Abstract
Cellular-FLICE inhibitory protein (c-FLIP) is an inhibitor of apoptosis downstream of the death receptors Fas, DR4, and DR5, and is expressed as long (c-FLIPL) and short (c-FLIPS) splice forms. We found that the knockdown of c-FLIP using small interfering RNA (siRNA) triggered ligand-independent caspase-8- and -9-dependent spontaneous apoptosis and decreased the proliferation of MCF-7 breast cancer cells. Further analysis revealed that an apoptotic inhibitory complex (AIC) comprised of DR5, FADD, caspase-8, and c-FLIPL exists in MCF-7 cells, and the absence of c-FLIPL from this complex induces DR5- and FADD-mediated caspase-8 activation in the death inducing signaling complex (DISC). c-FLIPS was not detected in the AIC, and using splice form-specific siRNAs we showed that c-FLIPL but not c-FLIPS is required to prevent spontaneous death signaling in MCF-7 cells. These results clearly show that c-FLIPL prevents ligand-independent death signaling and provides direct support for studying c-FLIP as a relevant therapeutic target for breast cancers.
1. Introduction
Apoptosis is an essential process in human development, immunity, and tissue homeostasis. One mechanism by which chemotherapeutic agents kill tumor cells is by inducing the apoptotic death pathways. The two primary apoptotic pathways are the death receptor and mithochondrial pathways [1, 2]. Apoptotic signaling and execution through these two pathways is dependent on caspases, a group of cysteine proteases that degrade a critical set of cellular proteins near specific aspartic acid residues [3]. In the death receptor pathway, the apoptosis-promoting members of the tumor necrosis factor superfamily, such as Apo2L/TRAIL and Fas ligand, engage their respective death receptors, DR4/DR5 or Fas, which homotypically bind to the adaptor protein FADD. FADD then recruits the initiating caspases-8 and -10 through homophilic death effector domain (DED) interactions to form the death inducing signaling complex (DISC) [4, 5]. The close proximity of the initiator caspases in the DISC facilitates their dimerization and activation. At the DISC, caspase-8 is initially processed into a 10 kDa species that is released from the DISC and an intermediate 43/41 kDa species that remains tethered to the DISC. The 43/41 kDa intermediate form is subsequently cleaved to give an 18 kDa species that associates with the 10 kDa species to form the active caspase-8 protease, which is released into the cytosol. The active caspase-8 cleaves downstream caspases such as caspases-3, -6, and -7, as well as the pro-apoptotic protein Bid which initiates the apoptotic process [6]. Cleaved Bid activates downstream pro-apoptotic proteins such as Bax and Bak which promote cytochrome c release from the mitochondria into the cytosol, thereby linking the two pathways [7]. Cytochrome c associates with caspase-9, dATP, and APAF-1 forming the apoptosome complex leading to the activation of caspase-9, which cleaves the downstream effector caspases-3, -6, and -7 [8, 9]. Effector caspase activation promotes cellular disintegration by the cleavage of multiple cellular proteins such as fodrin, protein kinase C, gelsolin, and poly(ADP-ribose) polymerase (PARP) [7, 10].
Cellular FLICE inhibitory protein (c-FLIP) is expressed in various cancers and is an inhibitior of death ligand-induced apoptosis downstream of death receptors and FADD [11]. Additionally, c-FLIP expression is associated with enhanced tumorgenicity and poor clinical outcome in many types of cancers because of chemotherapeutic drug and TRAIL resistance [12–18]. So far, 11 distinct c-FLIP splice variants have been reported, three of which are expressed as proteins: the 55 kDa long form (c-FLIPL), 26 kDa short form (c-FLIPS), and a 24 kDa form (c-FLIPR). All c-FLIP isoforms contain two N-terminal DED domains but only c-FLIPL harbors a C-terminal caspase domain with structural similarity to caspase-8. In the caspase-like domain of c-FLIPL, the catalytically active cysteine is replaced by tyrosine, rendering the molecule proteolytically inactive [19]. c-FLIP proteins are recruited to the DISC by their DED domains and compete with the initiator caspases for FADD binding sites [11]. c-FLIPS and c-FLIPR both inhibit death signaling at the DISC by inhibiting caspase-8 activation [20, 21]. The function of c-FLIPL at the DISC is controversial because studies have shown that it can be anti-apoptotic like c-FLIPS, or pro-apoptotic by directly activating caspase-8 [22, 23]. The pro-apoptotic role is supported by studies showing that c-FLIP-deficient mice die early in embryonic development due to heart failure, which is a defect also observed in caspase-8- and FADD-deficient mice [24, 25]. However, the majority of reports encompassing diverse types of cancers indicate that the role of c-FLIPL is generally anti-apoptotic in cancer cells. For instance, the silencing of c-FLIP expression using small interfering RNAs (siRNAs) has been shown to promote spontaneous apoptosis in colorectal, lung, and lymphoma cancer cell lines and augments TRAIL- and chemotherapy-induced apoptosis in various cancer cell types [23, 26–31].
In addition to c-FLIP’s role in apoptosis, c-FLIP proteins have also been shown to be involved in proliferation, cell cycle progression, and carcinogenesis. The overexpression of c-FLIPL inhibited the proteosomal degradation of β-catenin and the elevated β-catenin levels were shown to either promote Wnt signaling or induce cyclin D expression, leading to the proliferation and cell cycle progression of cancer cells [32, 33]. In both studies, the c-FLIP/β-catenin signals contributing to cell growth were reversed by the selective silencing of c-FLIP expression. Recently, a study showed that the caspase-8-dependent cleavage of c-FLIP produced an N-terminal p22 fragment that directly induced NF κB activation and promoted the proliferation of lymphocytic cells [34]. Furthermore, several studies have shown that c-FLIP overexpression can promote carcinogenesis and aggressiveness of endometrial and cervical cancers [35, 36]. These studies highlight the functional importance of c-FLIP in the proliferation of cancer cells.
The aim of this study was to elucidate the mechanism of how the silencing of c-FLIP triggers spontaneous apoptosis in breast cancer cells. We discovered that silencing the c-FLIP gene leads to DR5-, FADD-, caspases-8- and -9-induced apoptosis in MCF-7 breast cancer cells. Furthermore, we observed that c-FLIPL interacts with DR5, FADD, and caspase-8 forming an apoptotic inhibitory complex (AIC) in these cells. Collectively, this study shows the knockdown of c-FLIP expression triggers spontaneous apoptosis by activating both the death receptor and mitochondrial pathways and inhibiting breast cancer cell proliferation.
2. Materials and Methods
2.1 Cell culture, materials, and antibodies
The MCF-7 human breast cancer cell line was obtained from American Type Culture Collection (ATTC, Manassas, VA), and was maintained in RPMI 1640 medium containing 10% fetal calf serum (FCS) and 100 ng/ml each of penicillin and streptomycin (Invitrogen, Inc., Carlsbad, CA) at 37°C in 5% CO2. The caspase-8 inhibitor (z-IETD-fmk) and the caspase-9 inhibitor (z-LEHD-fmk) were purchased from R&D Systems (Minneapolis, MN). The inhibitors were dissolved in DMSO and added to the cultured cells so that the final concentration of DMSO was 0.1%. In this study the following primary antibodies were used: anti-c-FLIP clone G-11 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-c-FLIP clone Dave-2 (ProSci Inc., Poway, CA), anti-DR5 clone B-D37 (IgG2b) (Santa Cruz Biotechnology), anti-DR5 (Cell Signaling Technology, Danvers, MA), anti-caspase-8 clone 3-1-9 (BD Biosciences, San Jose, CA), anti-caspase-6 clone B93-4 (BD Biosciences), anti-caspase-7 clone 10-1-62 (BD Biosciences), anti-caspase-9 clone 5B4 (MBL International Corp., Woburn, MA), anti-FADD clone 1 (BD Biosciences), anti-BID clone 5C9 (Santa Cruz Biotechnology), Anti-DR4 (BD Biosciences), and anti-β-actin clone AC-74 (Sigma-Aldrich, St. Louis, MO).
2.2. Western blotting and immunoprecipitations
MCF-7 cells were harvested, rinsed in cold PBS, and lysed in M-PER (Pierce Biotechnology, Rockford, IL) containing 1% protease inhibitor cocktail (Sigma). Protein concentration was determined by using the BCA/Cu2SO4 protein assay (Sigma) as described by the manufacturer. One hundred micrograms of lysate were separated on a NuPAGE 4–12% or 10% Bis-Tris gel (Invitrogen) and transferred to an Immobilon-P membrane (Fisher Scientific, Pittsburgh, PA). Membranes were incubated in blocking buffer (PBS, 0.1% Tween 20, 5% skim milk) and then incubated first with specific antibodies in blocking buffer followed by the addition of horseradish peroxidase-conjugated sheep anti-mouse or anti-rabbit secondary antibodies (Amersham Biosciences, Piscataway, NJ). For detection of the anti-c-FLIP clone Dave-2 antibody, a horseradish peroxidase-conjugated goat anti-rat secondary antibody was used (Santa Cruz). Immunoreactive proteins were visualized by using SuperSignal West Pico solutions (Pierce Biotechnology).
