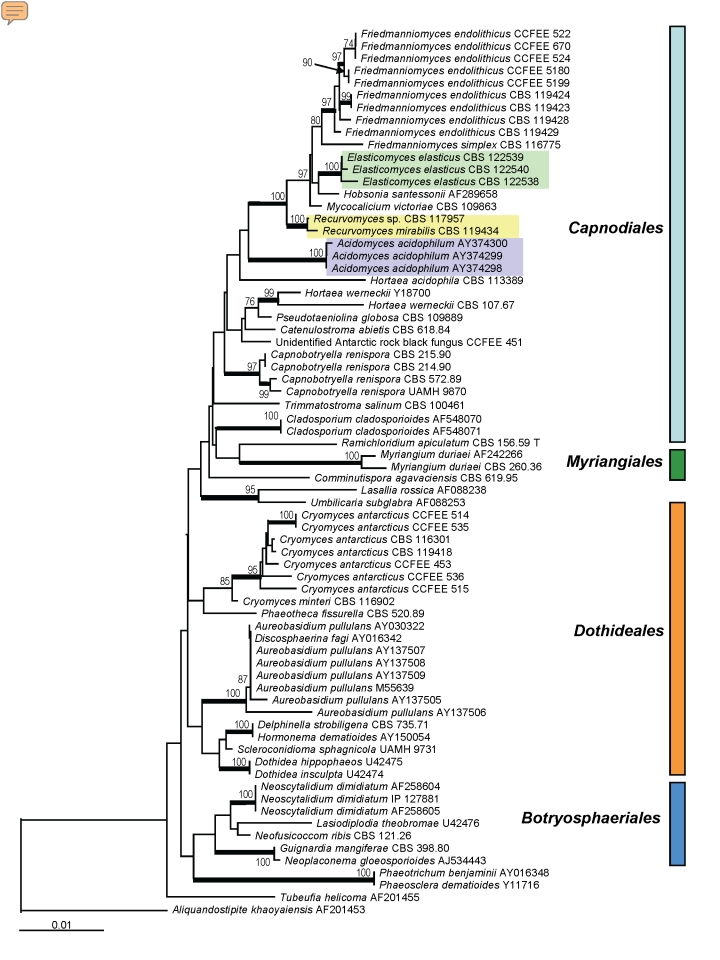
SSU sequences were analysed for 71 strains of ascomycetous black yeasts and relatives belonging to the orders Capnodiales, Dothideales, Myriangiales as well as the recently proposed new order Botryosphaeriales (Schoch et al. 2006). The recently described taxon Baudoinia compniacensis (Richon) J.A. Scott & Unter. (Scott et al. 2007) with an SSU similarity around 97 % with some capnodialean/dothidealean strains, was not included in this comparison. Fig. 1 shows a Neighbour Joining tree based on the SSU comparison where the outgroup is represented by Aliquandostipite khaoyaiensis (Jahnulales).
Fig. 1.
Molecular phylogeny based on SSU sequences indicating the positions of the clades in Dothideomycetidae; the described new genera were highlighted with coloured rectangles. The tree has been built with neighbour-joining algorithm in ARB package with 100 replications. Branches of the clades supported by a bootstrap value above 95 % are in bold.
Three Antarctic rock strains (CBS 122538, 122539 and 122540), with nearly identical sequences, were included in a group paraphyletic to Friedmanniomyces endolithicus Onofri. The strains shared a remarkable morphology, with straight fertile hyphae of which fragments detached, sometimes by basauxically expanding connectives.
Strain CBS 119434 clustered in a clade composed of mainly meristematic species, which included the Antarctic rock-inhabiting genus Friedmanniomyces. This strain also had characteristic morphology, showing a unique pattern of recurved hyphal branching at conidiation. Another strain with nearly identical sequence, CBS 117957 from Mediterranean rocks, showed a simple, undiagnostic meristematic micromorphology. The lichenicolous fungus Hobsonia santessonii Lowen & D. Hawksw. belonged to the same group, together with the black fungus Mycocalicium victoriae (C. Knight ex F. Wilson) Tibell.
The acidophilic species Hortaea acidophila Hölker et al. and Acidomyces acidophilum as “Acidomyces richmondensis” B.J. Baker et al. composed a sister clade to the Friedmanniomyces complex. The halophilic species Hortaea werneckii (Horta) Nishimura & Miyaji was found at a larger distance, in a heterogeneous clade with Pseudotaeniolina globosa De Leo et al., Catenulostroma abietis (Butin & Pehl) Crous & Braun and Coccodinium bartschii A. Massal. As the backbone of the tree shows low bootstrap values for most clades, the exact phylogenetic positions of the newly added Antarctic and acidophilic species are difficult to determine.
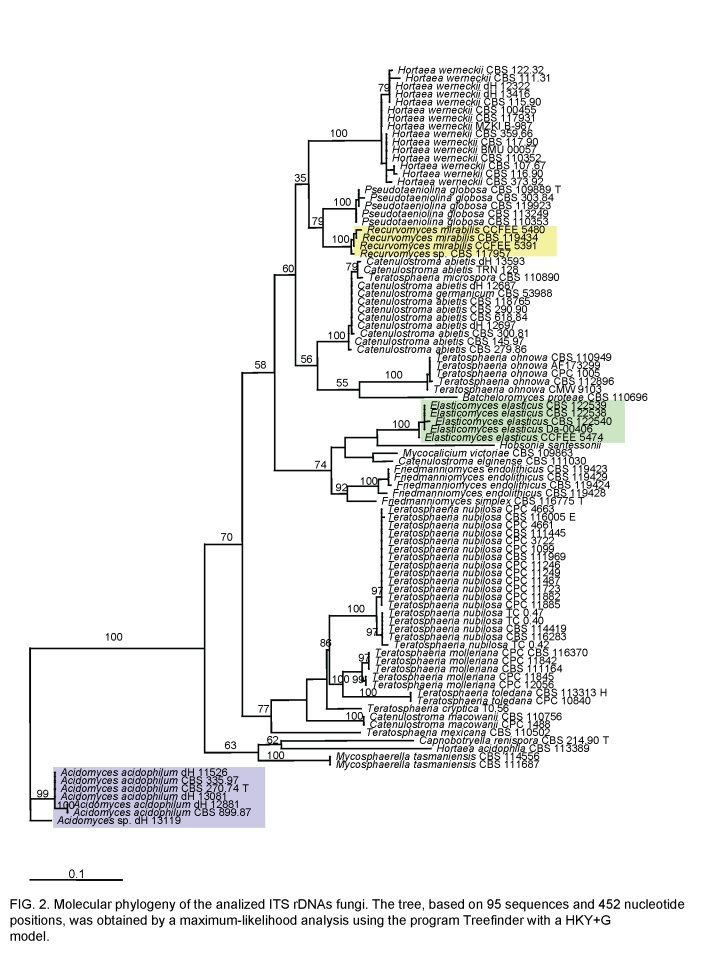
The ITS tree shown in Fig. 2 was generated on the basis of the manually optimised alignment containing 95 sequences of halophilic, acidophilic, rock and plant-pathogenic fungi; it is based on a length of 452 characters, including alignment gaps. The AICc selected HKY+G (Hasegawa et al. 1985) as the best model. The base frequencies was as follows: T = 0.2293, C = 0.3120, A = 0.2009, G = 0.2574, TC = 0.5413, AG = 0.4583. The entire ITS region showed too many polymorphisms to allow alignment with a sufficient degree of confidence. For this reason, DNAWD was applied, which is insensitive to alignment. Topology of this tree was identical (data not shown).
Fig. 2.
Molecular phylogeny of the analysed ITS rDNAs showing the relationship of the new genera described, highlighted with coloured rectangles, in the Capnodiales. The neighbour-joining tree, based on 95 sequences and 452 nucleotide positions, has been generated using HKY+G model; the model was calculated using ML in MrAIC software. Bootstrap values from 100 resampled data sets are shown.
In general the ITS trees showed excellent resolution of entities. Inter-group variability was constant and invariably significantly larger than intra-group variability. The tree contained anamorph taxa from extreme environments, as well as a number of plant-associated species of Teratosphaeria Syd. & P. Syd. Three main clades were discernible: an upper clade around Teratosphaeria microspora J.E. Taylor & Crous, a clade with T. nubilosa (Cook) Crous & U. Braun as central species, and an outgroup with strains isolated at very low pH (Fig. 2).
