Abstract
The cell wall mycolyl-arabinogalactan–peptidoglycan complex is essential in mycobacterial species, such as Mycobacterium tuberculosis and is the target of several antitubercular drugs. For instance, ethambutol targets arabinogalactan biosynthesis through inhibition of the arabinofuranosyltransferases Mt-EmbA and Mt-EmbB. A bioinformatics approach identified putative integral membrane proteins, MSMEG2785 in Mycobacterium smegmatis, Rv2673 in Mycobacterium tuberculosis and NCgl1822 in Corynebacterium glutamicum, with 10 predicted transmembrane domains and a glycosyltransferase motif (DDX), features that are common to the GT-C superfamily of glycosyltransferases. Deletion of M. smegmatis MSMEG2785 resulted in altered growth and glycosyl linkage analysis revealed the absence of AG α(1→3)-linked arabinofuranosyl (Araf) residues. Complementation of the M. smegmatis deletion mutant was fully restored to a wild-type phenotype by MSMEG2785 and Rv2673, and as a result, we have now termed this previously uncharacterized open reading frame, arabinofuranosyltransferase C (aftC). Enzyme assays using the sugar donor β-d-arabinofuranosyl-1-monophosphoryl-decaprenol (DPA) and a newly synthesized linear α(1→5)-linked Ara5 neoglycolipid acceptor together with chemical identification of products formed, clearly identified AftC as a branching α(1→3) arabinofuranosyltransferase. This newly discovered glycosyltransferase sheds further light on the complexities of Mycobacterium cell wall biosynthesis, such as in M. tuberculosis and related species and represents a potential new drug target.
Introduction
Tuberculosis (TB) affects a third of the world population and causes 1.8 million fatalities annually (Dye, 2006). The spread of TB has been facilitated in recent years due to the susceptibility of HIV-infected individuals to Mycobacterium tuberculosis, the aetiological agent of TB (Paolo and Nosanchuk, 2004). The problem has also been compounded by the emergence of multidrug-resistant TB (MDR-TB) (Kaye and Frieden, 1996) and extensively drug-resistant (XDR)-TB strains (Shah et al., 2007). M. tuberculosis and other mycobacteria have a distinct cell wall which has a lipid-rich outer layer that is highly impermeable (Minnikin, 1982). One of the major components of this outer envelope are mycolic acids, long-chain α-alkyl, β-hydroxy fatty acids that are essential for bacterial survival (Vilcheze et al., 2000; Portevin et al., 2004; Bhatt et al., 2005; Parish et al., 2007). These are found either esterified to the non-reducing termini of arabinogalactan (AG), or are present as trehalose esters, such as trehalose dimycolate (TDM) (Brennan and Nikaido, 1995; Dover et al., 2004).
A common feature of members of the Corynebacterianeae is that they all possess this unusual cell wall architecture (McNeil et al., 1990; 1991; Besra et al., 1995). Apart from mycolic acids, the cell wall is dominated by a second macromolecule, an essential heteropolysaccharide termed AG, which is linked to both mycolic acids and peptidoglycan, forming the mycolyl-arabinogalactan–peptidoglycan (mAGP) complex (Daffé et al., 1990; McNeil et al., 1990; 1991; Besra et al., 1995). The formation of the arabinan domain (α1→5, α1→3 and β1→2 glycosyl linkages) of AG results from the subsequent addition of arabinofuranose (Araf) residues by a set of unique arabinofuranosyltransferases including, the Emb proteins of which three paralogues exist in Mycobacterium avium (Belanger et al., 1996) and M. tuberculosis (Telenti et al., 1997), AftA (Alderwick et al., 2006a) and AftB (Seidel et al., 2007a). The lipid linked sugar donor β-d-arabinofuranosyl-1-monophosphoryldecaprenol (DPA; Wolucka et al., 1994; Lee et al., 1995; 1997) serves as the substrate molecule for these complex membrane-bound glycosyltransferases.