For immunoprecipitations, MCF-7 cells were rinsed in ice cold PBS and lysed in buffer containing 20 mM Tris-HCl [37], 1% NP-40, 150 mM NaCl, 10% glycerol, and 1% protease inhibitor cocktail (Sigma). Five hundred micrograms of cell lysate were incubated overnight with 5 μg of DR5 antibody at 4°C. The following day, 50 μL of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) were added and the mixture was incubated for an additional night at 4°C. The immunoprecipitates were collected, washed in PBS, and analyzed by SDS-PAGE as described above. To eliminate the interference of heavy and light chains of the immunoprecipitating antibody, the Mouse TrueBlot ULTRA secondary antibody (eBioscience, San Diego, CA) was used.
2.3. siRNA preparation and transfection
c-FLIP, FADD, and DR5 siRNA oligonucleotides were synthesized by Invitrogen according to published sequences [27, 38, 39]. The siCONTROL Non-Targeting siRNA (catalog number D-001210-03) was purchased from Dharmacon Research, Inc. (Lafayette, CO). MCF-7 cells were seeded at a density of 2 × 105 cells/mL in antibiotic-free medium one day prior to transfection. For transfection, 100 nM of siRNA was mixed with DharmaFECT 1 transfection reagent (Dharmacon) according to the manufacturer’s instructions. The cells were incubated with the siRNA-DharmaFECT 1 complexes for 48 or 72 h before further analysis.
2.3. Determination of apoptosis using Hoechst 33342 stain
To visualize nuclear morphology in MCF-7 cells by DNA staining, 1 × 105 cells were plated in Lab-Tek II chamber slides (Thermo Fisher Scientific, Waltham, MA) and treated with caspase inhibitors, siRNA, or caspase inhibitors and siRNA for 48 or 72 h. The cells were stained with 10 μM of Hoechst 33342 stain (Sigma) for 1 hour and viewed by fluorescence microscopy using a Nikon Diaphot 200 Inverted Microscope with a magnification of 40X. The images were captured using a Diagnostic Instruments SPOT color camera.
2.4. Annexin V binding assay to detect apoptotic cells
MCF-7 cells were plated at a density of 2 × 105 cells/mL and transfected with c-FLIP siRNA for 48 h, harvested, and stained with fluorescein isothiocyanate-labeled Annexin V (BD Biosciences) and propidium iodide according to the manufacturer’s protocol. The cells were analyzed using a FACScan (BD Biosciences) flow cytometry and CellQuest software (BD Biosciences). Apoptotic and necrotic cells were analyzed by quadrant statistics on the propidium iodide-positive, annexin V-positive, and propidium iodide- and annexin V-positive cells, respectively.
2.5 Mitochondrial staining
To observe the morphological changes in the mitochondria due to c-FLIP gene knockdown, 1 × 105 MCF-7 cells were plated in Lab-Tek II chamber slides (Thermo Fisher Scientific) and transfected with c-FLIP-specific siRNA for 48 h. Cells were stained with 10 μM Hoechst 33342 stain (Sigma) and 500 nM MiTotracker Red CMXRos (Invitrogen) for 1 hour, and viewed by fluorescence microscopy using a Nikon Diaphot 200 Inverted Microscope with a magnification of 40X. The images were captured using a Diagnostic Instruments SPOT color camera.
2.6 Clonogenic assay
MCF-7 cells were transfected with c-FLIP siRNAs for 48 h, harvested, rinsed in PBS, and 1,000 cells were added to each well of a 6-well plate containing complete growth medium. The plates were incubated for 5 nights and colonies were counted.
3. Results
3.1. Knockdown of the c-FLIP gene triggers spontaneous caspase-8 and -9-dependent apoptosis in MCF-7 breast cancer cells
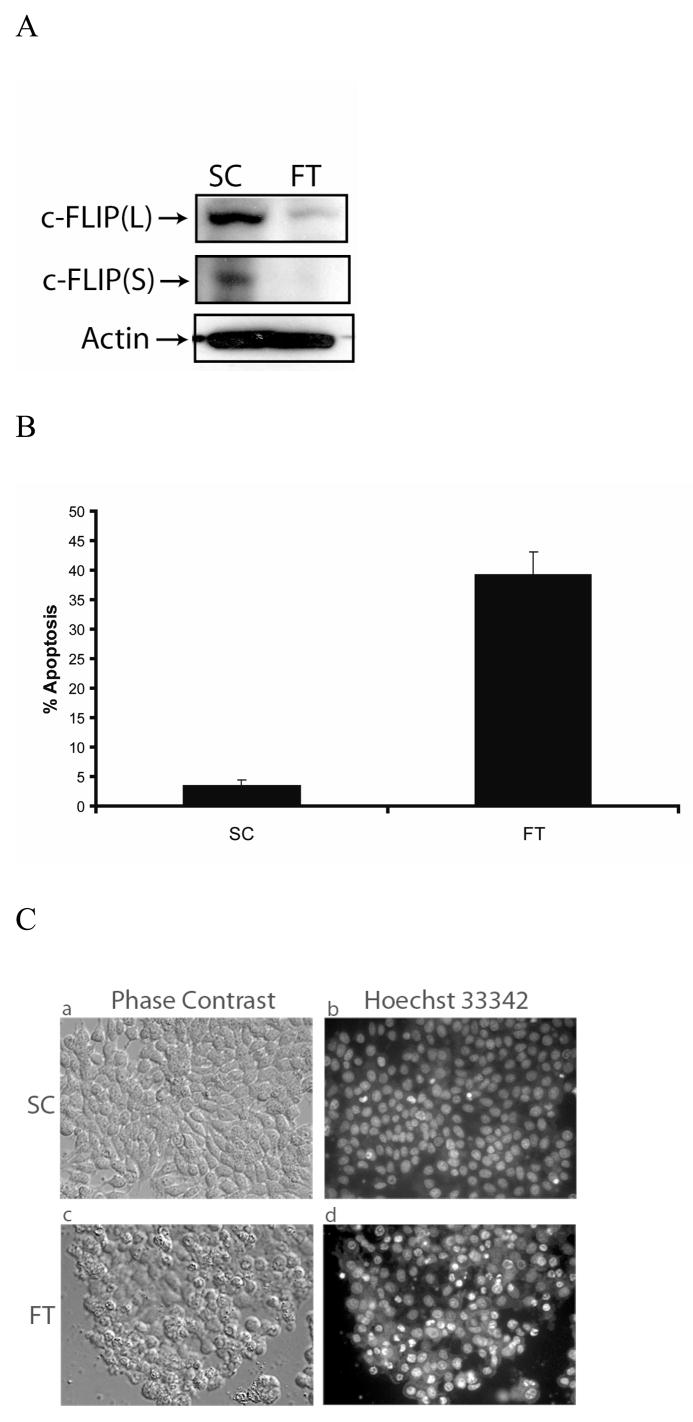
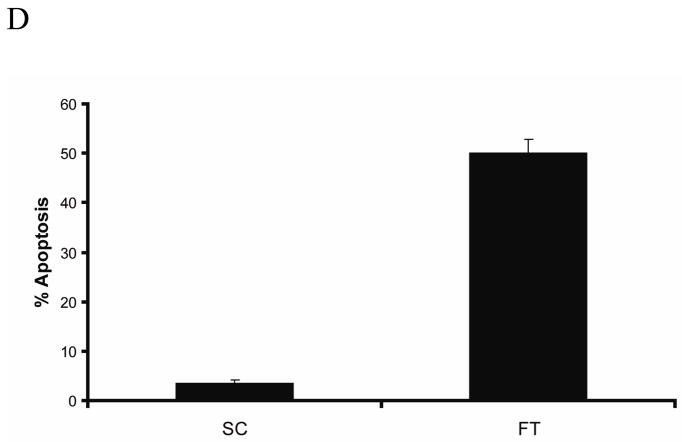
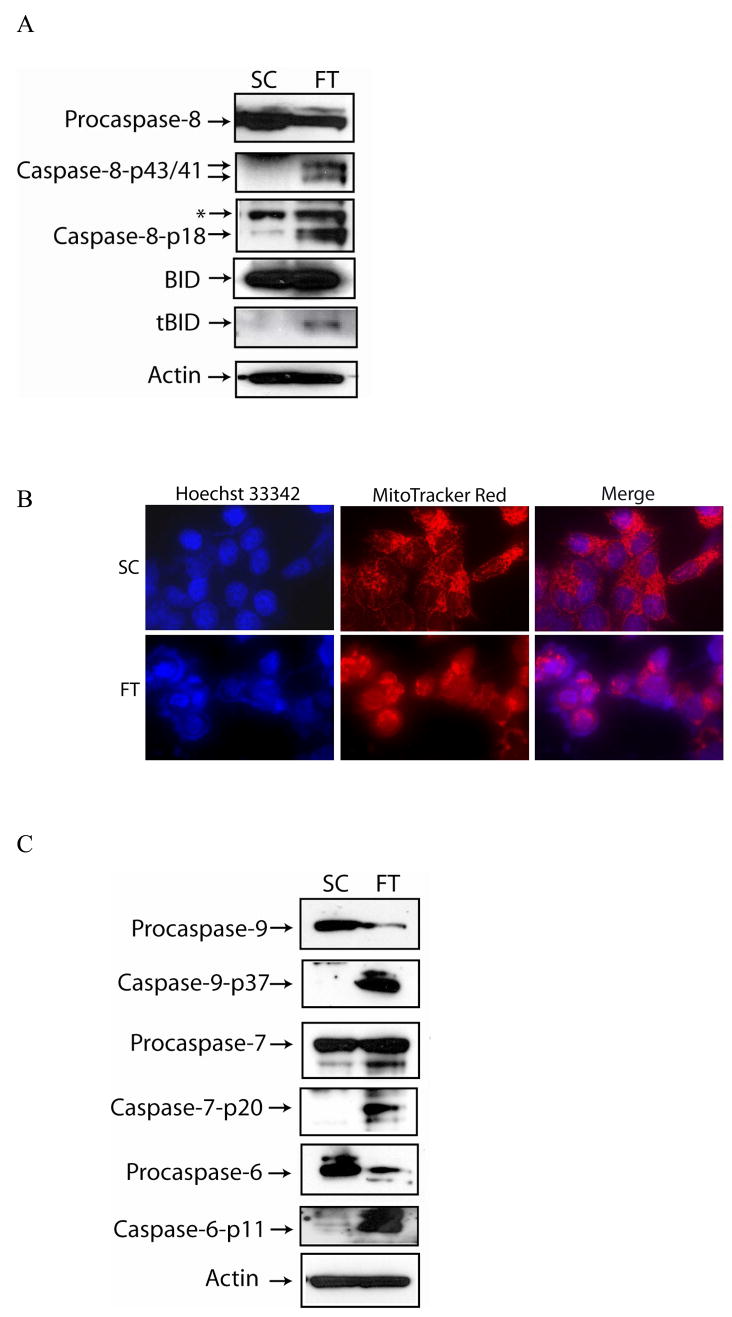
MCF-7 cells were transfected with c-FLIP-specific siRNA and immunoblot analysis showed the expression of both c-FLIP isoforms were silenced (FIG. 1A). An early event in the apoptotic process is the disruption of phospholipid asymmetry in the plasma membrane and the exposure of phosphatidylserine on the outer leaflet of the plasma membrane. Annexin V is a protein that binds to negatively charged phospholipids such as phosphatidylserine and is used to detect apoptotic cells. Double staining the cells with annexin V and propidium iodide allowed us to detect apoptotic cells by flow cytometry. Furthermore, we also identified apoptotic cells by fluorescence microscopy of Hoechst 33342-stained MCF-7 cells, since