Summary
Population-based plasmacytoma incidence and survival data are sparse. We analyzed incidence rates (IRs), IR ratios (IRRs), and 5-year relative survival for plasmacytoma overall and by site – bone (P-bone) and extramedullary (P-extramedullary) – in the Surveillance, Epidemiology and End Results (SEER) Program (1992−2004). For comparison, we included cases of multiple myeloma (MM) diagnosed over the same time period in SEER. Incidence of MM (n=23,544; IR 5.35/100,000 person-years) was 16-times higher than plasmacytoma overall (n=1,543; IR=0.34), and incidence of P-bone was 40% higher than P-extramedullary (p<0.0001). The male-to-female IRRs for P-bone, P-extramedullary, and MM were 2.0, 2.6, and 1.5, respectively. For plasmacytoma and MM, IRs were highest in Blacks, intermediate in Whites, and lowest in Asian/Pacific Islanders. Compared with Whites, the Black IR was ∼30% higher for P-extramedullary and P-bone and 120% higher for MM. IRs for all neoplasms increased exponentially with advancing age, less prominently at older ages for plasmacytoma than MM. Distinct age, gender, and race incidence patterns of plasma cell disorders suggest underlying differences in clinical detection, susceptibility, disease biology and/or aetiologic heterogeneity. Five-year relative survival for P-bone, P-extramedullary, and MM varied significantly by age (<60/60+ years), supporting age-related differences in disease burden at presentation, disease biology, and/or treatment approaches.
Plasmacytomas are clonal proliferations of plasma cells that are cytologically and immunophenotypically identical to plasma cell myeloma but manifest a localized osseous or extraosseous growth pattern (Jaffe, et al 2001). Solitary plasmacytoma of bone (P-bone) and solitary extramedullary plasmacytoma (P-extramedullary) are rare diseases, and our understanding of their epidemiologic features and clinical outcomes are largely derived from compilations of cases reported in the literature and small clinical series (Alexiou, et al 1999, Bataille and Sany 1981, Bolek, et al 1996, Brinch, et al 1990, Chak, et al 1987, Chao, et al 2005, Dingli, et al 2006, Frassica, et al 1989, Galieni, et al 1995, Galieni, et al 2000, Holland, et al 1992, Jackson and Scarffe 1990, Knobel, et al 2006, Knowling, et al 1983, Liebross, et al 1998, Liebross, et al 1999, Ozsahin, et al 2006, Shih, et al 1995, Wiltshaw 1976). Among the larger series of P-bone to date, approximately 100−200 cases diagnosed over a period of 25 years or more have been included (Bataille and Sany 1981, Dingli, et al 2006, Knobel, et al 2006, Ozsahin, et al 2006). Based on cases reported in the literature between 1905 and 1995, the most extensive series of P-extramedullary included nearly 900 patients (Alexiou, et al 1999). However, population-based data on plasmacytomas are scant, with reports of incidence (Pahor 1977) and survival (Pahor 1977, Strojan, et al 2002) based on fewer than 50 cases. Furthermore, some descriptive studies have considered plasmacytoma and multiple myeloma (MM) in combination, thus making it impossible to disentangle potential differences in disease patterns (Gebregziabher, et al 2006).
To further our understanding of the epidemiologic features of plasmacytoma overall and according to primary site, we used data from the population-based cancer registries of the Surveillance, Epidemiology and End Results (SEER) Program in the United States to evaluate incidence and survival patterns among more than 1,500 patients diagnosed with plasmacytoma. As a point of reference, we analyzed over 23,000 cases of MM diagnosed in SEER over the same time period.
Methods
We included all cases of plasmacytoma and MM diagnosed among residents of 12 SEER population-based cancer registries (SEER-12) during 1992−2004 (SEER-12 2007). SEER-12 includes the states of Connecticut, Hawaii, Iowa, New Mexico and Utah, and the areas of Detroit, MI; San Francisco, Los Angeles, and San Jose-Monterey, CA; Seattle-Puget Sound, WA; Atlanta and rural GA. These geographic areas include approximately 14% of the U.S. population.
The SEER Program currently classifies histology and topography information according to the International Classification of Diseases for Oncology (ICD-O), 3rd edition (ICD-O-3) (Fritz, et al 2000). Prior to the 2nd edition of ICD-O (Percy, et al 1990), plasmacytoma, not otherwise specified (NOS) (ICD-O-3 morphology code, M-9731) was not considered malignant (behavior code /1) and thus was not reportable to the SEER Program. In the ICD-O 2nd edition, which was first used in the SEER Program for classifying cases diagnosed during 1992, plasmacytoma, NOS, was reclassified as a malignant disease (behavior code /3). In the ICD-O-3, first used for SEER cases diagnosed during 2001, a code specific for P-extramedullary (M-9734/3) was added. Prior to 2001, the histology code for plasmacytoma, NOS (M-9731/3) included P-bone and P-extramedullary. In contrast to plasmacytoma, MM (M-9732 /3) has been considered a malignancy for decades.
