Abstract
Polymorphonuclear neutrophils (PMN) are critical innate immune effector cells that either protect the host or exacerbate organ dysfunction by migrating to injured or inflamed tissues. Resuscitated hemorrhagic shock (HS/R) following major trauma promotes the development of organ inflammation by priming PMN migration and activation in response to a second, often trivial, stimulus (a so-called “two hit” phenomenon). PMN mobilization from bone marrow (BM) supports a sustained, HS/R-primed migration of PMN. We addressed the role and mechanism of HS/R in regulating PMN egress from BM. We demonstrate that HS/R through the alarmin HMGB1 induces IL-23 secretion from macrophages in an autocrine and TLR4 signaling-dependent manner, in turn, IL-23, through an IL-17-G-CSF-mediated mechanism induces PMN egress from BM. We also show that β-adrenergic receptor activation by catecholamine of macrophages mediates the HS/R-induced release of HMGB1. These data indicate that HS/R, a global ischemia/reperfusion stimulus, regulates PMN mobilization through a series of interacting pathways that include neuro-endocrine and innate and acquired immune systems. Blocking this novel signaling axis may present a novel therapeutic target for post-trauma inflammation.
Keywords: hemorrhage, neutrophils, bone marrow, HMGB1, IL-17, IL-23, TLR
Introduction
Hemorrhagic shock/resuscitation (HS/R) promotes the development of multiple organ dysfunction by priming the innate immune system for an exaggerated inflammatory response, which contributes to high mortality in trauma patients. In post-hemorrhagic shock organ injury, polymorphonuclear neutrophils (PMN) sequestration is a hallmark and plays a central role in the development of the organ injury. We have shown that HS/R primes for PMN accumulation in the lung, resulting in an influx of PMN that exceeds the total number of PMN in circulation, in response to a second insult, e.g. a small dose of intratracheal LPS administration (1). Circulating PMN numbers are under tight homeostatic regulation. The HS/R-enhanced PMN tissue/organ sequestration suggests a yet unknown HS/R-regulated mechanism of PMN mobilization, by which a sustained local PMN infiltration can be maintained.
The IL-17-granulocyte colony-stimulating factor (G-CSF) signaling axis has been identified as an important signaling for the dynamic regulation of PMN production and release from the bone marrow (BM) in response to environmental stresses (2, 3). IL-17 is a proinflammatory cytokine expressed and secreted by specific activated γδ+ T cells subsets, CD4+ CD8− αβhigh T cells (also termed Th17 cells), and CD4− CD8− αβlow T cells (4–8). Additionally, IL-17 can induce G-CSF-dependent neutrophilia when expressed in mice (9–12). G-CSF is a member of a family of hematopoietic growth factors that selectively stimulates the proliferation of neutrophilic precursor cells, augments their activation and release from BM stores, and prolongs their in vivo survival (13). Studies have shown that levels of G-CSF expression in a rat model of HS correlated with severity of shock, PMN infiltration, and lung injury (14).
IL-23 is an important upstream regulator of IL-17 production (8, 15), and is required for neutrophil homeostasis in normal and neutrophilic mice (16). IL-23 is produced by activated myeloid antigen-presenting cells, such as macrophages and dendritic cells (17), in response to bacteria and their products (18).
Toll-like receptor 4 (TLR4) recognizes both pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP), and, hence, is involved in the immune response during both infection and injury (19–22). Recently, the role of TLR4 in following nominally sterile injury has been revealed in studies showing that the lack of functional TLR4 or impairment of TLR4 signaling results in reduced tissue damage and an attenuated inflammatory response in HS (21, 23–25) and organ ischemia/reperfusion (I/R) (26–29).
High-mobility group box 1(HMGB1) is a nuclear protein that functions to stabilize nucleosome formation, acts as a transcription factor that regulates the expression of several genes, and is now recognized to be a DAMP(30–32). HMGB1 can be secreted by innate immune cells in response to microbial products or other inflammatory stimuli (33, 34), and released by injured cells(35–37). There is also evidence that HMGB1 can be released in a regulated manner by parenchyma cells in response to cytokines (38) or redox stress/hypoxia (39). HMGB1 can act as an early mediator of inflammation contributing to the development of acute lung injury after hemorrhage(22, 40), and hepatic injury after liver I/R (26). HMGB1 signals via TLR4 in HS-primed acute lung injury (22), and hepatic I/R (39), and others have provided evidence for direct interaction of HMGB1 with the TLR4 receptor complex in vitro (31, 32, 41).
In the present study, we addressed the role of HS/R in regulating PMN egress from BM, and the mechanism that underlies the regulation. We demonstrate that HS/R through HMGB1induces IL-23 secretion from macrophages in a manner that depends on TLR4 signaling. In turn, IL-23 induces PMN egress from BM through a mechanism that includes IL-17 and G-CSF. We also show that β-adrenergic receptor activation by catecholamines in macrophages mediates the HS/R-induced release of HMGB1. These data raise the possibility of unifying the main mechanisms associated with HS/R (inflammatory, neuroendocrine, and DAMP).
Materials and Methods
Materials
Recombinant HMGB1 was purchased from R & D systems (Minneapolis, MN). Stimulating activity of the recombinant HMGB1 was confirmed in mouse macrophages by assay of TNF release, with an ED50 of 3 ~ 12 μg/ml. Polyclonal neutralizing antibody against HMGB1 prepared as described previously (34) was provided by Dr. K. J. Tracey (Feinstein Institute for Medical Research, North Shore-LIJ Health System, Manhasset, NY). Polyclonal anti-HMGB1 antibody for Western blotting, and kinase assay kits for IRAK4 were purchased from Cell Signaling Technology (Danvers, MA). Polyclonal rabbit anti-IRAK-4 antibody and MyD88 homodimerization inhibitory peptide set were purchased from Imgenex (San Diego, CA). Nonimmune rabbit IgG (item I5006) and all other chemicals were obtained from Sigma-Aldrich, except where noted.
