Abstract
Pregnant rats were flown on the NASA Space Shuttle during the early developmental period of their fetuses’ vestibular apparatus and onset of vestibular function. The authors report that prenatal spaceflight exposure shapes vestibular-mediated behavior and central morphology. Postflight testing revealed (a) delayed onset of body righting responses, (b) cardiac deceleration (bradycardia) to 70° head-up roll, (c) decreased branching of gravistatic afferent axons, but (d) no change in branching of angular acceleration receptor projections with comparable synaptogenesis of the medial vestibular nucleus in flight relative to control fetuses. Kinematic analyses of the dams’ on-orbit behavior suggest that, although the fetal otolith organs are unloaded in microgravity, the fetus’ semicircular canals receive high levels of stimulation during longitudinal rotations of the mother’s weightless body. Behaviorally derived stimulation from maternal movements may be a significant factor in studies of vestibular sensory development. Taken together, these studies provide evidence that gravity and angular acceleration shape prenatal organization and function within the mammalian vestibular system.
Keywords: microgravity, development, fetus, maternal, otoliths
Sensory deprivation during ontogenesis has proven to be a highly effective tool for investigating sensory development. Studies employing dark-rearing (Hubel & Wiesel, 1982), plugging the ears (Batkin, Groth, Watson, & Ansberry, 1970), obstructing the nares (Kucharsky & Hall, 1987), or eliminating tactile input normally supplied by the vibrissae (Woolsey & Wann, 1976) have yielded important insights into how stimulation plays an active, formative role during sensory ontogenesis. Sensory deprivation during early life produces enduring deficits in sensory functions. For example, clinical observations (Peters, Litovsky, Parkinson, & Lake, 2007; Rubinstein & Miller, 1999) have shown that proper acoustic input is critical for the development of hearing; delayed provision of a cochlear implant in children can prevent acquisition of proper understanding of spoken words. Similarly, understanding the roles of early stimulation has augmented clinical approaches in the treatment of visual impairments (Maurer, Lewis, Brent, & Levin, 1999). In contrast to other sensory systems, our understanding of neurovestibular ontogeny has progressed slowly, due, in part, to the historic difficulty of depriving young organisms of the Earth-constant stimulus of gravity. A growing number of techniques are proving their utility for depriving the immature mammalian vestibular system of sensory input. For example, the semicircular canals (Geisler & Gramsbergen, 1998; Geisler, IJkema-Paassen, Westerga, & Gramsbergen, 2000) of young rats have been plugged as early as the 5th postnatal day. Genetically altered mouse strains that fail to develop otoconia (tlt; tilted) may nevertheless compensate for much of this loss (Crapon de Crapona, Beisel, Nichols, & Fritzsch, 2004). Other mutants show depletions of sensory cells in both otoconia and semicircular canals (Hmx2 and Hmx3, members of the Hmx homeobox gene family), or other selective losses within the vestibular end organs (Fritzsch, Pauley, & Beisel, 2006) are beginning to yield a new understanding of the development and evolution of the vertebrate vestibular system, as its cellular and molecular bases are delineated (cf. Beisel, Wang-Lundberg, Maklad, & Fritzsch, 2005).
Relatively little work has focused on the emergence of vestibular function. Yet within the sequentially fixed, highly conserved pattern of sensory ontogenesis in birds and mammals, vestibular function is one of the first modalities to emerge (Fritzsch, 1998; Gottlieb, 1971). Onset but not final maturation of vestibular function always occurs before birth or hatching (Alberts, 1984). Thus, responses to vestibular perturbation are first observed prenatally (Gottlieb, 1971; Ronca & Alberts, 1994), after the neurovestibular circuitry is largely in place, albeit while still developing. Table 1 presents the neurodevelopmental milestones of the vestibular system in mice and rats. As evidenced by observations of prenatal responses to angular acceleration (Gottlieb, 1971; Ronca & Alberts, 1994), the neural substrate, although immature, is clearly capable of transducing vestibular input. This tenet is central to the studies described herein in which rats underwent a portion of their prenatal development in microgravity. Not only are the major milestones of vestibular morphological development occurring prenatally, the system is functioning before birth as well.
Table 1.
Milestones of Neuroanatomical and Neurobehavioral Vestibular Development in Mouse (Mus musculus) and Rat (Rattus norvegicus)
| Mouse embryonic age | Rat embryonic age | Rat gestational age | Developmental milestone |
|---|---|---|---|
| 9-13a | 11-15 (Altman & Bayer, 1980) | G11.5-15.5 | Differentiation of neurons within vestibular nuclei |
| 9.5 (Theiler, 1989; Sher, 1971) | 11.5 (Altman & Bayer, 1982) | G12 | Formation of otocyst |
| 10-12 (Ruben, 1967) | 12-14 (Altman & Bayer, 1982) | G13.5-14.5 | Formation of vestibular (otic) ganglion cells |
| 10.5 (Sher, 1971; Tello, 1931) | 13 (Ashwell & Zhang, 1998) | G13.5 | Afferent processes of vestibular ganglion cells invade the macula utricle and saccule and cristae of the semicircular canals |
| 11-12 (Fritzsch & Nichols, 1993) | 13-14a | G13.5-14.5 | Efferent nerve endings first approach hair cells |
| 13-17 (Ruben, 1967) | 15-19 (Altman & Bayer, 1980) | G15.5-19.5 | Peak hair cell mitosis in crista ampullaris, maculae of saccules, and utricles |
| 15 (Mbiene, Favre, & Sans, 1988) | 17a | G17.5 | Afferent synaptogenesis with hair cells begins |
| 18-P10 (Rusch, Lysakowski, & Eatock, 1998) | 20-P12a | G20.5-P12 | Morphological differentiation into Type I and Type II hair cells |
| P28 (Rusch, Lysakowski, & Eatock, 1998) | P30a | P30 | Fully mature morphological and physiological innervation |
Note. G = gestational age (days); P = postnatal age (days).
Data not available in either mouse or rat, so the approximate age is estimated based on data from the other species.
In the past 2 decades, we have entered a new era in the field of developmental space biology. Until recently, fetal mammals and their prenatal development had been exposed only once to the microgravity of space. In 1982, the unmanned Soviet biosatellite, Cosmos-1514, carried 10 pregnant female rats into low-Earth orbit on the 13th day of the rat’s 22-day gestation period. The mission lasted 4.5 days, about 20% of rat gestation. At satellite recovery, half of the flight dams were sacrificed and the other half were permitted to undergo parturition. Postflight analyses of fetal tissue revealed mitotic figures in cerebral cortex that were interpreted as signs of retarded cellular development and migration. Flight off-spring showed no obvious deficits in natural, postnatal behaviors involving vestibular and proprioceptive controls, but did show unusual responsiveness to angular acceleration in a rotation test (Alberts, Serova, Keefe, & Apanasenko, 1985).