A single cluster is composed of strains listed as Teratosphaeria microspora, Catenulostroma abietis (Butin & Pehl) Crous & U. Braun and C. germanicum Crous & U. Braun. The strain TRN 128 from Mediterranean rock is found in a paraphyletic position. Pseudotaeniolina globosa is a member of the same clade, while the endemic Antarctic Friedmanniomyces species are members of a neighbouring clade which includes Mycocalicium victoriae CBS 109663 and the lichenicolous fungus Hobsonia santessonii, without any known teleomorph. Five further undescribed Antarctic strains, among which was CBS 122539, constituted a sister clade (Fig. 2), and phenetically all showed conidial dehiscence with basauxically expanding connectives; we here describe a new genus, Elasticomyces Zucconi & Selbmann for this group. Another, separate cluster of four strains from Antarctic and Mediterranean rocks was found around CBS 119434, strains microscopically either showing recurved conidial branches or a reduced, meristematic morphology; for which we describe a new genus, Recurvomyces Selbmann & de Hoog. The halo-respectively acidophilic Hortaea species, H. werneckii and H. acidophila, were located at clearly separate positions. The same clade included also the plant-pathogenic teleomorph species Teratosphaeria ohnowa (Crous & M.J. Wingf.) Crous & U. Braun occurring on leaves of Myrtaceae. A basal cluster comprised some strains, among which Scytalidium acidophilum CBS 270.74 was selected as outgroup. The clade contained the plant-pathogenic Mycosphaerella nubilosa (Cooke) Hansf. and, as sister clade, a group of acidophilic fungi which included the ex-type strain of Scytalidium acidophilum Sigler & J.W. Carmich. CBS 270.74, isolated from acidic soil, was found to be identical to that species. No teleomorph relationships are known for this group. S. acidophilum was found to be identical to strains of the the invalidly described genus “Acidomyces” B.J. Baker et al. That genus is validated below as Acidomyces B.J. Baker et al. ex Selbmann et al.
DESCRIPTIONS
Recurvomyces Selbmann & de Hoog, gen. nov. – MycoBank MB511293.
Ad fungos anamorphos, hyphomycetes pertinens. Coloniae in agaro maltoso lente crescentes, compactae, velutinae, nigrae vel olivaceo-nigrae. Mycelium ex hyphis longis, levibus, pallide brunneis et crassitunicatis compositum. Hyphae torulosae interdum productae, brunneae, crassitunicatae, enteroblastice proliferantes. Conidiophora macronematosa vel semi-macronematosa, saepe ramosa, cum ramis lateralibus saepe angulo recto dispositis deorsumque inflexis. Conidia enteroblastica, schizolytice secedentia. Ramoconidia interdum producta. Teleomorphosis ignota.
Species typica: Recurvomyces mirabilis Selbmann & de Hoog, sp. nov.
Anamorphic fungi, hyphomycetes. Colonies very slowly growing, compact, heaped, black or olive-black. Mycelium composed of long, smooth, yellowish brown and thick-walled hyphae. Torulose hypae sometimes present, brown, thick-walled, smooth to verrucose, enteroblastically proliferating. Conidiophores macronematous or semi-macronematous, often branched with lateral branches mostly at roughly right angle and bent down. Conidia produced by enteroblastic proliferation released by schizolytic secession. Ramoconidia sometimes present.
Teleomorph: Unknown; phylogenetic affinity to the ascomycete order Capnodiales.
Type species: Recurvomyces mirabilis Selbmann & de Hoog, sp. nov.
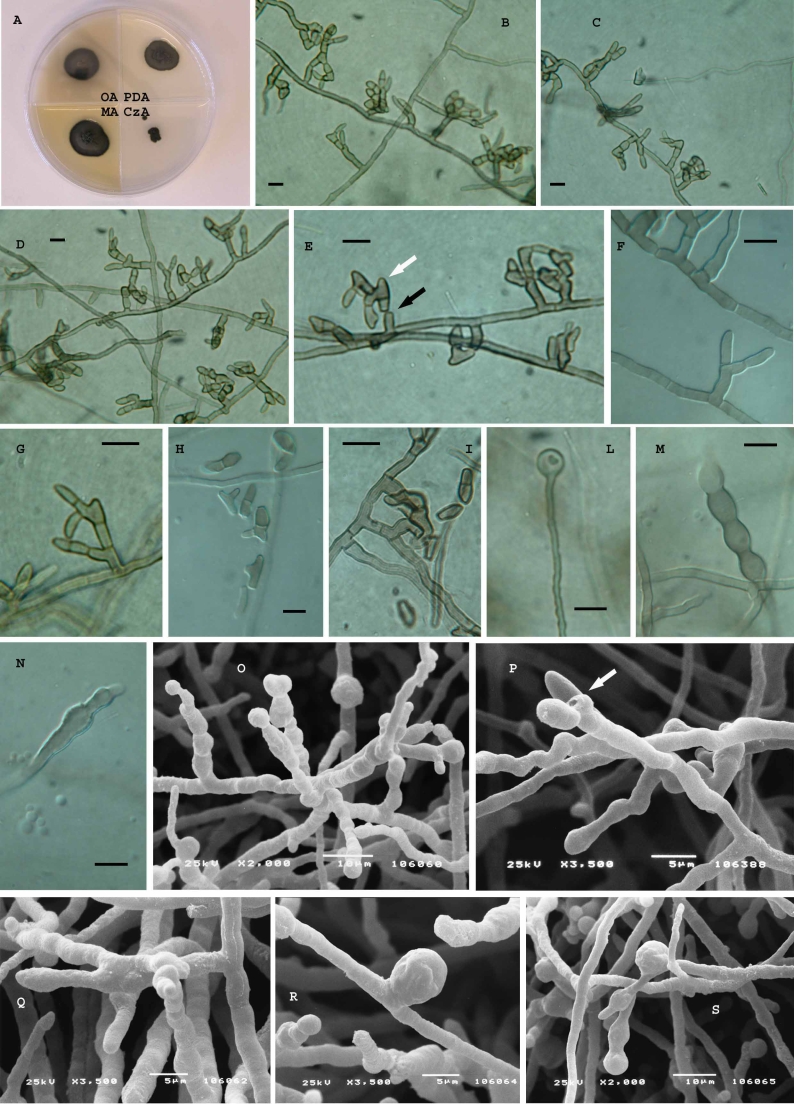
Recurvomyces mirabilis Selbmann & de Hoog, sp. nov. – MycoBank MB511294, Figs 3, 4, 5.
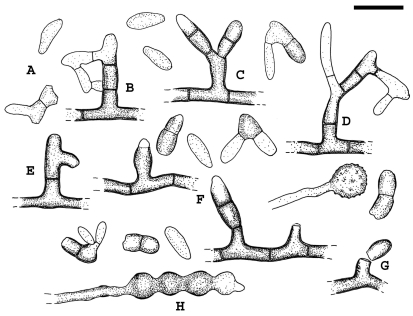
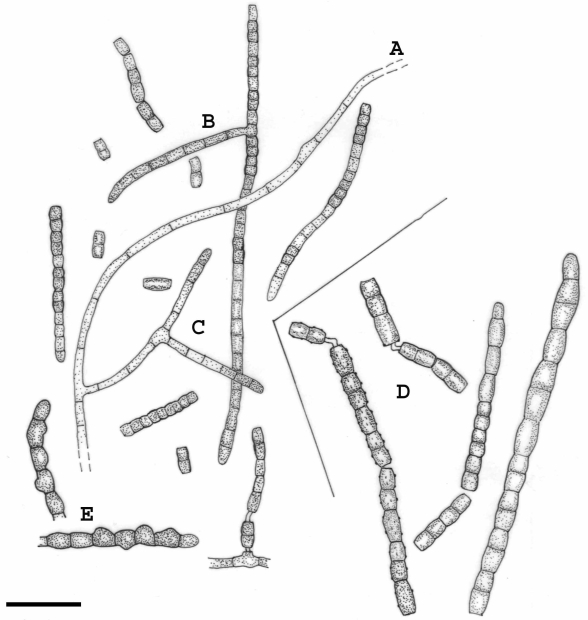
Fig. 3.
Recurvomyces mirabilis. A. 1- celled conidia and ramoconidia. B–D. Septate and branched conidiophores; branches forming right angle and bent down. E. Conidiophore with recurved hyphal branching. F. Conidiogenous cells producing conidia by enteroblastic proliferation. G. Schyzolytic conidial secession. H. Swelling cells. Scale bar = 10 μm.