apoptosis is characterized by changes in nuclear morphology [40]. As shown in Figure 1B, C, and D, the silencing of c-FLIP coincided with a 45% increase in apoptosis as compared to cells treated with non-targeting siRNA. Immunoblot analysis of c-FLIP siRNA-treated whole cell lysates showed that caspase-8 was activated and Bid was cleaved forming tBid (Fig. 2A). Moreover, when the non-targeting siRNA-treated cells were co-stained with Hoechst 33342 and MitoTracker Red, the stain was sequestered in the nucleus and mitochondria, but c-FLIP siRNA-treated cells undergoing apoptosis gave diffuse staining showing that the nuclear and mitochondrial structures were disrupted, resulting in cytochrome c release and activation of caspases-9, -6 and -7 (Fig. 2B and C). These results show that c-FLIP knockdown promotes signaling through both the death receptor and mitochondrial apoptotic pathways.
Fig.1. c-FLIP gene silencing triggers spontaneous apoptosis in MCF-7 cells.
A, Immunoblot analysis of c-FLIPL, c-FLIPS, and β-actin in cell lysates following the treatment with 100 nM of non-targeting siRNA (SC) or c-FLIP-targeting siRNA (FT) for 48 h. B, Cells were transfected with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h, and cells were harvested and apoptosis was determined by FACS analysis as described in the Materials and Methods section. Compensation was executed for each experiment using untreated cells stained with Annexin V and propidium iodide. Error bars show standard deviation from duplicate measurements. C, Cells were transfected with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h, and Phase contrast (a and c) and fluorescence microscopy images (b and d) are shown after staining the DNA with Hoechst 33342. D, Cells were transfected with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h, and the percentage of apoptotic cells was determined by counting fragmented nuclei stained with Hoechst 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts.
Fig. 2. c-FLIP knockdown induces the activation of caspases, Bid, and mitochondrial disintegration.
A, Immunoblot analysis of caspase-8, BID, and β-actin in cell lysates following the transfection with non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h. The asterisk (*) represents a non-specific protein that is recognized by the capase-8-specific antibody. B, Cells were transfected with non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h, stained with 10 μM of Hoechst 33342 and 500 nM MitoTracker Red, and visualized by fluorescent microscopy. C, Immunoblot analysis of caspase-9, caspase-7, caspase-6, and β-actin in cell lysates following the transfection of MCF-7 with non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) for 48 h.
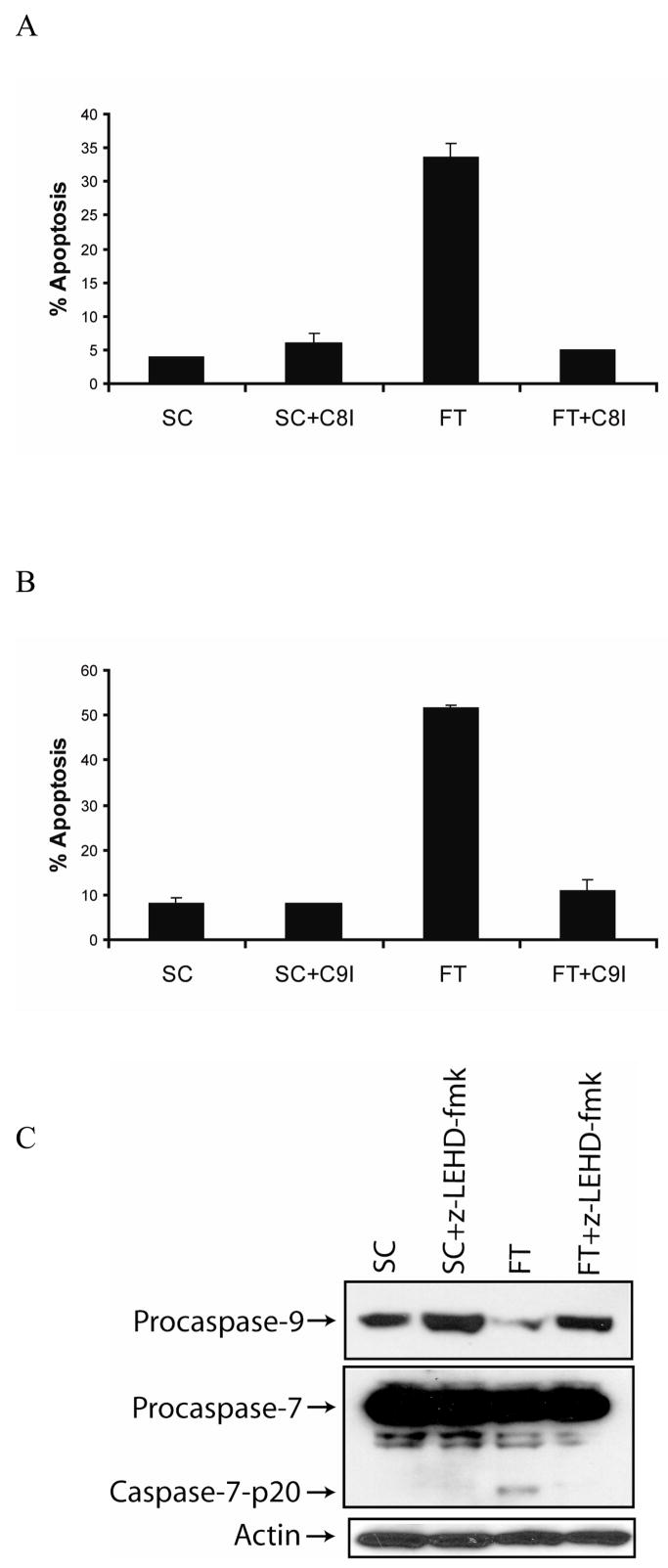
To determine whether the apoptosis mediated by the c-FLIP knockdown was caspase-8-dependent, we treated cells with c-FLIP siRNA in the presence or absence of the caspase-8 inhibitor z-IETD-fmk. As shown in Fig. 3A, the inhibition of caspase-8 blocked the spontaneous apoptosis induced by c-FLIP gene knockdown. These results reveal that silencing the c-FLIP gene triggers apoptosis by a caspase-8-dependent mechanism. Since c-FLIP knockdown leads to caspase-8 activation and Bid cleavage, we next examined whether c-FLIP siRNA-induced apoptosis is dependent on the activation of caspase-9. Transfection of cells with c-FLIP siRNA followed by the addition of the caspase-9 inhibitor z-LEHD-fmk protected the cells from spontaneous apoptosis, revealing that c-FLIP siRNA-triggered apoptosis occurred by a caspase-9-dependent process (Fig. 3B). Next, we determined if c-FLIP siRNA-mediated caspase-7 activation was dependent on caspase-8 or caspase-9. Transfection of cells with c-FLIP siRNA followed by the addition of the caspase-9 inhibitor z-LEHD-fmk prevented caspase-7 activation, revealing that caspase-7 was activated by caspase-9 in the absence of c-FLIP (Fig. 3C). Since MCF-7 cells do not express caspase-3, these results reveal that caspase-7 can substitute for caspase-3 and mediate apoptosis downstream of caspase-9 activation in the absence of c-FLIP. Collectively, these results show that silencing of c-FLIP induces apoptosis by activating both the death receptor (caspase-8) and the mitochondrial (caspase-9) pathways in MCF-7 cells.