Diagnostic criteria for plasmacytoma have varied over time. Recent guidelines from the International Myeloma Working Group (International Myeloma Working Group, (2003) and the Guidelines Working Group of the United Kingdom Myeloma Forum (Soutar, et al 2004) have suggested that the P-extramedullary should be associated with a normal bone marrow (International Myeloma Working Group, (2003, Soutar, et al 2004) and that P-bone should have a bone marrow that is normal (Soutar, et al 2004) or not consistent with MM (International Myeloma Working Group, (2003). Prior to these guidelines, diagnostic criteria for threshold of bone marrow plasmacytosis varied, including up to 10% bone marrow plasmacytosis (International Myeloma Working Group, (2003, Dimopoulos, et al 2000). In contrast, the minimum diagnostic threshold for MM has consistently included bone marrow plasmacytosis of ≥10% (International Myeloma Working Group, (2003, Kyle 1992).
We included all cases of MM (ICD-O-3 morphology code M-9732) and all solitary plasmacytoma (M-9731 and M-9734) with malignant behavior code (/3) (Fritz, et al 2000). Because our study largely covers the pre-guideline calendar years for plasmacytoma, it is not surprising that some cases of plasmacytoma were coded to bone marrow as the primary site. However, given that plasmacytomas with bone marrow involvement may also represent misclassified MM, we considered all cases of plasmacytoma coded to haematopoietic and reticuloendothelial systems (C42, n=401) and unspecified sites (C76 and C80, n=10) in a separate category entitled “P-unspecified.” We assessed plasmacytoma overall (all topography codes) and according to primary site: bone (P-bone, topography codes C40−41), extramedullary (P-extramedullary, all topography codes excluding C40−42, C76, and C80), and P-unspecified. We further categorized P-bone and P-extramedullary according to subsite, including appendicular (C40) and axial (C41) skeleton, mouth and pharynx (C00-C14), respiratory tract (C30−39), and other sites.
Incidence
We calculated incidence rates (IRs), IR ratios (IRRs), and associated 95% confidence intervals (CIs) in SEER-12 using the Rate Session in SEER*Stat (version 6.3.6). All IRs were age-adjusted to the 2000 U.S. standard population and expressed per 100,000 person-years. Rates for plasmacytoma and MM were calculated according to sex (females, males), race (Whites, Blacks, Asian/Pacific Islanders (APIs), and other/unspecified), age group (<60, 60+ years), calendar year (1992−1997, 1998−2004), and primary site (P-bone, P-extramedullary, P-unspecified). Age-specific IRs (<35, 35−44, 45−54, 55−64, 65−74, 75+ years) were plotted using log-linear scales as previously described (Devesa, et al 1995).
Survival
Using the Survival Session in SEER*Stat, we used the actuarial method to estimate the relative survival and 95% CI of patients diagnosed with plasmacytoma or MM. We considered only cases diagnosed among individuals of known age in the 12 SEER registries during 1992−2003 that were actively followed for vital status through 2004. Among 23,047 cases of plasmacytoma and MM eligible for study, we excluded cases of second or later primary cancers (n=3,306), those diagnosed by death certificate or autopsy (n=408), those with unknown survival time (n=21), and those with unspecified race (n=127). Relative survival is defined as the ratio of the proportion of observed survivors in a cohort of patients to the proportion of expected survivors in a comparable cohort of the general population (Ries, et al 2007). As detailed in a recent SEER-based survival monograph, for certain racial-ethnic groups, the life table calculations providing the expected survival are not available (Ries, et al 2007). Therefore, we limited relative survival rates by race to Whites and Blacks. Comparison of relative survival among two or more groups were based on methods by Brown (Brown 1983). Statistical significance was considered at the 0.05 level (two-sided).
Results
During 1992−2004, 1,543 plasmacytoma and 23,544 MM cases were diagnosed among residents of the 12 SEER areas (Table I). MM was 16 times more frequent than plasmacytoma (IRs 5.35 and 0.34 per 100,000 person-years, respectively), and incidence of P-bone was approximately 40% higher than P-extramedullary (IRR 1.41, 95% CI 1.25−1.59). Rates for plasmacytoma and MM were significantly higher among males than females: male-to-female IRR 2.14 for plasmacytoma and 1.49 for MM; and somewhat higher for P-extramedullary than for P-bone (male-to-female IRR 2.64 and 2.04, respectively). Incidence of P-extramedullary was lower than P-bone among females (IRR 0.60, 95% CI 0.48−0.73) and males (IRR 0.77, 95% CI 0.66−0.90). Significantly higher IRs were observed among Blacks compared to Whites, but the difference was less striking for plasmacytoma (Black-to-White IRR 1.30, 95% CI 1.10−1.53) than for MM (IRR 2.23, 95% CI 2.15−2.31). Among APIs, IRs for plasmacytoma and MM were significantly lower than among Whites or Blacks. Whereas the Black-to-White IRR was similar for P-bone and P-extramedullary (1.25 and 1.32, respectively), based on small numbers, APIs had significantly lower IRs of P-bone than Whites but not of P-extramedullary. In addition, P-bone predominated among Whites and Blacks (P-extramedullary-to-P-bone IRR 0.68, 95% CI 0.59−0.78 and IRR 0.72, 95% CI 0.49−1.04, respectively), but the converse was observed for APIs (P-extramedullary-to-P-bone IRR 1.57, 95% CI 0.93−2.70).
