Abstract
The olfactory system sensitively discerns scents from many small molecules as the brain analyses signals from nasal receptors. These receptors are selective to some degree, though the mechanism for selectivity is still controversial. Enantiomers, chiral pairs of left- and right-handed structures, are an important class of molecules in assessing proposed mechanisms. We show that there is a correlation between molecular (structural) flexibility and whether or not the left- and right-handed enantiomers smell the same. In particular, for the fairly extensive class of enantiomers with six-membered ring flexibility, enantiomers do not smell the same. There are, of course, significant experimental uncertainties, which we discuss here. We discuss models of receptor selectivity, both those based on shape and those where discrimination is based on other factors, such as electron affinity, proton affinity or vibration frequencies. The differences in scent of these enantiomers appear to be consistent with simple generalizations of a ‘swipe card’ model in which, while the shape must be good enough, critical information for actuation is a separate factor.
Keywords: enantiomers, flexibility, olfaction, olfactants, inelastic electron tunnelling spectra
1. Introduction
Our sense of smell enables us to detect and, up to a point, identify small volatile molecules in our environment. How this is achieved is still enigmatic, though the broad features are clear. Scent molecules must reach nasal receptors. Some of these receptors will be actuated, initiating signals to the brain where the signals are analysed and integrated and hence a particular smell is perceived.
Following Buck & Axel (1991), winners of the 2004 Nobel Prize in Physiology or Medicine, it is generally agreed that certain types of receptor are tuned to certain odorant properties. The receptor responses initiate signals to the olfactory bulb in so-called ‘zone-to-zone’ projection (Simoes de Souza & Antunes 2007), when a pattern of receptor response maps directly onto corresponding locations in the bulb, whence information defining the scent is communicated as a combinatorial code to the brain. There is no doubt that key steps in olfaction occur within the brain (Wilson & Stevenson 2006). But the brain must have something to work on. If two molecules smell different, somehow they must access or actuate receptors differently, however ignorant we may be of receptor structure or atomic-scale mechanisms.
When left- and right-handed chiral molecules smell different, somehow they must initiate different signals to the brain. While the brain works with inputs from a number of receptors, it can only distinguish between scent molecules if the receptors are themselves selective to some degree. It is the molecular mechanisms underlying selectivity that tend to be controversial. We identify an unexpected rule as to whether such pairs smell the same or different, and analyse molecular features that might underlie this seemingly counter-intuitive behaviour. Thus, our prime concern is with how the odorant accesses and actuates receptors.
Enantiomers provide interesting tests of various models of receptor selectivity. For example, if shape were the only olfactant characteristic recognized by the receptors and if the olfactant molecules were rigid, then left- and right-handed forms should always smell different unless receptors were always present in pairs of opposite chirality. If, however, an olfactant is identified entirely by a quantity that is invariant under a reflection, such as a characteristic energy (ionization potential or electron affinity for charge transfer, proton chemical potential or vibration frequencies), then left- and right-handed forms should smell the same. In practice, features of both mechanisms are likely to be employed. We shall group the models into two main classes. One is the familiar ‘lock and key’ picture, including mechanisms in which shape dominates selectivity. Such situations appear to describe many cases of large drug molecules interacting with receptors. In the other class, the ‘swipe card’ (key card) picture (Brookes et al. 2007), the geometric fit of the odorant with the receptor has to be good enough, but shape does not provide the information leading to actuation. An example would be Turin's proposal that selectivity arises from inelastic electron tunnelling, the receptor acting like a vibrational spectrometer, sensitive to the vibrational frequencies of the olfactant.
It is very well established (Leffingwell & Associates, http://www.leffingwell.com) that, for humans, some enantiomers smell different. Thus, (4R)-(−)carvone smells minty, whereas (4S)-(+)-carvone smells like caraway. Non-olfactory examples include the effects of cocaine (1R, 2R, 3S, 5S is psychoactive, while 1S, 2S, 3S, 5R is inactive), the hormone thyroxine (R is inactive, while S is active) and the gypsy moth pheromone disparlure (7R, 8S activates a response, whereas 7S, 8R inhibits a response). A distinction is also made in taste. In a few cases, d-amino acids have a sweet taste, whereas the enantiomers are tasteless. As with odour, the situation is complex and there are exceptions such as l-alanine that has a sweet taste. It is therefore not surprising that the chiral helices of the olfactory G-protein-coupled receptors (GPCRs) are also enantioselective.
The resolution of some aspects of olfaction awaits full X-ray crystallography or NMR data for the 347 or so functioning human olfactory receptor (OR) types. There is some information on the anatomy of the human olfactory system (Buck & Axel 1991), and residue sequences are known for the receptor proteins, but there is little information on secondary and tertiary receptor structures at the right level of detail to be helpful to us. Our analysis therefore concentrates on the olfactant molecules themselves.
2. Classifying enantiomers and their odours
It is prudent to treat all experimental data on smell with caution. First, there is some subjectivity in how people describe smells, though there is usually broad agreement. Second, the character of a smell can depend on the strength of the source, sometimes dramatically. Third, purity is very important: even small traces of some strong-smelling impurity can transform an odour. Even with 98% enantiomeric purity, incorrect results occur if one enantiomer is significantly stronger (e.g. with a 0.0002 ppb threshold): the smell character of the weaker can be easily dominated by a very small fraction of the stronger component. This is especially an issue for enantiomers, since impurities may arise in synthesis, or during storage before use, as a result of reactions that are chirally sensitive. Such reactions could misleadingly make enantiomers smell different. Data produced using gas chromatography should be used. There should also be checks that the sample indeed contains just one enantiomer (say at least 99% pure). For trademarked odorants, the in-house quality procedures may suffice.
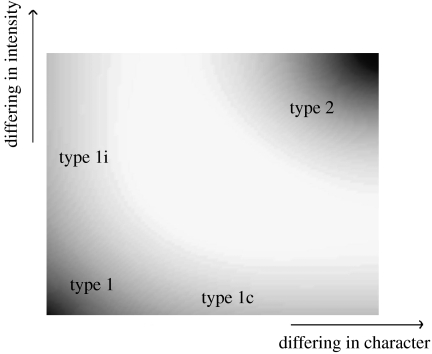
A major source of information, readily available, is that published by Leffingwell (Leffingwell & Associates, http://www.leffingwell.com), whose data we shall use. All odour descriptors in this paper are taken from Leffingwell's site and all odorants are categorized into the same groups Leffingwell uses. Similar to all large databases, the information may be uneven in quality. If we analyse the Leffingwell data, without being too critical about what could well be trace contaminants (i.e. we ignore small differences in odour character or intensity), then 59% of enantiomer pairs smell the same and 41% different. This predominance of pairs that smell largely the same should be borne in mind when we discuss differences in detail. However, if we demand odour character and intensity to be essentially identical, then only 5% of enantiomer pairs smell the same. This is illustrated in figure 1. In the interest of scientific rigour, we shall err on the side of caution and define four classes of enantiomer pairs, which are as follows.