Fig. 3. c-FLIP knockdown triggers caspase-8- and caspase-9-dependent apoptosis.
A, Cells were transfected with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) and treated with or without 20 μM of caspase-8 inhibitor (z-IETD-fmk) for 48 h, and apoptosis was determined by counting fragmented nuclei after staining with Hoecsht 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts. B, Cells were transfected with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) and incubated with or without 20 μM of caspase-9 inhibitor (z-LEHD-fmk) for 48 h, and apoptosis was determined by counting fragmented nuclei after staining with Hoecsht 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts. C, Immunoblot analysis of caspase-9, caspase-7, and β-actin in cell lysates after transfecting with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT) and incubated with or without 20 μM of the caspase-9 inhibitor (z-LEHD-fmk) for 48 h.
3.2. Silencing of c-FLIP induces FADD-mediated apoptosis
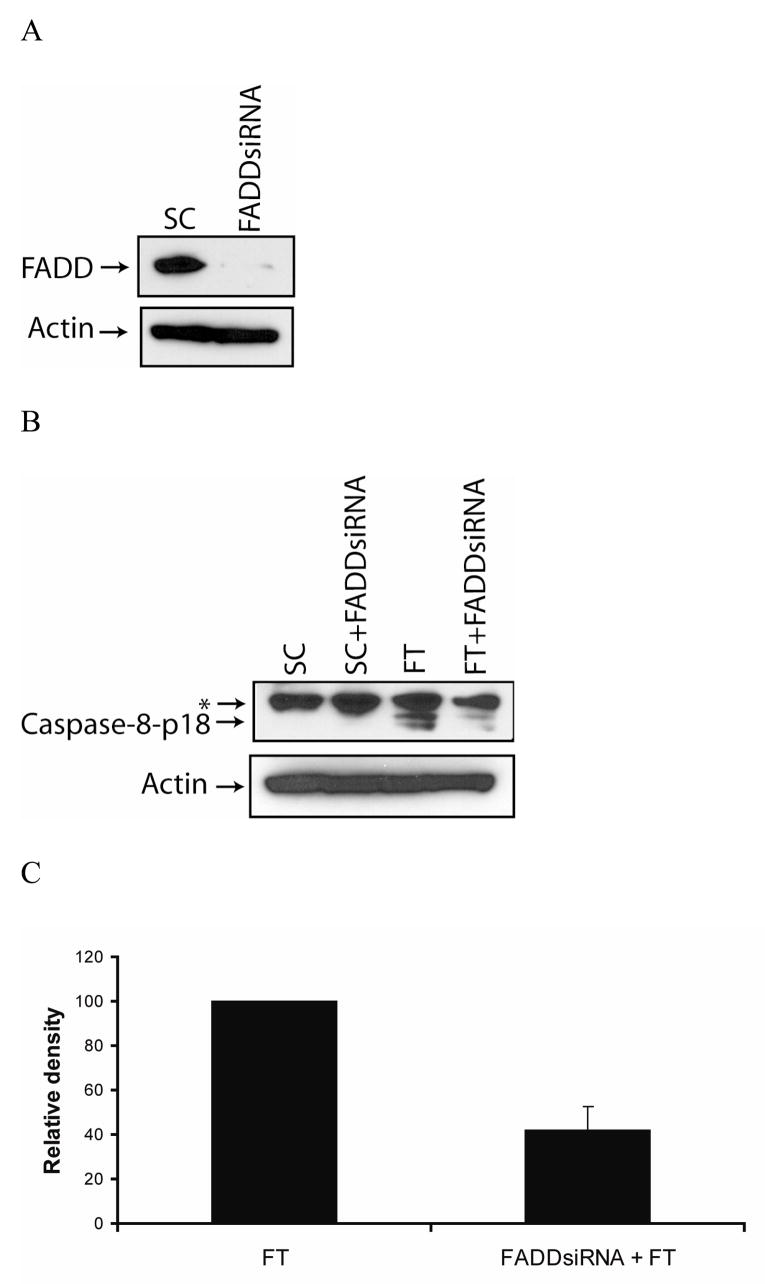
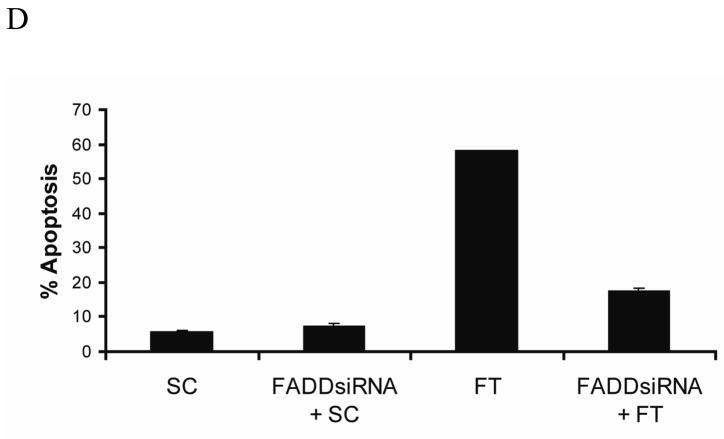
To investigate the role of FADD in c-FLIP siRNA-triggered apoptosis, we silenced the FADD gene by using a FADD-specific siRNA (Fig. 4A). The knockdown of FADD expression reduced the c-FLIP siRNA-mediated production of the caspase-8-p18 active subunit by 60% and decreased apoptosis from 55% to 15%, revealing that FADD is involved in the processing of caspase-8 (Fig. 4B, C, and D). The spontaneous apoptosis in FADD- and c-FLIP-siRNA treated cells as compared to FADD- and non-targeting siRNA-treated cells was most likely due to incomplete silencing of the FADD gene. These results reveal that in the absence of c-FLIP, FADD is required for the processing and activation of caspase-8.
Fig. 4. Silencing of c-FLIP triggers FADD-dependent apoptosis.
A, Immunoblot analysis of FADD and β-actin in cell lysates following the transfection with 100 nM of non-targeting siRNA (SC) or FADD-specific siRNA for 72 h. B, Immunoblot analysis of caspase-8 and β-actin in cell lysates treated with 100 nM FADD-specific siRNA for 24 h, and treated for an additional 48 h with 100 nM of non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT). The asterisk (*) represents a non-specific protein that is recognized by the capase-8-specific antibody. C, The density of the caspase-8-p18 band in the c-FLIP-specific siRNA (FT) or FADD siRNA and FT siRNA treated cell lysates was quantified using the Image J 1.37c software (values +/− s.d.). D, Cells were treated with 100 nM FADD-specific siRNA for 24 h and treated for an additional 48 h with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT), and apoptosis was determined by counting fragmented nuclei after staining with Hoecsht 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts.