The antituberculosis drug ethambutol (EMB) was shown to specifically inhibit AG biosynthesis (Takayama and Kilburn, 1989). The precise molecular target of EMB occupies the embCAB locus in M. tuberculosis (Telenti et al., 1997). To further define the role of EmbCAB proteins in cell wall arabinan biosynthesis, embA, embB and embC were individually inactivated in Mycobacterium smegmatis (Escuyer et al., 2001; Zhang et al., 2003). All three mutants were viable; however, the non-reducing terminal Ara6 motif which is the template for mycolylation in AG (McNeil et al., 1991) was altered in both the M. smegmatis (Ms)-embA and Ms-embB mutants (Escuyer et al., 2001), while Ms-embC was shown to be involved in the formation of the arabinan domains of lipoarabinomannan (LAM; Zhang et al., 2003). Attempts to obtain deletion mutants of embA (Amin et al., 2008) and embB in M. tuberculosis and embAB in M. smegmatis have proved unsuccessful (G.S. Besra, unpubl. results). In contrast, deletion of the single C. glutamicum (Cg)-emb orthologue and chemical analysis of the cell wall revealed a novel truncated AG structure possessing only terminal (t)-Araf residues with a corresponding loss of cell wall-bound mycolic acids (Alderwick et al., 2005). The presence of a novel enzyme responsible for ‘priming’ the galactan domain for further elaboration by Emb proteins led to the identification of AftA (Alderwick et al., 2006a). Recently, a retaining GT-C enzyme was identified, now termed AftB, which is responsible for the attachment of terminal β(1→2) Araf residues, and marks the ‘end-point’ for AG arabinan biosynthesis (Fig. 1) before decoration with mycolic acids (Seidel et al., 2007a).
Fig. 1.
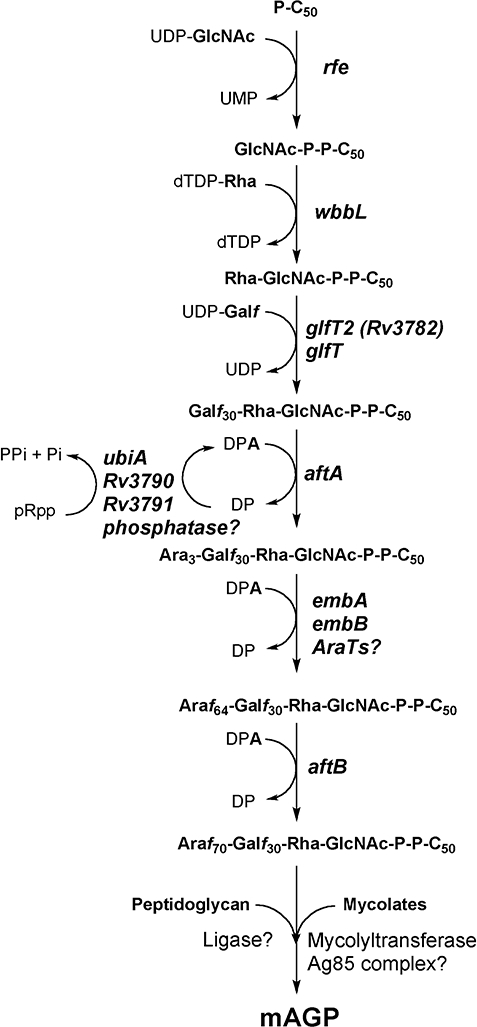
Biosynthetic pathway leading to arabinan formation in M. tuberculosis AG.
It is clear that additional arabinofuranosyltransferases involved in AG and LAM biosynthesis still remain to be identified. Indeed, Liu and Mushegian (2003) identified 15 members of the GT-C superfamily residing in M. tuberculosis, representing candidates involved in the biosynthesis of cell wall-related glycans and lipoglycans (Liu and Mushegian, 2003). We have continued our earlier studies (Alderwick et al., 2006a,b; Seidel et al., 2007a) to identify genes required for the biosynthesis of the core structural elements of the mAGP complex by studying mutants of M. smegmatis and the orthologous genes and enzymes of M. tuberculosis and Corynebacterium glutamicum. Herein, we present MSMEG2785, Rv2673 and NCgl1822 as a new distinct arabinofuranosyltransferase of the GT-C superfamily, which is responsible for the transfer of Araf residues from DPA to the arabinan domain to form α(1→3)-linked Araf residues, which result in the branched arabinan domain distal to the non-reducing terminal Ara6 motif characteristic of mycobacterial AG.