Pregnant mammals were not flown again in space until NASA and National Institutes of Health jointly sponsored two “small payloads” on the Space Shuttle, which provided the data for this report. For both missions, 10 pregnant rat dams were launched during midpregnancy on either their 9th (NIH.Rodent 1) or 11th (NIH.R2) gestational day and landed near term (G20 of the rats’ 22-day pregnancy; Ronca & Alberts, 2000b). Shuttle logistics were favorable: By 2 hr postflight, we had access to live fetuses, thus minimizing likely effects of 1 - g readaptation. A second advantage over the Cosmos flight was the presence on the shuttle of crew who provided daily videorecordings of the rat mothers during spaceflight.
In this article, we present a synthesis of behavioral and neuroanatomical experiments of pregnant rat dams (Rattus norvegicus), fetuses, and neonates flown on the Space Shuttle. Neurovestibular connectivity and onset of vestibular function of the fetal rats were established within the microgravity of space. Within the inner ear, the otoconia (macule and saccule) respond to gravitational stimuli (i.e., linear acceleration), and the semicircular canals respond to velocity of head rotation (angular acceleration). Therefore, prenatal microgravity exposure would be expected to differentially stimulate these two major subdivisions of the mammalian vestibular system. We hypothesized that gestation in microgravity would deprive the developing fetuses of gravistatic input to the otoconia, yet augment their exposure to angular acceleration because of increased rotations of the mother’s weightless body in space. The latter hypothesis was predicated on the idea that microgravity exposure would change the behavioral repertoire of the pregnant dams. We predicted that the dams, free-floating within the microgravity of space, would traverse all of the walls and surfaces of their enclosure. In contrast to Earth-bound controls, we expected the flight dams to turn and rotate their bodies more frequently and in orientations that would be uncommon on Earth. The dams’ behavior is biologically meaningful because their body movements create numerous episodes of angular acceleration that can be detected by fetuses and evoke responses from them (Ronca, Lamkin, & Alberts, 1993). We predicted neurovestibular and behavioral changes in offspring secondary to the pregnant dams’ behavior in microgravity. We used inflight videorecordings of the flight dams and corresponding videos of the synchronous controls to evaluate maternally instigated sensory input to prenatal semicircular canals.
We further predicted correlated changes in function and in neuroanatomical projections from the vestibular end organs. In other words, we expected a postflight phenotype comprising reduced sensitivity to gravistatic input with a corresponding reduction in projections from gravistatic receptors and increased sensitivity to angular acceleration with increased projections from semicircular canals.
Portions of these data have been published elsewhere (e.g., Bruce & Fritzsch, 1997; Ronca & Alberts, 2000a), and additional data are reported here for the first time. Together, this corpus of work provides preliminary evidence that gravity and angular accelerations, produced by the mother’s movements, may shape prenatal organization and function within the developing mammalian vestibular system.
Materials and Method
Subjects
Subjects were pregnant Sprague-Dawley rats and their offspring (Taconic Farms, Germantown, NY) air-shipped on Gestational Day (G) 2 (where G1 = day of conception) to Kennedy Space Center (Cape Canaveral, FL). On G7, dams sustained preflight laparotomies to confirm pregnancy, then were assigned to one of three treatment conditions (n = 10 per group): (a) Flight dams were housed in groups of 5 in rodent flight caging (animal enclosure modules; AEMs) situated on the shuttle middeck, the primary area occupied by the astronauts; (b) synchronous ground control dams were housed in identical AEMs in the orbital environment simulator (OES) at Kennedy Space Center, a chamber permitting simulation of thermal and lighting characteristics onboard the shuttle without exposure to acceleration or microgravity; and (c) vivarium ground control dams were individually housed and maintained under standard colony conditions.
Flight Housing
Each AEM comprised stainless steel mesh (23.5 × 35.6 × 21.6 cm [h]; Earth-gravity orientation). Food bars [2.52 × 20 cm], fabricated from a spoilage-resistant rodent diet (Teklad, Madison, WI), were attached to the sides of the AEM. Water was provided through a centrally located, stainless steel water reservoir (21 × 11.1 × 15.2 cm) with four Lixit valves designed to avoid leakage from the water spouts. Airflow was controlled by external fans that create a ceiling-to-floor (in normal Earth-gravity orientation) nearlaminar flow; the effluent airstream moves through a waste tray containing an activated charcoal and absorbent filter. A 24- to 48-hr delay imposed in the timed breeding of control relative to flight rats allowed duplication of environmental parameters (i.e., temperature, humidity, and lighting) onboard the shuttle in the synchronous control AEMs housed in the OES via daily down-linking of critical data.
Kinematic Analyses of Pregnant Dams’ Movement in Space
To quantify the mothers’ behavioral contributions to the prenatal vestibular sensory environment, we used the inflight videos to conduct a kinematic analysis of the dams’ body movements onboard the space shuttle. We devised a self-referencing kinematic code to classify and quantify the movements made by dams in microgravity and in the Earth-gravity control condition. The movements of individual dams were encoded in relation to their posture at immediately preceding time points.
Contact and Water Immersion Righting Responses of Postnatal Rats
These data, derived from neonatal rats flown prenatally as part of the NIH.R1 experiment, are reported elsewhere (Ronca & Alberts, 2000a). Briefly, we used two methods for analyzing gravistatic function in neonatal rats following prenatal exposure to microgravity. First, we used a contact righting test in which a Postnatal Day (P) 0 pup is placed on a flat surface in the supine position. Newborn pups typically perform a “corkscrew” maneuver that enables them to achieve the prone position. Second, we used a water immersion righting test (Pellis, Pellis, & Teitelbaum, 1991) to analyze vestibular-mediated body righting responses of neonatal rats. In this test, each neonate is gently held in the supine position just below the surface of a heated (38 °C) water bath and then released. On reaching the base of the tank, pups are quickly retrieved from the water. For both tests, righting success was quantified by assigning a score of 2 for complete righting (i.e., the neonate rotated fully from supine to prone); a score of 1 if a corkscrew pattern was observed, but righting was not achieved; and a score of 0 if the animal made no attempt to right itself.
Whereas righting responses performed on a solid surface rely on a combination of vestibular, tactile, and proprioceptive cues, water immersion righting is triggered predominantly by vestibular sensory input by virtue of the relative absence of tactile and proprioceptive sensory input along the dorsum. In developing rats, contact righting responses are present at birth. In contrast, water immersion righting responses are generally absent on the day of birth, but begin to emerge on P1 and mature by P5. We therefore tested the pups’ contact righting responses on the day of birth and water immersion righting responses on P1, P3, and P5. Both types of behavioral tests were videorecorded on high speed (1/1,000 s) using a camcorder (8mm) under high illumination; during subsequent review, they were analyzed in slow motion. Rat pups’ eyes are closed until P14 -P18, and they do not exhibit cardiac responses to a light stimulus until P14 (Haroutunian & Campbell, 1981), thereby eliminating the need to control directional cues to the gravity vector arising from lighting, a measure that is vital to vestibular assessments of older subjects.