Hemorrhagic shock and resuscitation
Male C3H/HeJ mice, which are not responsive to LPS because of a point mutation of tlr4 affecting the TIR domain(42, 43) and control wild-type (WT) C3H/HeOuJ mice were purchased from the Jackson Laboratory. All experimental protocols involving animals were approved by Institutional Animal Care and Use Committee of VA Pittsburgh Healthcare System. Mice were 12–14 weeks of age at the time of experiments. Animals were anesthetized with 50 mg/kg of ketamine and 5 mg/kg of xylazine via intraperitoneal (i.p.) administration. Femoral arteries were cannulated for monitoring of mean arterial pressure (MAP), blood withdrawal and resuscitation. Hemorrhagic shock was initiated by blood withdrawal and reduction of the MAP to 40 mmHg within 20 min. Blood was collected into 1 ml syringe and heparinized to prevent clotting. In order to exclude the effect of heparin on immune processes, equal amounts of heparin (10 U) was injected into sham animals through the cannulated femoral artery during the sham operation. After a hypotensive period of 1 hour, animals were resuscitated by transfusion of the shed blood and Ringer’s Lactate (RL) in a volume equal to that of shed blood, over a period of 60 min. The catheters were then removed, the femoral artery was ligated, and the incisions were closed. Sham animals underwent the same surgical procedures without hemorrhage and resuscitation. In some experiments, one of the neutralizing antibodies against HMGB1 (600 μg per mouse), IL-23 (100 μg per mouse, BioLegend, San Diege, CA), IL-17 (100 μg per mouse, BioLegend, San Diego, CA), G-CSF (100 μg per mouse, Abcam, Cambridge, MA) or nonimmune control IgG was injected intraperitoneally into the mice 10 min before hemorrhage respectively. At various time points after resuscitation, cells were harvested from bone marrow and peripheral blood for flow cytometry, and serum was collected for ELISA or Western blot.
AM isolation
In this study AM were used to elucidate the role of macrophage-derived IL-23 in mediating HS/R-induced PMN mobilization based on the following considerations: 1) AM possess the basic characteristics of macrophages and respond to in vitro stimulation (1, 21); 2) collection of AM from BAL does not need induction of cell emigration, thus eliminates possible artifacts of macrophage activation; and 3) the amount of AM harvested from HS/R and sham-operated animals is comparable, which provides ideal controls. BAL was performed as previously described (1). Normally the BAL fluid contains ~91% of AM, and ~9% of other cells including PMN, lymphocytes, and erythrocytes. The immunomagnetic separation system as described above was used to isolate AM from BAL fluid. Magnetic nanoparticle-conjugated antibodies (anti-mouse Gr-1, anti-CD4, anti-CD8, and anti-CD45R/B220 antibodies; BD Biosciences Pharmingen, San Diego, CA) were chosen to label and remove PMN and lymphocytes. The resulting sample contained ~1 ×106 cells, which consisted of >98% macrophages, and cell viability was >95%.
Flow cytometry
The procedures of flow cytometry analysis were performed as described elsewhere(44). Briefly, 100ul peripheral blood or 1 × 106 bone marrow nucleated cells were stained with anti-CD11b-PE antibody (eBioscience, La Jolla, CA) and anti-Gr-1-FITC antibody (eBioscience, La Jolla, CA). The red cells in the blood were lysed by FACS Lysing Solution (BD Biosciences, San Jose, CA). The stained cells were applied for data acquisition on Coulter EPICS XL Cytometer (Beckman Coulter) and re-analyzed with software WinMDI (Version 2.9).
IRAK4 kinase assay
Equal amounts of whole AM lysates were incubated with polyclonal rabbit anti-IRAK-4 antibody for 2 h at 4°C on a rotor, after which 50 μl of 50% protein G plus agarose was added to each sample and incubated for an additional 2 h at 4°C. The samples were precipitated in a microcentrifuge, and the beads were washed twice with lysis buffer and twice with kinase buffer following the kit instruction. The beads were incubated at 25°C for 30 min in a final volume of 37.5 μl of kinase buffer in the presence of biotinylated ezrin/radixin/moesin peptide as a substrate (1.5 μM/sample) and 200 μM ATP, both of which were provided in the HTScan IRAK4 kinase assay kit (Cell Signaling Technologies). After adding 50 μl/sample stop buffer (50 mM EDTA, pH 8), 25 μl of each reaction and 75 μl dH2O were transferred to 96-well streptavidin-coated plate (PerkinElmer Life Sciences) and incubated at room temperature for 60 min. IRAK4 activity was then measured following manufacture’s instructions for the kit using primary anti-phospho- ezrin/radixin/moesin antibody (Cell Signaling Technologies) and secondary Europium labeled anti-rabbit antibody (PerkinElmer Life Sciences) with DELFIA enhancement solution (PerkinElmer Life Sciences). Fluorescence emission at 615 nm was detected with SpectraMax M2™ Multi-detection reader (Molecular Devices, Sunnyvale, CA).
ELISA
IL-23, IL-17 and G-CSF levels in cell culture media and serum were evaluated by ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Reverse transcription and PCR
Total RNA from alveolar macrophages and blood T cells was isolated using the TRI-REAGENT (Molecular Research Center, Cincinnati, OH) following manufacture’s instructions. Total RNA was then reverse-transcribed using a SuperScript™ Preamplification kit (Invitrogen, Carlsbad, CA). Primers for IL-23 P19 amplification were: position 790 forward 5′-AACCCATTAGGACTTGTGC-3′, position 1084 reverse 5′-CTGAGCCACCCAGGAAAG-3′, amplifying 313 bp. Primers for IL-17 mplification were: position 310 forward 5′-CCTCTGTGATCTGGGAAGC-3′, position 699 reverse 5′-CACGAAGCAGTTTGGGAC-3′, amplifying 309 bp. Primers for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from R&D Systems, Inc. (Minneapolis, MN). The product of reverse transcription was amplified following the kit instructions. PCR products were separated using 1.2% agarose gel and identified by ethidium bromide staining. Expression of mRNA was quantitated using Scion Image® software (Scion Corp., Frederick, MD) and normalized by the GAPDH signal.
Statistics
The data are presented as mean ± SEM of n determinations as indicated in the figures. Data were analyzed by one-way analysis of variance; post hoc testing was performed using the Bonferroni modification of the t test. When individual studies are demonstrated, these are representative of at least three independent studies.