Table I.
Age-adjusted plasmacytoma and multiple myeloma incidence rates and incidence rate ratios according to gender, race, calendar period, and age group, SEER-12, 1992−2004*
|
Plasmacytoma |
Multiple myeloma |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Total |
Extramedullary |
Bone |
Unspecified |
Total |
|||||||||||
|
Median age at diagnosis (range) |
64 years (16−95 years) |
62 years (20−95 years) |
65 years (16−95 years) |
68 years (20−95 years) |
71 years (16−104 years) |
||||||||||
| Characteristic | No. | IR | IRR (95% CI) | No. | IR | IRR (95% CI) | No. | IR | IRR (95% CI) | No. | IR | IRR (95% CI) | No. | IR | IRR (95% CI) |
| Total | 1,543 | 0.34 | NA | 474 | 0.10 | NA | 658 | 0.15 | NA | 411 | 0.09 | NA | 23,544 | 5.35 | NA |
| Gender | |||||||||||||||
| Females | 561 | 0.23 | 1.00 (reference) | 148 | 0.06 | 1.00 (reference) | 247 | 0.10 | 1.00 (reference) | 166 | 0.07 | 1.00 (reference) | 11,136 | 4.44 | 1.00 (reference) |
| Males | 982 | 0.49 | 2.14 (1.93−2.38) | 326 | 0.16 | 2.64 (2.16−3.23) | 411 | 0.20 | 2.04 (1.74−2.40) | 245 | 0.13 | 1.86 (1.52−2.28) | 12,408 | 6.60 | 1.49 (1.45−1.52) |
| Race | |||||||||||||||
| Whites | 1,258 | 0.35 | 1.00 (reference) | 375 | 0.10 | 1.00 (reference) | 545 | 0.15 | 1.00 (reference) | 338 | 0.09 | 1.00 (reference) | 17,876 | 4.98 | 1.00 (reference) |
| Blacks | 179 | 0.45 | 1.30 (1.10−1.53) | 54 | 0.14 | 1.32 (0.97−1.76) | 75 | 0.19 | 1.25 (0.96−1.59) | 50 | 0.13 | 1.37 (0.99−1.85) | 4,032 | 11.11 | 2.23 (2.15−2.31) |
| Asian/Pacific Islanders | 84 | 0.19 | 0.54 (0.43−0.68) | 41 | 0.09 | 0.88 (0.62−1.22) | 26 | 0.06 | 0.38 (0.24−0.56) | 17 | 0.04 | 0.43 (0.24−0.70) | 1,361 | 3.21 | 0.64 (0.61−0.68) |
| Other/unspecified | 22 | ~ | ~ | 4 | ~ | ~ | 12 | ~ | ~ | 6 | ~ | ~ | 275 | ~ | ~ |
| Calendar period | |||||||||||||||
| 1992−1998 | 750 | 0.33 | 1.00 (reference) | 214 | 0.09 | 1.00 (reference) | 290 | 0.13 | 1.00 (reference) | 246 | 0.11 | 1.00 (reference) | 10,417 | 5.45 | 1.00 (reference) |
| 1999−2004 | 793 | 0.36 | 1.10 (1.00−1.22) | 260 | 0.12 | 1.28 (1.06−1.54) | 368 | 0.17 | 1.32 (1.13−1.55) | 165 | 0.08 | 0.69 (0.57−0.85) | 13,127 | 5.27 | 0.97 (0.94−0.99) |
| Age (years) | |||||||||||||||
| <60 | 576 | 0.15 | 1.00 (reference) | 215 | 0.05 | 1.00 (reference) | 241 | 0.06 | 1.00 (reference) | 120 | 0.03 | 1.00 (reference) | 5,232 | 1.35 | 1.00 (reference) |
| 60+ | 967 | 1.34 | 9.20 (8.29−10.22) | 259 | 0.36 | 6.70 (5.57−8.07) | 417 | 0.58 | 9.40 (8.00−11.06) | 291 | 0.41 | 13.14 (10.59−16.40) | 18,312 | 25.51 | 18.84 (18.27−19.43) |
Abbreviations: No., number of cases; IR, incidence rate; IRR, IR ratio; CI, confidence interval; NA, not applicable
incidence rates not calculated for other/unspecified race.
Incidence rates are age-adjusted to the 2000 US standard population and expressed per 100,000 person-years. Incidence rate ratios are based on unrounded rates.