Heart Rate Responses of Rat Fetuses to Head-up Tilt
Immediately on recovery from spaceflight, prenatal rats from the NIH.R2 mission were exposed to repeated vestibular perturbations consisting of a roll stimulus (70° head-up tilt from the side-lying position). The roll stimulus is predominantly (but not purely) angular acceleration: Because of the eccentricity of the labyrinths, stimulation of gravistatic receptors as well as the semicircular canals may occur during tilt. Using established procedures (Ronca & Alberts, 1994), we tested one fetus per litter for the flight, synchronous, and vivarium conditions for a total of four dams per condition. Briefly, the dams’ lower body sensation was eliminated using spinal anesthesia via chemomyelotomy (80 μl ethyl alcohol administered via intrathecal injection at vertebral level T10-11 with an effective level of transection at about T6). The uterus was externalized into a heated (37.5 °C) bath of buffered physiological saline and a small (2-cm) incision made along the antimesometrial border. A single fetus was then gently floated into the bath and positioned laterally within a tilt apparatus (36 mm × 27 mm; see Ronca & Alberts, 1994). Figure 1 shows the fetus in the test apparatus. The fetus’ head and body were aligned horizontally with the nose directed perpendicular to the body’s axis. Care was taken to ensure that umbilical connections between fetus and dam remained intact throughout the 10-min test. EKG electrodes (40 ga teflon-insulated nickel, Pelican Wire Company, Naples, FL) were implanted subcutaneously at the xiphoid process and at the nape of the neck. EKG signals were amplified online (Grass Model P15 preamplifier coupled to a Teac Model R-61 FM data recorder). Stimulus onset was time-locked to EKG measures using a recorded signal mark. Baseline EKG was collected for 60 s, followed by a single 10-s tilt comprising head-up acceleration from 0° to 70°, then accelerating back to 0°. EKG data collection was continuous with three stimuli administered at 90-s intervals. EKG signals were subsequently amplified and recorded (Grass Model 79D polygraph). The EKG signal was digitized for measurement of interbeat intervals (ms), then converted to beats per minute.
Figure 1.
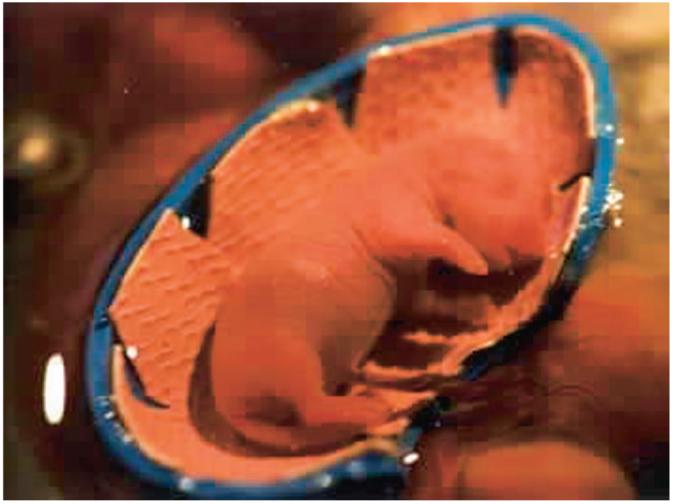
Photograph of a Gestational Day 20 rat fetus positioned in the test apparatus during the 70° head-up tilt (roll) stimulus. The fetus is externalized from the uterus into a heated bath with intact umbilical connections to the dam.
Neuroanatomical Labeling Technique
Two G20 fetuses from each rat dam were deeply anesthetized (Beauthanasia) and subsequently fixed by transcardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The heads of the fixed animals were hemisected sagittally, the ears surgically opened, and the sensory organ exposed. The tracer, DiI (1,1,-dioctadecyl-3,3,3,3,-tetramethylindocarbocyanine, perchlorate, Molecular Probes, OR), is a lipophilic dye that diffuses along the lipid bilayer of membranes and also as micelles within the cytoplasm (Bruce, Christensen, & Fritzsch, 1997). DiI was dissolved in dimethylformamide, and nylon-filter strips were soaked and then inserted into the gravistatic vestibular receptors (saccule or utricle, left side) or into one of the nongravistatic vestibular receptors (posterior vertical canal, right side) to label the sensory neurons projecting into the vestibular nuclei from the application sites.
After an appropriate diffusion period of 2-4 weeks at 37 °C, the brains were dissected from the bony capsule, embedded in 10% gelatin, and hardened in 4% paraformaldehyde. The blocks were sectioned on a vibratome at 100 μm, mounted on a slide and coverslipped, and then examined with epiflourescent microscopy. To determine whether gravistatic and angular sensory neurons formed similar types of synapses in both flight and synchronous control fetuses, DiI-labeled neurons in the medial vestibular nucleus were photoconverted and processed for transmission electron microscopy (Bruce et al., 1997).
The vestibular and cochlear epithelia were examined to verify injection sites and identify peripheral arborizations as previously reported (Maklad & Fritzsch, 2003). Numbers of synapses in the medial vestibular nuclei were quantified using electron microscopy.
Statistical Analysis
For statistical purposes, behavioral data were expressed as litter averages. Analyses of variance and t tests were performed, and Newman-Keuls post hoc tests were used.
Results
Flight Outcome
As we reported previously (Ronca & Alberts, 2002b), pre- and postflight body weights of dams and the body weights of the offspring used in these experiments were comparable across treatment groups. Within 48-72 hrs following landing, the rat dams that contributed offspring to the postnatal studies gave birth to healthy offspring.
Contact and Water immersion Righting Responses of Postnatal Rats
These data are reported in far greater detail elsewhere (Ronca & Alberts, 2000a). Results of the vestibular righting tests are shown in Table 2. The percentage of neonates that showed successful (i.e., achieved a score of 2) contact (P0) or water immersion (P1, P3, P5) righting in each condition is shown in Table 1. No group differences were observed in contact righting success. For water immersion righting, similar proportions of synchronous and vivarium neonates showed complete righting responses at each age. On P1, fewer flight neonates successfully righted themselves as compared with synchronous or vivarium control neonates, and twice as many flight as compared with synchronous neonates made no attempt to right themselves. On P3, fewer flight neonates responded (i.e., achieved a score greater than 0) as compared with synchronous and vivarium neonates. The water immersion test revealed clear deficits in righting responses among the neonatal rats that underwent prenatal development in space. By P5 (7 days following landing), flight pups righted themselves 100% of the time, matching the response level of control pups, indicating recovery of the behavioral impairments observed on P1 and P3.