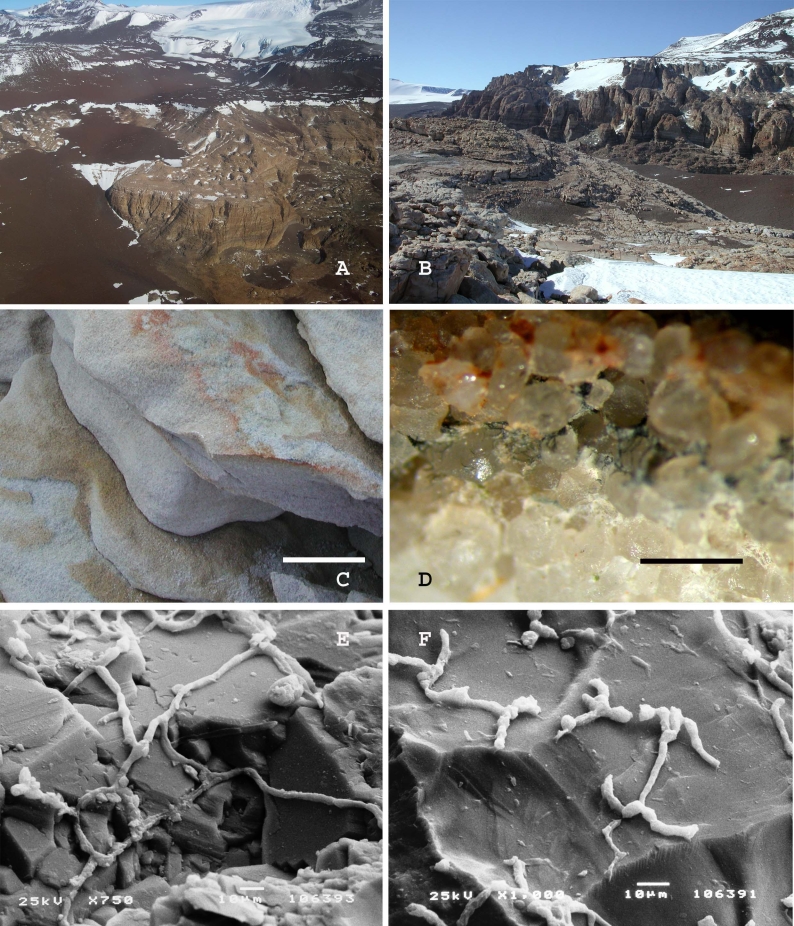
Fig. 4.
A. Battleship Promontory, Southern Victoria Land, Antarctica, view from the helicopter. B. Battleship Promontory, Southern Victoria Land, Antarctica, landscape. C. Sandstone sample from which Recurvomyces mirabilis CBS 119434 has been isolated. The surface is weathered by the activity of cryptoendolithic microorganisms. Scale bar = 5 cm. D. Magnification at the dissecting microscope of the black fungi endolithic colonization inside the sample of sandstone used for the isolation. Scale bar = 2 mm. E, F. Rock colonised by the fungus observed at the Scanning Electron Microscope.
Fig. 5.
Recurvomyces mirabilis, CBS 119434 (= CCFEE 5264). A. Strain grown on different media after two mo of incubation at 15 °C. B–D. Hyphae with branched and unbranched conidiophores producing 0–1 septated conidia. E. Curved branched conidiophores schyzolytically seceding (black arrow) showing enteroblastic elongation (white arrow) at the apex. F, G. high magnification of branched conidiophores producing 1-celled conidia. H, I. 1- and 2-celled conidia and ramoconidia. L. Terminal swelling cell. M, N. swelling hyphae showing enteroblastic elongation. O, P. Unbranched conidiophores producing 1-celled conidia, scar is visible after schizolytic secession (P, arrow). Q. Branched conidiophore. R, S. swelling cell at intercalary (R) and terminal position (S). B–N. Light microscopy; Scale bars = 10 μm. O–S. SEM.
Coloniae in agaro maltoso acervatae, compactae, margine regulari atque plano, velutinae, nigrae vel olivaceo-nigrae, reversus nigrum, lente crescentes, post tres menses usque ad 18 mm diam. Mycelium ex hyphis longis, levibus, pallide brunneis et crassitunicatis, apicem versus gradatim pallidioribus et subtilius tunicatis, subhyalinis, interdum anastomoses ferentibus, septatis, ramosis compositum. Torulosae hypae interdum productae, brunneae, crassitunicatae, leves vel verrucosae, partibus inflationis 4.5–7.5 μm crassis, proliferatione apicali enteroblastica ortae. Conidiophora ex mycelio superficiali orientia, macronematosa vel semi-macronematosa, mononematosa, erecta, brevia, septata, levia, crassitunicata, brunnea, apicem versus gradatim pallidiora et tenuitunicata, ex proliferatione enteroblastica percurrente orientia, saepe ramosa, cum ramis lateralibus saepe angulo recto dispositis, deorsum inflexis. Cellulae conidiogenae polyblasticae, integratae, terminales vel intercalares, tenuitunicatae, brunneae, enteroblasticae, post schizolyticam secessionem conidiorum tenui cicatrice praeditae. Conidia enteroblastica, sicca, solitaria, 0–1-septata, subhyalina vel dilute brunnea, tenuitunicata, levia, ellipsoidea vel obovoidea, raro modice ad septum constricta, ad apicem rotundata, plano hilo basilari incospicuo praedita post schizolyticam secessionem, 7.2–11.2 × 2.5–4.7 μm. Propagula liberata e partibus terminalibus conidiophororum vel cellulis conidiogenis orientia; ramoconidia etiam producta, saepe 1-septata, brunnea, crassitunicata, forma irregulare, quae a latere enteroblastica conidia gignere possunt. Teleomorphosis ignota.
Holotypus: CBS H-20178, cultura ex-typo CBS 119434 = CCFEE 5264, in Promontorio Pugnae Navalis, Terra Victoriae Meridionalis, Antarctica, isolatus ex arenari saxo. L. Zucconi legit, 24.I.2004.
Cultural characteristics: Description based on strain CBS 119434 at 10 °C.
Colonies on MEA and PDA heaped, with flat and regular margin, on OA remaining almost completely flat; growth very slow: diameter after two mos 18 mm on MEA2, 16.5 mm on OA, 15 mm on PDA, and 5 mm on CzA. Colony obverse olive-black, compact, felty; reverse black. Mycelium composed of long, smooth, yellowish brown and thick-walled hyphae, paler and thinner-walled towards the subhyaline apices, septate, branched, with occasional anastomoses. Torulose hyphae sometimes present, brown, thick-walled, smooth to verrucose, with swellings 4.5–7.5 μm wide, formed by enteroblastic apical proliferation. Conidiophores erect, semi-macronematous, mononematous, short, septate, smooth, thick-walled, brown, paler and thinner towards the apex, developing from percurrent enteroblastic proliferations of short simple conidiophores, repeatedly branched, with lateral branches mostly at roughly right angle and bent downward. Conidiogenous cells polyblastic, integrated, terminal or intercalary, thin-walled, brown, producing conidia by enteroblastic proliferation, with slight abscission scars remaining after schizolytic conidial secession.
Conidia enteroblastic, dry, solitary, 0–1-septate, subhyaline to yellowish brown, thin-walled, smooth, ellipsoidal to obovoidal, sometimes slightly constricted at the median septum, rounded at the apex and with a flat scar at the base, 7.2–11.2 × 2.5–4.7 μm; conidial secession schizolytic. Terminal parts of conidiophores or apical conidiogenous cells generally breaking off producing propagules; ramoconidia also present, formed by schizolitic secession, mostly 1-septate, brown, thick-walled, irregular in shape, laterally producing conidia by enteroblastic proliferation.
Teleomorph: Unknown.
Holotype: CBS H-20178, culture ex-type CBS 119434 = CCFEE 5264, Battleship Promontory, McMurdo Dry Valleys, Southern Victoria Land, Antarctica, isolated from sandstone. Leg. L. Zucconi, 24 Jan. 2004.
Strains examined: CBS 119434; CCFEE 5480; CCFEE 5391; CBS 117957.
Elasticomyces Zucconi & Selbmann, gen. nov. – MycoBank MB511296.
Ad fungos anamorphos, hyphomycetes pertinens. Coloniae compactae, coactae, cumulatae, nigrae, lente crescentes. Mycelium ex hyphis longis, septatis, ramosis, tenuitunicatis, hyalinis vel pallide pigmentatis compositum. Hyphae fertiles obscuriores et magis crassitunicatae, repetite ramosae, septatae, fissione ad septa in conidia disarticulantes. Arthroconidia catenata, uni- vel pluricellularia, cylindrica, utrimque secessione schizolytica truncata. Teleomorphosis ignota.
Species typica: Elasticomyces elasticus Zucconi & Selbmann, sp. nov.
