Introduction
Body workers from all disciplines appreciate that maneuvers that move nerves often reproduce radiating pain. This symptom reproduction has important implications for the diagnosis and management of radiating pain symptoms.
In the presentation at Fascia 2007 from which this manuscript is derived, two videos that were obtained with high resolution diagnostic ultrasound were presented that clearly showed median nerve gliding during normal finger and wrist movements. The movements were independent of the movements of the other surrounding structures. Such movements of the interface between the nerve and the surrounding structures constitute but one mechanical stimulus that nerves are susceptible to. Nerves are also bent around various structures, and indented by external pressures. Nerves have many anatomical features that allow them to accommodate such movements and mechanical stimuli. The reader is directed to books by Shacklock (Shacklock 2005) and Butler (Butler 2000) for full descriptions of nerve biomechanics.1
Nerve fascial anatomy
Nerves possess a complicated, 3-layer fascial structure (Fig. 1). Axons are covered by endoneurium, which offers little mechanical support. Groups of endoneurium-covered axons are bundled by perineurium, which is a thin but dense epithelium. The perineurium offers strength in tension, and also maintains the so-called blood-nerve barrier (if the perineurium is cut into, the axons herniate because of the positive pressure inside the nerve). The perineurium is covered by the thicker epineurium, an areolar tissue that acts as a cushion for the nerves. The epineurium is highly vascular, making it difficult to render any part of a peripheral nerve ischemic. The blood vessels are slightly coiled to adapt to excursion of the nerves. Feeder vessels from the epineurial blood vessels course to all inner parts of the nerve. Importantly to the discussion to follow, all layers of the nerve are innervated, and have a thin but potentially important plexus of nociceptors.
Figure 1.
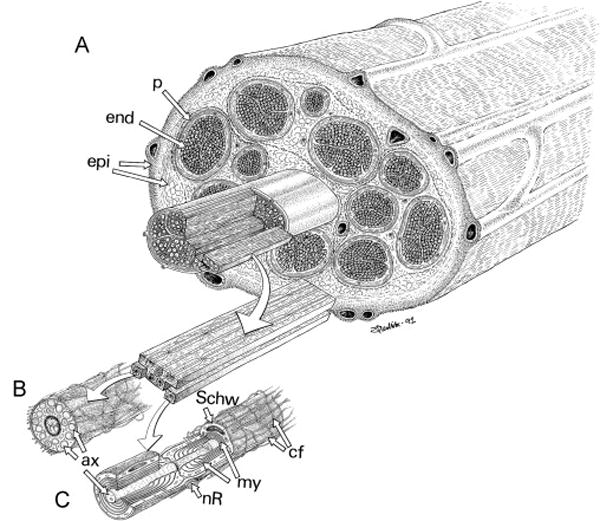
Peripheral nerve anatomy. A. Endoneurium-covered axons (end) are bundled by perineurium (p) into fascicles. These fascicles are in turn covered by epineurium (epi). B, C: Specializations of the Schwann cells that surround unmyelinated (B) and myelinated (C) axons. Numerous unmyelinated axons (ax) are embedded by invagination into the cytoplasm of a single Schwann cell (Sch). Each myelinated axon has multiple layers of Schwann cell membrane wrapped around it, forming myelin (my), interrupted at regular intervals by nodes of Ranvier (nR). (From Lundborg G 1988, used with permission.)
Nerve trunk pain and dysesthetic pain
Nociceptive pain is considered to be pain mediated by noxious stimuli to a structure that is innervated by nociceptors. A good example is the immediate pain that is felt when one stubs their toe. In this type of pain, the mechanical stimuli are transduced by the normal neural elements into electrical impulses that then are conducted to the central nervous system for perception. However there are types of pain that do not rely on normal transduction. Two major categories are nerve trunk pain and dysesthetic pain (Asbury & Fields 1984). A simple exercise can often potentially reveal the difference between these pain types. The ulnar nerve is located just posterior to the medial humeral condyle (the so-called “funny bone”). Here it can be easily manually stimulated. If the reader presses on their nerve, they may feel local sensations, and/or they may feel sensations in their medial hand and fingers (the distal innervation territory of the ulnar nerve). Local sensations, if painful, are properly considered nerve trunk pain. Such pain requires local transductive elements on the nerve that allow perception of pain coming from the area being stimulated. This is a form of nociceptive pain. Distal sensations, if painful, are called dysesthetic pain.2 To perceive any sensation distal to such a stimulus, there must be local transductive elements in addition to the normal transductive elements found more distally. A perfect clinical example of this phenomenon is the pain that is reproduced by the straight leg raising test in a patient with radicular pain. This test primarily moves the sciatic nerve and contributing roots, thus mechanically stimulating them. The nerve is stimulated in a place that normally does not evoke sensations, but the distal symptoms are reproduced.