Table 2.
Percentage of Postnatal Rats in the Flight, Synchronous, and Vivarium Conditions That Showed Successful Contact Righting (P0) and Water Immersion Righting (P1, P3, P5)
| Condition |
||||
|---|---|---|---|---|
| Test | Age | Flight | Synchronous | Vivarium |
| Contact righting | P0 | 53 | 45 | 52 |
| Water immersion righting | P1 | 1* | 3 | 3 |
| P3 | 60* | 90 | 90 | |
| P5 | 100 | 100 | 100 | |
Note. P = postnatal age (days).
p < .05.
Heart Rate Responses of Fetal Rats to Head-up Tilt
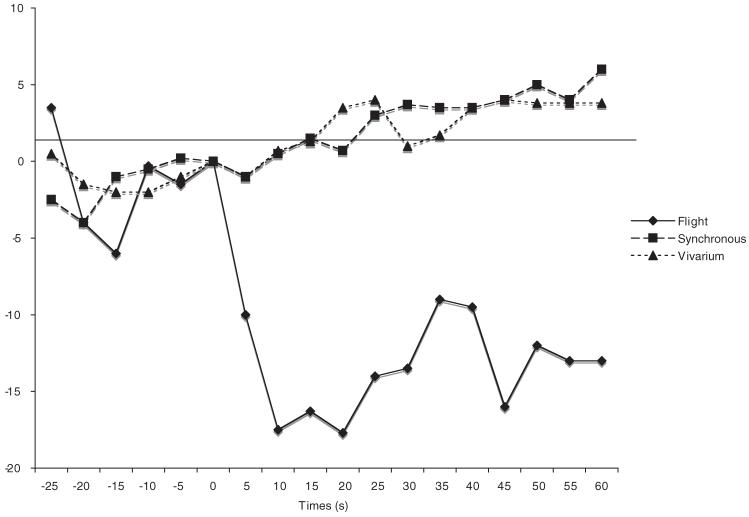
Figure 2 shows that flight fetuses responded to the first presentation of the roll stimulus with dramatic bradycardiac responses, t(3) = -5.7, p = .01. These responses were large and long lasting. In contrast, control subjects did not respond, t(5) = 0.312, ns. The first trial responses of flight fetuses were statistically different from those of control fetuses: Gravity × Trial, F(2, 16) = 6.7, p < .01; Newman Keuls, p > .05. The robust cardiac decelerations of flight fetuses closely resemble those observed in ground studies using older (G21) fetuses (Ronca & Alberts, 1990, 1994), suggesting that flight fetuses displayed more pronounced, and possibly precocial, responses to angular acceleration. Successive presentations of the roll stimulus did not elicit cardiac responses (Table 3).
Figure 2.
Average heart rate response of Gestational Day 20 fetuses in flight (n = 4) and synchronous control (n = 3) and vivarium control (n = 3) conditions to the 10-s 70° head-up tilt. Heart rate (beats/min [bpm]) is plotted as the difference from prestimulus baseline (time = 0 s). The horizontal axis (time = 0 s) corresponds to stimulus onset.
Table 3.
Resting Heart Rate (RHR) and Responses to 70° Head-up Roll (Expressed as Change From Baseline [BSL]) in Prenatal Rats Gestated During Spaceflight and 1 - g Controls
| Gravity condition |
||||||||
|---|---|---|---|---|---|---|---|---|
| Spaceflight |
1 - g Controls |
|||||||
| RHR |
Δ BSL |
RHR |
Δ BSL |
|||||
| Trial | M | SD | M | SD | M | SD | M | SD |
| 1 | 262.1 | 31.0 | -23.8a* | 8.4 | 302.1 | 31.7 | +0.9b | 3.5 |
| 2 | 260.2 | 38.9 | -0.4 | 3.1 | 305.4 | 35.8 | +1.4 | 7.5 |
| 3 | 259.7 | 34.7 | +1.1 | 8.3 | 303.3 | 40.0 | +5.3 | 12.8 |
Note. Synchronous and vivarium control animals did not differ. Their data were therefore combined for statistical analysis. Heart rate (beats per minute) was calculated for 30 s following stimulus onset minus prestimulus baseline (time = 0 s). Means with differing subscripts are different at p < .05.
Differed from prestimulus values, p < .05.
DiI Studies
DiI applications to the posterior vertical canals labeled sensory ganglion neurons that had elaborate branching patterns throughout all vestibular nuclei, as did similar applications to synchronous fetuses (n = 6). Thus, the canals projected to overlapping vestibular nuclei and vestibulocerebellar areas (Figure 3A). In contrast, the gravistatic sensory neurons of synchronous control fetuses extended more medially than did those of flight fetuses. This was particularly evident in the medial vestibular nucleus (Figure 3B). In both flight and synchronous control fetuses, labeled axons from sensory neurons of the posterior vertical canal typically had multiple, short side branches and synapse-like swellings. The same pattern of axonal growth and arborization was seen in saccular afferents of the synchronous control fetuses (Figure 4A). In contrast, DiI applications to saccular afferents in five of the six microgravity-exposed fetuses revealed that their axons were largely unbranched and generally ended in growth cones (Figure 4B), although one of the six fetuses exhibited axons with branching and arborization patterns more similar to the synchronous controls. The immature appearance of the flight fetuses’ axons suggest that microgravity during gestation may delay the development of gravistatic sensory neurons. We suggest that gravity-sensing sensory neurons of flight fetuses may have fewer or less mature synapses than ground controls.
Figure 3.