Results
Genome comparison of the Rv2673 locus
The arabinofuranosyltransferases EmbA, EmbB and EmbC are vital for M. tuberculosis and represent a target for the established drug EMB (Mikusova et al., 1995; Belanger et al., 1996; Telenti et al., 1997). Structural considerations of these proteins and a search for new drug targets resolved that more than 16 related proteins are present in M. tuberculosis, possibly also acting as glycosyltransferases (Liu and Mushegian, 2003). In our systematic analysis of GT-C glycosyltransferases, focusing on those present in M. tuberculosis and C. glutamicum, we have previously identified the arabinofuranosyltransferases AftA (Alderwick et al., 2006a) and AftB (Seidel et al., 2007a), as well as several α-mannosyltransferases (Mishra et al., 2007; 2008). Each of these glycosyltransferases plays a specific yet decisive role in cell wall biosynthesis and assembly. In silico analysis of one of the putative glycosyltransferases of M. tuberculosis, Rv2673, highlighted that orthologues are present in a range of species belonging to the suborder Corynebacterianeae, including the families Mycobacteriaceae, Corynebacteriacea and Nocardiaceae (Fig. 2A). Furthermore, the organization of the gene locus is largely retained. The adjacent genes are largely of unknown function. RibD encodes a bifunctional deaminase–reductase domain, followed by a gene product containing a hydrolase domain, which is however, absent in Corynebacterium, and downstream of Rv2673 a gene of unknown function is present. The wide distribution of Rv2673, its syntenic organization, and the fact that it is retained even in M. leprae, strongly indicates a fundamental function of its product. According to our experimental analysis (see below) we annotated this gene arabinofuranosyltransferase C (aftC).
Fig. 2.
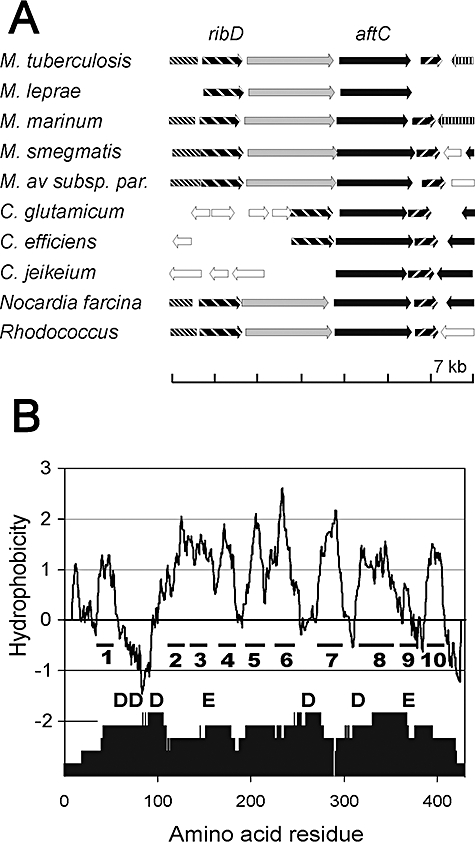
Comparison of the aftC locus within the Corynebacterianeae.
A. The locus in the bacteria analysed consists of aftC which in M. tuberculosis has the locus tag Rv2673 and in C. glutamicum NCgl1822. The genomic region displayed encompasses 7 kb, and orthologous genes are highlighted accordingly. M. marinum, Mycobacterium marinum; M. av ssp. par., Mycobacterium avium ssp. paratuberculosis; C. efficiens, Corynebacterium efficiens; C. jeikeium, Corynebacterium jeikeium; Nocardia farcina, Nocardia farcina IFM 10 152; Rhodococcus, Rhodococcus sp. strain RHA1.
B. AftC is a hydrophobic protein predicted to span the membrane 10 times and the transmembrane helices are numbered accordingly. The lower part of the figure shows the degree of conservation of the orthologues given in A as analysed by the DIALIGN method (Brudno et al., 2003). Also shown is the approximate position of the fully conserved aspartyl (D) and glutamyl (E) residues.