Results
PMN mobilization following HS/R
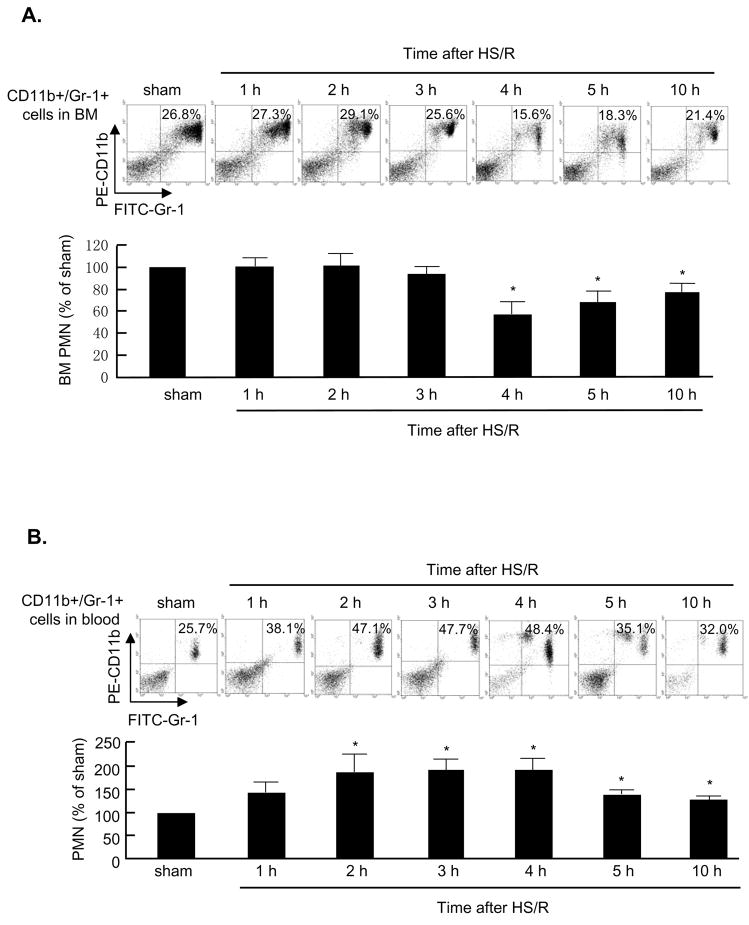
We quantified CD11b+/Gr-1+ PMN in BM and blood by flow cytometry. The CD11b+/Gr-1+ cells that were collected by cell-sorting and stained with Wright-Giemsa staining displayed a typical PMN morphology (data not shown). HS/R induced a 42% decrease in the CD11b+/Gr-1+ cell level in BM by 4h after HS/R, as compared to sham-cannulated animals. These cells remained at a low level for 10 h in HS/R animals (Fig. 1A). However, no changes in CD11b+/Gr-1+ cell level in BM was observed in sham-operated animals up to 10 h (data not shown). In the peripheral blood, the CD11b+/Gr-1+ cells were increased at as early as 2 h after HS/R, and remained 1.3 to 1.9 times higher than those in the sham animals up to at least 10 h. Notably, a group of CD11b+/Gr-1+ cells that expressed less Gr-1, possibly a group of immature myeloid cell population, started to appear in the peripheral blood at 4 h after resuscitation (Fig. 1B).
Figure 1. Dynamic alterations in CD11b+/Gr-1+ cells distribution in BM and circulating blood.
A and B: Representative density plots of CD11b+/Gr-1+ cells in mouse BM and blood. Mice were subjected to sham operation for 5 h or hemorrhagic shock/resuscitation (HS/R). Femoral bone and whole blood were harvested at the time as indicated, and cells in the BM and blood were collected and stained with anti-CD11b-PE and anti-Gr-1-FITC followed by flow cytometry analysis. The graphs depict means and SE from 3 mice. *P< 0.01 compared with the sham group.
TLR4 and HMGB1 are required for HS/R-induced PMN mobilization
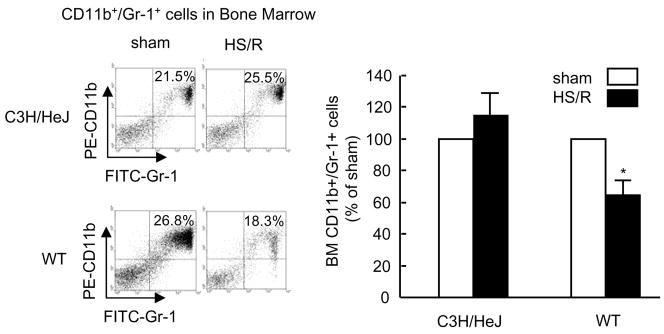
We have shown previously that TLR4 signaling plays an important role in mediating HS/R-induced activation of innate immunity (21, 25, 45). To address the role of TLR4 in HS/R-induced PMN mobilization, TLR4-mutant C3H/HeJ mice were subjected to HS/R. Four hours after resuscitation, BM CD11b+/Gr-1+ cells were quantified. As shown in Fig. 2, HS/R failed to decrease the BM CD11b+/Gr-1+ cells in TLR4-mutant mice as compared to TLR4 WT mice, indicating an essential role for TLR4 in HS/R-induced PMN egress from BM.
Figure 2. TLR4 is required for HS/R-induced CD11b+/Gr-1+ cells mobilization from BM.
WT (C3H/HeOuJ) and TLR4-mutant (C3H/HeJ) mice were subjected to HS/R or sham operation. Cells in femoral BM were recovered 4 hours after HS/R or sham operation, CD11b+/Gr-1+ cells were detected by flow cytometry. The graph depicts the mean and SE of the changes in BM CD11b+/Gr-1+ cells from three mice. * P< 0.01 compared with sham group.