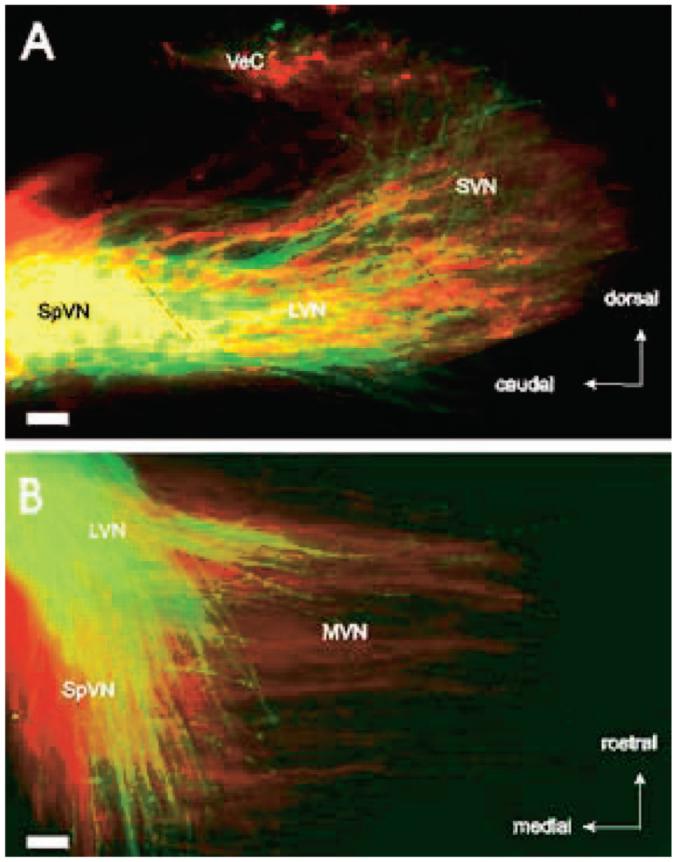
Superimposed branching patterns of posterior vertical canal (A) and saccular (B) axons in the vestibular nuclei of fetuses in flight (green) and control (red) conditions. (A) Parasagittal sections show afferent axons from the posterior vertical canal project to overlapping vestibular areas in both control and microgravity-exposed fetuses. (B) Horizontal sections demonstrate that afferent axons from the sacculus project more medially in control than microgravity-exposed fetuses. LVN = lateral vestibular nucleus; MVN = medial vestibular nucleus; SpVN = spinovestibular nucleus; SVN = superior vestibular nucleus; VeC = vestibulocerebellum. Bar = 100 μm.
Figure 4.
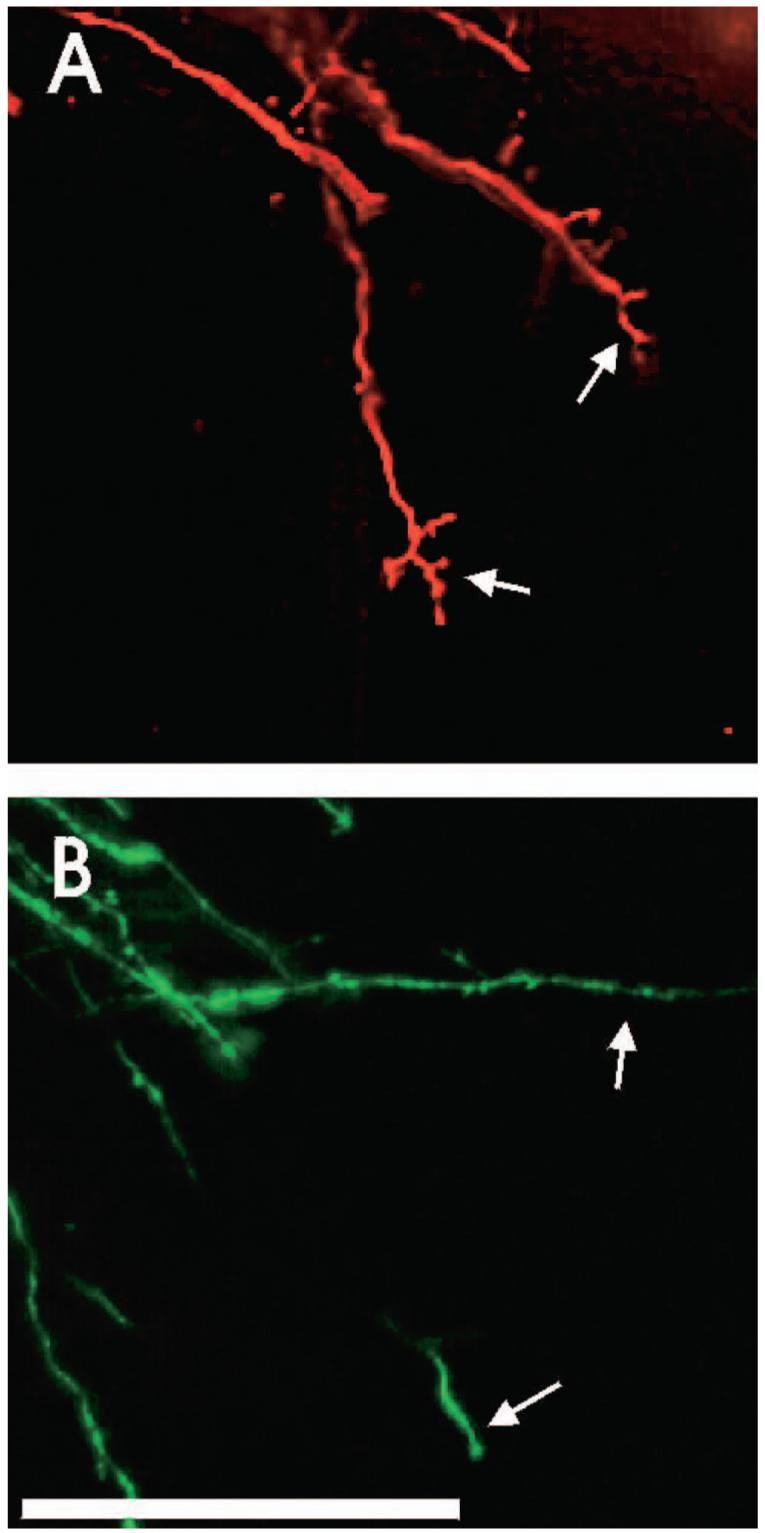
Saccular axon arbors in the medial vestibular nuclei of control animals (top) have more short side branches than those of flight animals (bottom), indicating that the development of flight gravistatic receptor afferents is delayed compared with those of controls. Bar = 100 μm.
Labeled axons with synapses consisting of synaptic vesicles, distinct synaptic clefts, apposed to neurons with postsynaptic densities were observed in posterior canal axons of both flight and control fetuses and in saccular axons of control fetuses, but were rarely observed in saccular axons of flight fetuses. Predominantly immature contacts with close appositions but no apparent synaptic clefts or postsynaptic specializations were observed in saccular axons of flight animals (Figure 5).
Figure 5.