Anamorphic fungi, hyphomycetes. Colonies compact, felted, clumped, black, slow growing. Mycelium composed of long, branched, septate, thin-walled, colourless or yellowish to pale brown hyphae. Fertile hyphae more pigmented and thicker-walled, repeatedly branched at roughly right angle, septate, forming conidia by fragmentation. Arthroconidia catenate, one or pluricellular, cylindrical, with truncated ends due to schizolytic secession.
Teleomorph: Unknown.
Phylogenetic affinity to the ascomycete order Capnodiales.
Type species: Elasticomyces elasticus Zucconi & Selbmann, sp. nov.
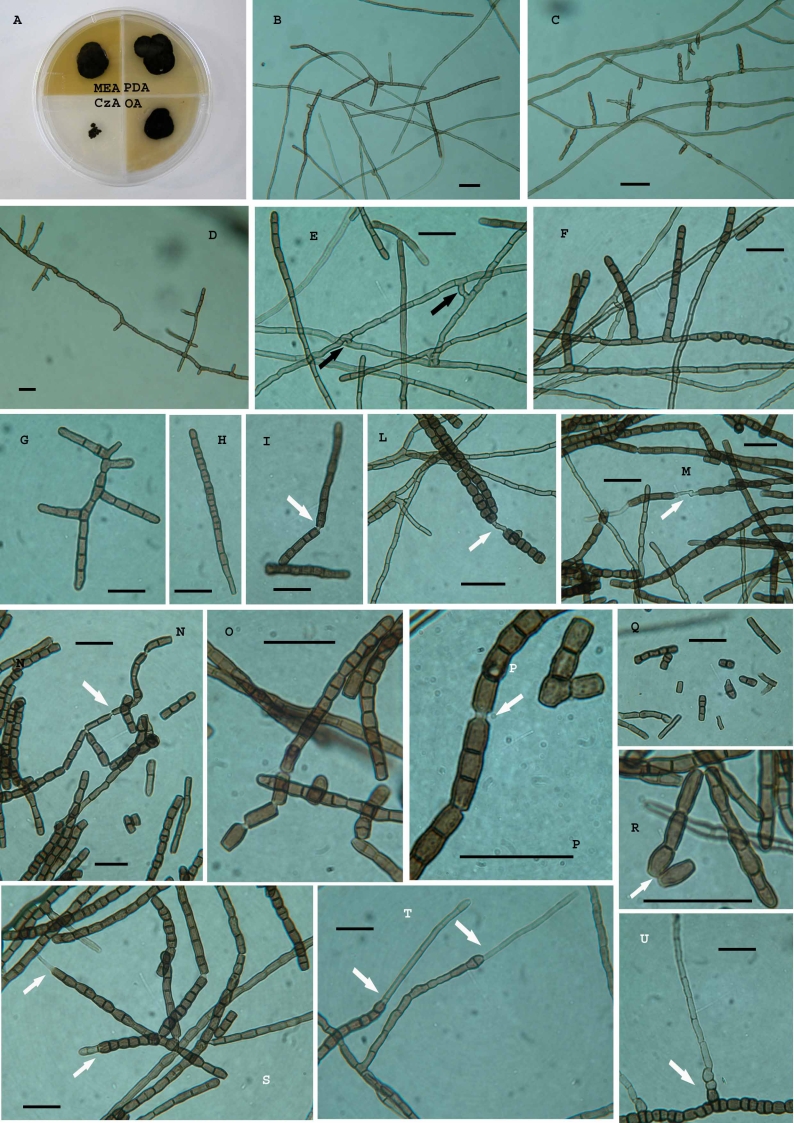
Elasticomyces elasticus Zucconi & Selbmann, sp. nov. – MycoBank MB511297, Figs 6, 7.
Fig. 6.
Elasticomyces elasticus. A. Vegetative hyphae. B, C. Branched fertile hyphae producing conidia by fragmentation. D. Disarticulating fertile hyphae where cells remain joint by connectives. E. Swelling hyphae. Scale bar = 20 μm.
Fig. 7.
Elasticomyces elasticus, CCFEE 5313. A. Strain grown on different media after 1.5 mo of incubation at 15 °C. B–D. Vegetative and fertile hyphae. E–H. High magnification of fertile hyphae; anastomoses in the vegetative hyphae (black arrows in E). I–O. Uncompleted disarticulation of artic conidia and hyphal fragments remaining joint by connectives (white arrows). P. High magnification of conidia remaining joint after disarticulation. Q, R. 1- and 2-celled conidia produced after schyzolithic secession (white arrow). S–U. Enteroblastic elongation at the apexes (white arrows). Scale bars = 20 μm.
Coloniae in agaro maltoso compactae, coactae, partim immersae, margine plerumque irregulari, cumulatae, obversum reversumque nigra, lente crescentes, post duos menses usque ad 15 mm diam. Mycelium ex hyphis longis, septatis, levibus, tenuitunicatis, luteolis vel pallide brunneis, ramosis, anastomosantibus, 3.5–5.3 μm latis compositum. Hyphae fertiles brunneae, crassitunicatae, ad angulo recto repetite ramosae, septatae, primum leves, demum crenulatae, fissione ad septa in numerosa brevia segmenta, ex uno vel pluribus conidiis composita, disarticulantes, interdum connectivo coniuncta. Arthroconidia catenata, plerumque bicellularia, rarius unicellularia, levia vel crenulata, cylindrica, truncata et magis crassitunicata ad apices secessione schizolytica, modice ad septum constricta, 12.5–16 × 3.5–5.3 μm. Cellulae intercalares chlamydosporarum similes interdum orientes, parietibus crassis et brunneis. Teleomorphosis ignota.
Holotypus: CBS H-20177, cultura ex-typo CBS 122538 = CCFEE 5313, Insula Kay, Terra Victoriae Settentrionalis, Antarctica, isolatus ex thallo Usneae antarcticae. L. Zucconi legit, 30.I.2004.
Cultural characteristics: Description based on strain CBS 122538 at 20 °C.
Colonies compact, felted, partially immersed in the agar, margin rather irregular, obverse and reverse black, clumped on MEA2 % and PDA, almost completely flat on OA; slow growth: diameter after two mos 15 mm on MEA2 % and OA, 17 mm on PDA, and 5 mm on CzA. Mycelium composed of long, septate, branched, smooth, thin-walled, yellowish to pale brown hyphae, 3.5–5.3 μm wide, with anastomoses. Fertile hyphae more pigmented, thicker-walled, at roughly right angle repeatedly branched, septate, at first smooth, then crenulate, forming by fragmentation numerous short segments, composed of one or more conidia, sometimes joined by connectives. Arthroconidia catenate, mostly bicellular, rarely aseptate, smooth or crenulate, cylindrical, with thickened and truncated ends due to schizolytic secession, slightly constricted at the septum, 12.5–16 × 3.5–5.3 μm. Intercalary chlamydospore-like cells, with thickened and brown wall, sometimes present.
Teleomorph: Unknown.
Holotype: CBS H-20177, culture ex-type CBS 122538 = CCFEE 5313, Kay Island (75°04'13.7”S, 165°19'0.2.0”E), Northern Victoria Land, Antarctica, isolated from lichen thallus (Usnea antarctica Du Rietz). Leg. L. Zucconi, 30 Jan. 2004.
Strains examined: CBS 122538; CBS 122539; CBS 122540; Da-004-06; CCFEE 5474.
Notes: The conidium ontogeny is holoarthric, involving an irregular basipetal maturation of cells and fragmentation of fertile hyphae. Often short portions of fertile hyphae are released by fragmentation of longer hyphae, functioning as propagules. Apical growth of fertile hyphae occurs concomitantly with holoarthric development. A circumscissile scar remains at both ends, after schizolytic secession of adjacent conidia; complete disarticulation is retarded by the presence of thin strands of wall material at the central convexity of septa. Sometimes conidial secession is not completed; new wall material is laid down in the existing septum, and adjacent cells remain connected by narrow and pale connectives. Connectives can eventually elongate to form new pale hyphae, sometimes evolving in new fertile hyphae.
Acidomyces B.J. Baker, M.A. Lutz, S.C. Dawson, P.L. Bond & J.F. Banfield ex Selbmann, de Hoog & De Leo, gen. nov. – MycoBank MB511298.
Ad fungos anamorphos, hyphomycetes pertinens. Coloniae lente crescentes, celerius in acido agaro, compactae, nigrae. Mycelium ex septatis, interdum ramosis, brunneis crassitunicatisque hyphis compositum, demum meristematice increscens. Conidia arthrice secedentia. Teleomorphosis ignota.