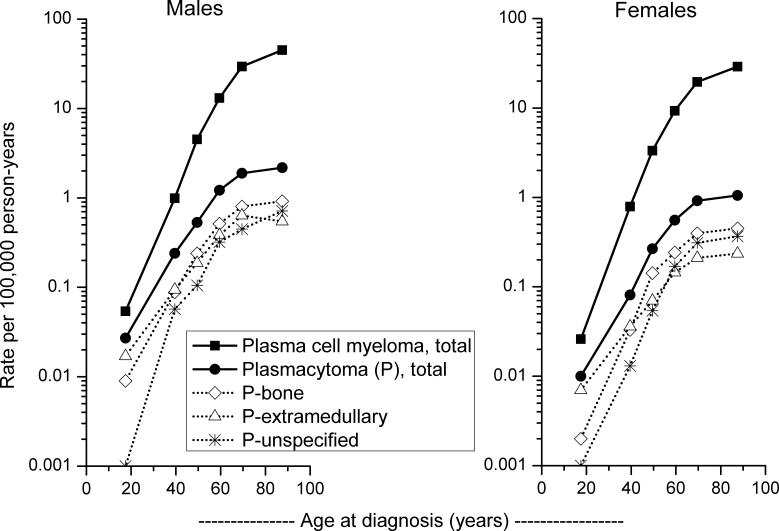
Total plasmacytoma incidence increased by 10% in 1999−2004 relative to the 1992−1998 calendar period, whereas IRs of MM decreased slightly (3%). Compared to individuals <60 years of age at diagnosis, IRs of plasmacytoma were 9-fold higher among those 60+ years of age, whereas MM IRs were nearly 19-fold higher in the older compared to the younger age group. Among men and women, an exponential rise in incidence with age was observed for MM, with IRs rising less steeply at older ages for plasmacytoma overall as well as P-bone and P-extramedullary (Fig 1). About one-quarter of plasmacytomas were not specified as bone or extramedullary; the IRR patterns of P-unspecified were intermediate to those of the specified plasmacytomas and those of MM.
Fig 1.
Age-specific plasmacytoma and multiple myeloma incidence rates according to gender, SEER-12, 1992−2004.
For all individuals with plasma cell disorders, 5-year survival was most favourable among the younger age group (<60 years) and poorest among the older age group (60+ years), decreasing from 78.9% to 70.5% for P-extramedullary (p=0.023), from 76.8% to 53.3% for P-bone (p<0.0001), from 72.1% to 40.1% for P-unspecified (p<0.0001), and from 43.0% to 25.9% for MM (p<0.0001) (Table II). For both age groups, there was no significant difference in survival between between males and females or Whites and Blacks for plasmacytoma overall, P-extramedullary, and P-bone. From 1992−1997 to 1998−2003, survival increased significantly only among patients <60 years with MM (p<0.0001).
Table II.
Five-year relative survival among patients diagnosed with plasmacytoma or multiple myeloma according to age group, gender, race, and calendar period, SEER-12, 1992−2004*
|
Plasmacytoma |
Multiple myeloma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Total |
Extramedullary |
Bone |
Unspecified |
Total |
||||||
| |
No. |
RS (95% CI) |
No. |
RS (95% CI) |
No. |
RS (95% CI) |
No. |
RS (95% CI) |
No. |
RS (95% CI) |
|
<60 years at diagnosis | ||||||||||
| Total | 479 | 76.6 (72.1−81.1) | 182 | 78.9 (71.6−86.2) | 199 | 76.8 (69.9−83.7) | 98 | 72.1 (62.1−82.1) | 4,477 | 43.0 (41.2−44.8) |
| Gender | ||||||||||
| Females | 139 | 72.0 (63.2−80.8) | 49 | 85.9 (73.7−98.1) | 60 | 72.8 (59.9−85.7) | 30 | 46.2 (23.1−69.3) | 1,876 | 42.8 (40.3−45.3) |
| Males | 340 | 78.5 (73.4−83.6) | 133 | 76.4 (67.8−85.0) | 139 | 78.7 (70.7−86.7) | 68 | 81.9 (71.1−92.7) | 2,601 | 43.1 (40.9−45.3) |
| Race | ||||||||||
| Whites | 390 | 77.1 (72.2−82.0) | 152 | 77.5 (69.3−85.7) | 161 | 78.1 (70.5−85.7) | 77 | 73.7 (62.5−84.9) | 3,119 | 43.6 (41.6−45.6) |
| Blacks | 53 | 66.4 (52.1−80.7) | 15 | 73.9 (48.6−99.2) | 24 | 64.3 (42.3−86.3) | 14 | 58.5 (31.1−85.9) | 1,046 | 39.4 (35.9−42.9) |
| Calendar period | ||||||||||
| 1992−1997 | 206 | 76.4 (70.1−82.7) | 73 | 78.5 (68.5−88.5) | 82 | 77.4 (67.8−87.0) | 51 | 71.3 (57.9−84.6) | 2,036 | 39.4 (37.2−41.6) |
| 1998−2003 |
273 |
75.5 (68.4−82.6) |
109 |
77.7 (66.5−88.9) |
117 |
74.6 (63.8−85.4) |
47 |
73.1 (56.2−90.0) |
2,441 |
47.0 (44.3−49.7) |
|
60+ years at diagnosis | ||||||||||
| Total | 704 | 53.6 (48.5−58.7) | 183 | 70.5 (60.5−80.5) | 302 | 53.3 (45.3−61.3) | 219 | 40.1 (31.7−48.5) | 13,525 | 25.9 (24.9−26.9) |
| Gender | ||||||||||
| Females | 288 | 50.6 (42.8−58.4) | 66 | 74.2 (58.3−90.1) | 126 | 46.8 (34.6−59.0) | 96 | 37.7 (25.5−49.9) | 6,696 | 24.9 (23.5−26.3) |
| Males | 416 | 55.7 (48.8−62.6) | 117 | 67.3 (54.8−79.8) | 176 | 57.1 (46.3−67.9) | 123 | 42.0 (30.6−53.4) | 6,829 | 26.9 (25.5−28.3) |
| Race | ||||||||||
| Whites | 578 | 54.3 (48.6−60.0) | 138 | 71.3 (59.7−82.9) | 256 | 54.0 (45.2−62.8) | 184 | 41.0 (31.8−50.2) | 10,568 | 25.4 (24.2−26.6) |
| Blacks | 82 | 45.3 (31.6−59.0) | 26 | 47.6 (22.3−72.9) | 33 | 46.0 (24.2−67.8) | 23 | 40.7 (16.2−65.2) | 2,062 | 28.8 (26.3−31.3) |
| Calendar period | ||||||||||
| 1992−1997 | 330 | 51.5 (44.8−58.2) | 65 | 68.0 (52.9−83.1) | 137 | 50.1 (39.7−60.5) | 128 | 43.0 (32.6−53.4) | 6,751 | 26.1 (24.9−27.3) |
| 1998−2003 | 374 | 56.5 (48.1−64.9) | 118 | 71.7 (58.2−85.2) | 165 | 56.7 (42.8−70.6) | 91 | 34.5 (19.4−49.6) | 6,774 | 25.3 (23.7−26.9) |
Abbreviations: No., number of cases; RS, 5-year relative survival (%); CI, confidence interval.