AftC of M. tuberculosis is 433 amino acid residues long. It is a hydrophobic protein and is predicted to possess 10 transmembrane-spanning segments (Fig. 2B). However, in contrast to AftA, AftB or EmbC, it is characterized by the absence of a periplasmic carboxyterminal extension. The amino acid sequence among the Corynebacterianeae is very well conserved, and there are 43% identical residues shared by the M. tuberculosis and C. glutamicum proteins. The degree of conservation is particularly high in the loop regions, for instance between helixes 1 and 2, 3 and 4, or 6 and 7 (Fig. 2B). The fully conserved aspartyl (D) and glutamyl (E) residues, which we propose to be involved in catalysis or substrate binding, are located in the first extended loop region (Liu and Mushegian, 2003), as we have demonstrated for similarly located aspartyl (D) residues of Cg-Emb and AftB (Seidel et al., 2007a,b). Interestingly, the long transmembrane helix 8 is well conserved and it is within this region that there is a strong identity to a membrane protein of Vibrio parahaemolyticus (CpsG). Furthermore, this gene is located in a gene cluster involved in the biosynthesis of a capsular polysaccharide within this pathogen (Guvener and McCarter, 2003).
Construction and growth of mutants
In order to delete aftC and study for possible consequences we generated a null mutant of M. smegmatis mc2155 MSMEG2785 (orthologue of Rv2673) using specialized transduction (Fig. 3A). In contrast to our C. glutamicum studies (see below) growth of M. smegmatisΔaftC in comparison to M. smegmatis was poor in liquid medium (Fig. 3B) and sensitive to the addition of Tween-80 on agar plates (> 0.005%). Complementation of M. smegmatisΔaftC with either pMV261-Ms-aftC or pMV261-M. tuberculosis (Mt)-aftC restored the mutant to a wild-type phenotype (Fig. 3B). On solid media M. smegmatisΔaftC had a smooth and glossy appearance in comparison to the typical crenulated colony morphology found for wild-type M. smegmatis (Fig. 3C) and failed to stain as ‘acid-fast’ positive (data not shown). In addition, susceptibility of M. smegmatisΔaftC to EMB and the hydrophobic antibiotics rifampicin and chloramphenicol was enhanced (minimal inhibitory concentration of 2, 100 and 10 μg ml−1 for wild-type M. smegmatis in comparison to 0.4, 4 and 5 μg ml−1 for M. smegmatisΔaftC respectively), indicating increased permeability and that M. smegmatisΔaftC had an altered cell wall. To study the function of the corynebacterial AftC the non-replicative plasmid pK19mobsacBΔaftC was constructed. This was used to transform C. glutamicum to kanamycin resistance, indicating integration in its chromosome (Fig. S1A). Loss of vector was obtained by selection for sucrose-resistance yielding clones with aftC deleted. A PCR analysis with primer pairs P5 and P6 resulted in the expected fragment of 2160 bp for the wild-type and of 1065 bp for the deletion mutant, which was termed C. glutamicumΔaftC. Colonies of this mutant were more erose compared with the usual glossy appearance of the wild-type colony (data not shown). In contrast to M. smegmatisΔaftC the growth of the C. glutamicumΔaftC mutant on the salt medium CGXII possessed only a slightly reduced growth rate of 0.32 h−1, whereas, that of the wild-type C. glutamicum was 0.39−1 h (Fig. S1B).
Fig. 3.
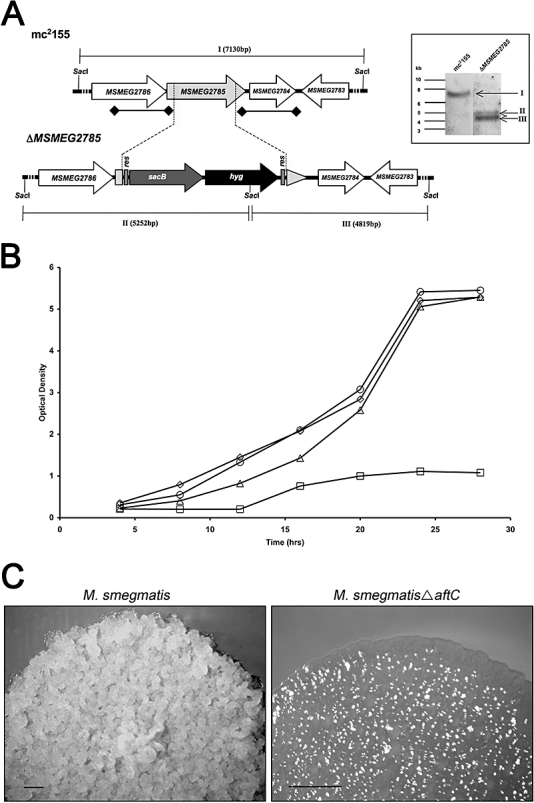
Generation of a MSMEG2785 null mutant.
