Abstract
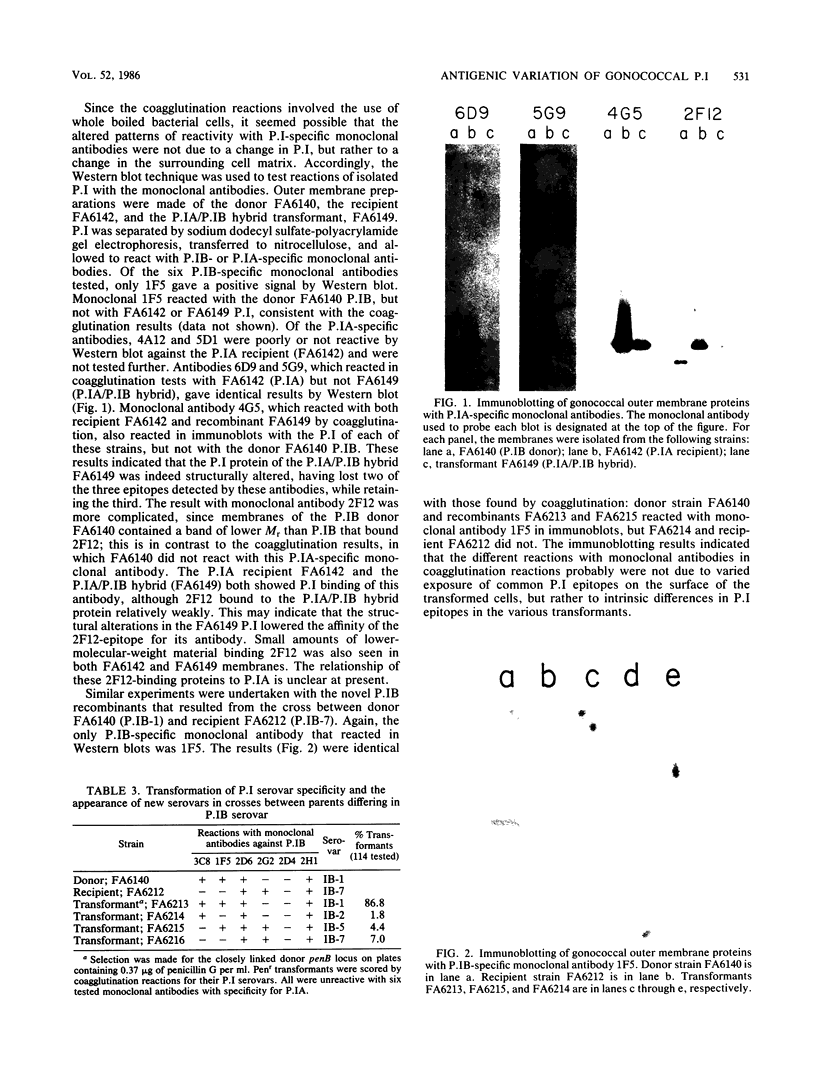
In gonococci, the nonspecific antimicrobial resistance locus penB is known to be closely linked to loci designated nmp that alter the Mr and antigenicity of the outer membrane porin protein I (P.I). We report that after selection for the linked donor penB locus, occasional recombinants expressed P.I with some epitopes from each parent. These hybrid P.I antigens were stable on subculture and were transformed at a locus closely linked to penB. The hybrid P.I antigens were detected with monoclonal antibodies in both coagglutination and Western blot assays. The alterations of P.I antigenicity may have resulted from recombination between structural genes for P.I that are closely linked to penB.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bygdeman S., Bäckman M., Danielsson D., Norgren M. Genetic linkage between serogroup specificity and antibiotic resistance in Neisseria gonorrhoeae. Acta Pathol Microbiol Immunol Scand B. 1982 Jun;90(3):243–250. doi: 10.1111/j.1699-0463.1982.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Klapper D. G., Blackman E. Y., Sparling P. F. Genetic locus (nmp-1) affecting the principal outer membrane protein of Neisseria gonorrhoeae. J Bacteriol. 1980 Aug;143(2):847–851. doi: 10.1128/jb.143.2.847-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Lee T. J., Guymon L. F., Sparling P. F. Genetics of serum resistance in Neisseria gonorrhoeae: the sac-1 genetic locus. Infect Immun. 1981 May;32(2):547–552. doi: 10.1128/iai.32.2.547-552.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Faruki H., Kohmescher R. N., McKinney W. P., Sparling P. F. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally mediated resistance). N Engl J Med. 1985 Sep 5;313(10):607–611. doi: 10.1056/NEJM198509053131004. [DOI] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982 Aug;37(2):481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Guymon L. F., Sparling P. F. Identification of a new genetic site (sac-3+) in Neisseria gonorrhoeae that affects sensitivity to normal human serum. Infect Immun. 1982 Mar;35(3):764–769. doi: 10.1128/iai.35.3.764-769.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]