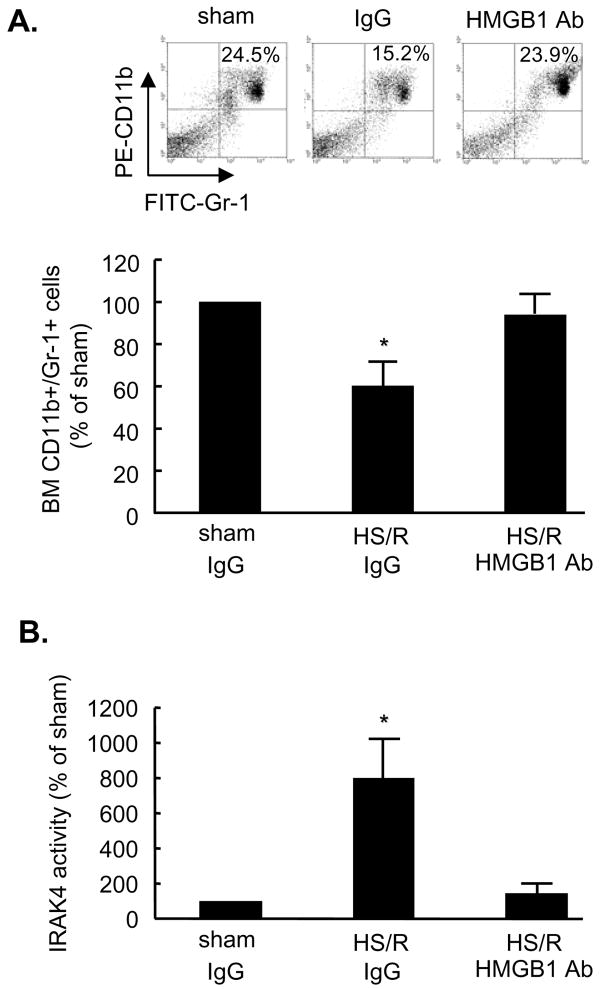
TLR4 recognizes a variety of endogenous ligands including HMGB1 (30, 46). We observed in our previous studies that HS/R caused an increase of HMGB1 in the serum, lung, and liver at 2 h after HS/R (22). To determine if endogenous HMGB1 contributes to HS/R-induced PMN mobilization, neutralizing antibody to HMGB1 was administered to mice 10 min before HS/R. Treatment with anti-HMGB1 antibody prevented the HS/R-induced reduction of the CD11b+/Gr-1+ cells in BM as compared to non-specific IgG-treated animals (Fig. 3A), suggesting that HMGB1 contributes to the regulation of PMN mobilization. To further determine whether the administration of anti-HMGB1 antibody actually attenuates TLR4 activation, IRAK4 activity, as a marker of TLR4 signaling in the PMN isolated from mouse blood, was analyzed. As shown in Fig. 3B, 2 h after HS/R, IRAK4 activity was significantly suppressed in the PMN from anti-HMGB1 antibody- treated animals as compared to that from the nonimmune IgG-treated group, indicating that the anti-HMGB1 antibody decreased the availability of HMGB1 for TLR4 activation. Thus, both HMGB1 and TLR4 are required for HS/R-induced PMN mobilization.
Figure 3. Pretreatment with neutralizing antibody to HMGB1 prevents HS/R-induced CD11b+/Gr-1+ cells mobilization from BM and TLR4 signaling activation.
WT mice received anti-HMGB1 antibody (600 μg per mouse) or nonimmune control IgG by i.p. injection 10 min before HS/R or sham operation. A. Femoral bones from the mice were harvested at 4 h after HS/R or sham operation, and BM cells were then collected for detection of CD11b+/Gr-1+ cells by flow cytometry. Representative density plots of BM CD11b+/Gr-1+ cells are shown. The graph depicts the mean and SE of the changes in BM CD11b+/Gr-1+ cells from three mice. * P< 0.01 compared with other groups. B. Blood PMN were isolated from the mice at 2 h after HS/R or sham operation for detection of IRAK4 activity. The graph depicts the mean and SE of the changes in IRAK4 activity from three mice. * P< 0.01 compared with other groups.
HMGB1-TLR4 signaling induces IL-23 and IL-17secretion
IL-23 derived from myeloid antigen-presenting cells is an important regulator of the IL-17-G-CSF axis. Accordingly, we sought to determine if HMGB1 acts through TLR4 to induce IL-23 release from AM. AM were isolated from the BAL fluid of WT and TLR4-mutant mice, the cells were treated with HMGB1 for 0 to 6 h, and the levels of IL-23 in the medium were measured. HMGB1 induced IL-23 release in WT AM as early as 1 h, and reached a peak at 2 h, as shown in Fig. 4A. However, HMGB1 failed to induce a significant release of IL-23 in TLR4-mutant AM.
Figure 4. Recombinant HMGB1 induces IL-23 release through TLR4-MyD88-dependent pathway.
A, TLR4 mutation or MyD88 suppression diminishes HMGB1-induced IL-23 release in AM. AM (5 × 106 cells/ml) isolated from WT and TLR4-mutant (C3H/HeJ) mice were incubated with recombinant HMGB1 (0.5 μg/ml) for 0 to 6 hours. In some experiments, WT AM were pre-incubated with MyD88 inhibitory peptide (100 μM) for 2 h, and then treated with HMGB1 (0.5 μg/ml) for 0 to 6 hours. IL-23 concentrations in the media were then determined using ELISA. The graphs show the mean and SE of the IL-23 levels from three independent experiments. * P<0.01 compared with the value at time=0. B, HMGB1 induces IRAK4 activation in the AM. The AM were treated as described in “A”, and IRAK4 activity was detected as described in the Methods. Data represent means + SE (n=3), * P< 0.01 compared with the value at time=0.
Previous studies have shown that TLR4 can signal through both MyD88-dependent and MyD88-independent pathways (47). To determine if IL-23 release induced by HMGB1/TLR4 is a MyD88-dependent event, we made use of the MyD88 inhibitor homodimerization inhibitory peptide (48). AM recovered from TLR4 WT mice were pre-incubated with MyD88 inhibitory peptide (100 μM) for 2 h and subsequently treated with HMGB1 for 0 to 6 h. MyD88 inhibitor eliminated the effect of HMGB1 on IL-23 release from the AM (Fig. 4A), indicating that HMGB1/TLR4 induces IL-23 release through a MyD88-dependent pathway.
We further examined the activation of IRAK4 in the HMGB1-treated WT AM. HMGB1 induced an 8.3-fold increase in IRAK4 activity by 1 h as compared to baseline (time = 0), and kept the IRAK4 activity at a 3.6-fold increase from the basal level at 6 h (Fig. 4B). HMGB1-induced IRAK4 activation was significantly suppressed in both TLR4-mutant AM and WT AM treated with MyD88 inhibitor (Fig. 4B). These results suggest an important role for TLR4-MyD88-IRAK4 signaling in mediating HMGB1-induced IL-23 secretion.