A. A map of the MSMEG2785 region in the parental M. smegmatis strain and its corresponding region in the ΔMSMEG2785 mutant. res, γδ resolvase site; hyg, hygromycin resistance gene from Streptomyces hygroscopicus; sacB, sucrose counter-selectable gene from Bacillus subtilis. Digoxigenin-labelled probes were derived from ∼1 kb upstream and downstream flanking sequences that were used to construct the knockout plasmid, and are indicated by thick lines with square ends. SacI-digested bands expected in a Southern blot are indicated in roman numerals with sizes in brackets. The inset shows the Southern blot of SacI-digested genomic DNA from the two strains with expected bands indicated by arrows.
B. Growth of wild-type M. smegmatis (◊), M. smegmatisΔaftC (□), M. smegmatisΔaftC pMV261-Ms-aftC (▵) and M. smegmatisΔaftC pMV261-Mt-aftC (○) in TSB medium.
C. Colony morphology of wild-type M. smegmatis and M. smegmatisΔaftC on TSB-agar plates. Black bar represents 1 mm.
mAGP analyses from M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC, M. smegmatisΔaftC pMV261-Mt-aftC, C. glutamicum and C. glutamicumΔaftC
To study the function of mycobacterial aftC deletion, defatted cells were analysed qualitatively for AG esterified mycolic acids and cell wall-associated lipids from an equivalent starting amount of biomass for each strain due to differences in growth rate (Fig. 3B). As expected, M. smegmatis exhibited a typical profile of cell wall-bound α, α′ and epoxy-mycolic acid methyl esters (MAMEs), whereas, these products were drastically reduced in M. smegmatisΔaftC (Fig. 4A). In addition, complementation of M. smegmatisΔaftC with either pMV261-Ms-aftC or pMV261-Mt-aftC (Fig. 4A), led to the restoration of normal ‘levels’ of cell wall-bound mycolic acids. Analysis of cell wall-associated lipids in several independent experiments highlighted an apparent increase in TDM for the aftC deletion mutant. This was confirmed quantitatively through [14C]acetate labelling of cultures and equal loading of radioactivity of extractable free lipids from M. smegmatis, M. smegmatisΔaftC and the complemented M. smegmatisΔaftC strain using plasmids pMV261-Ms-aftC and pMV261-Mt-aftC (Fig. 4B). Typically, wild-type M. smegmatis synthesized 5250 cpm, whereas M. smegmatisΔaftC afforded 14 676 cpm of TDM after equivalent loading of radioactivity and quantitative analysis by phosphorimaging. Complementation of M. smegmatisΔaftC with either pMV261-Ms-aftC or pMV261-Mt-aftC restored the phenotype of the deletion mutant back to that of wild-type M. smegmatis (Fig. 4B). These results demonstrated that Ms-aftC and Mt-aftC are involved in a key aspect of arabinan biosynthesis, whereby deletion substantially perturbs tethering of mycolic acids to AG, which results in an increase in TDM production.
Fig. 4.