Relative survival is based on cases diagnosed during 1992−2003 and followed through 2004.
The majority of P-bone arose in the axial skeleton (83%), with 17% arising in the appendicular skeleton (Table III). P-bone arising in the axial skeleton occurred at a slightly younger age than cases arising in the appendicular skeleton (median age 64 years compared to 69 years). Survival rates did not differ significantly for axial (64.8%) and appendicular (59.4%) P-bone (p=0.8).
Table III.
Age-adjusted plasmacytoma incidence rates and relative survival according to primary site, SEER-12, 1992−2004*
|
Incidence |
5-year relative survival |
||||||
|---|---|---|---|---|---|---|---|
| Median |
(All ages) |
||||||
| Site | No. | (%) | age (years) | IR | No. | RS | (95% CI) |
| Bone | |||||||
| Axial skeleton (C41) | 547 | (83.1) | 64 | 0.12 | 422 | 64.8 | (58.7−70.9) |
| Appendicular skeleton (C40) | 111 | (16.9) | 69 | 0.03 | 79 | 59.4 | (43.9−74.9) |
| Extramedullary | |||||||
| All respiratory (C30−39) | 133 | (28.1) | 60 | 0.03 | 109 | 83.0 | (72.8−93.2) |
| Mouth and pharynx (C00−14) | 115 | (24.3) | 62 | 0.03 | 91 | 78.2 | (66.2−90.2) |
| Connective and soft tissues (C49) | 67 | (14.1) | 65 | 0.02 | 49 | 56.7 | (37.7−75.7) |
| Eye, brain, CNS (C69−72) | 41 | (8.6) | 59 | 0.01 | 32 | 48.1 | (25.2−71.0) |
| All digestive (C15−26) | 35 | (7.4) | 64 | 0.01 | 25 | 60.3 | (38.0−82.6) |
| Skin (C44) | 26 | (5.5) | 62 | 0.01 | 21 | 92.8 | (77.5−100.0) |
| Lymph nodes (C77) | 25 | (5.3) | 58 | 0.01 | 17 | 98.0 | (83.3−100.0) |
| All other sites | 32 | (6.8) | 61 | 0.01 | 21 | 73.2 | (46.0−100.0) |
Abbreviations: No., number of cases; IR, incidence rate; RS, 5-year relative survival (%); CI, confidence interval; CNS, central nervous system.
Incidence rates are age-adjusted to the 2000 US standard population and expressed per 100,000 person-years. Relative survival is based on cases diagnosed during 1992−2003 and followed through 2004. ICD-O-3 topography codes are specified adjacent to each site category.
Nearly 30% of P-extramedullary were of respiratory origin, and 24% occurred in the mouth and pharynx. The median age at diagnosis for P-extramedullary ranged from 58 years (lymph nodes) to 65 years (connective and soft tissue). Survival rates ranged from more than 90% among patients with P-extramedullary arising in the skin or lymph nodes to 48% for those with eye/brain/central nervous system (CNS) tumours.
Discussion
Although plasmacytoma and MM are plasma cell disorders that share cytologic and immunophenotypic characteristics, their predilection to affect distinct sites suggests that differences between these entities might also exist. Furthermore, both P-bone and P-extramedullary can progress to MM, although with disparate frequencies of >75% and <30%, respectively (Soutar, et al 2004). Recent molecular studies of P-extramedullary and MM have also shown that despite cytogenetic similarities between these entities, differences also exist (Bink, et al 2008). With more than 1,500 cases diagnosed in the SEER Program during 1992−2004, in the largest population-based study of plasmacytoma reported to date, we found similarities and differences in incidence and survival patterns of plasmacytoma and MM. Prior population-based studies have not been sufficiently large to assess incidence and survival patterns of plasmacytoma according to gender, race, age, and disease site.