Anamorphic fungi, hyphomycetes. Colonies growing slowly, faster in acidic medium, compact, dark. Mycelium composed of septate, scarcely branched, brown and thick-walled hyphae, eventually converting into a meristematic mycelium. Conidia produced by arthric disarticulation of hyphae.
Teleomorph: Unknown; phylogenetic affinity to the ascomycete order Capnodiales.
Type species: Acidomyces acidophilus (Sigler & J.W. Carmich.) de Hoog & De Leo
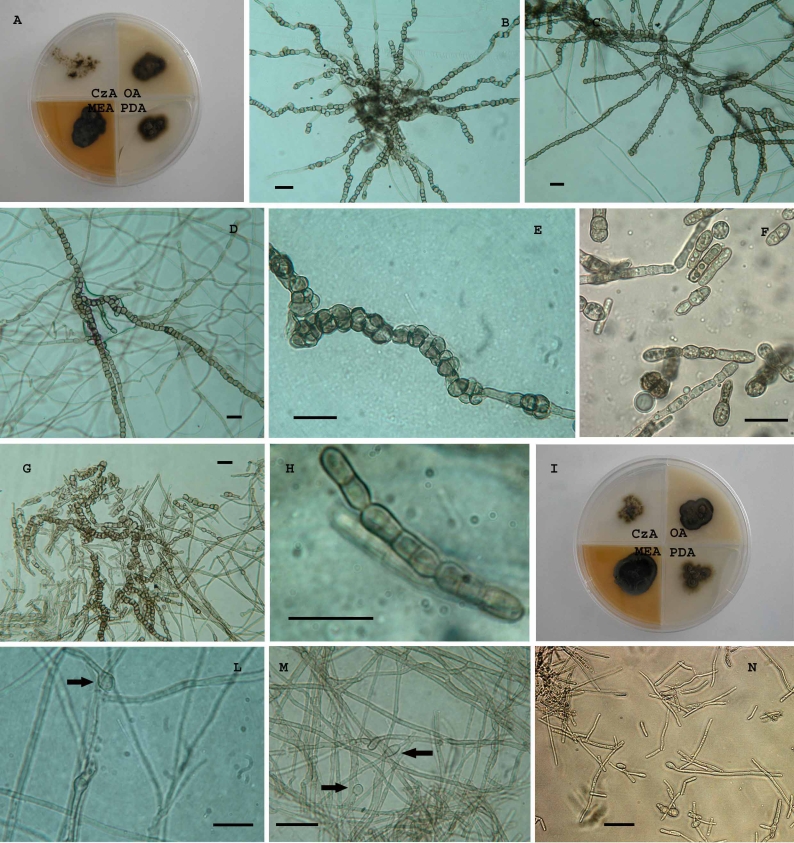
Acidomyces acidophilus (Sigler & J.W. Carmich.) Selbmann, de Hoog & De Leo, comb. nov. – MycoBank MB511856, Fig. 8.
Fig. 8.
Acidomyces acidophilus. A. Strain CBS 899.87 grown on different media after 1 mo of incubation at 25 °C. B–D, G. Toruloid unbranched hyphae with melanised and thick-walled cells. E. Meristematic development of the hyphae. F. Fungus grown at pH 1 in liquid culture. H. Chain of 1- 2- and 3- celled conidia. I. Strain CBS 335.97 grown on different media after 1 mo of incubation at 25 °C. L, M. Filamentous hyphae with intercalary and terminal swelling cells (black arrows). N. Fungus grown at pH 1 in liquid culture. Scale bars = 20 μm.
Basionym: Scytalidium acidophilum Sigler & J.W. Carmich., Canad. J. Microbiol. 20: 267, 1974.
≡ `Fungus D', Starkey & Waksman, J. Bacteriol. 45: 512, 1943 (without description, without type).
≡ `Acidomyces richmondensis' B.J. Baker, M.A. Lutz, S.C. Dawson, P.L. Bond & J.F. Banfield, Appl. Environm. Microbiol. 70: 6270, 2004 (nom. inval., Arts 36.1, 37.1 ICBN).
Type: UAMH 3460, from field soil, adjacent to elementary sulphur stockpile from natural gas purification plant, Bowden, Alberta, Canada [as Scytalidium acidophilum].
Ex-type strain: CBS 270.74 (= ATCC 26772 = IMI 183518 = UAMH 3460).
Additional strains examined: See Table 2.
DISCUSSION
Melanised rock-inhabiting fungi are phylogenetically quite heterogeneous and have been grouped at least in four different fungal orders: Capnodiales, Dothideales, Chaetothyriales and Pleosporales (Sterflinger et al. 1997, Selbmann et al. 2005, Ruibal et al. 2008). Now that suitable isolation and identification techniques have been developed for these fungi (Wollenzien et al. 1995, Ruibal et al. 2005), they appear to be very common in arid climates, from Polar Regions to the subtropics. Stringent environmental conditions include high solar radiation (Urzì et al. 1995), strongly fluctuating temperatures (Nienow & Friedmann 1993), repeated freezing and thawing cycles (Selbmann et al. 2002), low water activity (Sterflinger 1998, Zalar et al. 1999), acidity (Price 2000), and nutrient deficiency (Sterflinger et al. 1999).
The physiological studies indicated that all the strains studied were well adapted to the particular stressing conditions characterising their natural environments. For instance, the strains belonging to the newly validated genus Acidomyces, all isolated from very acidic environments (see Table 1), showed an acidophilic profile being able to grow very well at pH 1 and with optimum well below the neutral value (pH 3–5). This means that most probably they are scarcely competitive in other environments and remain trapped in the extremely acidic ones where they have been isolated. Furthermore, they showed only a moderate tolerance to salinity. Similar data have been already reported for other acidophilic fungi phylogenetically distant from Acidomyces strains; for instance, Hortaea acidophila, was reported to be very sensitive to osmotic stresses being unable to grow at NaCl concentration above 2 % (Hölker et al. 2004). These data suggest that the adaptation to acidic environments does not require a concomitant tolerance to osmotic stresses. The situation seems to be different for fungi colonising rocks; Sterflinger (1998) observed a certain degree of halotolerance for some fungal strains in a selected, phylogenetically heterogeneous, group of rock fungi. Halotolerance has been proven to be particularly pronounced in some fungi isolated from the exposed rocks of the Antarctic desert (Onofri et al. 2007), among the driest terrestrial ice-free areas on Earth; there, the high evaporation leads also to salt accumulation on rock surfaces and fungi adapted to that environment have to cope with both water deficiency and salinity (Ruisi et al. 2007).
All Antarctic strains studied can also be referred to as psychrophilic according to criteria outlined by van Uden (1984) and Vishniac (1987), having an optimum at 15 °C and being unable to grow at temperatures above 20–25 °C. This result confirms what has been recently published for other strains isolated from Antarctic rocks (Selbmann et al. 2005). Remarkable is the finding that strains belonging to the genus Elasticomyces here described, isolated from Antarctic lichens, showed a wider temperature range for growth with respect to the Antarctic cryptoendolithic strain of Recurvomyces mirabilis Selbmann & de Hoog. Their ability to grow at 0 as well as at 25 °C can be an adaptive strategy to withstand not only the very low temperatures characterising their natural environment but also the very wide thermal fluctuations, much more marked in the epilithic rather than in the endolithic environment.
Common features enhancing survival are high degrees of melanisation and thick cell walls (Figueras et al. 1996), slow, isodiametric growth, isodiametric expansion ensuring an optimal surface/volume ratio (Wollenzien et al. 1995), ability to change cellular polarity (Yoshida et al. 1996), and hence little differentiation although with great polymorphism. These characters seem to favour a marked degree of convergent evolution.
In the SSU phylogeny (Fig. 1) a clade is recognisable, containing a large number of melanised fungi that can be isolated from bare rocks, among which there is Pseudotaeniolina globosa isolated from rock surfaces in Sicily (De Leo et al. 2003). The clade also contains some Teratosphaeria species inhabiting leathery plant leaves, mostly in semi-arid climates (Crous et al. 2004); this genus was re-established as separate from Mycosphaerella because of this ecology and because of its phylogenetic position (Crous et al. 2007). The phylogenetic relationship to numerous extremotolerant species is remarkable. Most plant-associated fungi are known with their teleomorph, while for the epi- and endolithic species no teleomorphs are known. Teratosphaeria microspora can be found both on rock and plant leaves, suggesting that adaptation to a life at the extreme starts with dispersal under semi-arid conditions.
In general the ITS tree shows excellent resolution of species. Recognised entities were ecologically consistent (Fig. 2), such as the halophilic species Hortaea werneckii and the acidophilic Acidomyces acidophilus.