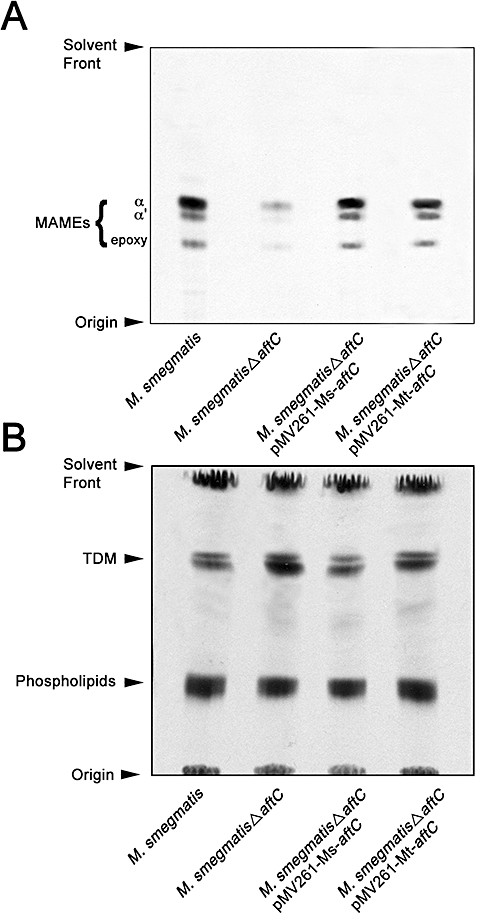
Analysis of cell wall-associated lipids and bound MAMEs from M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC and M. smegmatisΔaftC pMV261-Mt-aftC.
A. Analysis of cell wall-bound MAMEs from M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC and M. smegmatisΔaftC pMV261-Mt-aftC. The bound mycolic acids from an equivalent amount of freeze-dried cells (100 mg), which were initially de-lipidated using two consecutive extractions of CHCl3/CH3OH/H2O (10/10/3; v/v/v) at 50°C for 4 h, were released by the addition of tetra-butylammonium hydroxide at 100°C overnight, and methylated as described in the Experimental procedures. An equivalent aliquot from each strain was subjected to TLC using silica gel plates (5735 silica gel 60F254, Merck), and developed in petroleum ether/acetone (95:5, v/v) and charred to reveal MAMEs and compared with known standards (Gande et al., 2004).
B. Quantitative analysis of extractable [14C]-lipids from M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC and M. smegmatisΔaftC pMV261-Mt-aftC. Lipids were extracted from cells by a series of organic washes as described in Experimental procedures (Seidel et al., 2007a). An equivalent aliquot (50 000 cpm) from each strain was subjected to TLC using silica gel plates (5725 silica gel 60F254, Merck) developed in CHCl3/CH3OH/NH4OH (80:20:2, v/v/v) and quantified using phosphorimaging and compared with known standards (Mikusova et al., 1995) after exposure to Kodak X-Omat film for 24 h.
The cell wall core (mAGP) was prepared from M. smegmatis and M. smegmatisΔaftC as described (Daffé et al., 1990; Besra et al., 1995; Alderwick et al., 2005) and the ratio of Ara to Gal in mAGP determined by gas chromatography (GC) analysis of alditol acetates (Daffé et al., 1990; Besra et al., 1995; Escuyer et al., 2001; Alderwick et al., 2005) (Fig. 5). The glycosyl composition was calculated based on a single rhamnosyl (Rha) residue per AG chain (McNeil et al., 1990). The glycosyl compositional analysis revealed a relative molar ratio of Rha : Ara : Gal of 1:71:31 and an Ara : Gal ratio of 2.3:1, which is in accord with previous data (Escuyer et al., 2001). The M. smegmatisΔaftC mutant yielded AG with a significant reduction in Ara content concomitant with a relative increase in the amount of Gal. The M. smegmatisΔaftC yielded an AG with an Rha : Ara : Gal ratio of 1:22:56 and an Ara : Gal ratio of 0.4:1. Complementation of M. smegmatisΔaftC with either pMV261-Ms-aftC or pMV261-Mt-aftC, restored the Rha : Ara : Gal ratio to that of wild-type M. smegmatis. Gas chromatography mass spectrometry (GC/MS) analysis of per-O-methylated alditol acetate derivatives prepared from M. smegmatis and M. smegmatisΔaftC indicated the complete absence of 3,5-Araf branching residues and a significant reduction in t-Araf, 2-Araf and 5-Araf-linkages (Fig. 6). Complementation of M. smegmatisΔaftC with either plasmid encoding Ms-aftC or Mt-aftC restored the glycosyl linkage profile to that of wild-type M. smegmatis (Fig. 6). These results demonstrate that MSMEG2785 and Rv2673 are functionally equivalent and are involved in the synthesis of 3,5-Araf branching residues. Interestingly, LAM preparations from M. smegmatisΔaftC were truncated in size on SDS-PAGE analysis to ‘full-size’ LAM from wild-type M. smegmatis. Further purification and detailed chemical analyses of LAM from the aftC mutant strain are currently being undertaken and will be reported separately (H.L. Birch, unpubl. results).