A larger relative male excess was observed for plasmacytoma than MM, and among plasmacytoma, male predominance was greater for P-extramedullary than P-bone. These findings are in keeping with studies from Canada and the United Kingdom that have described the three disease entities within one population (Knowling, et al 1983, Pahor 1977). Similarly, among the larger series of plasmacytoma, sex ratios of 1.5 to 2.4 have been reported for P-bone (Bataille and Sany 1981, Knobel, et al 2006, Ozsahin, et al 2006). In the largest study of P-extramedullary reported to date, a 3:1 male predominance was noted for P-extramedullary of the upper aerodigestive tract and a ratio of 1.4:1 was observed for all other P-extramedullary (Alexiou, et al 1999). Taken together these findings suggest that sex-specific differences in susceptibility to MM and plasmacytoma exist and/or that aetiologic exposures may differ among men and women.
Although based on small numbers of non-Whites, we describe incidence and survival patterns of plasmacytoma and MM within the same population according to race. High, intermediate, and low IRs of MM among Blacks, Whites and Asians, respectively, have been well documented, and differences in genetic susceptibility may contribute to these observations (Linet, et al 2005, Morton, et al 2006). Monoclonal gammopathy of undetermined significance (MGUS), a precursor of MM, has similarly been associated with a Black predominance and is characterized by a 2−3-fold greater prevalence among Blacks than Whites (Landgren, et al 2006, Landgren, et al 2007). Compared to Whites, and similar to patterns of MM, incidence of plasmacytoma was significantly higher among Blacks and significantly lower among Asians. Further racial-ethnic differences were suggested by the relatively lower incidence of P-extramedullary compared to P-bone among Whites and Blacks whereas the opposite pattern was observed among Asians. Given the limited data available on incidence of plasmacytoma by race, additional studies will be needed to confirm our findings.
Similar to MM, the incidence of plasmacytoma increased with age, although more markedly for MM than plasmacytoma. The slowing in rise in incidence of plasmacytoma at older ages may account for the generally younger mean or median age at diagnosis reported for plasmacytoma than for MM (Dimopoulos, et al 2000, Knowling, et al 1983, Pahor 1977, Soutar, et al 2004). Among studies comparing age at diagnosis of P-bone and P-extramedullary, results have varied (Knowling, et al 1983, Pahor 1977, Soutar, et al 2004). Although we did not detect a significant difference in incidence between P-bone and P-extramedullary prior to age 60 years, at older ages incidence of P-bone significantly exceeded that of P-extramedullary. These findings raise the possibility of aetiologic heterogeneity, and/or age-related differences in susceptibility in P-bone, P-extramedullary, and MM. Alternatively, differential detection of disease may exist, with skeletal abnormalities of P-bone possibly being detected more readily than extraosseous lesions of P-extramedullary at older ages. In addition, under-diagnosis of plasmacytoma compared to MM may exist given that MM is often associated with systemic abnormalities (i.e., anaemia, renal failure, hypercalcaemia) that would precipitate further diagnostic evaluation, whereas plasmacytoma is not.
Incidence of total plasmacytoma increased and MM decreased from 1992−1998 to 1999−2004, although the changes were small. P-bone and P-extramedullary IRs increased 0.03 and 0.04, respectively, coincident with a decline of 0.03 in P-unspecified, suggesting that part of the observed increases in P-bone and P-extramedullary may have been due to improved specificity of primary site. Additional years of data collection will be necessary to further assess temporal patterns.
Significant differences in survival were observed for individuals with P-bone, P-extramedullary, and MM for individuals diagnosed prior to age 60 years compared to those diagnosed at older ages. Younger age has been found to be a favourable prognostic feature in clinical and population-based studies of MM (Brenner, et al 2008, Kristinsson, et al 2007, Kumar, et al 2007, Ludwig, et al 2008) and plasmacytoma (Knobel, et al 2006, Ozsahin, et al 2006). Among the younger and older age groups in the SEER Program, survival was superior for plasmacytoma than for MM. More favourable overall survival for P-extramedullary than P-bone has been reported in a multi-institutional study of plasmacytoma (Ozsahin, et al 2006), and based on small numbers of cases, other studies have described longer overall and median survival for P-extramedullary than P-bone (Galieni, et al 1995, Knowling, et al 1983).
Women with MM have been reported to have longer survival than men in some (Kristinsson, et al 2007, Kumar, et al 2007), but not all (Ludwig, et al 2008), studies. In a large international study, 10-year overall survival of P-bone did not differ by gender (Knobel, et al 2006). Significant gender differences in survival of P-bone and P-extramedullary also were not evident in the SEER population.
The significant improved survival in more recent calendar years in MM at ages <60 years is consistent with other investigations and has been attributed to novel therapies and more intensive therapeutic approaches in recent years (Brenner, et al 2008, Kristinsson, et al 2007, Kumar, et al 2007). Although similar improvement in survival was not observed for plasmacytoma, this may reflect that existing therapy over the past decades (e.g., radiation therapy) has been generally effective in the management of solitary plasmacytoma, as supported by the relatively high 5-year survival rates we observed.