Trimmatostroma Corda, with the generic type species T. salicis Corda, was recently treated as a genus of Leotiales, while the capnodialean species were reclassified in Catenulostroma Crous & U. Braun (Crous et al. 2007). Teleomorph connections of Catenulostroma are in Teratosphaeria. Crous et al. (2007) mentioned differences in ecology and geography, as Teratosphaeria microspora would be an endemic species of Protea in South Africa, while other taxa within the T. microspora complex were observed on conifer needles and rocks in the Northern Hemisphere. Members of the complex are commonly observed on rocks and other relatively inert surfaces in temperate climates, but on conifer needles they produce well-defined acervuli (Butin et al. 1996). This suggests that superficial rock-colonising strains of this group may have originated from leathery plant leaves; we did not find any match between observed ITS polymorphisms and geography or ecology (Table 1). Crous et al. (2007) distinguished two additional anamorph species in the complex of T. microspora, viz. Catenulostroma abietis and C. germanicum. ITS sequences of these species are nearly identical (Fig. 2). At reduced water activity, C. abietis adapts with a meristematic form (Figueras et al. 1996). Catenulostroma germanicum was claimed to be different from C. abietis in having occasional oblique conidial septa, in contrast to the remaining species. In addition, in C. abietis the transformation to meristematic morphology reported above can be reproduced in vitro when reaching the stationary phase (Figueras et al. 1996, Yoshida et al. 1996), leading to the formation of septa in all directions, as in C. germanicum.
The genus Friedmanniomyces consists merely of species occurring cryptendolithically in rocks in the Antarctic, suggesting a further degree of extremotolerant specialisation (Selbmann et al. 2005). The strains belonging to the new genus and species here described, Elasticomyces elasticus, are located as a sister group of Friedmanniomyces. They have been firstly isolated from Antarctic lichens, but later on also from Antarctic lichen-dominated cryptoendolithic communities, i.e. microorganisms living inside rocks in the airspaces between crystals (Friedmann & Ocampo 1976; Friedmann 1982), as well as from Andean rocks (4885 a.s.l.) colonised by epilithic lichens. In this respect lichens seem to be a recurrent element in the environments where these strains have been found. Therefore, Elasticomyces seems to be particularly sensitive to the oligotrophic conditions of rocks and the epi- or endolithic lichens could play a pivotal role as nutrient suppliers. The peculiar ability to produce connectives seems a distinctive feature of the genus, being observed both in strains from the Antarctic and the Andes.
The Antarctic strain CBS 119434 is at some distance from Friedmanniomyces. The group to which the strain belongs is described as Recurvomyces mirabilis, a further cryptoendolithic member of the Capnodiales. The ex-type strain and the strain CCFEE 5480 were both isolated from inside sandstone as a member of an Antarctic lichen-dominated cryptoendolithic community. Additional, sterile strains with a nearly identical ITS sequence, CBS 117957 (TRN 491) and CCFEE 5391, were isolated from Spanish rocks (Ruibal 2004) and the Alps (unpublished data) respectively. Recurvomyces mirabilis thus is an example of a rock-inhabiting fungus with a distribution spanning both hemispheres. Antarctic and Mediterranean environments have very different temperature regimens, but share high solar radiation and water deficiency at least during part of the yr, suggesting that tolerance to such stresses is promoted by the same set of morphological and physiological factors (Ruisi et al. 2007). No cryptoendolithic behaviour is known for Mediterranean rock-colonisers, but their prevalent mode of action is superficial biopitting (Sterflinger et al. 1997). Their phylogenetic affinity could be related to the production of easily air-dispersed propagules. Particularly the catenate conidia of R. mirabilis resemble Cladosporium, which is abundantly present in Antarctic air (Marshall 1997). Otherwise air-dispersed conidia are uncommon among black rock-inhabiting fungi.
Hortaea werneckii was found in derived position in the tree (Fig. 2). The species is one of the most pronounced halophilic fungi known to date (Sterflinger 1998, Zalar et al. 1999). It has a growth optimum at 17 % salt and still shows good growth near the saturation point of NaCl (Plemenitaš & Gunde-Cimerman 2005). Its natural niche is in waters of solar salterns worldwide, reaching its optimum distribution during the hot summer period.
Hortaea acidophila is a further related species with a very peculiar ecology. It was isolated from a lignite extract at pH 0.6, using humic and fulvic acids as carbon sources. It was placed in the monotypic genus Hortaea which only included the halophilic species H. werneckii, in the order Dothideales, based on SSU rDNA sequences (Hölker et al. 2004).
The outgroup of the tree is composed of the genus Acidomyces which includes a single acidophilic species. The name Acidomyces was invalidly introduced (Baker et al. 2004) for a fungus, named “Acidomyces richmondensis”, isolated from warm (35 to 57 °C) pyrite ore mine drainage at pH between 0.5 and 0.9. A fungus with identical properties is Scytalidium acidophilum (Sigler & Carmichael 1974), which was invariably isolated from extremely acidic environments (Table 1). Sequencing reference and additional strains of this species, we noticed that “Acidomyces richmondensis” is indeed identical to S. acidophilum. The phylogenetic position of the latter fungus is far away from that of the generic type species of Scytalidium, S. lignicola Pesante. The ex-type strain of that fungus, CBS 233.57 = UAMH 1502, was recently proven to belong to the subclass Leotiomycetidae (Hambleton & Sigler 2005), while the present study highlighted that S. acidophilum phylogenetically belongs to the Dothideomycetidae, order Capnodiales. This result justifies the validation of the genus Acidomyces and the synonymy of A. richmondensis with S. acidophilum.
Acidomyces acidophilus has a remarkable ecology. Starkey & Waksman (1943) first found it in extremely acidic, sulphate-containing industrial water. Gould et al. (1974) reported the species as the only organism isolated from a sulphur-containing soil at a pH of 1.1, where it occurred at high CFU counts. Harrison et al. (1966) found it in uranium mine drain water and Gimmler et al. (2001) on an acidophilic moss species. Ivarsson & Morita (1982) showed that acidity is a crucial factor in the ecology of this fungus, obtaining good growth when adjusting the pH to 0.5 with HCl. In pre-molecular times strains of this fungal species – defined by slow-growing cultures producing arthric conidia – frequently were not recognised, because strains tend to convert to meristematic growth, reluctantly disarticulating clumps of cells being produced, or remain entirely hyphal, without conidiation. ITS-sequencing proved the strict identity of all these strains. All positively identified strains originated from environments with pH of 2.0 or below (Table 1).
All the fungi described above are highly melanised. Melanin is frequently viewed as a virulence factor playing a role in fungal pathogenicity to humans (Wheeler & Stipanovic 1985, Schnitzler et al. 1999, Paolo et al. 2006). Increasing amounts of melanin made Madurella mycetomatis (Laveran) Brumpt more virulent, apparently scavenging oxygen radicals (van de Sande et al. 2006). Meristematic growth also is a known virulence factor (Matsumoto et al. 1984). Nevertheless, members of the subclass Dothideomycetidae are only exceptionally encountered as agents of infection (Clark et al. 1995, Caporale et al. 1996, Kurzai et al. 2003). In contrast, a large number of agents of severe mycoses is known in black yeasts belonging to the subclass Chaetothyriomycetidae, order Chaetothyriales (de Hoog et al. 2001). All factors discussed above of melanisation, meristematic morphology, growth at low water activity and at high/low temperature, and acid tolerance, are encountered in Dothideales as well as in Chaetothyriales, in varying combinations, but only in Chaetothyriales they play a role in infection. For example, meristematic morphology, particularly expressed at low pH (Mendoza et al. 1993), determines the invasive form in humans with the black yeast-specific skin disease chromoblastomycosis. The natural habitat of one of the agents of this disease, Cladophialophora carrionii (Trejos) de Hoog et al., was found to be in cactus debris in semi-arid climates (de Hoog et al. 2007), where the same morphology is expressed as prevalent in human skin. This suggests that in C. carrionii the extremotolerant morphology directly enhances human invasion. Nevertheless, de Hoog et al. (2005) noticed that human pathogenicity is associated with a stress-factor like osmotolerance at the order level, but the two factors are nearly mutually exclusive at the species level. This means that extremotolerance may facilitate pathogenic evolution, but this has to be additive to other factors, such as, in the case of Chaetothyriales, oligotrophy with the ability to assimilate monoaromates (Prenafeta-Boldú et al. 2006). We therefore consider the characters listed above as primarily suited for growth on exposed surfaces under harsh climatic conditions, rather than for the capacity to evade immune cells.