Fig. 5.
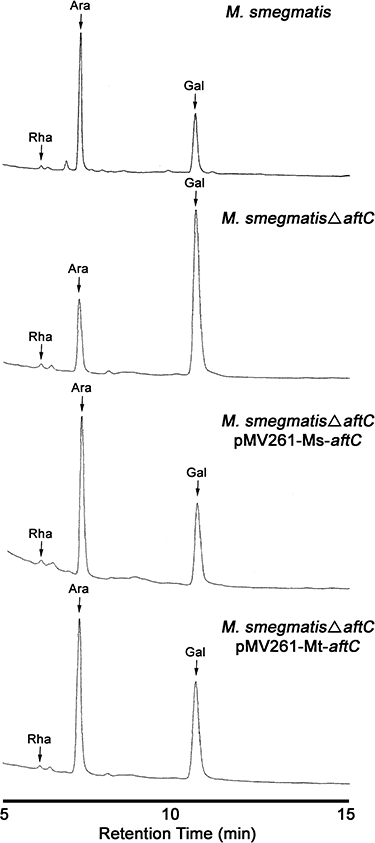
GC analysis of cell walls of M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC and M. smegmatisΔaftC pMV261-Mt-aftC. Samples of purified cell walls were hydrolysed with 2 M TFA, reduced, per-O-acetylated and analysed as described under Experimental procedures (Besra et al., 1995; Alderwick et al., 2005).
Fig. 6.
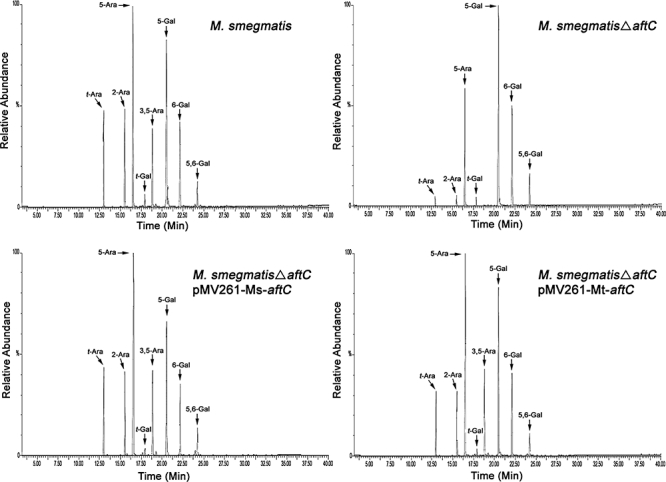
GC/MS analysis of cell walls of M. smegmatis, M. smegmatisΔaftC, M. smegmatisΔaftC pMV261-Ms-aftC and M. smegmatisΔaftC pMV261-Mt-aftC. Samples of per-O-methylated cell walls were hydrolysed with 2 M TFA, reduced, per-O-acetylated and analysed as described under Experimental procedures (Besra et al., 1995; Alderwick et al., 2005).
In contrast to the mycolic acid studies performed with the mycobacterial aftC deletion mutant, C. glutamicumΔaftC cells were analysed quantitatively for AG esterified corynemycolic acids due to similar growth rates between strains (Fig. S1B). Wild-type C. glutamicum exhibited the known profile of corynomycolic acid methyl esters (CMAMEs, 35 345 cpm; Fig. S2), whereas, cell wall-bound CMAMEs were significantly reduced in C. glutamicumΔaftC (8023 cpm). The above data were reassuring as the qualitative (M. smegmatisΔaftC) and quantitative (C. glutamicumΔaftC) analyses were comparable in terms of a reduction in cell wall-bound mycolic acids (Fig. 4A and Fig. S2). Importantly, these results have also shown that Cg-aftC is involved in a key aspect of arabinan biosynthesis, whereby deletion perturbs tethering of corynomycolic acids to AG. The GC/MS profiles of per-O-methylated alditol acetate derivatives of C. glutamicum and C. glutamicumΔaftC are shown in Fig. S3 with C. glutamicumΔaftC also clearly devoid of 3,5-Araf branching residues.