Type 1. The left- and right-handed versions smell the same in character, with similar thresholds. Presumably both enantiomers have very similar affinity and efficacy for the same receptors. An example from table 2 is (1R,4S)-(+)- and (1S,4R)-(−)-fenchone; both smell ‘camphoraceous, strong, diffusive, sweet odour for racemate’. For another type 1 example, see tetrahydronootkatone (figure 10).
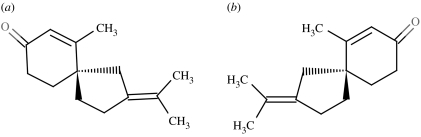
Type 2. The left- and right-handed forms are clearly distinct. Type 2 enantiomer pairs activate different receptors through different affinity or efficacy or both. An example from table 3 is (4R)-(−)- and (4S)-(+)-carvone, which is shown in figure 4. For further examples, see cis- and trans-p-menthan-8-thiol-3-ones (figure 2), γ-methyl cyclogeranates (figure 3) and nootkatones (figure 11).
Type 1c (character). The left- and right-handed forms smell much the same, but have small, sometimes subtle, differences in character. Clearly, one possibility is that these type 1c odorants should be type 1 but impurity species are involved somehow. Many odorants fall into this category, and it is hard to give an unambiguous example.
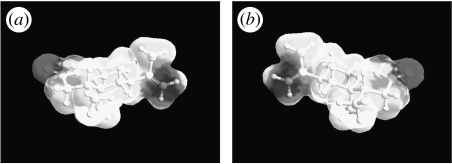
Type 1i (intensity). The left- and right-handed versions smell the same, but have very different thresholds. Since, presumably, several different receptors are actuated, but the only difference is in intensity, there is an implication that both affinity and efficacy simply scale on going from one enantiomer to another. An example shown in figure 9 is (4S,4aS,8aR)-(−)-geosmin and (4R,4aR,8aS)-(+)-geosmin; both smell ‘earthy, musty’, but are known to be differentiated by their thresholds.
Figure 1.
An odorant square representing in shades odorant classifications, within the strong regime, where type 1 is 5%. The bottom-left corner represents type 1, the top-right corner type 2, along the x-axis represents type 1c and along the y-axis represents type 1i. Graduations in shade are meant to indicate areas of ambiguity and show an overlap between the four categorizations. While graduated areas are contentious, we note that all examples examined in this paper are taken from odorants that lie within one of the corners. In other words, where it may be disputable how enantiomer pairs may smell different from one another, it is not disputable whether they smell the same or whether they smell different.
Table 2.
Type 1 odorants, seeking a possible counter example. (Type 1's: ‘plus’ denotes a positive response and ‘minus’ a negative response to whether the odorant is flexible.)
| section | enantiomer pair | no. | flexible? | notes: a counter example? |
|---|---|---|---|---|
| cyclic terpenoids | (2S,4S)-2-acetyl-p-menth-6-ene and (2R,4R) | 1 | + | no, the (2S,4R) and (2R,4S) isomers smell different |
| bicyclic terpenoids | (S)- and (R)-2-methylborneol | 2 | − | |
| (1R,4S)- and (1S,4R)-fenchone | 3 | − | ||
| (1R,4R)- and (1S,4S)-camphor | 4 | − | ||
| (1S,4R,5R)- and (1R,4S,5S)-5-acetoxy-1,8-cineole | 5 | − | virtually too weak to evaluate | |
| ionones, irones, damascones and structurally related odorants | (1′S,2S,2′R,5′S)- and (1′R,2R,2′S,5′R)-5-methyl ambercore | 6 | + | no, (1′R,2S,2′S,5′R) and (1′S,2R,2′R,5′S) smell different and comes with the description ‘vague’ |
| acyclic terpenoids | (2R,3S)-, (2S,3R)-, (2S,3S)- and (2R,3R)-3-mercapto-2-methyl-pentane-1-ol | 7 | − | note all four are type 1; the odour description appears to be for the four isomer mixtures, but the enantiomers are differentiated by their threshold data |
| lactones | (4R)- and (4S)-γ-octathionolactone | 8 | − | |
| (3R)- and (3S)-butylphthalide | 9 | − | ||
| sesquiterpenoid | (4R,4aS,6R,8aS)- and (4S,4aR,6S,8aR)-tetrahydronootkatone | 10 | + | possible counter example |
| related odorants | (5S)- and (5R)-10-demethyl-β-vetivone | 11 | + | possible counter example |
| (4R,5R)- and (4S,5S)-3,5-dimethyl-4-(4-methyl-pent-3-enyl)-cyclohex-2-enone | 12 | + | no, ‘nearly odourless’ and their isomers smell different, a partial structure of β-vetivone | |
| steroids and sandalwood-type odorants | (1R,2′R,3′R)- and (1S,2′S,3′S)-ebanol | 13 | − | not very reliable; the only descriptor is ‘no sandalwood relevance’ |
| musks | (3S)- and (3R)-3-methyl-1,4-dioxacyclopentadecan-2-one | 14 | − | |
| miscellaneous | (R)- and (S)-lilial | 15 | − | |
| (4S,4aS,8aR)- and (4R,4aR,8aS)-geosmin | 16 | + | possible counter example | |
| (R)- and (S)-2-methoxy-3-[1-methylpropyl] pyrazine | 17 | − |
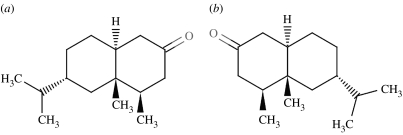
Figure 10.
Type 1, (a) (4R,4aS,6R,8aS)-(+)-tetrahydronootkatone and (b) (4S,4aR,6S,8aR)-(−)-tetrahydronootkatone smell ‘dusty-woody, fresh, green, sour, spicy, herbal, slightly fruity, animal, erogenic’.
Table 3.
Type 2 odorants, seeking possible examples. (Type 2's: ‘plus’ denotes a positive response and ‘minus’ denotes a negative response to whether the odorant is flexible.)