The basis of nerve trunk pain: the nervi nervorum
Nerve trunk pain is mediated by nervi nervorum, a term that means “the nerve of the nerve.” Although recognized as an entity for more than a century (Horsley 1884, Sappey 1867), an good anatomical description did not appear in the literature until 1963 (Hromada 1963). In the 1990s we performed a series of experiments that documented the functional properties of these neural elements. While characterizing paraspinal nociceptors, we observed nociceptor receptive fields located on neurovascular bundles and often extending to muscles of tendon (Bove & Light 1995b). Using immunohistochemistry, we revealed peptidergic fine-caliber axons in the epineurium and perineurium, consistent with nociceptive function (Bove & Light 1995a; Fig. 2). We also performed in-vitro electrical and noxious chemical stimuli of nerves, nerve sheaths, and isolated axons and documented that nervi nervorum can evoke neurogenic inflammation (Sauer et al 1999). Collectively, these data support that nervi nervorum are nociceptive and also nocifensive, meaning that they are responsive to damaging stimuli by contributing to local inflammation, thus helping to defend and maintain the nerve’s local environment (Lembeck 1983, Light 2004).
Figure 2.
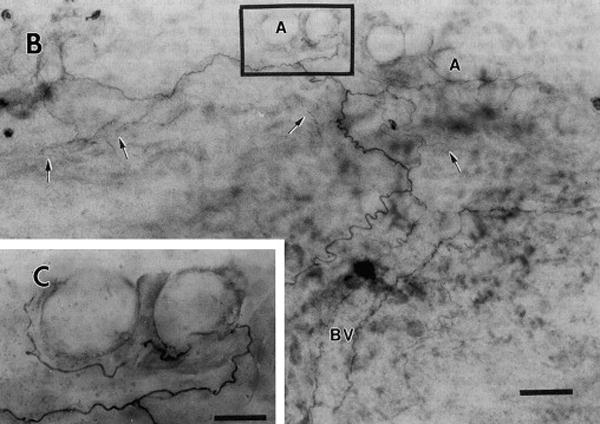
Peripherin-like immunostaining in nervi nervorum and vasa nervorum of rat sciatic nerve (whole mount). a. Montage demonstrating nerves following a blood vessel (BV, outlined by the fibers flanking the label) and leaving it to terminate in epineurium (arrows) and associated with adipose tissue (A). Note extensive branching. b, enlargement of area marked in a, detailing adipose innervation. M = mast cell, A = adipocyte. Scale bars in a = 50 μm, b = 20 μm. (From Bove GM & Light AR 1995a, used with permission.)
The basis of mechanically evoked dysesthetic pain: ectopic axonal mechanical sensitivity
Mechanically evoked dysesthetic pain seems more difficult to understand, because neural impulses have to be generated in a structure not normally capable of initiating action potentials. We have reported that normal axons are not mechanically sensitive (Bove et al 2003). This is good, because axons are mechanically stimulated during most movements. We also know that when diseased or broken, axons can become mechanically sensitive (Chen & Devor 1998). However, if substantial damage is done, there will be areas of sensory loss, a potentially important diagnostic metric. Demyelination also causes spurious activity in axons, but this does not necessarily affect nociceptors, which are not myelinated. Moreover, clinical observations support that dysesthetic pain in the absence of apparent or overt nerve injury can often resolve rapidly (faster than possible by regeneration). Again, for intact axons to become mechanically sensitive there must be a transductive element present.
