Abstract
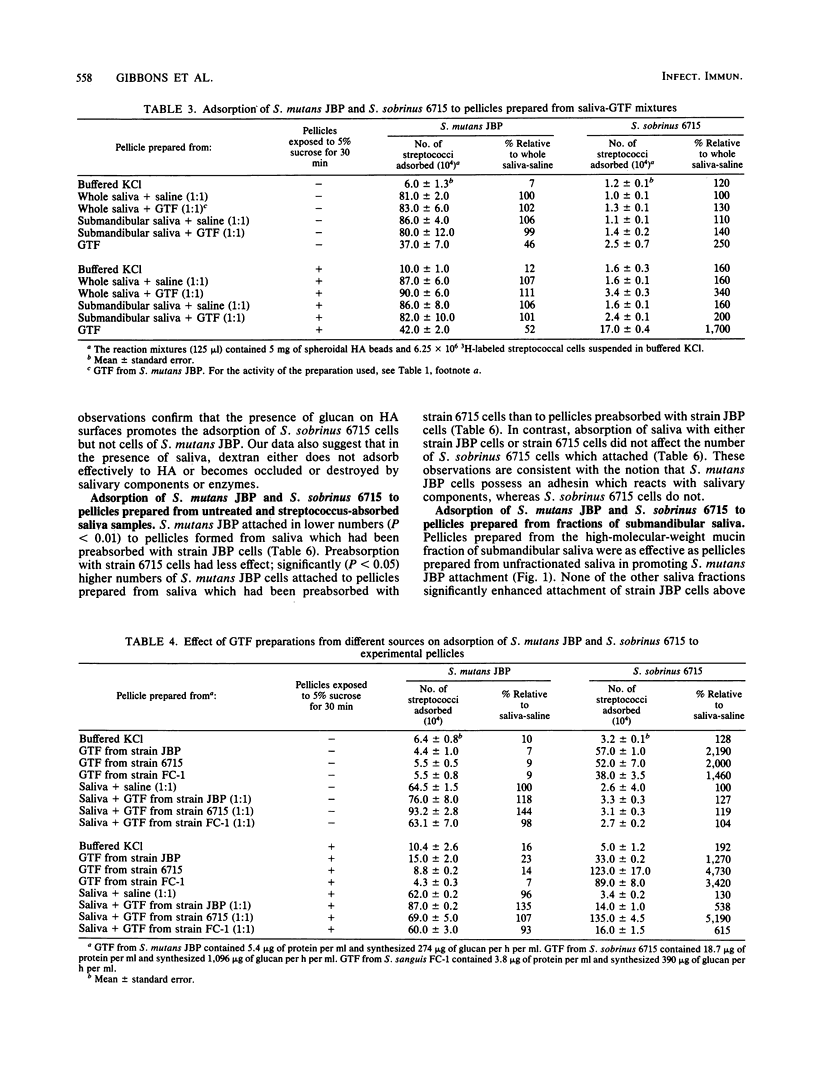
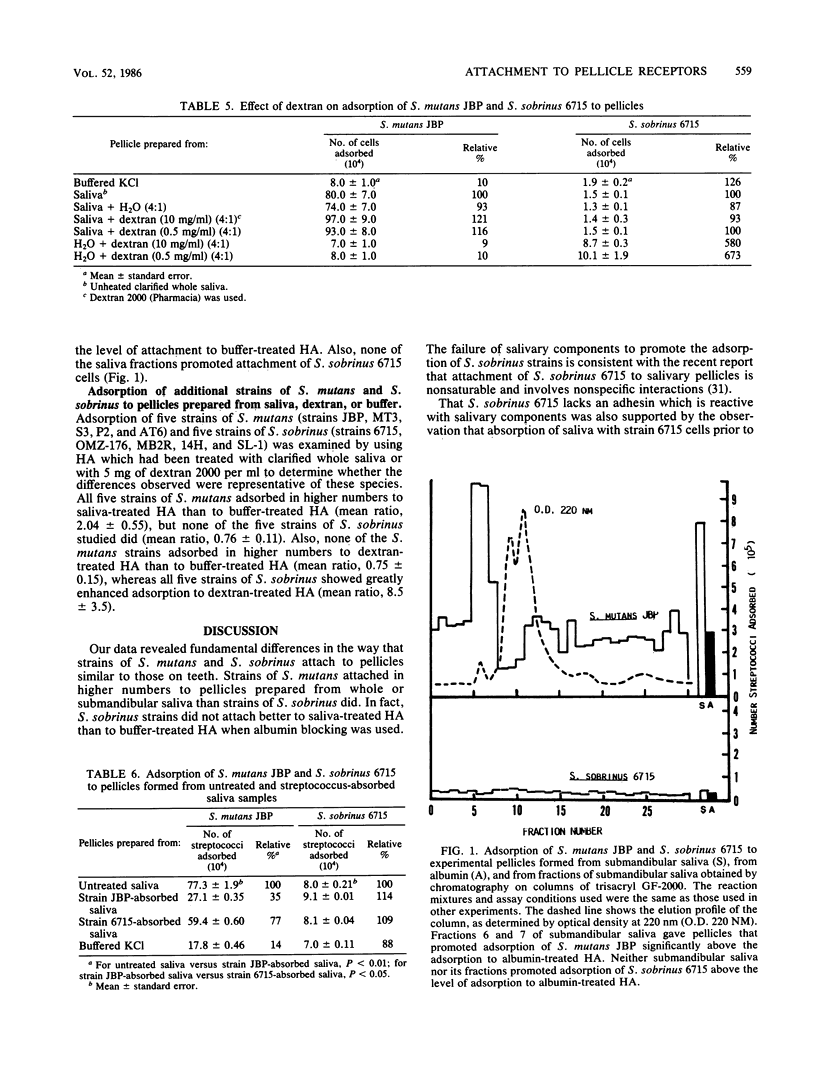
We compared the levels of adsorption of Streptococcus mutans JBP and Streptococcus sobrinus 6715 to experimental pellicles formed from unsupplemented and glucosyltransferase (GTF)-supplemented saliva. Pellicles formed on hydroxyapatite beads from GTF or from saliva-GTF mixtures possessed detectable GTF activity. Low levels of GTF activity were also detected in clarified whole human saliva, but not in samples of submandibular saliva. The adsorptive behavior of S. mutans JBP to pellicles formed from saliva or saliva-GTF mixtures was strikingly different from that of S. sobrinus 6715. S. mutans JBP adsorbed in higher numbers to pellicles formed from whole or submandibular saliva than to buffer-treated hydroxyapatite under the assay conditions used, in which blocking with albumin was used. In contrast, S. sobrinus 6715 attached in lower numbers and did not show enhanced adsorption to pellicles prepared from saliva. Pellicles prepared from the high-molecular-weight mucin fraction of submandibular saliva effectively promoted adsorption of S. mutans JBP, but none of the saliva fractions tested enhanced the attachment of S. sobrinus 6715 above the levels of buffer controls. Exposure of pellicles which contained GTF to sucrose to permit in situ synthesis of glucan markedly enhanced attachment of S. sobrinus 6715 but not attachment of S. mutans JBP. Also, the presence of sucrose throughout the adsorption period did not enhance attachment of S. mutans JBP. Both organisms possessed cell-associated GTF, and GTF preparations derived from S. sobrinus 6715 and Streptococcus sanguis FC-1 behaved like GTF derived from S. mutans JBP. S. sobrinus 6715 attached in high numbers to dextran-treated hydroxyapatite, whereas S. mutans JBP did not. These observations suggest that S. mutans JBP cells possess an adhesin which binds to salivary components in the pellicles. In contrast, S. sobrinus 6715 cells appear to possess an adhesin which binds to glucan in the pellicles. Four additional strains of S. mutans and four additional strains of S. sobrinus behaved qualitatively like strains JBP and 6715, respectively, and thus the differences observed