| section | enantiomer pair | no. | flexible? | planar? | chiral centre? | electron rich? | other? |
|---|---|---|---|---|---|---|---|
| cyclic terpenoids | (1R,4S)- and (1S,4R)-cis- and trans-p-menthan-8-thiol-3-ones | 1 | + | — | 1,4 | 3,4 | — |
| linalool oxides (pyranoids) | 2 | + | — | 1,4 | 1,4 | 2,3 | |
| R- and S-carvones | 3 | + | — | 4 | 2,4 | 1 | |
| carvone epoxides | 4 | + | — | 1,2,5 | 1,2, 3,5 | — | |
| carveols | 5 | + | 2 | 2,4 | 2,4 | 1 | |
| trans-dihydrocarvones | 6 | + | 1 | 1,4 | 2,4 | — | |
| carvotanacetols | 7 | + | 2 | 2,4 | 2 | 1 | |
| (2S,4R)- and (2R,4S)-2-acetyl-p-menth-6-enes | 8 | + | 4 | 2,4 | 2 | 1 | |
| bicyclic terpenoids | 4-cyclohexylmethylene-isopinocamphones | 9 | + | 1 | 1 | 2, (E–Z)3 | 5 |
| ionones, irones, damascones and structurally related odorants | methyl-α-cyclogeranates | 10 | + | — | 2 | 2 | 1,3 |
| methyl-γ-cyclogeranates | 11 | + | — | 2 | 2,3 | 1 | |
| trimethyl-cyclohexyl-pentan-3-ones | 12 | + | 2 | 2,3 | 2 | 1 | |
| trimethylcyclohexyl-pent-1-en-3-ones | 13 | + | 2 | 2,3 | 2 | 1 | |
| tetrahydroirones | 14 | + | 1,3 | 1,3 | 3 | 2 | |
| trans-γ-irones | 15 | + | 1 | 1,3 | 3,4 | 2 | |
| theaspiranes | 16 | + | — | 2 | 2 | 1,3 | |
| acyclics (alcohols, esters, acids, aldehydes) | methylhexanols | 17 | no ring | — | — | — | — |
| 2-methylbutanoic acids | 18 | no ring | — | — | — | — | |
| 2-pentanols | 19 | no ring | — | — | — | — | |
| 3-hydroxyheptanes | 20 | no ring | — | — | — | — | |
| 1-hexen-3-ols | 21 | no ring | — | — | — | — | |
| 1-hepten-3-ols | 22 | no ring | — | — | — | — | |
| 1-octen-3-ols | 23 | no ring | — | — | — | — | |
| 1-nonen-3-ols | 24 | no ring | — | — | — | — | |
| 1-decen-3-ols | 25 | no ring | — | — | — | — | |
| 3-mercaptohexanals | 26 | no ring | — | — | — | — | |
| 3-acetylthiohexanals | 27 | no ring | — | — | — | — | |
| miscellaneous | methyl-1,4-dimethylcyclohex-3-ene-1-carboxylates | 28 | + | — | 4 | 4 | 1 |
| 2-methylbutyl-2-methylpyrolidines | 29 | pentane ring | — | — | — | 1,2 | |
| 2-methyl-N-(3′-methylbutylidene)butan-1-amines | 30 | no ring | — | — | — | 1,2 |
Figure 4.
Type 2 (a) equatorial and (b) axial (4R)-(−)-carvone smells ‘sweet spearmint, fresh herbal’ and (4S)-(+)-carvone smells ‘caraway, fresh herbal’.
Figure 2.
Type 2 cis- and trans-p-menthan-8-thiol-3-ones. (a) (1S,4R)-cis smells ‘blackcurrant leaf, tropical note of passion fruit, intensive fruit note’. (1R,4S)-cis smells ‘rubber, mercaptan note, isopulegone note, sulphurous, disagreeable’. (b) (1R,4R)-trans smells ‘onion-like, weak fruity, tropical, dirty’. (1S,4S)-trans smells ‘stronger than (1R,4R)-isomer, tropical, sulphurous, pronounced buchu leaf oil notes’.
Figure 3.
Type 2 (a) chair and (b) boat (1R)-γ-methyl cyclogeranate smells ‘camphoraceous, corky, cellar’ and (1S)-γ-methyl cyclogeranate smells ‘aromatic, damascone-like, thujone, fruity’.
Figure 11.
Type 2, nootkatones: (a) (4R,4aS,6R)-(+)-nootkatone smells of grapefruit (0.8 ppm) and (b) (4S,4aR,6S)-(−)-nootkatone is ‘woody, spicy’ (600 ppm) (Bentley 2006). Note also that the (+) enantiomer is approximately 750 times more potent than the (−) enantiomer (Eliel & Wilen 1993).
Figure 9.
Type 1i, (a) (4R,4aR,8aS)-(+)-geosmin and (b) (4S,4aS,8aR)-(−)-geosmin smell ‘earthy, musty’.
We shall be scrupulous when identifying enantiomers as type 1 or type 2. For example, if one enantiomer is described as ‘amber, woody, cedar wood, animal, strong’ and the other member of the pair as ‘woody, camphoraceous, amber, spicy’, there are very strong similarities (woody, amber), but we have regarded the scents as different, so the pair is classed as type 2. We shall be equally scrupulous in distinguishing type 2 from type 1i or type 1c, since they have their own characteristic features. Therefore, as above, we distinguish between strong similarity (type 1, 5% and other, 95%) and weak similarity (types 1, 1c, 1i the same, 59%, and type 2, 41%). We emphasize this owing to the subjectivity objection that is often voiced in olfactory research. This study uses objective information, i.e. while the descriptions of differences may be subjective, the fact of whether they are the same or not is much less. The schematic of these odorant classifications is shown in figure 1.
Despite the contentious issue of purity, it is well established that it is the case that (one way or another) many enantiomer pairs smell different (Sell 2006). We might postulate four possible explanations. First, there may be discrimination prior to actuation through metabolism en route. One isomer only might break into chiral parts, so the nose smells different molecules, and thus perceives them differently. Also, diffusion processes to the cilia may be chirally biased, much like gas chromatography. Second, there may be discrimination during actuation of a receptor that may depend, in the majority, on adaptability of fit. Perhaps rigid molecules only activate receptors where there is a good fit, while flexible molecules can activate even when there is a poor fit: the poorness of the fit then means that the difference between left- and right-handedness actually matters, whereas for a good fit it might not. Third, there might be allosteric regulation (one binding site regulates the response of a second binding site) of GPCRs. For example, one odorant could change a key geometry or energy probed by the second odorant. Allosteric regulation is known to occur in other GPCRs (e.g. muscarinic acetylcholine receptors). Fourth, there may be discrimination post-actuation in terms of how the brain might interpret the combinations of signals from a number of receptors. However, given the zone-to-zone evidence, and the lack of evidence for metabolizing enzymes, it seems likely that the discrimination of enantiomer pairs occurs because the receptor somehow differentiates these molecules.
3. Olfactant structures: flexible and inflexible molecules
An examination of the Leffingwell data for May 2006 (Leffingwell & Associates, http://www.leffingwell.com) shows that a majority (52%) of enantiomer odorants contain six-membered rings. We are interested in the flexibility or rigidity of these rings, finding an apparently significant correlation between such flexibility and enantiomer class.
We shall usually infer flexibility from the molecular structure; however, we have calculated energies for a number of changes of geometry and identified probable low-energy structural changes on the basis of the bonding. We describe as flexible those olfactants containing six-membered rings for which any one of the following three possible structural changes is easy. The first group of flexible molecules includes those having cis–trans transitions about the six-membered ring (figure 2). These correspond to molecules where two functional groups lie across the plane of the ring, and where it is feasible that these groups can isomerize by switching between sides of the plane. Second, there are molecules whose flexibility stems from cyclohexane ring twist transitions from chair to boat conformations, as shown in figure 3. Third, there are those molecules for which cyclohexene ring twists are possible, similar to cyclohexane chair–boat, but more strained, as shown in figure 4. We shall continue to make comparisons with cyclohexane, partly because it has been so thoroughly investigated, and partly because it exhibits the type of flexibility we think is also exhibited by odorants. Cyclohexane is not chiral but it is useful as a good control and measure of flexibility. In figures 2–4, we show minimum energy geometries of chiral olfactants. If the nose discerns seemingly subtle enantiomeric conformations, then we must also consider all other stereoisomeric conformations. Such a full analysis is not only important in the study of olfactants, but also in steroid compounds. Thus, Galigniana et al. (2004) noted that pregnanesteroids can vary drastically in their biological effect (mineralcorticoid potency) when two molecules have exactly the same functional groups but different conformations: contrast 11,19-oxidoprogesterone with 6,19-oxidoprogesterone and 5α-dihydroprogesterone with high doses of 5β-dihydroprogesterone.