More favourable prognosis has been reported for P-bone of the appendicular than axial skeleton in some (Bataille and Sany 1981), but not all studies (Knobel, et al 2006). We did not discern a substantial difference in 5-year relative survival between P-bone occurring in the axial or appendicular skeleton. P-extramedullary has been associated with more favourable overall and disease-free survival than P-bone (Ozsahin, et al 2006), and smaller plasmacytoma size has been associated with more favourable survival (Knobel, et al 2006, Ozsahin, et al 2006). Although we were unable to assess size of plasmacytoma, based on small numbers, we found that 5-year relative survival for P-extramedullary varied by site, with plasmacytoma involving skin and lymph nodes having the best survival and plasmacytoma of the eye/brain/CNS having the poorest survival. These findings raise the possibility that tumours that are more amenable to clinical detection (e.g., skin) may be detected earlier (and at smaller sizes) and/or that aetiology or disease biology may differ according to primary site.
Strengths of our study include the use of population-based data that make our findings representative of and generalizable to the U.S. population, and the large number of cases that allowed us to assess incidence and survival patterns of total plasmacytoma, P-bone, P-extramedullary, and MM within the same population. A constraint of this study is that we did not undertake a pathologic review of cases nor were we able to assess extent of bone marrow or other end-organ involvement, tumour size, or specific treatment of these plasma cell neoplasms. It is possible that some cases of MM were misclassified as plasmacytoma. Nevertheless, given that we only included cases diagnosed from 1992 onward when modern diagnostic testing was widely available in the United States, including sensitive testing for monoclonal paraproteins and magnetic resonance imaging (MRI), the likelihood of misclassification is less than if cases diagnosed prior to 1992 had been included. In addition, misclassification of MM would tend to attenuate differences between MM and plasmacytoma. In fact, the patterns for P-unspecified were intermediate to those of P-extramedullary or P-bone and MM.
In summary, this is the first large-scale, population-based study to assess incidence and survival patterns of plasmacytoma, including both P-bone and P-extramedullary, according to age at diagnosis, gender, and race. The predominance among males, Blacks, and older individuals was evident for plasmacytoma and MM. Although these similarities in incidence patterns may reflect shared risk factors, varying gender and racial patterns suggest that aetiologic heterogeneity, differences in susceptibility, disease biology, and/or differential detection among these plasma cell disorders may also exist. Continued monitoring of incidence patterns will be necessary to better assess temporal patterns of P-bone and P-extramedullary. In contrast to incidence patterns, gender and racial differences in 5-year relative survival were not significant for plasmacytoma, indicating that the disease may be comparably sensitive to therapy. Alternatively, our ability to detect statistical differences may be limited by the relatively small number of cases available for evaluation. Notably, older age at diagnosis had a stark impact on survival of P-bone, P-extramedullary, and MM. Future studies that incorporate clinical, molecular, and epidemiologic data should provide further insight into the aetiology and biology of these plasma cell neoplasms and also whether these factors in turn affect or predict response to treatment and survival.
Acknowledgements
This research was supported by the Department of Veterans Affairs and the Intramural Research Program of the National Cancer Institute/National Institutes of Health.
Bibliography
- Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, Arnold W. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- Bataille R, Sany J. Solitary myeloma: clinical and prognostic features of a review of 114 cases. Cancer. 1981;48:845–851. doi: 10.1002/1097-0142(19810801)48:3<845::aid-cncr2820480330>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Bink K, Haralambieva E, Kremer M, Ott G, Beham-Schmid C, de Leval L, Peh SC, Laeng HR, Jutting U, Hutzler P, Quintanilla-Martinez L, Fend F. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica. 2008;93:623–626. doi: 10.3324/haematol.12005. [DOI] [PubMed] [Google Scholar]
- Bolek TW, Marcus RB, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys. 1996;36:329–333. doi: 10.1016/s0360-3016(96)00334-3. [DOI] [PubMed] [Google Scholar]
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- Brinch L, Hannisdal E, Abrahamsen AF, Kvaloy S, Langholm R. Extramedullary plasmacytomas and solitary plasma cell tumours of bone. Eur J Haematol. 1990;44:132–135. doi: 10.1111/j.1600-0609.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Brown CC. The statistical comparison of relative survival rates. Biometrics. 1983;39:941–948. [PubMed] [Google Scholar]
- Chak LY, Cox RS, Bostwick DG, Hoppe RT. Solitary plasmacytoma of bone: treatment, progression, and survival. J Clin Oncol. 1987;5:1811–1815. doi: 10.1200/JCO.1987.5.11.1811. [DOI] [PubMed] [Google Scholar]
- Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35:211–215. doi: 10.1111/j.1445-5994.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037–2044. [PubMed] [Google Scholar]