To further confirm the role of HMGB1 in inducing IL-23 release and subsequent IL- 17 secretion, we treated the mice in vivo with an anti-HMGB1 neutralizing antibody prior to HS/R, and IL-23 and IL-17 serum levels were then assessed 2 h after resuscitation. HS/R induced a 4.4-fold increase in serum IL-23, while the neutralizing antibody to HMGB1 prevented the HS/R-induced increase in serum IL-23 (Fig. 5A). Likewise, the HS/R-induced increase in serum IL-23 was significantly attenuated in TLR4-mutant mice (Fig. 5A). Consistent with the changes in serum IL-23, HS/R induced a 7.3-fold increase in serum IL-17, as shown in Fig. 5A. However, the HS/R-induced elevation of serum IL-17 was also greatly attenuated in both TLR4-mutant mice and TLR4 WT mice pretreated with neutralizing antibody to HMGB1 (Fig. 5A).
Figure 5. Neutralizing antibody against HMGB1 prevents HS/R-induced increase in IL-23 and IL-17 serum level as well as mRNA expression.
A. WT and TLR4-mutant mice received anti-HMGB1 antibody (600 μg per mouse) or nonimmune control IgG by i.p. injection 10 min before HS/R or sham operation. Serums were then isolated from the mice 2 hours after HS/R or sham operation for detection of IL-23 and IL-17 using ELISA. The graph depicts the mean and SE of the changes from three mice. * P< 0.01 compared with all other groups. B. WT mice were injected with anti-HMGB1 antibody (600 μg per mouse) or nonimmune control IgG by i.p.10 min before HS/R. AM and T cells were isolated using an immunomagnetic separation system from BAL fluid and blood, respectively, at 0 to 6 h after HS/R. IL-23 P19 and IL-17 mRNA were then analyzed by RT-PCR. The pictures are representative of three independent studies.
To address whether the HS/R-induced secretion of IL-23 and IL-17 are results of de novo synthesis in response to HMGB1, IL-23 P19 mRNA in AM and IL-17 mRNA in blood T cells were detected using RT-PCR at 0 to 6 h after HS/R. As shown in Fig. 5B, increased IL-23 P19 mRNA expression in the AM was detected starting from 0.5 h after HS/R, and elevation of IL-17 mRNA in the T cells was observed starting from 1 h after HS/R. Administration of neutralizing antibody against HMGB1 exhibited a suppressive effect on both IL-23 and IL-17 mRNA expression.
Taken together, the results indicate an important role for HMGB1-TLR4 signaling in HS/R-regulated IL-23 and IL-17 release.
Signals from the HMGB1-IL-23-IL-17pathway trigger G-CSF-induced PMN mobilization
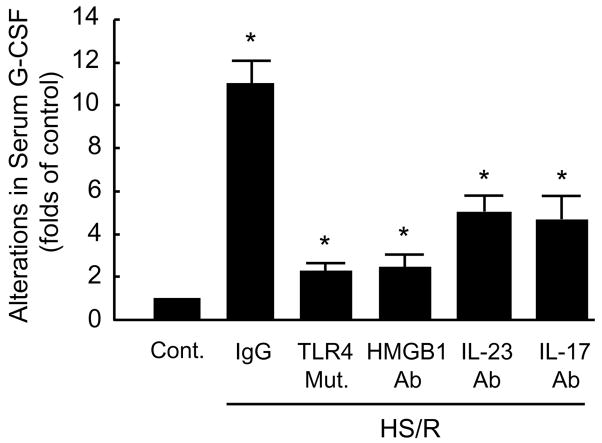
We next sought to determine if G-CSF, as a down-stream component of IL-17 signaling, mediates PMN egress from BM induced by HMGB1-TLR4-IL-23-IL-17 signaling. We first determined if HS/R could cause G-CSF release in a TLR4-dependent manor. Fig. 6 shows that HS/R induced an 11-fold increase in serum G-CSF at 4 h after HS/R as compared to the non-HS/R group; however, TLR4-mutation was associated with an 80% decrease in the HS/R-induced G-CSF release. Likewise, anti-HMGB1 antibody also markedly decreased G-CSF level by 79%. We then evaluated the effect of neutralizing antibodies to IL-23 and IL-17 on HS/R-induced G-CSF release. Both anti-IL-23 and anti-IL-17 antibodies significantly decreased the HS/R-induced increase in G-CSF serum levels (Fig. 6).
Figure 6. HS/R induces G-CSF release through HMGB1, TLR4, IL-23 and IL-17 signaling.
WT mice received neutralizing antibody against HMGB1 (600 μg per mouse), IL-23 (100 μg per mouse) or IL-17 (100 μg per mouse) or nonimmune control IgG by i.p. injection 10 min before HS/R or sham operation. The Serums from the mice were isolated at 4 h after HS/R or sham operation, and G-CSF levels were then detected using ELISA. To address the role of TLR4 in mediating HS/R-induced G-CSF release, TLR4-mutant mice were subjected to HS/R, and the serums were collected 4 hours after resuscitation, and G-CSF concentration was detected as well. Data represent mean ± SE (n=3). * P< 0.01 compared with control.