The energy differences between the alternative structural forms of the isolated molecule will usually be larger than thermal energies. For an idea of these magnitudes, we calculated the isolated gas-phase energies for initial- and final-state geometries and the transition states (table 1). Chair and boat geometries were optimized using B3LYP/6-31G* on Gaussian '03 and the single point energy found. Transition states were initially estimated from a potential energy scan implemented again using B3LYP/6-31G*. For each molecule a C–C–C–C dihedral angle is incremented by 5° over a range of approximately 100° with these atoms held fixed and the rest of the molecule is optimized partially. From these results, an initial guess was taken from the potential energy surface for the transition state, which is then implemented in a Gaussian ‘QST3’ calculation that uses the synchronous-transit-guided-quasi-Newton method. Thus, the transition state is found and verified by the analysis of the second derivatives to find the zero gradient and one imaginary frequency. We analyse cyclohexane, which is well established, as a test of the method, and compare our results with Peng et al. (1996) who found E1=0.2 eV, E2=0.5 eV and hence E3=0.3 eV using Hartree–Fock methods, where E1, E2 and E3 are the energies defined in table 1, and hence find a satisfactory agreement.
Table 1.
Ab initio energy differences between conformations in small molecules. (Comparison of the energy gaps exhibited by chair–boat flexible molecules. Energies are in eV. C, the energy of the chair configuration; B, the energy of the boat configuration; T, the energy of the transition state. Calculated using B3LYP/6-31G* on Gaussian '03. E1 is the difference from transition state to boat, E2 is the difference from chair to transition state and hence E3 is the difference from chair to boat.)
| molecule | T−B (E1) | T−C (E2) | C−B (E3) |
|---|---|---|---|
| cyclohexane | 0.22 | 0.50 | 0.28 |
| (1R)-γ-methyl cyclogeranates | 0.17 | 0.33 | 0.16 |
| (4R)-(−)-carvone | 0.27 | 0.34 | 0.07 |
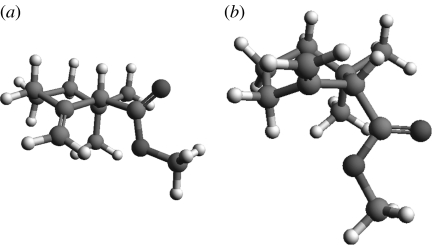
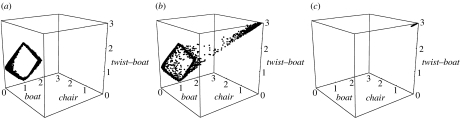
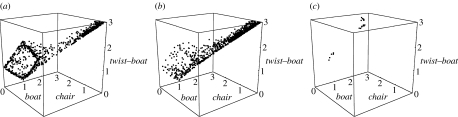
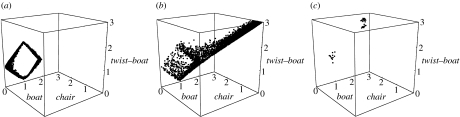
We see that the energy gaps for odorants are comparable to cyclohexane and small, certainly small enough for the molecule to explore a wide range of geometries. Therefore, we expect to see chair, boat and transition-state-like internal motion in odorants, reminiscent of the type of conformational sampling explored for cyclohexane at room temperature (Juaristi 1995). We used molecular dynamics (MD) simulations to investigate known results for cyclohexane and compare with type 1 (1R,4S)-(+)-fenchone (table 2, no. 3) and type 2 (4R)-(−)-carvone (table 3, no. 3). MD simulations were performed using Dl_poly v. 2.16. We use an NVE ensemble that has a constant number of particles (N), volume (V) and energy (E). We set a target temperature for the system about which it averages, and randomly orientated initial velocities are chosen accordingly. All calculations start with an energy minimum for the initial geometry found using DFT/B3LYP/6-31G*, as in previous ab initio calculations. The time step is set to 0.2 fs. This is a time interval that seems appropriate: it seems unlikely that within this short time (a fraction of an atomic vibrational period) events will be missed, thereby degrading the resolution. The Dreiding force field is implemented, which describes the intermolecular bonds harmonically and the intramolecular interactions (van der Waals) using a Lennard-Jones potential. We analyse the results by plotting for each configuration the minimum difference the six-membered ring has from the three stationary points, namely the chair, boat and transition state, as described by Dixon & Komornicki (1990). Conformations are recorded in xyz format at intervals of 100 steps, which corresponds to 0.02 ps. These results are depicted in a three-dimensional scatter plot (figures 5 and 6) for cyclohexane at 20 and 100 ps, respectively. The first 20 ps calculation represents an equilibration period, and the second 100 ps starts at the endpoint of the first. Figures 5a,b and 6a,b show that cyclohexane explores a wide conformational space even at around room temperature, while at 10 K, very little is happening and the molecule remains chair-like. In particular, we see that during a simulation, a distinct square forms, which indicates cyclohexane's preference to pseudorotate about boat and twist forms. The longer simulation shows that at a higher temperature of 600 K, the energy barrier required to approach this behaviour is easily surmounted. Figures 7 and 8 contrast the behaviour of (4R)-(−)-carvone and (1R,4S)-(+)-fenchone, and we see very clearly that (4R)-(−)-carvone (type 2) explores a much more conformational space than (1R,4S)-(+)-fenchone (type 1), as is expected because fenchone is kept rigid in the twist–boat-like geometry by a carbon bridge.
Figure 5.
Cyclohexane simulation of 20 ps, showing the conformational space of an isolated gas-phase molecule at (a) 600 K, (b) 300 K and (c) 10 K. We note that with increasing temperature, cyclohexane explores a much wider conformational space: at 600 K, more heavily populating pseudorotating states between boat and twist.
Figure 6.
Cyclohexane simulation of 100 ps, showing the conformational space of an isolated gas-phase molecule at (a) 600 K, (b) 300 K and (c) 10 K. We note that after the 20 ps equilibration at higher temperatures, cyclohexane will easily surmount the energy barrier and heavily populate pseudorotating states between boat and twist, throughout a longer simulation.
Figure 7.
(a) Cyclohexane, (b) (4R)-(−)-carvone (type 2) and (c) (1R,4S)-(+)-fenchone (type 1) at 600 K. The simulations ran for 20 ps. We clearly note that cyclohexane reaches the boat–twist pseudorotating barrier, (4R)-(−)-carvone approaches it, but (1R,4S)-(+)-fenchone does not.
Figure 8.
(a) Cyclohexane, (b) (4R)-(−)-carvone (type 2) and (c) (1R,4S)-(+)-fenchone (type 1) at 600 K. The simulations ran for 100 ps. We clearly note that cyclohexane easily reaches the boat–twist pseudorotating barrier, as does (4R)-(−)-carvone, but (1R,4S)-(+)-fenchone even after a longer simulation does not.