3.3. Knockdown of c-FLIP triggers DR5-mediated apoptosis
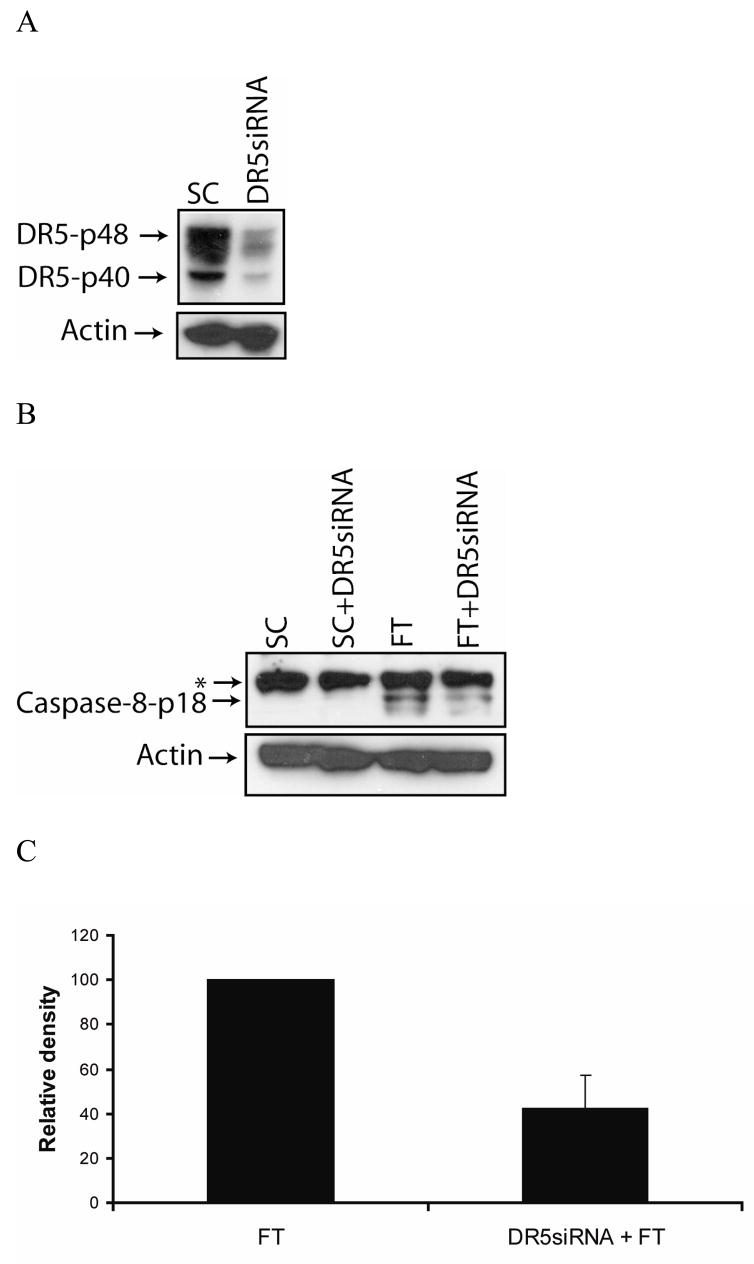
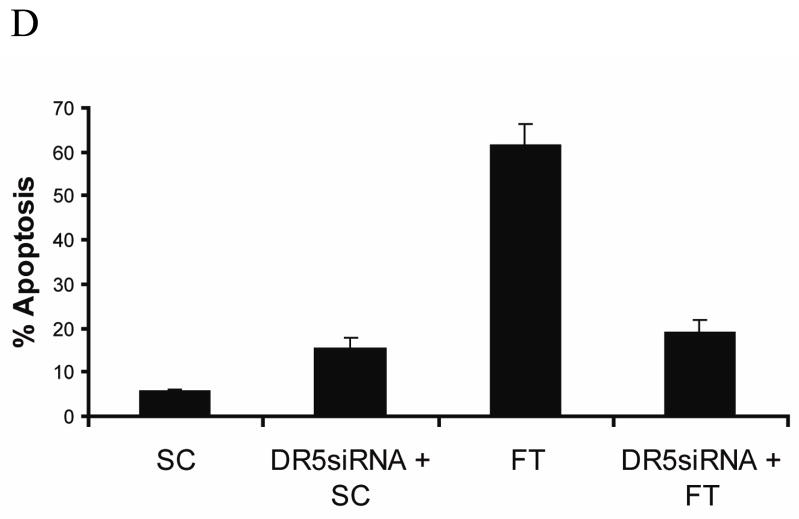
It is well documented that c-FLIP can inhibit caspase-8 activation and DISC formation induced by the TRAIL receptor DR5 [23, 27, 41, 42]. Therefore, to investigate the role of DR5 in c-FLIP siRNA-mediated apoptosis, we knocked down the expression of DR5 using a DR5-specific siRNA (Fig. 5A). We discovered that the knockdown of DR5 gene expression decreased the c-FLIP siRNA-triggered production of the caspase-8-p18 active subunit by 60% and reduced apoptosis from 55% to 22% (Fig. 5B, C and D). These findings show that DR5 is required for the processing and activation of caspase-8 in the absence of c-FLIP. The apoptosis in DR5- and c-FLIP-siRNA treated cells as compared to DR5- and non-targeting siRNA-treated cells is probably due to the FADD-induced activation of caspase-8 (see Fig. 4), as well as the incomplete knockdown of DR5 gene expression (Fig. 5A). These data clearly show that DR5 is involved in inducing spontaneous apoptosis in MCF-7 cells following the knockdown of c-FLIP.
Fig. 5. The knockdown of c-FLIP triggers DR5-dependent apoptosis.
A, Immunoblot analysis of DR5 and β-actin in cell lysates following the transfection with 100 nM of non-targeting siRNA (SC) or DR5-specific siRNA for 72 h. B, Immunoblot analysis of caspase-8 and β-actin in cell lysates treated with 100 nM DR5-specific siRNA for 24 h, and treated for an additional 48 h with 100 nM of non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT). The asterisk (*) represents a non-specific protein that is recognized by the capase-8-specific antibody. C, The density of the caspase-8-p18 band in the c-FLIP-specific (FT) siRNA or DR5-specific siRNA and FT siRNA treated cell lysates was quantified using the Image J 1.37c software (values +/− s.d.). D, Cells were treated with 100 nM DR5-specific siRNA for 24 h and treated for an additional 48 h with 100 nM non-targeting siRNA (SC) or c-FLIP-specific siRNA (FT), and apoptosis was determined by counting fragmented nuclei after staining with Hoecsht 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts.