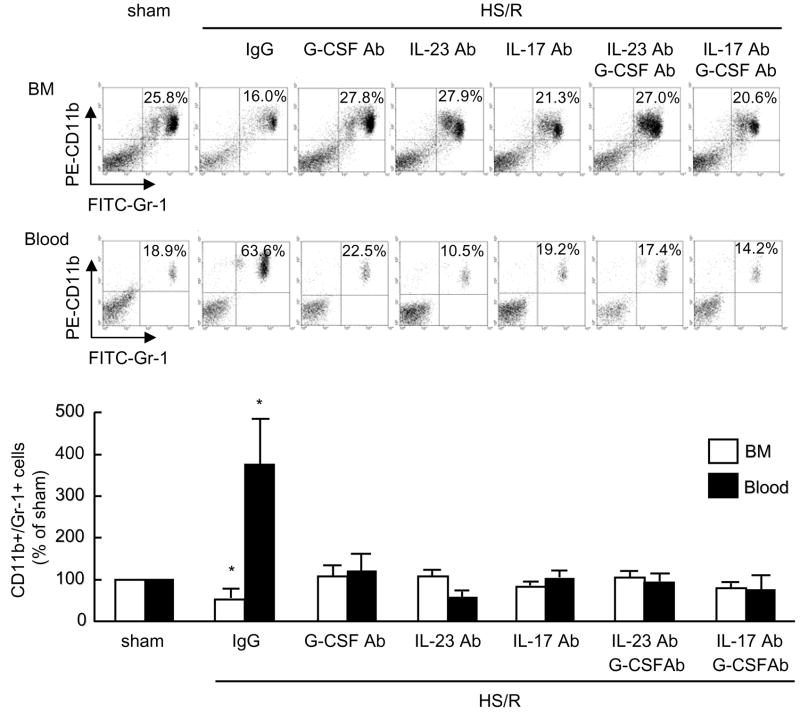
Next we assessed the role of G-CSF in inducing PMN mobilization from BM using a neutralizing anti-G-CSF antibody. As shown in Fig. 7, neutralization of G-CSF prevented the HS/R-induced decrease in BM CD11b+/Gr-1+ cells and concomitant increase in blood CD11b+/Gr-1+ cells, as compared to mice treated with non-specific IgG as a control. Furthermore, we injected the WT mice with neutralizing anti-G-CSF, IL-23 or IL-17 antibody prior to HS/R. Either single or combined use of the antibodies exhibited a significant suppressive effect on HS/R-induced PMN egress from BM as assessed by attenuation of the HS/R-induced decrease of CD11b+/Gr-1+ cells in BM and concomitant increase of CD11b+/Gr-1+ cells in blood (Fig. 7).
Figure 7. IL-23-IL-17-G-CSF axis mediates HS/R-induced CD11b+/Gr-1+ cells mobilization from BM.
Animals were injected intraperitoneally with one of the neutralizing antibodies against IL-23 (100 μg per mouse), IL-17 (100 μg per mouse), G-CSF (100 μg per mouse) or nonimmune control IgG, or a combination of anti-G-CSF Ab and anti-IL-23 Ab, or anti-G-CSF Ab and anti-IL-17 Ab 10 min before hemorrhage. Femoral bones and whole blood from the mice were harvested at 4 h after HS/R or sham operation, and cells in BM and blood were then collected for detection of CD11b+/Gr-1+ cells by flow cytometry. Representative density plots of CD11b+/Gr-1+ cells in BM (A) and blood (B) are shown. The graph depicts the mean and SE of the changes in CD11b+/Gr-1+ cells in BM and blood from three mice. * P< 0.01 as compared with sham groups.
Adrenergic stimulation induces HMGB1 release
To determine if adrenergic activation following HS/R is responsible for HMGB1 release, we evaluated the effect of the non-selective β-adrenergic receptor antagonist, β-propranolol, on HS/R-induced increase in serum HMGB1. At 2 h after HS/R, HMGB1, IL-23 and IL-17 were markedly increased in serum compared to those in sham animals. However, β-propranolol significantly decreased the HS/R-induced increase in the serum levels of HMGB1, IL-23 and IL-17 (Fig. 8A). We further observed that the injection of epinephrine in sham operated animals can directly increase serum HMGB1, IL-23, and IL-17 (Fig. 8A). These data suggests an important role for adrenergic stimulation in regulating HMGB1.
Figure 8. β-adrenergic receptor activation is responsible for HS/R-induced HMGB1 release.
A,β-adrenergic receptor antagonist prevents HS/R-induced increase in serum levels of HMGB1, IL-23 and IL-17. β-adrenergic receptor antagonist, propranolol (“Prop”, 2 mg/kg B.W., i.p.) or epinephrine (“Ep”, 2 mg/kg B.W., i.p.) was given to WT mice 10 min before HS/R or sham operation, respectively, and serums were collected 2 hours after HS/R or sham operation for HMGB1, IL-23, and IL-17 detection by Western blotting. Data are representative of 3 independent studies. B, epinephrine activation of β-adrenergic receptor induces HMGB1 and IL-23 release from AM. AM collected from WT and C3H/HeJ mice BAL fluid were stimulated with epinephrine (“Ep”, 2 μg/ml) or epinephrine plus propranolol (“Ep/Prop”, both in 2 μg/ml) for 2 h, HMGB1and IL-23 in the supernatant were analyzed using Western blotting, and IL-23 P19 mRNA in the AM was detected by RT-PCR. The blots shown are representative of three independent experiments with similar results.
To further confirm a direct role for adrenergic stimulation in inducing HMGB1 and IL-23 release from macrophages, AM collected from the BAL fluid of WT and TLR4-mutant mice were stimulated with epinephrine for 2 h, and HMGB1 and IL-23 in the supernatant as well as IL-23 P19 mRNA expression in the AM were detected. As shown in Fig. 8B, epinephrine caused a marked release of HMGB1 from both the WT and TLR4-mutant AM, whereas, β-propranolol blocked the epinephrine-induced release of HMGB1. Epinephrine also induced IL-23 release and P19 mRNA expression from WT AM but not from TLR4-mutant AM, and this induction was suppressed by β-adrenergic receptor antagonist, β-propranolol (Fig. 8B). Taken together, the results indicate a role for β-adrenergic receptor activation in stimulating HMGB1 release from macrophages, and subsequently leading to TLR4-dependent induction of IL-23 in an autocrine form.