appear to be representative of these species. Collectively, our data indicate that S. mutans and S. sobrinus attach to different receptors in experimental pellicles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Ability of Streptococcus mutans and a glucosyltransferase-defective mutant to colonize rodents and attach to hydroxyapatite surfaces. Infect Immun. 1978 Aug;21(2):681–684. doi: 10.1128/iai.21.2.681-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Bratthall D., O'Connor K., Dvarskas R. A. Serological and genetic examination of some nontypical Streptococcus mutans strains. Infect Immun. 1976 Sep;14(3):667–670. doi: 10.1128/iai.14.3.667-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Albumin as a blocking agent in studies of streptococcal adsorption to experimental salivary pellicles. Infect Immun. 1985 Nov;50(2):592–594. doi: 10.1128/iai.50.2.592-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Kobayashi Y., Slade H. D. Cell-bound synthesis and subsequent adherence of oral streptococci due to the binding of extracellular glucosyltransferase to the streptococcal cell surface. Microbiol Immunol. 1978;22(5):279–282. doi: 10.1111/j.1348-0421.1978.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Masuda N., Ooshima T., Sobue S., Kotani S. Epidemiological survey of Streptococcus mutans among Japanese children. Identification and serological typing of the isolated strains. Jpn J Microbiol. 1976 Feb;20(1):33–44. doi: 10.1111/j.1348-0421.1976.tb00905.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Tsuchitani Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect Immun. 1981 Apr;32(1):364–372. doi: 10.1128/iai.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Shibata H., Morioka T. Serotype specificity for some biochemical characteristics of Streptococcus mutans. Microbios. 1982;33(131):7–14. [PubMed] [Google Scholar]
- Kuramitsu H. K., Wondrack L., McGuinness M. Interaction of Streptococcus mutans glucosyltransferases with teichoic acids. Infect Immun. 1980 Aug;29(2):376–382. doi: 10.1128/iai.29.2.376-382.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N., Tsutsumi N., Sobue S., Hamada S. Longitudinal survey of the distribution of various serotypes of Streptococcus mutans in infants. J Clin Microbiol. 1979 Oct;10(4):497–502. doi: 10.1128/jcm.10.4.497-502.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Rolla G. Adsorption of dextran to saliva-treated hydroxyapatite. Arch Oral Biol. 1971 May;16(5):527–533. doi: 10.1016/0003-9969(71)90198-1. [DOI] [PubMed] [Google Scholar]