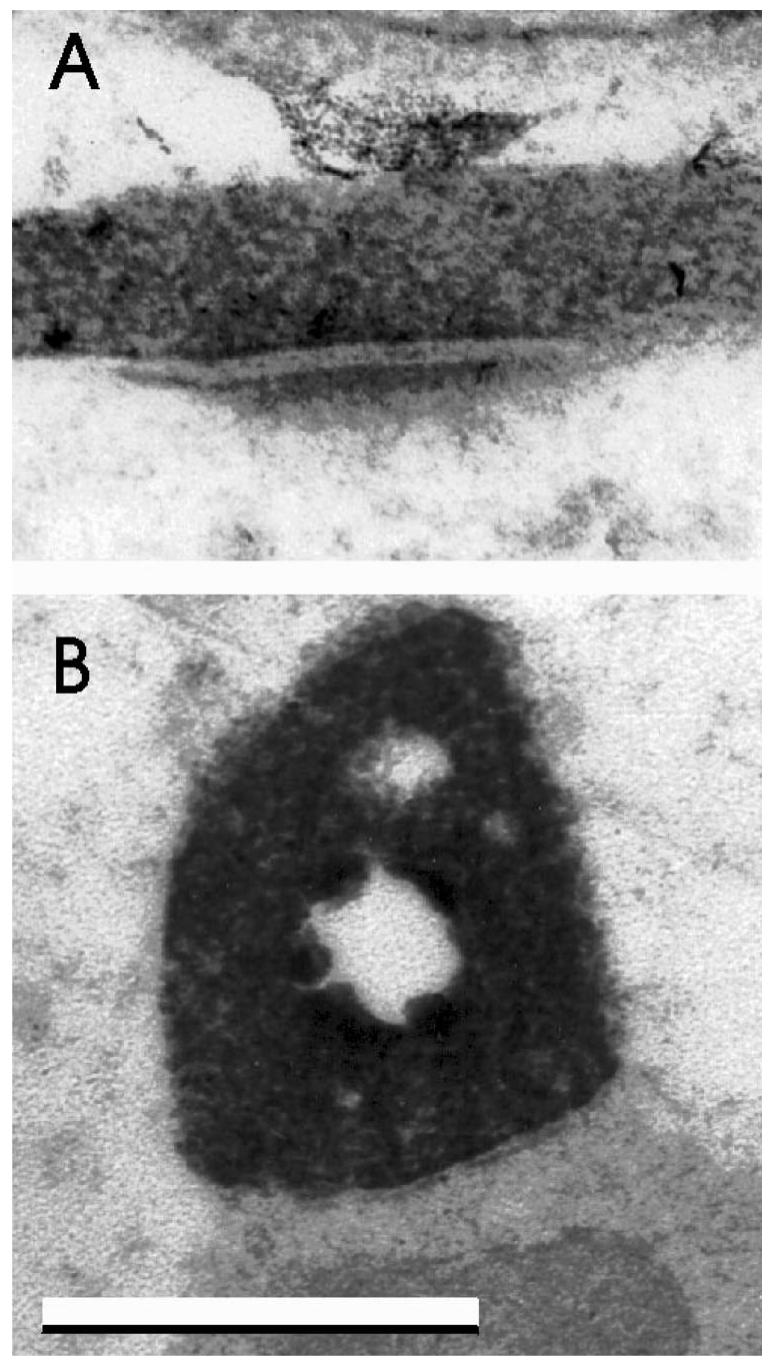
Labeled axosomatic synapses in the medial vestibular nuclei after 1,1,-dioctadecyl-3,3,3,3,-tetramethylindocarbocyanine applications to saccular (gravistatic) axons in control (top) and flight (bottom) fetuses. Labeled axons with synaptic clefts and apposed to postsynaptic specializations were present in control fetuses, but predominantly immature contacts were observed in flight fetuses. The cavity in the center of the lower synapse was caused by loss of the dense 3,3′-diaminobenzidine tetrahydrochloride reaction product during sectioning. Bar = 0.5 μm.
We performed double-blind counts of the relative number of all identifiable synapses in the medial vestibular nucleus of four flight and four synchronous control fetuses to determine whether the delayed development of fetal gravistatic neurons was associated with an overall decrease in number of synapses, or whether angular neurons (and/or other vestibular afferents) would increase their synaptic number. Only structures with a distinct synaptic cleft, post-ynaptic thickening, and at least one synaptic vesicle were counted as synapses. We found that average number of synapses/18,060 μm2 in flight animals (mean ± SD = 43.5 ± 18.5) and in synchronous control animals (mean ± SD = 39.6 ± 13.5) was not significantly different (p > .10). Thus, despite decreased numbers of gravistatic afferent synapses, the overall number of synapses in the medial vestibular nucleus did not change in flight animals compared with synchronous controls. This suggests that, in flight fetuses, other vestibular afferents, such as those transducing angular acceleration stimulation, may have compensated by increasing synaptic number.
Together, these data suggest that spaceflight conditions can delay the development of gravistatic connections compared with angular acceleration afferents. In light of the apparent neuroanatomical reorganization in these vestibular pathways, including the flight fetuses’ increased sensitivity to angular acceleration cues and the inferred increase in angular acceleration afferent synapses, we sought to identify a means by which the fetuses’ semicircular canals may have been differentially hyperstimulated in microgravity.
Kinematic Analyses of Pregnant Dams’ Movement in Space
We found that movements involving pitch and yaw were about equivalent in flight and synchronous dams. In contrast, flight dams displayed about 7 times more rolling movements than did controls (Figure 6). We believe that this dramatic difference reflected the increased numbers and orientations of surfaces available for walking and crawling in microgravity (“walls” and “ceiling” are akin to “floors” in a weightless environment). Many of the movements from surface-to-surface involved rolling movements about the dam’s longitudinal (x) axis. The fetuses residing in the mother’s body, naturally positioned in varying directions and orientations that change over time, are thus exposed to additional angular accelerations.
Figure 6.
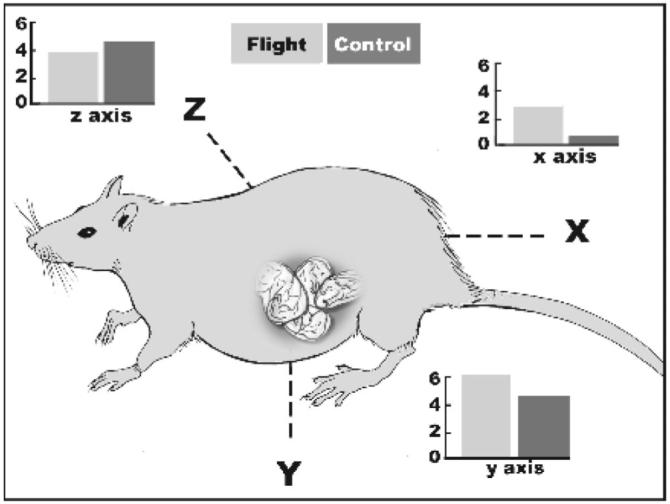
Relative rates of transitions of body movements over three vectors (x, y, z) shown by rat dams (flight and synchronous control). Numbers of changes in each vector are expressed in degrees (angle) per second (time) as coded from inflight videorecordings.