3.4. DR5 binds to c-FLIPL, caspase-8, and FADD independently of receptor ligation
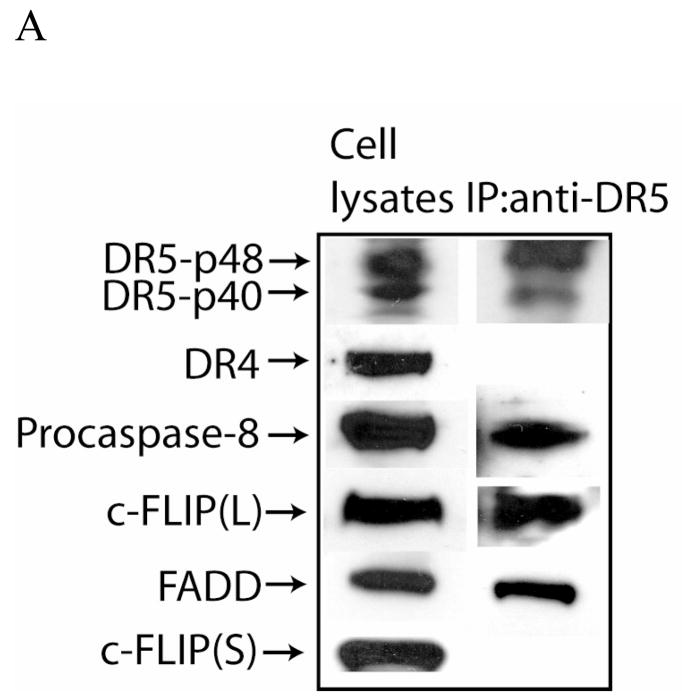
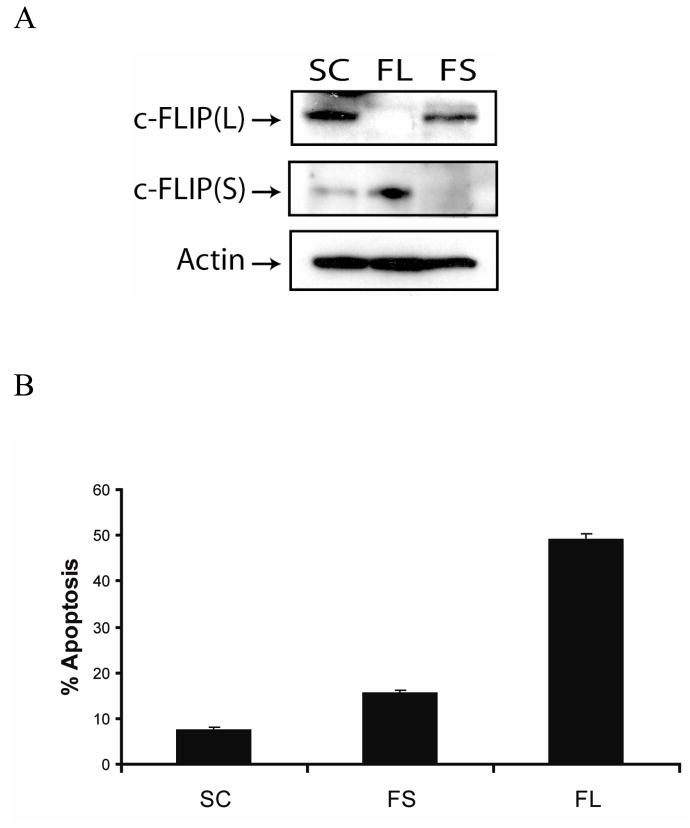
Because c-FLIP siRNA-triggered apoptosis was partially dependent on the expression of DR5, we determined whether c-FLIPL interacts with DR5 in MCF-7 cells. As shown in Figure 6, c-FLIPL, caspase-8, and FADD, but not DR4 and c-FLIPS, were immunoprecipitated from whole cell lysates using an anti-DR5 monoclonal antibody. These results reveal that c-FLIPL interacts with DR5, FADD, and caspase-8 to form an apoptotic inhibitory compex (AIC) that inhibits spontaneous DISC signaling. However, when c-FLIPL is absent from the AIC, activation of caspase-8 via DR5 and FADD triggers DISC activation and apoptosis. We did not detect any DR4 in the DR5 immunocomplexes, indicating that DR5 and DR4 do not exist as a heterocomplex in MCF-7 cells. Additionally, since c-FLIPS was not detected in the immunoprecipitated complex, we determined whether c-FLIPS expression was involved in regulating apoptosis in these cells. Transfection of cells with c-FLIPL- or FLIPS-specific siRNAs silenced the expression of each gene (Fig. 7A), and induced apoptosis in 50% and 15% of the cells, respectively, as compared to 8% in non-targeting siRNA-treated cells (Fig. 7B). These results clearly show that c-FLIP-specific siRNA-triggered apoptosis is primarily due to the silencing of the c-FLIPL gene and not the c-FLIPS gene, and that c-FLIPL expression is required to prevent spontaneous caspase-8 activation from occurring at the DISC. Furthermore, the small increase in spontaneous apoptosis following the knockdown of the c-FLIPS gene may be due to its role in regulating the formation and activation of the DISC, even though it does not bind directly to DR5.
Fig. 6. DR5 binds to FADD, caspase-8, and c-FLIP.
Input cell lysates and anti-DR5 immunoprecipitates were analyzed by Western blotting using DR5-, DR4-, caspase-8-, c-FLIP-, and FADD-specific antibodies. No proteins were immunoprecipitated using a control isotype-specific antibody.
Fig. 7. c-FLIPL inhibits spontaneous apoptosis.
A, Immunoblot analysis of c-FLIPL, c-FLIPS, and β-actin in cell lysates treated with 100 nM of non-targeting siRNA (SC), c-FLIPL-targeting siRNA (FL), or c-FLIPS-targeting siRNA (FS) for 48 h. B, Cells were treated with 100 nM of non-targeting siRNA (SC), c-FLIPL-targeting siRNA (FL), or c-FLIPS-targeting siRNA (FS) for 48 h, and apoptosis was quantified by counting fragmented nuclei after staining with Hoecsht 33342 among 200 cells. All values are representative of three independent experiments, and error bars show standard deviation from triplicate counts.
3.5. Knockdown of c-FLIP decreases the proliferation of MCF-7 cells
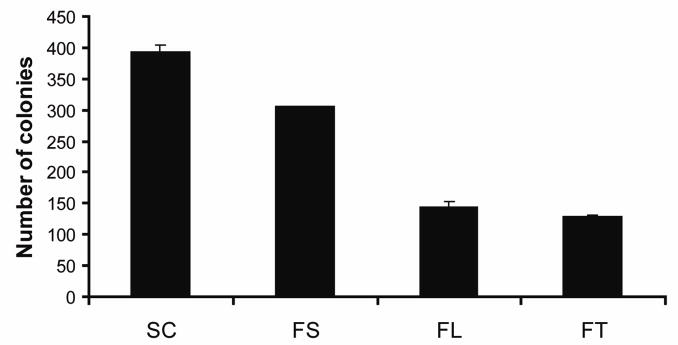
To examine whether silencing the c-FLIP gene affects the proliferation and long term survival of MCF-7 cells, we executed a colony forming assay. The results presented in Fig. 8 show that cells transfected with c-FLIP-specific siRNA, c-FLIPL-specific siRNA, or c-FLIPS-specific siRNA reduced the number of colonies by 67%, 63%, and 22%, respectively. These data show that both c-FLIP isoforms promote the proliferation of MCF-7 cells, but c-FLIPL appears to be more important than c-FLIPS in promoting long term survival. Collectively, these results reveal that c-FLIPL inhibits spontaneous DISC formation and promotes breast cancer cell proliferation.
Fig. 8. Knockdown of c-FLIP decreases the proliferation of MCF-7 cells.
Cells were transfected with 100 nM of non-targeting siRNA (SC), c-FLIP-specific siRNA (FT), c-FLIPL-targeting siRNA (FL), or c-FLIPS-targeting siRNA (FS) for 48 h. The cells were harvested and 1,000 cells were transferred to 6-well plates containing complete media. Colonies were counted after incubation for 5 days. The values are representative of three independent experiments, and error bars show standard deviation from triplicate counts.
4. Discussion
c-FLIP is a regulator of apoptosis that is mediated by the activation of death receptors TNFR1, Fas, DR4, and DR5 [43, 44]. Following death receptor engagement, c-FLIP inhibits caspase-8 binding to FADD and prevents DISC formation and apoptosis. Several studies have shown that silencing c-FLIP expression by using c-FLIP-specific siRNA induces spontaneous apoptosis in lung, colorectal, and lymphoma cancer cell lines, and sensitizes cells to TRAIL- and chemotherapy-mediated apoptosis [23, 26, 28, 41]. However, detailed molecular mechanisms by which downregulation of c-FLIP triggers spontaneous apoptosis have not been reported. In the present study, we found that the knockdown of the c-FLIP gene triggers ligand-independent DR5-, FADD-, caspases-8-, and -9-induced apoptosis in MCF-7 breast cancer cells. Our data revealed that a ligand-independent pre-associated apoptotic inhibitory complex (AIC) containing DR5, FADD, caspase-8, c-FLIPL, but not DR4 or c-FLIPS exists in MCF-7 cells, and the absence of c-FLIPL from the complex leads to caspase-8 activation and apoptosis. In support of our findings, several studies have shown that DR5 can recruit caspase-8 and FADD independently of DR4 after receptor activation [45, 46], and current studies are underway to determine if DR4 plays a role in apoptosis following the knockdown of c-FLIP. However, studies performed using neutralizing antibodies or receptor-selective mutants to DR4 and DR5, identified DR5 as having a more prominent role in death receptor-mediated apoptosis [47–49].
In agreement with a previous study, our data showed that FADD is required for activating caspase-8 and triggering apoptosis in the absence of c-FLIP [29]. c-FLIPL may inhibit FADD activity in the DISC by a similar mechanism that has been shown for the viral FLIP, M159, which cooperatively assembles with FADD and prevents FADD self-association that is required for caspase activation in the DISC [50]. By using DR5-specific siRNA, our data showed that DR5 is required for processing procaspase-8 to active caspase-8 following knockdown of the c-FLIP gene. Previously, c-FLIPL has been shown to bind directly to DR5 by a FADD-independent mechanism; therefore, the interaction of c-FLIPL with DR5 may prevent DR5-mediated caspase-8 activation in the DISC [42]. In this paper, only caspase-8 was shown to be involved in spontaneous apoptosis due to c-FLIPL knockdown; however, caspase-10 may also be involved in triggering apoptosis in cells that express caspase-10 [51]. Together, these experiments provide direct support for the conclusion that c-FLIPL is required to prevent constitutive DISC signaling in MCF-7 cells.