Many other types of flexibility are possible, though we shall not consider them here. They include the entgegen–zusammen (E–Z) transitions for which cis–trans cannot be unambiguously defined. We note that an (E–Z) transition across a double bond in a carbon chain can make a vast difference to odour perception (Sell 2006). One may contrast the ‘creamy, butter, odour’ of Z-4-heptanal with the ‘aggressive, green and putty-like’ odour of its E-isomer. There may also be transitions across five-membered rings or odorant rings with more than six atoms (e.g. table 2, no. 14) that are not unlike cis–trans or chair–boat flexibilities observed in six-membered rings. Such transitions may be of interest in another study.
We shall focus on odorants similar in form to 11-cis-retinal, partly because receptors evolved from rhodopsin could be sensitive to the structural changes for these types of transitions. The conformational change in 11-cis-retinal to all-trans-retinal upon photoexcitation is the initiating step in visual signal transduction, with photons of several electronvolts being needed to trigger the protein–ligand conformation change. Olfaction involves rather different energies (probably of the order of 0.1 eV as illustrated above) from the optically induced processes. However, it is possible that in olfaction, the molecular environment might reduce the energy difference between alternative structures, for example by stabilizing a higher energy form through interactions with molecular components of the receptor. This could be achieved through favourable binding and folding. It is common that GPCRs change conformation upon binding. For example, the intercellular loop 3 (IL3) is known to be important in G-protein activation (Day et al. 2007) and Day et al. observed that IL3 in the GPCR β2AR connect and can move the transmembrane (TM) helices TM5 and TM6 such that they realign with TM3, producing relatively different responses depending on the agonist that induced the changes. If such properties of GPCRs are conserved in the OR repertoire, similar activation should not be ruled out. The Leffingwell database contains many odorants that are structurally similar to 11-cis-retinal, such as the strong-smelling irones, ionones and damascones. It is reported that the β-ionone ring of 11-cis-retinal exists 8 Å from a zinc coordination site within the binding site in rhodopsin (Stojanovic et al. 2004). With zinc deficiency, the receptor rhodopsin malfunctions; similarly, a lack of zinc causes anosmia. It seems the six-membered ring is an important feature in olfaction, and so its conformational properties may explain certain observations. Thus, cis- and trans-1,2-difluoroethenes have differing dipole moments as one conformer exists out of the plane (is non-planar; Allan et al. 2002). This affects their observed IR spectra, and could be relevant in any model that relates selectivity to odorant vibrations (Turin 1996). It is interesting that in figures 2–4, these types of geometry changes produce the biggest changes in positions of various dipole moments within the molecule. Furthermore, these regions that change position probably define how the molecule binds to the receptor (see §4.1). Therefore, there are several reasons to look at the flexibilities of six-membered ring molecules. The data analysed (Leffingwell & Associates, http://www.leffingwell.com) included 428 enantiomer pairs (hence 856 molecules). Of these, 450 molecules were flexible, following the above definition; 404 molecules were rigid, and 2 were ones for which we were unsure.
4. Structure and character for enantiomer pairs
Our analysis of data from the Leffingwell list, summarized in tables 2 and 3, shows a novel systematic feature. The type 2 enantiomer pairs are distinctive. It seems a universal result that flexible enantiomers (at least those with the six-membered ring flexibility discussed previously) are type 2, i.e. produce two different scents. We now examine the conjecture that flexibility implies type 2 and rigidity implies type 1. We shall avoid cases that are type 1i or type 1c, since the data raise other questions.
4.1 Are all type 1's rigid?
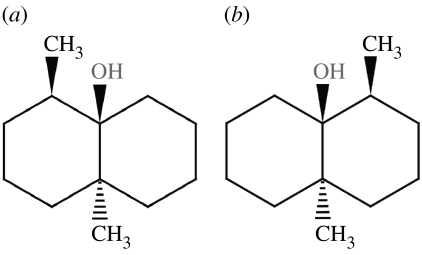
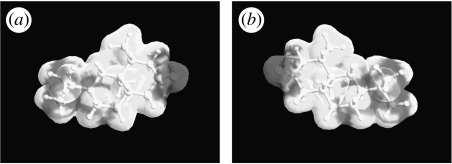
Our conjecture implies that there should be few, if any, flexible enantiomers that smell the same. The data agree. From table 2, it certainly seems that most of the rather few clear type 1's are rigid by our definition. Of the 11 definite type 1's (see the notes for why we exclude nos. 1, 5, 6, 7, 12 and 13), three are rigid through constraint of the carbon ring at the 1 and 4 carbon position restricting twist (see 2-methylborneol, fenchone and camphor), and the rest have more exotic geometries (table 2, no. 14). We consider the three possible counter examples (table 2, nos. 10, 11 and 16). Do these molecules contradict our conjecture?
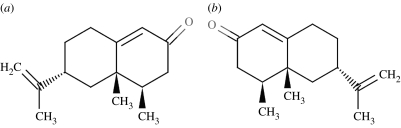
It is interesting to compare the type 1's geosmin and tetrahydronootkatone with type 2 nootkatone, as they are structurally similar (figures 9–11).
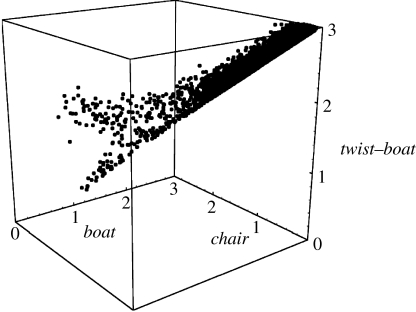
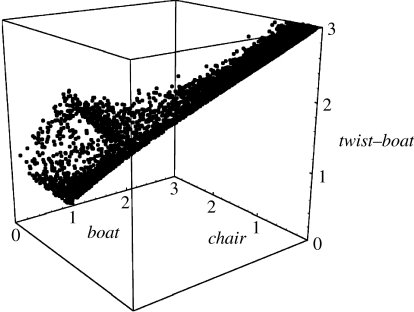
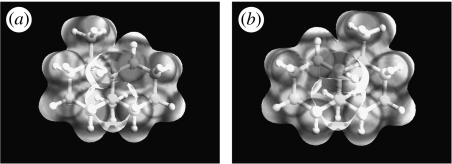
Prima facie, one might expect all these odorant pairs to be all type 1 or all type 2. Closer inspection shows that, in fact, the first two examples are rigid, while the third is not. Although all three exhibit six-membered rings, the presence of two rings, combined with the transpositions of groups across the join exhibited in the first two, produces a restriction in movement not exhibited by the third. The two groups, trans across the join, occupy definite axial or equatorial positions (H is equatorial and CH3 is axial) as in turn do all substituents. The rings are locked in two conformationally rigid chair–chair formations (Eliel & Wilen 1993). If the configuration is cis (e.g. if both H and CH3 were axial), then the rings have the ability to interconvert from chair–chair to the alternative chair–chair (as cyclohexane does, via boat and twist), and the substituent groups are mobile about axial–equatorial positions. We use the same method described in §3 to evaluate type 1 (4R,4aS,6R,8aS)-(+)-tetrahydronootkatone (figure 12) and type 2 (4R,4aS,6R)-(+)-nootkatone (figure 13), finding that, indeed, tetrahydronootkatone is rigid whereas nootkatone is flexible approaching the same type of pseudorotation exhibited by cyclohexane (figure 6). The possible counterexample, examined in detail, actually reinforces a strong correlation between flexibility and discrimination. We find, to the authors' knowledge, a new observation that though nootkatone is not cis because it does not possess two groups, it exhibits a similar type of conformational freedom when compared to the trans version.