Discussion
Our findings indicate that, during spaceflight, the fetuses’ gravistatic receptors are effectively unloaded, whereas their angular acceleration detectors (the semicircular canals) are hyperstimulated by the dams’ increased rolling movements, likely resulting also in linear accelerations of otoconia as a consequence of the centrifugal movements acting on them. This contrasting distinction, to our knowledge, has been overlooked in previous analyses of spaceflight results. The present data and observations suggest that behaviorally derived stimulation may be an important variable in studies of mammalian development in altered gravity fields.
Our neuroanatomical findings indicate that, despite a reduction in gravistatic afferent synapses, the overall number of synapses in the medial vestibular nucleus did not change in flight animals compared with synchronous controls. This suggests that, in flight fetuses, other vestibular afferents, such as those transducing angular acceleration stimulation, may have compensated by increasing synaptic number.
The behavioral data provide a compelling connection between functional and neuroanatomical observations concerning early vestibular development. First, the limited stimulation of gravistatic receptors during development in microgravity, which likely reflects centrifugal acceleration of otoconia generated during the dam’s rotation, was associated with delayed postnatal emergence of behavioral responses that rely predominantly on the otoliths. We found dramatic impairments in a test involving gravistatic detection in the neonatal rats born at 1 - g following gestation in space. Reduced righting success was correlated with decreased projections from the saccule to the medial vestibular nucleus and with reduced branching of gravistatic afferent axons in five of six rat fetuses gestating in space.
Second, kinematic analyses of the weightless mothers’ behavior revealed 7 times more rolling movements in space compared with 1 - g control dams. These increased angular accelerations, delivered to the fetuses in utero, were related to the precocial emergence of bradycardiac orienting responses in the fetuses during immediate postflight tests. We had expected, but did not observe, increased branching of angular acceleration receptor projections in flight fetuses. Importantly, we did observe comparable synaptogenesis of the medial vestibular nucleus in flight relative to control fetuses, suggesting that, in flight fetuses, other vestibular afferents, possibly those transducing angular acceleration stimulation, may have compensated by increasing synaptic number. One possibility is that inferior development of linear acceleration (gravity) sensing may allow angular acceleration afferent neurons to form greater numbers of synapses in the vestibular nuclei, consequently augmenting fetuses’ bradycardiac orienting responses. In adult animals, vestibular inputs elicited by nose-up movements of the head act to rapidly increase blood pressure, a reflex mechanism that counteracts orthostatic hypotension induced by head-up rotation in quadripeds (Woodring, Rossiter, & Yates, 1997). This compensatory response to orthostatic intolerance (a response that is absent in prenatal and newborn rats) is likely to develop far differently under conditions of either increased or reduced vestibular input, possibly leading to major changes in the development of cardiovascular regulation.
In this study, fetuses were exposed to microgravity during the latter half of gestation, coincident with the earliest phases of functional and neuroanatomical vestibular development. It is astounding that, following landing on G20, the developing rats were exposed to the Earth’s gravity for a full week (in utero and postpartum) before righting impairments were no longer observed. It is also noteworthy that the flight pups showed an accelerated development of vestibular righting from P3 to P5 (Table 1), rather than a phase shift in which the achievement of asymptote was delayed by 1 or 2 days. The differentiation of neurons of the vestibular nuclei follows a latero-medial and rostro-caudal progression (Altman & Bayer, 1980; Fritzsch, Tessarollo, Coppolla, & Reichardt, 2004), and the lateral nucleus is involved in postural stabilization at birth. Postflight recovery of the righting reflex may have been accelerated rather than delayed because of the relatively advanced maturity of the underlying neural substrates and their sensitivity to sensory tuning during early postnatal period, a phase during which neurotrophins (e.g., brain-derived nerve growth factor) appear to play important roles in plasticity of afferant connections (Fritzsch, Pirvola, & Ylikoski, 1999). Collectively, these observations suggest that exposure to microgravity throughout the entirety of neurovestibular development would likely produce enduring and possibly irreversible deficits in adaptive vestibular responses with correlative changes in receptor projections and synaptogenesis within central vestibular nuclei.
Flight fetuses, but not controls, responded to the first presentation of the roll stimulus with dramatic and long-lasting bradycardia. Successive presentations of the roll stimulus did not elicit a response (Table 2), suggesting either sensory fatigue or possibly rapid habituation (Sokolov, 1963) given that habituation characteristically occurs very quickly in immature organisms (Richardson, Hayne, & Campbell, 1992). In either case, the lack of continued responding to the roll stimulus indicates that the bradycardiac response was not merely a byproduct of cardiovascular reflexes. One explanation of the bradycardiac responses in flight fetuses is that microgravity exposure during gestation alters the neuroanatomical substrate by promoting the development of neurons stimulated by angular acceleration and simultaneously delaying the development of those stimulated (on Earth) by gravistatic acceleration.
In this article, we have provided preliminary evidence that the presence of gravity during early development may be a neurobehavioral determinant of form and function in the mammalian vestibular system by coupling coordinated maturation of angular and linear acceleration systems. Yet alternate interpretations should be considered. For example, microgravity exposure could induce a high degree of stress that induces biochemical changes in the dam that indirectly affect pup development. However, we found that only vestibular righting was delayed. Pups were similar in body weight and gross morphological development, and contact righting was not impaired. It would be useful to test the hypothesis that restricted or enhanced maternal movements during prenatal life (such as that which occurs during bedrest in humans) lead to neurovestibular alterations or through manipulations mimicking the additional rotation in microgravity. This could be accomplished experimentally through environmental or surgical means, and we look forward to pursuing these hypotheses. Regardless, our present findings provide a tantalizing example of how the Earth’s gravity may have shaped neurobehavioral and physiological adaptations in mammals.
Acknowledgments
We wish to acknowledge Regina A. Abel, Michael A. Armbruster, Karen Cabell, and Cheryl Galvani for their assistance with data collection and analysis. G. G. Berntson wrote and supplied the Heart V6.0 software (©1990). NASA astronaut Joe Tanner, NASA Ames Research Center staff Sharon Yavrom, Debra Reiss-Bubenheim, Paula Dumars, Carol Elland, Dana Leonard, Nichola Hawes, and Vera Vizar, and Kennedy Space Center staff Brad Birch, William McLamb, Ramona Bober, and John Carver assisted with the experiments. We also acknowledge the comments of Gary Berntson, Richard Boyle, Golda Leonard, and Charles Wade on draft of this article.