These results are intriguing considering the classical model of death receptor-induced apoptosis that requires the binding of extracellular ligands, such as Fas ligand or TRAIL, to their cognate receptors, Fas and DR4/DR5, respectively, leading to the trimerization of the receptors and recruitment of cytosolic death domain containing proteins which trigger downstream signaling events. However, a recent study showed that TNFα receptors pre-associate as homotrimers on the cell surface prior to ligand binding [52]. In fact, various stimuli have been shown to mediate the clustering of pre-associated homotrimers on the cell membrane without ligand binding [53–56]. Moreover, our results show that c-FLIPL prevents the activation of pre-associated DISCs, and thereby protects the cells from spontaneous apoptosis. Therefore, c-FLIPL serves as a cytoprotective protein by preventing apoptotic signaling pathways in the absence of death-ligand engagement. The cytoprotective role of c-FLIP is supported by several studies that have shown c-FLIP can regulate the NFκB, ERK, and Wnt signaling pathways [32, 34, 57]. In fact, our clonogenic assay results reveal that the knockdown of c-FLIP decreases the proliferation and long term survival of MCF-7 cells.
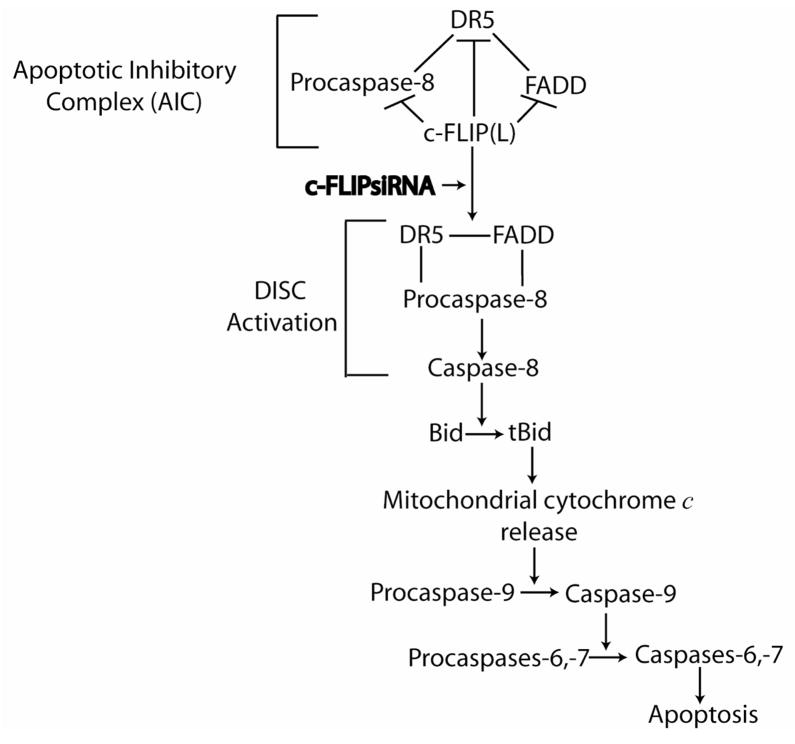
Based on the results from this study, we have proposed an apoptotic signaling pathway following the knockdown of c-FLIP in MCF-7 cells (Fig. 9). From our findings, an apoptotic inhibitory complex (AIC) comprised of DR5, FADD, caspase-8, and c-FLIPL exists, and the knockdown of c-FLIPL triggers DISC activation leading to FADD- and DR5-mediated caspase-8 activation. Active caspase-8 cleaves Bid to form truncated Bid resulting in mitochondrial disintegration and caspase-9 activation. Activation of caspase-9 leads to the activation of the downstream effector caspases-6 and -7 triggering apoptosis.
Fig. 9. Proposed signaling pathway of c-FLIP siRNA-triggered spontaneous apoptosis in MCF-7 cells.
In this signaling pathway, we propose that an apoptotic inhibitory complex (AIC) comprised of DR5, FADD, caspase-8, and c-FLIPL exists, and c-FLIPL inhibits caspase-8 activation. The knockdown of c-FLIPL using a c-FLIP-specific siRNA induces DISC activation by triggering DR5- and FADD-mediated activation of procaspase-8, forming active caspase-8. Active caspase-8 cleaves Bid to form tBid, leading to the release of cytochrome c from the mitochondria. Mitochondrial cytochrome c release activates procaspase-9 producing active caspase-9, which activates downstream caspases-6 and -7 thereby inducing apoptosis. The solid lines indicate protein binding partners and the brackets show protein complexes that comprise the AIC and DISC.
There does not appear to be a “handle” to inhibit c-FLIP function with small molecule ligands, since its cytoprotective DISC binding is mediated by highly conserved DEDs which function by homotypic binding. However, small molecules that induce c-FLIP downregulation and sensitize neoplastic cells to apoptosis induction by the cytokine TRAIL have been identified [58–61]. Moreover, several studies have shown that silencing c-FLIP expression by using c-FLIP-specific siRNA induces apoptosis in lung, colorectal, and lymphoma cancer cell lines, and sensitizes cells to TRAIL- and chemotherapy-mediated apoptosis. Therefore, the development of gene therapy strategies to knockdown c-FLIP expression should overcome the barrier of dose-limiting toxicity associated with cancer chemotherapy. In conclusion, this study adds key mechanistic insight into how c-FLIP prevents ligand-independent death signaling and validates c-FLIP as a relevant therapeutic target for breast cancers.
Acknowledgments
We would like to thank Dr. Mary D Kraeszig for editorial assistance and Sherry Clendenon for assistance with fluorescent microscopy. This work was supported by a research grants from the National Cancer Institute (R01 CA 101743) and the Indiana University Cancer Center Translational Research Acceleration Collaboration (ITRAC) initiative to A.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–24. doi: 10.1038/sj.onc.1209605. [DOI] [PubMed] [Google Scholar]
- 2.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–66. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 3.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 7.Gogvadze V, Orrenius S. Mitochondrial regulation of apoptotic cell death. Chem Biol Interact. 2006;163:4–14. doi: 10.1016/j.cbi.2006.04.010. Epub 2006 Apr 30. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 10.Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 11.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Wang H, Lee P, Melamed J, Li CX, Zhang F, et al. Androgen receptor and prostate apoptosis response factor-4 target the c-FLIP gene to determine survival and apoptosis in the prostate gland. J Mol Endocrinol. 2006;36:463–83. doi: 10.1677/jme.1.01991. [DOI] [PubMed] [Google Scholar]
- 13.Morales JC, Ruiz-Magana MJ, Ruiz-Ruiz C. Regulation of the resistance to TRAIL-induced apoptosis in human primary T lymphocytes: role of NF-kappaB inhibition. Mol Immunol. 2007;44:2587–97. doi: 10.1016/j.molimm.2006.12.015. Epub 007 Jan 25. [DOI] [PubMed] [Google Scholar]
- 14.Valnet-Rabier MB, Challier B, Thiebault S, Angonin R, Margueritte G, Mougin C, et al. c-Flip protein expression in Burkitt’s lymphomas is associated with a poor clinical outcome. Br J Haematol. 2005;128:767–73. doi: 10.1111/j.1365-2141.2005.05378.x. [DOI] [PubMed] [Google Scholar]
- 15.Steele LP, Georgopoulos NT, Southgate J, Selby PJ, Trejdosiewicz LK. Differential susceptibility to TRAIL of normal versus malignant human urothelial cells. Cell Death Differ. 2006;13:1564–76. doi: 10.1038/sj.cdd.4401846. Epub 2006 Jan 13. [DOI] [PubMed] [Google Scholar]
- 16.Mezzanzanica D, Balladore E, Turatti F, Luison E, Alberti P, Bagnoli M, et al. CD95-mediated apoptosis is impaired at receptor level by cellular FLICE-inhibitory protein (long form) in wild-type p53 human ovarian carcinoma. Clin Cancer Res. 2004;10:5202–14. doi: 10.1158/1078-0432.CCR-03-0537. [DOI] [PubMed] [Google Scholar]
- 17.Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007;13:5070–5. doi: 10.1158/1078-0432.CCR-06-2547. [DOI] [PubMed] [Google Scholar]
- 18.Song JH, Song DK, Herlyn M, Petruk KC, Hao C. Cisplatin down-regulation of cellular Fas-associated death domain-like interleukin-1beta-converting enzyme-like inhibitory proteins to restore tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human melanoma cells. Clin Cancer Res. 2003;9:4255–66. [PubMed] [Google Scholar]
- 19.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–13. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 21.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–40. doi: 10.1074/jbc.M101780200. Epub 2001 Mar 05. [DOI] [PubMed] [Google Scholar]
- 22.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–71. doi: 10.1074/jbc.M206882200. Epub 2002 Sep 4. [DOI] [PubMed] [Google Scholar]
- 23.Sharp DA, Lawrence DA, Ashkenazi A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem. 2005;280:19401–9. doi: 10.1074/jbc.M413962200. Epub 2005 Mar 10. [DOI] [PubMed] [Google Scholar]