- Rolla G., Ciardi J. E., Schultz S. A. Adsorption of glucosyltransferase to saliva coated hydroxyapatite. Possible mechanism for sucrose dependent bacterial colonization of teeth. Scand J Dent Res. 1983 Apr;91(2):112–117. doi: 10.1111/j.1600-0722.1983.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Rölla G., Ciardi J. E., Bowen W. H. Identification of IgA, IgG, lysozyme, albumin, alpha-amylase and glucosyltransferase in the protein layer adsorbed to hydroxyapatite from whole saliva. Scand J Dent Res. 1983 Jun;91(3):186–190. doi: 10.1111/j.1600-0722.1983.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Simonson L. G. Distribution and frequency of streptococcus mutants in caries-active individuals. J Dent Res. 1972 May-Jun;51(3):882–882. doi: 10.1177/00220345720510034201. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Preparation of glucosyltransferase from Streptococcus mutans by elution from water-insoluble polysaccharide with a dissociating solvent. Infect Immun. 1979 Feb;23(2):446–452. doi: 10.1128/iai.23.2.446-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Dzink J. L., Smith C. M. Chemically defined medium for oral microorganisms. J Clin Microbiol. 1985 Aug;22(2):303–305. doi: 10.1128/jcm.22.2.303-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Peyton J. C. Adherence of oral streptococci: evidence for nonspecific adsorption to saliva-coated hydroxylapatite surfaces. Infect Immun. 1984 Jun;44(3):653–659. doi: 10.1128/iai.44.3.653-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinanoff N., Tanzer J. M., Freedman M. L. In vitro colonization of Streptococcus mutans on enamel. Infect Immun. 1978 Sep;21(3):1010–1019. doi: 10.1128/iai.21.3.1010-1019.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren G., Olsson J. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect Immun. 1983 Apr;40(1):432–435. doi: 10.1128/iai.40.1.432-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]