We have modeled dysesthetic pain using a nerve inflammation model (Bove et al 2003). In this model, the sciatic nerve of a rat is inflamed using complete Freund’s adjuvant (CFA), which induces a robust immune mediated inflammation without leading to axonal breakage or other overt nerve injury (Bove et al 2003, Eliav et al 1999). We have recorded from nociceptors with axons passing through such inflamed areas, and have reported that at least a subpopulation of inflamed axons with intact nociceptive receptive fields becomes mechanically sensitive at the inflamed site (Bove et al 2003; Fig. 3). Put otherwise, after a few days of inflammation, the axons became capable of generating action potentials with similar mechanical forces that also caused their terminal receptive fields to generate action potentials. This axonal mechanical sensitivity (AMS) is referred to as “ectopic electrogenesis.” We subsequently performed a time course of this change (Dilley & Bove 2007). We have reported that the peak AMS occurs at 4–7 days post-initiation, and then decreases to insignificance or “heals” within 2 months of the initial insult. We have characterized the inflammatory response using the presence and numbers of macrophages and T-cells. We have found that the inflammatory response is limited to the epi- and perineurium, and outlasts the AMS by months (unpublished data). We hypothesize, but have not investigated fully, that the perineurial diffusion barrier is disrupted by inflammation, and heals faster than the immune system can clear the pro-inflammatory debris. This will be the subject of further study.
Figure 3.
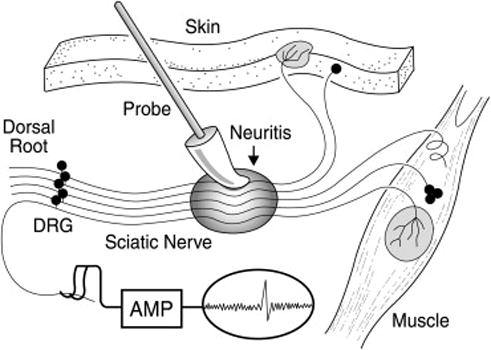
Axonal mechanical sensitivity is induced by nerve inflammation. The sciatic nerve is depicted in the middle of the image, with the shaded oval area indicating CFA-induced inflammation. The individual neurons lead to receptive fields in muscle or skin (right and top), and centrally to dorsal roots (left), for recording. When inflamed, the normally insensitive axons become mechanically sensitive. DRG = dorsal root ganglion. (From Bove GM et al 2003, used with permission.)
The role of axoplasmic transport
We also questioned the mechanism of induced ectopic AMS. It seems reasonable that the mechanical-electrical transductive elements normally present at nociceptor terminals become functional on the axons during inflammation. Unfortunately, the transductive element of nociceptors has eluded detection, making the search for the inflammation-induced element difficult. We do know that nerve inflammation interferes with normal fast axoplasmic transport (Armstrong et al 2004), and that the mechanical transductive elements are likely transported from the cell body to the terminal (Koschorke et al 1994). Therefore, we hypothesized that if axoplasmic transport was blocked in the absence of inflammation, we should still detect AMS. Thus, we applied the axoplasmic transport blockers colchicine and vinblastine to nerves, and tested for AMS. We found that such treatment evoked AMS in a majority of tested axons (Dilley & Bove 2008; Fig. 4). We thus concluded that the mechanical-electrical transductive element is transported by fast axoplasmic flow. The ion channel(s) responsible for AMS have yet to be determined.
Figure 4.
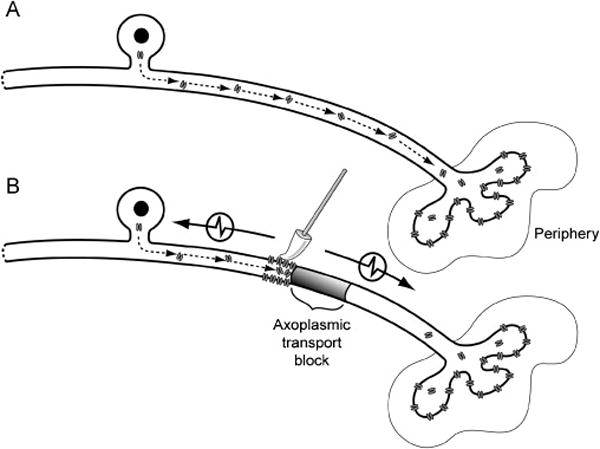
A. Components required for mechanical sensitivity are transported from the cell body of a single C-fiber neuron to the periphery for insertion at the terminals. B. Blocking axoplasmic transport leads to the accumulation and insertion of mechanosensitive components proximal to the site of axoplasmic blockade. Mechanical stimulation of the axon membrane therefore becomes an effective stimulus to generate action potentials that pass in both directions along the axon (denoted by arrows). (From Dilley A & Bove GM 2008, used with permission.)