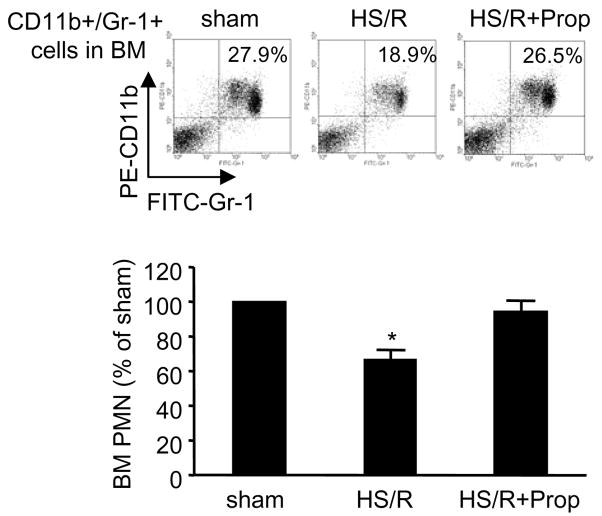
To further determine if β-adrenergic receptor activation is responsible for HS/R-induced PMN mobilization, β-propranolol was administered to mice 10 min before HS/R, and BM CD11b+/Gr-1+ cells were quantified 4 hours after resuscitation. Treatment with β-propranolol prevented the HS/R-induced reduction of the CD11b+/Gr-1+ cells in BM (Fig. 9). This result indicates an important role for β-adrenergic receptor activation in HS/R-induced PMN egress from BM.
Figure 9. β-adrenergic receptor antagonist prevents HS/R-induced CD11b+/Gr-1+ cells mobilization from BM.
β-adrenergic receptor antagonist, propranolol (“Prop”, 2 mg/kg B.W., i.p.) was given to WT mice 10 min before HS/R or sham operation, and femoral bones from the mice were harvested at 4 h after HS/R or sham operation, BM cells were then collected for detection of CD11b+/Gr-1+ cells by flow cytometry. Representative density plots of BM CD11b+/Gr-1+ cells are shown. The graph depicts the mean and SE of the changes in BM CD11b+/Gr-1+ cells from three mice. * P< 0.01 as compared with other groups.
Discussion
Tissue/organ PMN sequestration plays an important role in the development of multiple organ dysfunction following HS/R. Continuous mobilization of PMN from BM contributes to sustained PMN sequestration and the maintenance of normal hematopoiesis. In this study we sought to determine how the global ischemia/reperfusion injury initiated by resuscitated HS/R regulates PMN mobilization from BM. We show that HS/R, through HMGB1, induces IL-23 expression in a process that requires TLR4 signaling. In turn, IL-23 induces PMN egress from BM through a mechanism that involves IL-17 and G-CSF. In addition, HS/R-induced adrenergic activation is an important determinant for initiating HMGB1 secretion from macrophages.
HMGB1 is becoming recognized as the prototypic alarmin (49). Its cytokine-like properties were revealed in experiments showing that HMGB1 is released by activated macrophages and that it acts as a late mediator of lethality in animal modes of sepsis (33, 34, 50). Increasing evidence now indicates that HMGB1 also acts as an early inflammatory mediator in ischemia (26–29), hemorrhagic shock (22, 24), and non-infectious hepatitis (51). Recently, chemoattractant roles for HMGB1 in inducing migration of immature dendritic cell (49), smooth muscle cells(52), and mesoangioblasts (53, 54) have been reported. HMGB1 acts through the Receptor for Advanced Glycation End Products (RAGE) to induce the migration of these cells directly. However, no role had been ascribed to HMGB1 in the regulation of PMN mobilization.
In the present study, we show that HMGB1 plays an important role in inducing PMN egress from BM in a setting of HS/R through a mechanism, in which TLR4 signaling and the IL-23-IL-17-G-CSF axis are essential components. We found that HS/R leads to an increased HMGB1 level in serum, lungs and liver within 2 h after HS/R in mice (22), consistent with previous studies that demonstrated elevated HMGB1 in human HS/R (23), mouse ischemia/reperfusion injury (26, 39), and in acute lung injury(55). Further, we found that HMGB1 directly induces IL-23 expression in AM in a TLR4-dependent manner, and that a neutralizing antibody to HMGB1 blocks HS/R-induced release of IL-23 and IL-17 in vivo. Finally, neutralizing antibodies to HMGB1, IL-23, or IL-17 prevented HS/R-induced PMN egress from BM.
HMGB1 is increased in serum and various tissues following HS/R and/or ischemia/reperfusion injury (22, 25, 26, 39, 56). However, it was not clear from these studies whether HMGB1 was secreted actively or released passively from damaged cells following HS/R. In this study, we demonstrated that macrophage β-adrenergic receptor activation by catecholamines serves as an important mechanism for HS/R-induced HMGB1 secretion. Evidence for this mechanism comes from our finding that epinephrine, like HS/R, increases serum HMGB1, IL-23 and IL-17 in vivo. Further, a β-adrenergic receptor antagonist prevented HS/R-induced increase in serum HMGB1, IL-23, and IL-17. In vitro, we found that direct treatment of AM with epinephrine caused HMGB1 and IL-23 release from the AM, as well as IL-23 P19 mRNA expression in the AM, and this effect was suppressed by a β-adrenergic receptor antagonist. Epinephrine induced both HMGB1 and IL-23 release in WT AM, whereas epinephrine induced only HMGB1 release but no IL-23 secretion and expression in TLR4-mutant AM. These in vitro studies suggest that macrophages through possessing both β-adrenergic receptor and TLR4 are capable of responding to HMGB1 in an autocrine or juxtacrine fashion. However, other sources of HMGB1 may also exist. For example, hypoxia hepatocytes have been shown to release HMGB1 in a TLR4- and reactive oxygen species- dependent manner (39).
A recent report has shown that norepinephrine signaling via the sympathetic nervous system regulates hematopoietic stem cell egress from BM through a mechanism that involves G-CSF-induced suppression of bone-lining osteoblasts and downregulation of the chemokine SDF-1 (also called CXCL12) (57). We have previously shown that G-CSF is increased in HS/R and that G-CSF administration in the airway leads to PMN accumulation in the lung (14, 58). Clearly, HS/R causes a broad range of physiological changes in multiple systems to initiate complex, self-regulatory mechanisms (59). The HS/R-induced egress of PMN from BM is therefore likely also affected by the activation and interaction of multiple systems. Indeed, other mechanisms may also contribute to the regulation of neutrophilic homeostasis in HS/R. For example, we observed in this study that the increase of blood PMN appeared sooner than the decrease of BM PMN after HS/R (Fig. 1), suggesting that PMN driven from terminal microcirculation, possibly as a result of adrenergic activation and micro-vascular contraction, also contribute to the increase in circulating PMN in the early stage of HS/R.