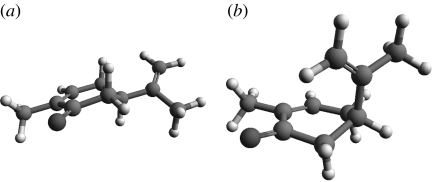
Figure 12.
Type 1, (4R,4aS,6R,8aS)-(+)-tetrahydronootkatone, 600 K simulation for 100 ps. This odorant does not reach the pseudorotating contour as shown in cyclohexane (figure 6). It is not flexible.
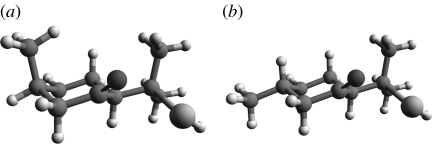
Figure 13.
Type 2, (4R,4aS,6R)-(+)-nootkatone, 600 K simulation for 100 ps. This odorant does reach the pseudorotating contour as shown in cyclohexane (figure 6). It is flexible.
So, how does this rigidity, or rather its absence, affect important groups on the olfactant? ‘Osmophoric groups’ have been coined as a phrase to describe polar parts of an odorant molecule. Usually, odorants have one strongly polar group and a secondary weaker polar group (Sell 2006). Having discovered one correlation between two rare odorant qualities, namely that there are very few true type 1's and that they are rigid, it is interesting to observe that the same molecules seem only to have one osmophoric group. Here, an osmophoric group means one that is electronegative, and an osmophore is a region rich in such groups. This was investigated by looking at the electrostatic potential (ESP) that gives a measure of which parts of the molecule can participate in electrostatic non-bonding interactions, crucial to the binding and alignment of the odorant in the receptor. The ESP, calculated using ArgusLab (Thompson 2004), is that felt by a positive point charge, and is mapped onto the electron density using ZINDO for several odorant pairs at their isolated phase equilibrium geometries (figures 14–16). By contrast, it is observed that the type 2 nootkatone exhibits two areas of higher regions of electronegativity about opposite ends of the conjoined rings (at the C=O and C=C), whereas the type 1i geosmin and type 1 tetrahydronootkatone exhibit only one area of electronegativity (about the alcohol and carbonyl group, respectively). Thus, we propose that a combination of rigidity and a sole osmophoric group (Sell 2006) actually renders left- and right-handed odorants superimposable (in the sense that the same atoms exist in similar space) within a receptor. In this case, the receptor is unable to discriminate, thus these unlikely cases we perceive as type 1. The presence of two osmophoric groups, however, in particular at either side of a flexible ring, introduces a degree of complexity in signal generation that may arise from the alignment of these osmophoric groups with contacts in the receptor. This complexity is reflected in our ability to differentiate enantiomer pairs, as shown in figure 16 and table 3, and is discussed in §4.3.
Figure 14.
Type 1i, (a) (4R,4aR,8aS)-(+)-geosmin and (b) (4S,4aS,8aR)-(−)-geosmin: ESP fit to the electron density isosurface. Note that there is one main region of electronegativity, corresponding to the ‘osmophoric group’.
Figure 15.
Type 1, (a) (4R,4aS,6R,8aS)-(+)-tetrahydronootkatone and (b) (4S,4aR,6S,8aR)-(−)-tetrahydronootkatone: ESP fit to the electron density isosurface. Note that there is one main region of electronegativity.
Figure 16.
Type 2, (a) (4R,4aS,6R)-(+)-nootkatone and (b) (4S,4aR,6S)-(−)-nootkatone: ESP fit to the electron density isosurface: note that there are two main regions of negativity, one weaker than the other.
The final possible counterexample is shown in table 2 (no. 11) and figure 17. We suggest that, again, this structure is in fact rigid, as the five-membered ring stabilizes the six-membered ring in a way similar to that observed in the previous example. We conclude, then, that all probable counterexamples in fact are rigid. The significant conclusion from a thorough investigation is thus that there are few true type 1's, and what they all share is the property of rigidity and one osmophore. We have found no counterexample to this proposition. Furthermore, we suggest an inverse relationship between rigidity and the number of receptors activated, i.e. rigid molecules can actuate fewer receptors.
Figure 17.
Type 1, (a) (5R)- and (b) (5S)-10-demethyl-β-vetivone smells ‘intense cresolic’.
4.2 Are all type 2's flexible?
As noted previously, the division into type 1 and type 2 can be tricky, for there are various reasons as to why such a categorization could be unreliable and we have been very cautious in assigning enantiomers to type 1, and we should be equally scrupulous in deciding which are of type 2 (table 3).
For those pairs with six-membered rings, the positions of any functional groups have been counted in a clockwise direction for one hand; the four right-most columns of table 3 indicate the carbon atom number on the ring where such a group exists. The functional groups are categorized thus: ‘planar?’ indicates if a group is positioned away from the plane of the ring (into the page); ‘chiral centre?’ indicates which carbons have chirality; ‘electron rich?’ indicates if the functional group is electron rich (i.e. there exists a π-bond, or something electronegative such as oxygen and sulphur); and ‘other?’ indicates a functional group that exists but without these features. We anticipate that a combination of electron-rich groups and a chiral centre would make the receptor sensitive to chirality, as superimposability is then unlikely. This suggestion is based on three ideas. First, it seems likely that the electronically interesting part of an odorant to a receptor is the regions to which it may bind. Second, binding will be chiral sensitive. If the above parts are positioned chirally, they are opposite in space and thus different in character for each enantiomer unless a receptor is ambidextrous or equally and oppositely designed (chiral receptors exist for every chiral odorant). Third, binding and detection are further sensitive to conformational change. The receptor may be rendered even more sensitive to chirality when conformational changes affect chiral and electronic groups.
Excluding the acyclics category, table 3 indicates a typical type 2 odorant recurrent pattern. The position of certain important groups about the ring is highlighted for their role in chiral discrimination. This pattern is consistent with our discussion above. It is also consistent with the results from Laska et al. (2005), who found that wherever an odorant bears an isopropenyl group at the chiral carbon atom, a methyl group at the para-position and/or an oxygen containing group at the meta-position, the enantiomer pairs could be discriminated. Furthermore, as Laska et al. discussed, this feature gives some support for Ohloff's multipoint attachment theory, whereby the receptor is ‘two toothed’, binding by using at least two points on the odorant.