In summary, we determined a group with pronounced extremotolerance among semi-arid plant-associated fungi. It is probable that all these fungi share elaborate complexes of factors, as an adaptive response to these extreme conditions (Plemenitaš & Gunde-Cimerman 2005). Having acquired a basic set of vitality factors, a shift to a different habitat with a comparable degree of stress seems to be allowed. Phylogeny thus is predictive for ecology in that overall tendencies within a single clade are similar; the shifts to other extreme conditions may be possible provided that they fit the same framework of extremotolerance. In this group of fungi, the winning strategy consists in escaping competitors by colonising selective niches.
Acknowledgments
This research received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” Programme. The authors would like to thank PNRA (Italian National Program for Antarctic Research) for supporting samples collecting, Italian National Antarctic Museum “Felice Ippolito” for supporting CCFEE (Culture Collection of Fungi From Extreme Environments). The Alpine guides A Serafini and M Heltai are kindly acknowledged for collecting rock samples in the Alps and Aconcagua respectively.
Taxonomic novelties: Recurvomyces Selbmann & de Hoog, gen. nov.; Recurvomyces mirabilis Selbmann & de Hoog, sp. nov.; Elasticomyces Zucconi & Selbmann, gen. nov.; Elasticomyces elasticus Zucconi & Selbmann, sp. nov.; Acidomyces Selbmann, de Hoog & De Leo, gen. nov.; Acidomyces acidophilus (Sigler & J.W. Carmich.) Selbmann, de Hoog & De Leo, comb. nov.
References
- Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF (2004). Metabolically active eukaryotic communities in extremely acidic mine drainage. Applied and Environmental Microbiology 70: 6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills GF, Collado J, Ruibal C, Peláez F, Platas G (2004). Hormonema carpetanum sp. nov., a new lineage of dothideaceous black yeasts from Spain. Studies in Mycology 50: 149–157. [Google Scholar]
- Bogomolova EV, Minter DW (2003). A new microcolonial rock-inhabiting fungus from marble in Chersonesos (Crimea, Ukraine). Mycotaxon 86: 195–204. [Google Scholar]
- Bruns TD, Vilgalys R, Barns SM, Gonzalez D, Hibbett DS, Lane DJ, Simon L, Stickel S, Szaro TM, Weisburg WG (1992). Evolutionary relationships within the fungi: analyses of nuclear small subunit rRNA sequences. Molecular Phylogenetics and Evolution 1: 231–241. [DOI] [PubMed] [Google Scholar]
- Butin H, Pehl L, Hoog GS de, Wollenzien U (1996). Trimmatostroma abietis sp. nov. (Hyphomycetes) and related species. Antonie van Leeuwenhoek 69: 203–209. [DOI] [PubMed] [Google Scholar]
- Caligiorne RB, Licinio P, Dupont J, Hoog GS de (2005). Internal Transcribed Spacer rRNA gene-based phylogenetic reconstruction using algorithms with local and global sequence alignment for black yeasts and their relatives. Journal of Clinical Microbiology 43: 2816–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale NE, Calegari L, Perez D, Gezuele E (1996). Peritoneal catheter colonization and peritonitis with Aureobasidium pullulans. Peritoneal Dialysis International 16: 97–98. [PubMed] [Google Scholar]
- Clark EC, Silver SM, Hollick GE, Rinaldi MG (1995). Continuous ambulatory peritoneal dialysis complicated by Aureobasidium pullulans peritonitis. American Journal of Nephrology 15: 353–355. [DOI] [PubMed] [Google Scholar]
- Crous PW, Braun U, Groenewald JZ (2007). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME (2004). Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–193. [Google Scholar]
- Crous PW, Hong L, Wingfield BD, Wingfield MJ (2001). ITS rDNA phylogeny of selected Mycosphaerella species and their anamorphs occurring on Myrtaceae. Mycological Research 105: 425–431. [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ (2006). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 40: 783–791. [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1996). Phylip (Phylogeny Inference Package), v. 3.572. Department of Genetics, University of Washington, Seattle, WA, U.S.A.
- Figueras MJ, Hoog GS de, Take K, Guarro J (1996). Stationary phase development of Trimmatostroma abietis. Antonie van Leeuwenhoek 69: 217–222. [DOI] [PubMed] [Google Scholar]
- Friedmann EI (1982). Endolithic microrganisms in the Antarctic cold desert. Science 215: 1045–1053. [DOI] [PubMed] [Google Scholar]
- Friedmann EI, McKay CP, Nienow JA (1987). The cryptoendolithic microbial environment in the Ross Desert of Antarctica: satellite-transmitted continuous nanoclimate data, 1984-1986. Polar Biology 7: 273–287. [DOI] [PubMed] [Google Scholar]
- Friedmann EI, Ocampo-Friedmann R (1976). Endolithic blue-green algae in the dry valleys: primary producers in the Antarctic desert ecosystem. Science 193: 1247–1249. [DOI] [PubMed] [Google Scholar]
- Gimmler H, de Jesus J, Greiser A (2001). Heavy metal resistance of the extreme acidotolerant filamentous fungus Bispora sp. Microbial Ecology 42: 87–98. [DOI] [PubMed] [Google Scholar]
- Gould WD, Fujikawa JI, Cook FD (1974). A soil fungus tolerant to extreme acidity and high salt concentrations. Canadian Journal of Microbiology 20: 1023–1027. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Hambleton S, Sigler L (2005). Meliniomyces, a new anamorph genus for root-associated fungi with phylogenetic affinities to Rhizoscyphus ericae (= Hymenoscyphus ericae), Leotiomycetes. Studies in Mycology 53: 1–27. [Google Scholar]
- Harrison VF, Gow WA, Ivarsson KC (1966). Leaching of uranium from Elliott Lake ore in the presence of bacteria. Canadian Mining Journal 87: 64–67. [Google Scholar]
- Hasegawa M, Kishino H, Yano T-A (1985). Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22: 160–174. [DOI] [PubMed] [Google Scholar]
- Hölker U, Bend J, Pracht R, Müller T, Tetsch L, Hoog GS de (2004). Hortaea acidophila, a new acidophilic black yeast from lignite. Antonie van Leeuwenhoek 86: 287–294. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2001). Atlas of Clinical Fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Universitat Rovira i Virgili, Utrecht, Reus.
- Hoog GS de, Nishikaku AS, Fernández Zeppenfeldt G, Padín-González C, Burger E, Badali H, Gerrits van den Ende AHG (2007). Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Studies in Mycology 58: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Zalar P, Gerrits van den Ende AHG, Gunde-Cimerman N (2005). Relation of halotolerance to human-pathogenicity in the fungal tree of life: an overview of ecology and evolution under stress. In: Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya (Gunde-Cimerman N, Oren A, Plemenitaš A eds). Springer, Dordrecht, The Netherlands: 373–395.
- Hoog GS de, Zalar P, Urzì C, De Leo F, Yurlova NA, Sterflinger K (1999). Relationship of dothideaceous black yeasts and merismatic fungi based on 5.8S and ITS2 rDNA sequence comparison. Studies in Mycology 43: 31–37. [Google Scholar]
- Ivarsson KC, Morita H (1982). Single-cell protein production by the acid-tolerant fungus Scytalidium acidophilum from acid hydrolysates of waste paper. Applied and Environmental Microbiology 43: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobb GA, von Haeseler A, Strimmer K (2004). Treefinder: a powerful graphical analysis environment for molecular phylogenetics. BMC Evolutionary Biology 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kane J, Summerbell RC (1987). Sodium chloride as aid in identification of Phaeoanellomyces werneckii and other medically important dematiaceous fungi. Journal of Clinical Microbiology 25: 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzai O, Keith P, Hopp H, Hoog GS de, Abele-Horn M, Frosch M (2003). Post mortem isolation of Pseudotaeniolina globosa from a patient with aortic aneurysm. Mycoses 46: 141–144. [DOI] [PubMed] [Google Scholar]
- Küster FW, Thiel A (1990). Tabelle per le analisi chimiche e chimico-fisiche. Hopli Ed, Milano Italy, p. 303.