- 24.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 25.Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 26.Dutton A, O’Neil JD, Milner AE, Reynolds GM, Starczynski J, Crocker J, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin’s lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci U S A. 2004;101:6611–6. doi: 10.1073/pnas.0400765101. Epub 2004 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–36. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 28.Rogers KM, Thomas M, Galligan L, Wilson TR, Allen WL, Sakai H, et al. Cellular FLICE-inhibitory protein regulates chemotherapy-induced apoptosis in breast cancer cells. Mol Cancer Ther. 2007;6:1544–51. doi: 10.1158/1535-7163.MCT-06-0673. [DOI] [PubMed] [Google Scholar]
- 29.Wilson TR, McLaughlin KM, McEwan M, Sakai H, Rogers KM, Redmond KM, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67:5754–62. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- 30.Day TW, Najafi F, Wu CH, Safa AR. Cellular FLICE-like inhibitory protein (c-FLIP): A novel target for Taxol-induced apoptosis. Biochem Pharmacol. 2006;71:1551–61. doi: 10.1016/j.bcp.2006.02.015. Epub 2006 Mar 31. [DOI] [PubMed] [Google Scholar]
- 31.Ganten TM, Haas TL, Sykora J, Stahl H, Sprick MR, Fas SC, et al. Enhanced caspase-8 recruitment to and activation at the DISC is critical for sensitisation of human hepatocellular carcinoma cells to TRAIL-induced apoptosis by chemotherapeutic drugs. Cell Death Differ. 2004;11:S86–96. doi: 10.1038/sj.cdd.4401437. [DOI] [PubMed] [Google Scholar]
- 32.Naito M, Katayama R, Ishioka T, Suga A, Takubo K, Nanjo M, et al. Cellular FLIP inhibits beta-catenin ubiquitylation and enhances Wnt signaling. Mol Cell Biol. 2004;24:8418–27. doi: 10.1128/MCB.24.19.8418-8427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada K, Nakamura M, Matsuyoshi S, Ishida E, Konishi N. Specific positive and negative effects of FLIP on cell survival in human prostate cancer. Carcinogenesis. 2006;27:1349–57. doi: 10.1093/carcin/bgi380. Epub 2006 Mar 14. [DOI] [PubMed] [Google Scholar]
- 34.Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 2006;203:1295–305. doi: 10.1084/jem.20051556. Epub 2006 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Wang S, Song X, Sima N, Xu X, Luo A, et al. The relationship between c-FLIP expression and human papillomavirus E2 gene disruption in cervical carcinogenesis. Gynecol Oncol. 2007;105:571–7. doi: 10.1016/j.ygyno.2007.01.051. Epub 2007 Apr 12. [DOI] [PubMed] [Google Scholar]
- 36.Chen HX, Liu YJ, Zhou XD, Luo RY. Expression of cellular FLICE/caspase-8 inhibitory protein is associated with malignant potential in endometrial carcinoma. Int J Gynecol Cancer. 2005;15:663–70. doi: 10.1111/j.1525-1438.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhou XD, Yu JP, Chen HX, Yu HG, Luo HS. Expression of cellular FLICE-inhibitory protein and its association with p53 mutation in colon cancer. World J Gastroenterol. 2005;11:2482. doi: 10.3748/wjg.v11.i16.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rathore N, Matta H, Chaudhary PM. An evolutionary conserved pathway of nuclear factor-kappaB activation involving caspase-mediated cleavage and N-end rule pathway-mediated degradation of IkappaBalpha. J Biol Chem. 2004;279:39358–65. doi: 10.1074/jbc.M406712200. Epub 2004 Jul 13. [DOI] [PubMed] [Google Scholar]
- 39.Sun SY, Liu X, Zou W, Yue P, Marcus AI, Khuri FR. The farnesyltransferase inhibitor lonafarnib induces CCAAT/enhancer-binding protein homologous protein-dependent expression of death receptor 5, leading to induction of apoptosis in human cancer cells. J Biol Chem. 2007;282:18800–9. doi: 10.1074/jbc.M611438200. Epub 2007 May 9. [DOI] [PubMed] [Google Scholar]
- 40.Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem. 1999;47:1101–10. doi: 10.1177/002215549904700901. [DOI] [PubMed] [Google Scholar]
- 41.Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25:838–48. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 42.Jin TG, Kurakin A, Benhaga N, Abe K, Mohseni M, Sandra F, et al. Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5. J Biol Chem. 2004;279:55594–601. doi: 10.1074/jbc.M401056200. Epub 2004 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 2007;17:759–71. doi: 10.1038/cr.2007.52. [DOI] [PubMed] [Google Scholar]
- 44.Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 46.Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–55. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–12. doi: 10.1074/jbc.M410660200. Epub 004 Nov 1. [DOI] [PubMed] [Google Scholar]
- 48.Park SJ, Wu CH, Choi MR, Najafi F, Emami A, Safa AR. P-glycoprotein enhances TRAIL-triggered apoptosis in multidrug resistant cancer cells by interacting with the death receptor DR5. Biochem Pharmacol. 2006;72:293–307. doi: 10.1016/j.bcp.2006.04.024. Epub 2006 May 4. [DOI] [PubMed] [Google Scholar]
- 49.Wu C, Kao C, Safa AR. TRAIL recombinant adenovirus triggered robust apoptosis in multidrug resistant HL-60/Vinc cells preferentially through the death receptor DR5. Hum Gene Ther. 2008;13:13. doi: 10.1089/hum.2008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ, et al. Crystal structure of MC159 reveals molecular mechanism of DISC assembly and FLIP inhibition. Mol Cell. 2005;20:939–49. doi: 10.1016/j.molcel.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engels IH, Totzke G, Fischer U, Schulze-Osthoff K, Janicke RU. Caspase-10 sensitizes breast carcinoma cells to TRAIL-induced but not tumor necrosis factor-induced apoptosis in a caspase-3-dependent manner. Mol Cell Biol. 2005;25:2808–18. doi: 10.1128/MCB.25.7.2808-2818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–4. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 53.Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987–92. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- 54.Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320:165–9. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- 55.Martin S, Phillips DC, Szekely-Szucs K, Elghazi L, Desmots F, Houghton JA. Cyclooxygenase-2 inhibition sensitizes human colon carcinoma cells to TRAIL-induced apoptosis through clustering of DR5 and concentrating death-inducing signaling complex components into ceramide-enriched caveolae. Cancer Res. 2005;65:11447–58. doi: 10.1158/0008-5472.CAN-05-1494. [DOI] [PubMed] [Google Scholar]
- 56.Delmas D, Rebe C, Micheau O, Athias A, Gambert P, Grazide S, et al. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene. 2004;23:8979–86. doi: 10.1038/sj.onc.1208086. [DOI] [PubMed] [Google Scholar]
- 57.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640–8. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Yue P, Schonthal AH, Khuri FR, Sun SY. Cellular FLICE-inhibitory protein down-regulation contributes to celecoxib-induced apoptosis in human lung cancer cells. Cancer Res. 2006;66:11115–9. doi: 10.1158/0008-5472.CAN-06-2471. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Zhang X, Barrisford GW, Olumi AF. Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett. 2007;251:146–57. doi: 10.1016/j.canlet.2006.11.013. Epub 2006 Dec 20. [DOI] [PubMed] [Google Scholar]
- 60.Kabore AF, Sun J, Hu X, McCrea K, Johnston JB, Gibson SB. The TRAIL apoptotic pathway mediates proteasome inhibitor induced apoptosis in primary chronic lymphocytic leukemia cells. Apoptosis. 2006;11:1175–93. doi: 10.1007/s10495-006-8048-9. [DOI] [PubMed] [Google Scholar]
- 61.Guo F, Sigua C, Tao J, Bali P, George P, Li Y, et al. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004;64:2580–9. doi: 10.1158/0008-5472.can-03-2629. [DOI] [PubMed] [Google Scholar]