- Dingli D, Kyle RA, Rajkumar SV, Nowakowski GS, Larson DR, Bida JP, Gertz MA, Therneau TM, Melton LJ, 3rd, Dispenzieri A, Katzmann JA. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2006;108:1979–1983. doi: 10.1182/blood-2006-04-015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassica DA, Frassica FJ, Schray MF, Sim FH, Kyle RA. Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 1989;16:43–48. doi: 10.1016/0360-3016(89)90008-4. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. World Health Organization; Geneva (Switzerland): 2000. [Google Scholar]
- Galieni P, Cavo M, Avvisati G, Pulsoni A, Falbo R, Bonelli MA, Russo D, Petrucci MT, Bucalossi A, Tura S. Solitary plasmacytoma of bone and extramedullary plasmacytoma: two different entities? Ann Oncol. 1995;6:687–691. doi: 10.1093/oxfordjournals.annonc.a059285. [DOI] [PubMed] [Google Scholar]
- Galieni P, Cavo M, Pulsoni A, Avvisati G, Bigazzi C, Neri S, Caliceti U, Benni M, Ronconi S, Lauria F. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85:47–51. [PubMed] [Google Scholar]
- Gebregziabher M, Bernstein L, Wang Y, Cozen W. Risk patterns of multiple myeloma in Los Angeles County, 1972−1999 (United States). Cancer Causes Control. 2006;17:931–938. doi: 10.1007/s10552-006-0030-x. [DOI] [PubMed] [Google Scholar]
- Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992;69:1513–1517. doi: 10.1002/1097-0142(19920315)69:6<1513::aid-cncr2820690633>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Jackson A, Scarffe JH. Prognostic significance of osteopenia and immunoparesis at presentation in patients with solitary myeloma of bone. Eur J Cancer. 1990;26:363–371. doi: 10.1016/0277-5379(90)90235-l. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization classification of tumours: Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon: 2001. [Google Scholar]
- Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, Oner FD, Landmann C, Castelain B, Ozsahin M. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118. doi: 10.1186/1471-2407-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983;1:255–262. doi: 10.1200/JCO.1983.1.4.255. [DOI] [PubMed] [Google Scholar]
- Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25:1993–1999. doi: 10.1200/JCO.2006.09.0100. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2007 doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA. Diagnostic criteria of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:347–358. [PubMed] [Google Scholar]
- Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, Fears TR, Hoover RN, Linet MS. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, Biritwum RB, Tettey Y, Adjei AA, Larson DR, Dispenzieri A, Melton LJ, 3rd, Goldin LR, McMaster ML, Caporaso NE, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007;82:1468–1473. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:1063–1067. doi: 10.1016/s0360-3016(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol. 1999;52:245–249. doi: 10.1016/s0167-8140(99)00114-0. [DOI] [PubMed] [Google Scholar]
- Linet MS, Devesa SS, Morgan GJ. New perspectives on the epidemiology of hematological malignancies and related disorders. In: Shields PG, editor. Cancer Risk Assessment. Taylor and Francis; New York: 2005. pp. 671–716. [Google Scholar]
- Ludwig H, Durie BG, Bolejack V, Turesson I, Kyle RA, Blade J, Fonseca R, Dimopoulos M, Shimizu K, San Miguel J, Westin J, Harousseau JL, Beksac M, Boccadoro M, Palumbo A, Barlogie B, Shustik C, Cavo M, Joshua D, Attal M, Sonneveld P, Crowley J. Myeloma in patients under age 50 presents with more favorable features and shows better survival: an analysis of 10,549 patients from the International Myeloma Working Group. Blood. 2008 doi: 10.1182/blood-2007-03-081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992−2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsahin M, Tsang RW, Poortmans P, Belkacemi Y, Bolla M, Dincbas FO, Landmann C, Castelain B, Buijsen J, Curschmann J, Kadish SP, Kowalczyk A, Anacak Y, Hammer J, Nguyen TD, Studer G, Cooper R, Sengoz M, Scandolaro L, Zouhair A. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006;64:210–217. doi: 10.1016/j.ijrobp.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Pahor AL. Extramedullary plasmacytoma of the head and neck, parotid and submandibular salivary glands. J Laryngol Otol. 1977;91:241–258. doi: 10.1017/s0022215100083626. [DOI] [PubMed] [Google Scholar]
- Percy C, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology. World Health Organization; Geneva, Switzerland: 1990. [Google Scholar]
- Ries LAG, Young JL, Jr., Keel GE, Eisner MP, Lin YD, Horner M-J. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988−2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program; 2007. [Google Scholar]
- SEER-12 2007 Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 12 Regs Limited-Use, Nov 2006 Sub (1992−2004) - Linked To County Attributes - Total U.S., 1969−2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- Shih LY, Dunn P, Leung WM, Chen WJ, Wang PN. Localised plasmacytomas in Taiwan: comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer. 1995;71:128–133. doi: 10.1038/bjc.1995.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haematol. 2004;124:717–726. doi: 10.1111/j.1365-2141.2004.04834.x. [DOI] [PubMed] [Google Scholar]
- Strojan P, Soba E, Lamovec J, Munda A. Extramedullary plasmacytoma: clinical and histopathologic study. Int J Radiat Oncol Biol Phys. 2002;53:692–701. doi: 10.1016/s0360-3016(02)02780-3. [DOI] [PubMed] [Google Scholar]
- Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore) 1976;55:217–238. doi: 10.1097/00005792-197605000-00002. [DOI] [PubMed] [Google Scholar]