References
- Alberts JR. Sensory-perceptual studies in the Norway rat: A view toward comparative studies. In: Kail R, Spear NS, editors. Comparative perspectives on memory development. Erlbaum; Hillsdale, NJ: 1984. pp. 65–101. [Google Scholar]
- Alberts JR, Serova LV, Keefe JR, Apanasenko Z. Early postnatal development of rats gestated during flight of Cosmos 1514. The Physiologist. 1985;28:S81–82. [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat: III. Thymidine-radiographic study of the time of origin of neurons on the vestibular and auditory nuclei of the upper medulla. Journal of Comparative Neurology. 1980;194:877–904. doi: 10.1002/cne.901940410. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Zhang LI. Prenatal development of the vestibular ganglion and vestibulocerebellar fibres in the rat. Anatomy & Embryology (Berlin) 1998;198:149–161. doi: 10.1007/s004290050173. [DOI] [PubMed] [Google Scholar]
- Batkin S, Groth H, Watson JR, Ansberry M. Effects of auditory deprivation on the development of auditory sensitivity in albino rats. EEG & Clinical Neurophysiology. 1970;28:351–359. doi: 10.1016/0013-4694(70)90227-0. [DOI] [PubMed] [Google Scholar]
- Beisel KW, Wang-Lundberg Y, Maklad A, Fritzsch B. Development and evolution of the sensory apparatus of the mammalian ear. Journal of Vestibular Research. 2005;15:225–241. [PMC free article] [PubMed] [Google Scholar]
- Bruce LL, Christensen MA, Fritzsch B. Electron microscope differentiation of directly and transneuronally transported DiI and applications for studies of synaptogenesis. Journal of Neuroscience Methods. 1997;73:107–112. doi: 10.1016/s0165-0270(96)02218-2. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Fritzsch B. The development of vestibular connections in rat embryos in microgravity. Journal of Gravitational Physiology. 1997;4:P59–62. [PubMed] [Google Scholar]
- Crapon de Caprona M-D, Beisel KW, Nichols DH, Fritzsch B. Partial behavioral compensation is revealed in balance tasked mutant mice lacking otoconia. Brain Research Bulletin. 2004;64:289–301. doi: 10.1016/j.brainresbull.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Evolution of the vestibulo-ocular system. Otolaryngology, Head & Neck Surgery. 1998;119:182–192. doi: 10.1016/S0194-5998(98)70053-1. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst. Hearing Research. 1993;65:51. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- Fritzch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neutrotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: The making of the vertebrate ear. Brain Research. 2006;26:151–171. doi: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Progress in Brain Research. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Geisler HC, Gramsbergen A. Motor development after vestibular deprivation in rats. Neuroscience & Biobehavioral Reviews. 1998;22:565–569. doi: 10.1016/s0149-7634(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Geisler HC, IJkema-Paassen J, Westerga J, Gramsbergen A. Vestibular deprivation and the development of dendrite bundles in the rat. Neural Plasticity. 2000;7:193–203. doi: 10.1155/NP.2000.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The biopsychology of development. Academic Press; New York: 1971. pp. 67–128. [Google Scholar]
- Haroutunian V, Campbell BA. Development and habituation of the heart rate orienting response to auditory and visual stimuli in the rat. Journal of Comparative and Physiological Psychology. 1981;95:166–174. doi: 10.1037/h0077756. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier Lecture: Functional architecture of the macaque monkey visual cortex. Proceedings of the Royal Society London (Biology) 1982;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Kucharsky D, Hall WG. New routes to early memories. Science. 1987 November 6;238:786–788. doi: 10.1126/science.3672125. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Research Bulletin. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Brent HP, Levin AV. Rapid improvement in the acuity of infants after visual input. Science. 1999 October 1;286:108–110. doi: 10.1126/science.286.5437.108. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Favre D, Sans A. Early innervation and differentiation of hair cells in the vestibular epithelia of mouse embryos: SEM and TEM study. Anat Embryol (Berl) 1988;177:331–340. doi: 10.1007/BF00315841. [DOI] [PubMed] [Google Scholar]
- Pellis VC, Pellis SM, Teitelbaum P. A developmental analysis of postnatal development of contact righting in rats (Rattus norvegicus) Developmental Psychobiology. 1991;24:237–267. [Google Scholar]
- Peters BR, Litovsky R, Parkinson A, Lake J. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otology & Neurotology. 2007;28:649–657. doi: 10.1097/01.mao.0000281807.89938.60. [DOI] [PubMed] [Google Scholar]
- Richardson R, Hayne H, Campbell BA. The orienting response as a measure of attention and information processing in the developing rat. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. pp. 113–136. [Google Scholar]
- Ronca AE, Alberts JR. Heart rate development and sensoryevoked cardiac responses in perinatal rats. Physiology & Behavior. 1990;47:1075–1082. doi: 10.1016/0031-9384(90)90355-8. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Lamkin CA, Alberts JR. Maternal contributions to sensory experience in the fetal and newborn rat (Rattus norvegicus) J Comp Psychol. 1993;107:61–74. doi: 10.1037/0735-7036.107.1.61. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Alberts JR. Sensory factors associated with gestation and parturition evoke cardiac and behavioral responses in fetal rats. Psychobiology. 1994;55:270–282. [Google Scholar]
- Ronca AE, Alberts JR. Effects of prenatal spaceflight on vestibular responses of neonatal rats. Journal of Applied Physiology. 2000a;89:2318–2324. doi: 10.1152/jappl.2000.89.6.2318. [DOI] [PubMed] [Google Scholar]
- Ronca AE, Alberts JR. Physiology of a microgravity environment selected contribution: Effects of spaceflight during pregnancy on labor and birth at 1 G. Journal of Applied Physiology. 2000b;89:849–854. doi: 10.1152/jappl.2000.89.2.849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear in mice: A radiographic study of terminal mitoses. Acta Otolaryngology. 1967;220:1–44. [PubMed] [Google Scholar]
- Rubinstein JT, Miller CA. How do cochlear prostheses work? Current Opinions in Neurobiology. 1999;9:399–404. doi: 10.1016/S0959-4388(99)80060-9. [DOI] [PubMed] [Google Scholar]
- Rusch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: Acquisition of voltage-gated conductances and differentiated morphology. Journal of Neuroscience. 1998;18:7487–7501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Oto-Laryngol (Suppl) 1971;285:1–77. [PubMed] [Google Scholar]
- Sokolov YN. Perception and the conditioned reflex. Pergamon Press; New York: 1963. [Google Scholar]
- Tello JF. Le reticule des cellules ciliees du labyrinth chez la souris et son independence des terminaisons nerveuses de la huitime paire. Trav Lab Rech Biol. 1931;27:151–186. [Google Scholar]
- Theiler K. The house mouse: Altas of embryonic development. Springer; New York: 1989. [Google Scholar]
- Woodring SF, Rossiter CD, Yates BJ. Pressor response elicited by nose-up vestibular stimulation in cats. Experimental Brain Research. 1997;113:165–168. doi: 10.1007/BF02454153. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. Journal of Comparative Neurology. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]