Conclusion
In conclusion, we have shown that nerves possess an intrinsic innervation of their fascial layers, and that this innervation is nociceptive and likely responsible for nerve trunk pain. We have also shown that inflammation changes axons by making them sensitive to mechanical stimuli. This latter effect heals with inflammation resolution. We feel that these findings have immediate clinical implications, although we caution that these data were gathered using rats in a very controlled environment. It is however possible that patients with nerve trunk or dysesthetic pain have a persistent source of inflammation that is affecting a nerve. Symptoms can appear as local tenderness of a nerve, or a “doorbell” type of response, where a palpated spot leads to symptoms in a more distal region. Most peripheral nerves can be directly moved or palpated, and nerve courses are well described anatomically. Nerves and their fascia deserve full diagnostic attention.
Footnotes
Readers are cautioned regarding abnormal nerve movements, or nerve tethering, as contributory to nerve-related pain. Such aberrant biomechanics have yet to be documented, and in fact substantial evidence exists that refutes the presence of adverse nerve movements during disease states such as carpal tunnel syndrome and other repetitive motion disorders.
Other commonly used terms for this type of pain include radiating pain, and radicular pain. Radiating pain is not specific, and radicular pain is used when the structure affected is the nerve root, or radix.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong BD, et al. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Asbury AK, Fields HL. Pain due to peripheral nerve damage: an hypothesis. Neurology. 1984;34:1587–1590. doi: 10.1212/wnl.34.12.1587. [DOI] [PubMed] [Google Scholar]
- Bove GM, Light AR. Calcitonin gene-related peptide and peripherin immunoreactivity in nerve sheaths. Somatosensory and Motor Research. 1995a;12:49–57. doi: 10.3109/08990229509063141. [DOI] [PubMed] [Google Scholar]
- Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. Journal of Neurophysiology. 1995b;73:1752–1762. doi: 10.1152/jn.1995.73.5.1752. [DOI] [PubMed] [Google Scholar]
- Bove GM, et al. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. Journal of Neurophysiology. 2003;90:1949–1955. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- Butler DS. The Sensitive Nervous System. Noigroup Publications; Adelaide: 2000. [Google Scholar]
- Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. European Journal of Pain. 1998;2:165–178. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- Dilley A, Bove GM. Resolution of inflammation induced axonal mechanical sensitivity and conduction slowing in C-fiber nociceptors. Journal of Pain. 2007;9:185–192. doi: 10.1016/j.jpain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. Journal of Physiology. 2008;586:593–604. doi: 10.1113/jphysiol.2007.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliav E, et al. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:168–182. doi: 10.1016/s0304-3959(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Horsley V. Preliminary communication on the existence of sensory nerves in nerve-trunks, true “nervi nervorum”. British Medical Journal. 1884;1:166–166. [Google Scholar]
- Hromada J. On the nerve supply of the connective tissue of some peripheral nervous tissue system components. Acta Anatomica. 1963;55:343–351. doi: 10.1159/000142483. [DOI] [PubMed] [Google Scholar]
- Koschorke GM, et al. Cellular components necessary for mechanoelectrical transduction are conveyed to primary afferent terminals by fast axonal transport. Brain Research. 1994;641:99–104. doi: 10.1016/0006-8993(94)91820-1. [DOI] [PubMed] [Google Scholar]
- Lembeck F. Sir Thomas Lewis’s nocifensor system, histamine and substance-P- containing primary afferent nerves. Trends in Neurosciences. 1983;6:106–108. [Google Scholar]
- Light AR. “Nocifensor” system re-revisited. Focus on “Two types of C nociceptor in human skin and their behavior in areas of capaicin-induced secondary hyperalgesia”.[comment] Journal of Neurophysiology. 2004;91:2401–2403. doi: 10.1152/jn.00090.2004. [DOI] [PubMed] [Google Scholar]
- Lundborg G. Nerve Injuries and Repair. Churchill Livingstone; Edinburgh: 1988. [Google Scholar]
- Sappey MC. Recherches sur les nerfsdu névrilème ou nervi nervorum. CR Acad Sci. 1867;65:761–762. [Google Scholar]
- Sauer SK, et al. Rat peripheral nerve components release calcitonin gene-related peptide and prostaglandin E-2 in response to noxious stimuli: Evidence that nervi nervorum are nociceptors. Neuroscience. 1999;92:319–325. doi: 10.1016/s0306-4522(98)00731-3. [DOI] [PubMed] [Google Scholar]
- Shacklock M. Clinical Neurodynamics. Elsevier; Edinburgh: 2005. [Google Scholar]