RAGE had been originally identified as a receptor for HMGB1 in neurites and malignant cells (60–62). Recent studies have suggested that both TLR4 and TLR2 are also important in mediating HMGB1-induced inflammatory responses (26, 31, 32). Our in vivo study demonstrates that TLR4 mutation greatly attenuates the HS/R-induced release of IL-23 and IL-17 and PMN egress from BM. Our in vitro data further support the hypothesis that TLR4 signaling components, including MyD88 and IRAK4, are required for HMGB1-induced IL-23 secretion from AM. Thus, we implicate HMGB1-TLR4 on macrophages in a positive-feedback, pro-inflammatory loop. In support of this hypothesis, we found that the absence of functional TLR4 signaling greatly abolished the effect of HS/R on PMN mobilization as well as the effect of HMGB1 on inducing IL-23 secretion. However, TLR4 mutation appeared to be insufficient to completely eliminate the effect of HS/R on IL-23, IL-17 and G-CSF secretion in vivo, and therefore we cannot rule out the possibility that HMGB1 may also act through other receptors, e.g. TLR2 and RAGE, to regulate IL-23, IL-17, and G-CSF expression. Likewise, we observed that anti-IL-23 antibody and anti-IL-17 antibody did not exhibit a complete reversal effect on the HS/R-induced G-CSF secretion (Fig. 6) although both the antibodies demonstrated significant suppressive effect on PMN mobilization (Fig. 7), suggesting that the IL-23-IL-17 pathway is an important, but possibly not exclusive, signaling pathway in mediating HMGB1-TLR4-induced G-CSF secretion.
G-CSF has been identified as an important mediator of the proliferation, release from BM, and activation in circulation of PMN following trauma, resuscitated shock and sepsis in both experimental animal models and humans (58, 63–65). Earlier work demonstrated a critical role of IL-17 in the up-regulation of G-CSF, leading to the chemoattraction of PMN in settings of infectious diseases (4, 66, 67). This IL-17-G-CSF pathway appears to be operant also in the non-infectious setting of HS/R as shown in the current study. Recent studies have provided evidences in support of an important role for IL-23 in regulating IL-17 production from IL-17-producing cells, and driving local immune responses to pathogen infection (8, 68, 69). Indeed, without the acute IL-23-IL-17 response, mice rapidly succumb to lethal infection (8, 70). However, neither the regulation of the IL-23-IL-17 pathway in HS/R, nor the relationship of these cytokines to TLR4 and HMGB1 in this setting has been examined previously. This novel pathway appears to be at least partially responsible for the local sequestration of HS/R-primed PMN affecting inflammation and organ injury. Blocking the signaling axis may present a similarly novel therapeutic target.
Footnotes
This work was supported by the National Institutes of Health Grant R01-HL-079669 (J.F.), National Institutes of Health Center Grant P50-GM-53789 (T.R.B. and Y.V.) and a VA Merit Award (J.F.).
Abbreviations used in this paper: AM, alveolar macrophages; BM, bone marrow; G-CSF, granulocyte CSF; HMGB1, high-mobility group box 1; HS/R, hemorrhagic shock/resuscitation; I/R, ischemia/reperfusion; IRAK, IL-1R kinase; PMN, neutrophils; ROS, reactive oxygen species.
References
- 1.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- 2.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spriggs MK. Interleukin-17 and its receptor. J Clin Immunol. 1997;17:366–369. doi: 10.1023/a:1027360106635. [DOI] [PubMed] [Google Scholar]
- 6.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 7.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 8.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 11.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 13.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Hierholzer C, Kelly E, Lyons V, Roedling E, Davies P, Billiar TR, Tweardy DJ. G-CSF instillation into rat lungs mediates neutrophil recruitment, pulmonary edema, and hypoxia. J Leukoc Biol. 1998;63:169–174. doi: 10.1002/jlb.63.2.169. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 16.Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 Is Required for Neutrophil Homeostasis in Normal and Neutrophilic Mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 17.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L738–746. doi: 10.1152/ajplung.00280.2005. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 23.Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, Tracey KJ. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446–1447. doi: 10.1016/S0140-6736(99)02658-6. [DOI] [PubMed] [Google Scholar]
- 24.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, McIntyre RC. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 25.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–417. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 28.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 29.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van ’t Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 30.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 32.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 36.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 37.Zeh HJ, 3rd, Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290:C990–999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 39.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JY, Park JS, Strassheim D, Douglas I, Diaz Del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 42.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 45.Fan J, Kapus A, Marsden PA, Li YH, Oreopoulos G, Marshall JC, Frantz S, Kelly RA, Medzhitov R, Rotstein OD. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–5259. doi: 10.4049/jimmunol.168.10.5252. [DOI] [PubMed] [Google Scholar]
- 46.Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Henneke P, Golenbock DT. TIRAP: how Toll receptors fraternize. Nat Immunol. 2001;2:828–830. doi: 10.1038/ni0901-828. [DOI] [PubMed] [Google Scholar]
- 48.Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, Carminati P, Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-{kappa}B. J Biol Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 50.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 52.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 58.Hierholzer C, Kelly E, Tsukada K, Loeffert E, Watkins S, Billiar TR, Tweardy DJ. Hemorrhagic shock induces G-CSF expression in bronchial epithelium. Am J Physiol. 1997;273:L1058–1064. doi: 10.1152/ajplung.1997.273.5.L1058. [DOI] [PubMed] [Google Scholar]
- 59.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock states. Shock. 2005;24:7484. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 60.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 61.Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 62.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 63.Barth E, Fischer G, Schneider EM, Moldawer LL, Georgieff M, Weiss M. Peaks of endogenous G-CSF serum concentrations are followed by an increase in respiratory burst activity of granulocytes in patients with septic shock. Cytokine. 2002;17:275–284. doi: 10.1006/cyto.2002.1010. [DOI] [PubMed] [Google Scholar]
- 64.Hierholzer C, Kelly E, Billiar TR, Tweardy DJ. Granulocyte colony-stimulating factor (G-CSF) production in hemorrhagic shock requires both the ischemic and resuscitation phase. Arch Orthop Trauma Surg. 1997;116:173–176. doi: 10.1007/BF00426067. [DOI] [PubMed] [Google Scholar]
- 65.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood. 1999;93:425–439. [PubMed] [Google Scholar]
- 66.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 67.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 68.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]