- Larena I, Salazar O, Gonzalez V, Julian MC, Rubio V (1999). Design of a primer for ribosomal DNA internal trascribed spacer with 5enhanced specifity for ascomycetes. Journal of Biotechnology 75: 187–194. [DOI] [PubMed] [Google Scholar]
- Leo F de, Urzì C, Hoog GS de (1999). Two Coniosporium species from rock surfaces. Studies in Mycology 43: 77–85. [Google Scholar]
- Leo F de, Urzì C, Hoog GS de (2003). A new meristematic fungus, Pseudotaeniolina globosa. Antonie van Leeuwenhoek 83: 351–360. [DOI] [PubMed] [Google Scholar]
- Licinio P, Caligiorne RB (2004). Inference of phylogenetic distances from DNA-walk divergences. Physica A 341: 471–481. [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost S, Konig S, Liss T, Lussmann S, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004). ARB: a software environment for sequence data. Nucleic Acids Research 32: 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA (1997). Seasonality in Antarctic airborne fungal spores. Applied and Environmental Microbiology 63: 2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Matsuda T, McGinnis MR, Ajello L (1984). Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36: 145–155. [DOI] [PubMed] [Google Scholar]
- McGinnis MR (1979). Taxonomy of Exophiala werneckii and its relationship to Microsporum mansonii. Sabouraudia 17: 145–154. [PubMed] [Google Scholar]
- Mendoza L, Karuppayil SM, Szaniszlo PJ (1993). Calcium regulates in vitro dimorphism in chromoblastomycotic fungi. Mycoses 36: 157–164. [DOI] [PubMed] [Google Scholar]
- Mok WY, Castello FP, Barreto da Silva MS (1981). Occurrence of Exophiala werneckii on salted freshwater fish Osteogossum bicirrhosus. Journal of Food Technology 16: 505–512. [Google Scholar]
- Nienow JA, Friedmann EI (1993). Terrestrial lithophytic (rock) communities. In: Antarctic Microbiology (Friedmann EI ed.) Wiley-Liss, New York, U.S.A: 343–412.
- Nylander JAA (2004). Mr Aic.pl. Programme distributed by the author. Evolutionary Biology Centre, Uppsala University.
- Onofri S, Castagnola M, Rossi Espagnet S (1980). L'impiego della microscopia elettronica a scansione in micologia. Micologia Italiana 1: 29–32. [Google Scholar]
- Onofri S, Pagano S, Zucconi L, Tosi S (1999). Friedmanniomyces endolithicus (Fungi, Hyphomycetes), anam. gen. and sp. nov., from continental Antarctica. Nova Hedwigia 68: 175–181. [Google Scholar]
- Onofri S, Selbmann L, Hoog GS de, Grube M, Barreca D, Ruisi S, Zucconi L (2007). Evolution and adaptation of fungi at boundaries of life. Advances in Space Research 40: 1657–1664. [Google Scholar]
- Ooyen A van (2002). Theoretical aspects of pattern analysis. In: New Approaches for the Generation and Analysis of Microbial Typing Data (Dijkshoorn L, Towner KJ, Struelens M eds.). Elsevier, Amsterdam: 31–45.
- Page RDM (1996). Treeview: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Paolo WF Jr, Dadachova E, Mandal P, Casadevall A, Szaniszlo PJ, Nosanchuk JD (2006). Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella (Exophiala) dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiology 6: 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemenitaš A, Gunde-Cimerman N (2005). Cellular responses in the halophilic black yeast Hortaea werneckii to high environmental salinity. In: Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya (Gunde-Cimerman N, Oren A, Plemenitaš A eds.) Springer, Dordrecht, The Netherlands: 455–470.
- Prenafeta-Boldú FX, Summerbell RC, Hoog GS de (2006). Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard. FEMS Microbiology Reviews 30: 109–130. [DOI] [PubMed] [Google Scholar]
- Price PB (2000). A habitat for psychrophiles in deep Antarctic ice. Proceedings of the National Academy of Sciences 97: 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C (2004). Isolation and characterization of melanized, slow-growing fungi from semiarid rock surfaces of central Spain and Mallorca. Dissertation, Universidad Autónoma de Madrid.
- Ruibal C, Platas G, Bills GF (2005). Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycological Progress 4: 23–38. [Google Scholar]
- Ruibal C, Platas G, Bills GF (2008). High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21: 93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007). Fungi in Antarctica. Reviews in Environmental Science and Biotechnology 6: 127–141. [Google Scholar]
- Sande W van de, Kat J de, Ahmed A, Verbrugh H, Belkum A van (2006). Melanin protects Madurella mycetomatis against itraconazole and ketoconazole, first-line treatment agents against mycetoma. Nederlands Tijdschrift voor Medische Microbiologie 14: 520–521. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences 74: 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zündorf J, Lütticken R, Haase G (1999). Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infection and Immunity 67: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Scott JA, Untereiner WA, Ewaze JO, Wong B, Doyle D (2007). Baudoinia, a new genus to accommodate Torula compniacensis. Mycologia 99: 592–601. [DOI] [PubMed] [Google Scholar]
- Selbmann L, Hoog GS de, Mazzaglia A, Friedmann EI, Onofri S (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic deserts. Studies in Mycology 51: 1–32. [Google Scholar]
- Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M (2002). Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Research in Microbiology 153: 585–592. [DOI] [PubMed] [Google Scholar]
- Sigler L, Carmichael JW (1974). A new acidophilic Scytalidium. Canadian Journal of Microbiology 20: 267–268. [DOI] [PubMed] [Google Scholar]
- Sikaroodi M, Lawrey JD, Hawksworth DL, DePriest PT (2001). The phylogenetic position of selected lichenicolous fungi: Hobsonia, Illosporium, and Marchandiomyces. Mycological Research 105: 453–460. [Google Scholar]
- Starkey RL, Waksman SA (1943). Fungi tolerant to extreme acidity and high concentrations of copper sulphate. Journal of Bacteriology 45: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterflinger K (1998). Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie van Leeuwenhoek 74: 271–281. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, De Baere R, Hoog GS de, De Wachter R, Krumbein WE, Haase G (1997). Coniosporium perforans and Coniosporium apollinis, two new rock-inhabiting fungi isolated from marble in the Sanctuary of Delos (Cyclades, Greece). Antonie van Leeuwenhoek 72: 349–363. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, Hoog GS de, Haase G (1999). Phylogeny and ecology of meristematic ascomycetes. Studies in Mycology 43: 5–22. [Google Scholar]
- Taylor JE, Groenewald JZ, Crous PW (2003). A phylogenetic analysis of Mycosphaerellaceae leaf spot pathogens of Proteaceae. Mycological Research 107: 653–658. [DOI] [PubMed] [Google Scholar]
- Todaro F, Berdar A, Cavaliere A, Criseo G, Pernice L (1983). Gasophtalmus in blacksea bream (Sprodylinosoma cantharus) caused by Sarcinomyces crustaceus Lindner. Mycopathologia 81: 95–97. [DOI] [PubMed] [Google Scholar]
- Uden N van (1984). Temperature profiles of yeasts. Advances in Microbiological Physiology 25: 195–251. [DOI] [PubMed] [Google Scholar]
- Urzì C, Wollenzien U, Criseo G, Krumbein WE (1995). Biodiversity of the rock inhabiting microflora with special reference to black fungi and black yeasts. In: Microbial Diversity and Ecosystem Function (Allsopp D, Colwell RR, Hawksworth DL eds.). CAB International, Wallington, U.K.: 289–302.
- Vishniac HS (1987). Psychrophily and systematics of yeast-like fungi. In: The expanding Realm of Yeast-like (Hoog GS de, Smith MTh, Weijman ACM eds). Studies in Mycology 30: 389–402. [Google Scholar]
- Wheeler MH, Stipanovic RD (1985). Melanin biosynthesis and the metabolism of flaviolin and 2-hydroxyjuglone in Wangiella dermatitidis. Archives of Microbiology 142: 234–241. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee SB, Taylor JW (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for Phylogenetics. In: PCR Protocols, A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ eds.), Academic, San Diego, U.S.A.: 315–322.
- Wollenzien U, Hoog GS de, Krumbein WE, Uijthof JMJ (1997). Sarcinomyces petricola, a new microcolonial fungus from marble in the Mediterranean basin. Antonie van Leeuwenhoek 71: 281–288. [DOI] [PubMed] [Google Scholar]
- Wollenzien U, Hoog GS de, Krumbein WE, Urzì C (1995). On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Science of the Total Environment 167: 287–294. [Google Scholar]
- Yoshida S, Takeo K, Hoog GS de, Nishimura K, Miyaji M (1996). A new type of growth exhibited by Trimmatostroma abietis. Antonie van Leeuwenhoek 69: 211–215. [DOI] [PubMed] [Google Scholar]
- Zalar P, Hoog GS de, Gunde-Cimerman N (1999). Trimmatostroma salinum, a new species from hypersaline water. Studies in Mycology 43: 57–62. [Google Scholar]
- Zucconi L, Pagano S, Fenice M, Selbmann L, Tosi S, Onofri S (1996). Growth temperature preferences of fungal strains from Victoria Land, Antarctica. Polar Biology 16: 53–61. [Google Scholar]