At this juncture, odorants appear to fall into at least three natural categories: (i) true type 1's, (ii) true type 2's, and (iii) acyclics. In our quite substantial dataset, it is always the case that the two members of an enantiomer pair with the six-membered ring flexibility produce two different scents (type 2).
4.3 How might flexibility affect olfaction?
In normal thermal diffusion, processes occur during rare events, near critical values of reaction coordinates, and we suspect that relatively rare geometries have an important role in olfaction, just as in diffusion. If so, thermal fluctuations allow relatively soft molecules to explore a wider range of geometries. Such an exploration of molecular conformations permitted by flexibility might be important before docking or at the receptor, or both. Our discussion concentrates on situations made possible by the relative ease of larger structural changes, and the effect of this at the OR site.
Flexibility complicates the interpretation of both the lock and key and the swipe card descriptions. Intuitively, one would expect rigid molecules to be sensitive to receptor structure (and so type 2), whereas flexible molecules might be more able to evade chiral constraints (and so type 1). Theimer et al. (1977) suggested that very flexible enantiomers, such as the acyclics, should always smell the same because they are able to explore mutually similar and wide conformational spaces. These expectations seem plausible in almost any model of olfaction. Yet both expectations prove false. This is less surprising when we recognize that the conformational space explored by an odorant is likely to be limited by a chiral receptor. Within the chiral space defined by a receptor, the motion will be subject to restrictions, but a flexible molecule will still be able to explore a wider range of conformations than a rigid one, and so produce a more varied response.
We note also that it could only take a single extra receptor activated by just one hand (say, by L) for us to perceive a difference between L and R forms. Flexibility then offers the opportunity for the odorant to be at once promiscuous and selective in activating a receptor. This depends on the receptor type, an observation noted often in the literature (Breer 2003) and this brings us back to the issue of a ‘good’ fit. For the type 1 fenchone, (1R,4S) and (1S,4R) are a good fit: they appear superimposable, and so may activate the same receptors and smell the same. However, the type 2 carvone has 4R, 4R′, 4S and 4S′ forms (figure 4), where the prime symbol denotes distinct equilibrium geometries. These are non-superimposable, so 4R, 4R′ and 4S may adapt to fit and activate receptors equally, but 4S′ may not. If so, chiral pairs would be discriminated. Therefore, if the shapes, structures and positions of groups matter, which they clearly do, then flexibility must also be a factor in olfaction.
Consider, for example, the nose's ability to discriminate between 4R and 4S carvone (figure 4). If a molecule has n chiral centres, then there are always 2n forms in space. When we introduce flexibility, there are also what we may call pseudoequilibrium states, metastable, but likely to be achieved with a useful probability. With m such states, there will be (m+1)2n forms. For carvone, there are at least 22=4 geometries where the equatorial form is the equilibrium and the axial the pseudoequilibrium form. Two of these states are shown in figure 4. Statistically, such molecules as these should have more chances of activating an appropriate chiral receptor. Combinatorially, there are not enough types of receptor to accommodate all these structures, so it is not surprising there is an asymmetry favouring one hand to succeed and its enantiomer to fail. Rigid chiral molecules, however, should activate the same receptors with equal success owing to the superimposability of their fewer 2n forms.
Whether chair/boat-type conformations are preferred or not, however, will depend on the binding site and on the contacts the odorant may make with that site (figures 14–16). Binding hinders motion of the odorant, reducing its effective symmetry and so the number of ways a receptor can ‘see’ the odorant. Most odorants exhibit at least one polar group, the so-called osmophoric group or osmophore (Sell 2006), likely to make a hydrogen bond to the receptor. The combination of flexibility and the presence of more than one osmophoric group, as discussed in §4.1, defines the odorant's non-superimposability, and thus its type. Also important may be the time spent at the site. Lai et al. (2005; Sell 2006) showed that when ligands are fitted to a binding pocket, then, once natural vibratory motion was included, some odorants stayed within the pocket, whereas others moved out. Their results correlated with experiment, in that those that stayed within the domain activated the OR17 receptor, whereas the others did not.
Conformation change thus complicates simpler lock and key models because it becomes important to consider which is the structure that the receptor actually recognizes. There are also complications for swipe card models. In Turin's model (Turin 1996), for instance, some odorant orientations favour electron inelastic tunnelling, and some do not. The first requirement for a favourable conformation is alignment of dipoles within the odorant to maximize the change in force as the electron tunnels; the rate and intensity of the signal is sensitive to this. The overall distance the electron tunnels should be small, for tunnelling rates drop exponentially with distance. The odorant must have a mode with the right vibrational frequency for the conformations that satisfy the other criteria. This mode, directly involved in actuation in this model, is probably distinct from the motions associated with flexibility. However, the motions associated with flexibility could have another role. In solid-state physics, there is a well-known role for what are termed promoting modes, which affect the rate of some processes. The promoting mode (Stoneham 1981) is not the reaction coordinate, but makes a key step easier. In solid-state diffusion, a promoting mode might open a gap between atoms, so a diffusing atom can migrate more easily. Fluctuations associated with flexibility may have a role in enabling the actuation event, perhaps in making inelastic tunnelling more probable. Of course, analogous enhancements might be possible for other actuation mechanisms.
5. Conclusions
An examination of a very large number of enantiomer pairs, with a proper concern for the likely accuracy of the data, shows a general rule: the members of an enantiomer pair will smell alike (type 1) when the molecules are rigid, and will smell different (type 2) when they are flexible. The flexibility referred to is not related to the geometric changes needed to deform one enantiomer into the other. This result is counter-intuitive for both shape theories and those for which selectivity depends on some other factor.
It is possible that the processes leading to the type 2 differences could occur en route to the receptor, for example, by chirally selective catalysis or during diffusion steps through a chiral medium. For example, the acyclics category (table 3) does not share the features predominant in type 2 odorants containing six-membered rings; we suggest that these odorants may be metabolized by chirally biased enzymes. Though there is no evidence for this in olfaction, enantioselective enzyme reactivity is ubiquitous in other systems. Interestingly, there are many systems where enantiomers will simultaneously bind and superimpose within an enzyme binding site. For example, X-ray crystallographic data are available that show the evidence of binding of both (1R,2S) and (1S,2R) isocitric acids to isocitrate dehydrogenase (for a more complete review, see Bentley 2003). A specific flexibility is required to aid this superposition, so it may be the case that discrimination is dictated at this point. It is also possible that the critical step is docking at the receptor, where (for example) one member only of a flexible enantiomer pair might be able to wriggle into one or more receptors that the other member cannot access. This would lead to a different ensemble of signals being sent to the brain, and hence different smells. We cannot rule this out, but, again, the large number of type 2 pairs makes it seem improbable as the whole explanation of the rule.
Flexible molecules will be able to explore a wider range of conformations than their rigid counterparts. Our MD calculations for free molecules (and small groups of them) confirm that this is so. What seems probable to us is that the actuation of the receptor involves some of the rare conformations of the odorant that become possible for a flexible molecule, i.e. fluctuations from the average shape are important. This would be analogous to other situations. Thus, Luchinsky et al. (1998) noted that large, though infrequent, fluctuations are often the cause of important changes in many systems, for example ‘nucleation at phase transitions, chemical reactions, mutations in DNA sequences, protein transport in biological cells and failures of electronic devices’. Furthermore, as noted, the fluctuations associated with flexibility may act as promoting modes that enhance the electron tunnelling (Stoneham 1981).
It seems likely that constrained fluctuations within the chiral receptor are critical. We have rather little information on the receptor structure at the appropriate level of detail. However, we can ask about the likely way the olfactant will be tethered to the receptor, since this will limit some of the motions. If the left- and right-handed molecules do indeed enter the same receptors, then, when there, the type 1 odorants must ‘look’ the same. As discussed above, our particular concern is whether or not the two members of the enantiomer pair are superimposable or not, placing the active sites on the odorants in a precise way relative to the receptor.
If the olfactant is only bound to the receptor at a single point, then the molecule will have quite a lot of freedom to adjust its position and orientation. There is a significant possibility that the two members of the enantiomer pair will be superimposable, in the sense just mentioned. Rigidity assists superimposability (for flexible molecules left- and right-handed forms might be deformed differently), and having only one site makes it insensitive to handedness. However, if the olfactant is bound at two or more points, then it becomes more likely that the two members will not be superimposable. Many of the type 2 olfactants have electronegative groups placed so as to make two-point binding likely. Thus, conformational analysis must be considered in olfactory modelling because it appears that the OR is extremely sensitive to the state of the molecule it is detecting.
Our analysis supports the general swipe card picture of receptor actuation, in that actuation involves processes beyond the odorant having a good enough shape. It is surprising that molecular flexibility proves so crucial. The most probable reasons, we feel, suggest that fluctuations of molecular shape, not just the average shape, are very important.
Acknowledgments
We gratefully acknowledge illuminating discussions with Luca Turin, William Motherwell and Simon Gane, and the support to J.C.B. and A.P.H. by the EPSRC through the IRC in Nanotechnology. We would like to thank Dr Charles Sell for some stimulating comments. J.C.B. would also like to thank Chris Howard for much help with the calculations in conformational analysis.
References
- Allan M, Craig A.C, McCarty L.V. Vibrational excitation of cis- and trans-1,2-difluoroethenes by electron impact; effect of dipole moment on the threshold peaks. J. Phys. B: At. Mol. Opt. Phys. 2002;35:523–532. doi: 10.1088/0953-4075/35/3/307. [DOI] [Google Scholar]
- Bentley R. Diastereoisomerism, contact points, and chiral selectivity: a four-site saga. Arch. Biochem. Biophys. 2003;414:1–12. doi: 10.1016/S0003-9861(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Bentley R. The nose as a stereochemist. Enantiomers and odor. Chem. Rev. 2006;106:4099–4112. doi: 10.1021/cr050049t. [DOI] [PubMed] [Google Scholar]
- Breer H. Olfactory receptors: molecular basis for recognition and discrimination of odors. Anal. Bioanal. Chem. 2003;377:427–433. doi: 10.1007/s00216-003-2113-9. [DOI] [PubMed] [Google Scholar]
- Brookes J.C, Hartoutsiou F, Horsfield A.P, Stoneham A.M. Could humans recognize odor by phonon assisted tunneling? Phys. Rev. Lett. 2007;98:038 101. doi: 10.1103/PhysRevLett.98.038101. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multi-gene family may encode odorant receptors. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- Day P.W, et al. A monoclonal antibody for G-protein-coupled receptor crystallography. Nat. Methods. 2007;4:927–929. doi: 10.1038/nmeth1112. [DOI] [PubMed] [Google Scholar]
- Dixon D.A, Komornicki A. Ab initio conformational analysis of cyclohexane. J. Phys. Chem. 1990;94:5630–5636. doi: 10.1021/j100377a041. [DOI] [Google Scholar]
- Eliel E.L, Wilen S.H. Wiley; New York, NY: 1993. Stereochemistry of organic compounds. [Google Scholar]
- Galigniana M.D, Pilipuk G.P, Kanelakis K.C, Burton G, Lantos C.P. Molecular mechanism of activation and nuclear translocation of the mineralcorticoid receptor upon binding of pregnanesteroids. Mol. Cell. Endocrinol. 2004;217:167–179. doi: 10.1016/j.mce.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Juaristi, E. (ed.) 1995 Conformational behavior of six-membered rings, analysis, dynamics, and stereoelectronic effects. New York, NY: VCH Publishers.
- Lai C.P, Singer M.S, Crasto C.J. Structural activation pathways from dynamic olfactory receptor–odorant interactions. Chem. Senses. 2005;30:781–792. doi: 10.1093/chemse/bji070. [DOI] [PubMed] [Google Scholar]
- Laska M, Genzel D, Wieser A. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performances with enantiomers. Chem. Senses. 2005;30:171–175. doi: 10.1093/chemse/bji013. [DOI] [PubMed] [Google Scholar]
- Luchinsky D.G, McClintock P.V.E, Dykman M.I. Analogue studies of nonlinear systems. Rep. Prog. Phys. 1998;61:889–997. doi: 10.1088/0034-4885/61/8/001. [DOI] [Google Scholar]
- Peng C, Ayala P.Y, Schlegel H.B, Frisch M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996;17:49–56. doi: 10.1002/(SICI)1096-987X(19960115)17:1%3C49::AID-JCC5%3E3.0.CO;2-0. [DOI] [Google Scholar]
- Sell C.S. On the unpredictability of odor. Angew. Chem. Int. Ed. 2006;45:6254–6261. doi: 10.1002/anie.200600782. [DOI] [PubMed] [Google Scholar]
- Simoes de Souza F.M, Antunes G. Biophysics of olfaction. Rep. Prog. Phys. 2007;70:451–491. doi: 10.1088/0034-4885/70/3/R04. [DOI] [Google Scholar]
- Stojanovic A, Stitham J, Hwa J. Critical role of transmembrane segment zinc binding in the structure and function of rhodopsin. J. Biol. Chem. 2004;279:35 932–35 941. doi: 10.1074/jbc.M403821200. [DOI] [PubMed] [Google Scholar]
- Stoneham A.M. Non-radiative transitions in semiconductors. Rep. Prog. Phys. 1981;44:1251–1295. doi: 10.1088/0034-4885/44/12/001. [DOI] [Google Scholar]
- Theimer E.T, Yoshida T, Klaiber E.M. Olfaction and molecular shape. Chirality as a requisite for odor. J. Agric. Food Chem. 1977;25:1168–1177. doi: 10.1021/jf60213a029. [DOI] [PubMed] [Google Scholar]
- Thompson, M. A. 2004 ArgusLab, v. 4.0.1. Seattle, WA: Planaria Software, LCC. See http://www.arguslab.com.
- Turin L. A spectroscopic mechanism for primary olfactory reception. Chem. Senses. 1996;21:773–791. doi: 10.1093/chemse/21.6.773. [DOI] [PubMed] [Google Scholar]
- Wilson D.A, Stevenson R.J. The Johns Hopkins University Press; Baltimore, MD: 2006. Learning to smell. Olfactory perception from neurobiology to behavior. [Google Scholar]