In vitro arabinofuranosyltransferase activity with extracts of M. smegmatis, M. smegmatisΔaftC and complemented strains
Initial attempts to develop an in vitro assay using either purified recombinant expressed AftC or E. coli membranes expressing aftC, have thus far proved unsuccessful, probably due to the hydrophobic nature of the protein. In an alternative approach, we assessed the capacity of membrane preparations from M. smegmatis, M. smegmatisΔaftC and M. smegmatisΔaftC complemented with pMV261-Mt-aftC to catalyse arabinofuranosyltransferase activity in the presence of exogenous synthetic acceptors (Lee et al., 1997; Seidel et al., 2007a).
We first assessed whether M. smegmatisΔaftC was deficient in α(1→5) and β(1→2) arabinofuranosyltransferase activity using an α-d-Araf-(1→5)-α-d-Araf-O-(CH2)7CH3 (Ara2) synthetic acceptor (Lee et al., 1997) and DP[14C]A as a sugar donor based on an established assay format for determining α(1→5) and β(1→2) arabinofuranosyltransferase activities (Lee et al., 1998). TLC/autoradiographic analysis of the products which were only synthesized in the presence of Ara2, when assayed with M. smegmatis membranes resulted in the formation of two products (A and B) (Fig. 7A and B). The enzymatic synthesis of products A and B are consistent with our previous studies using mycobacterial (Lee et al., 1997) and corynebacterial (Seidel et al., 2007a) membrane preparations resulting in trisaccharide products as a result of α(1→5) and β(1→2) Araf linkages to the Ara2 acceptor (Fig. 7A). Addition of EMB in several experiments, even at high concentrations of up to 1 mg ml−1 to the reaction mixture, resulted in a decrease in only the in vitro synthesized α-d-[14C]Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-O-(CH2)7CH3 product A (Fig. 7A and B). Assays performed with membranes from M. smegmatisΔaftC and the pMV261-Mt-aftC complemented strain using the Ara2 synthetic acceptor gave a similar profile to that of wild-type M. smegmatis (Fig. 7B). The data clearly show that the M. smegmatisΔaftC strain possesses comparable levels of EMB-sensitive α(1→5) and EMB-resistant β(1→2) arabinofuranosyltransferase activity.
Fig. 7.
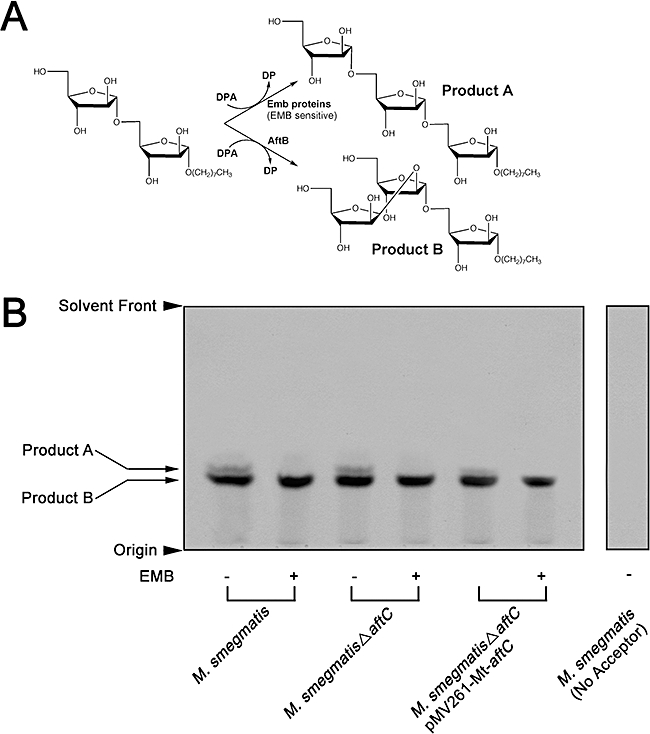
Arabinofuranosyltransferase activity utilizing an Ara2 acceptor and membranes prepared from M. smegmatis, M. smegmatisΔaftC and M. smegmatisΔaftC pMV261-Mt-aftC.
A. Biosynthetic reaction scheme of products A and B formed in arabinofuranosyltransferase assays using the neoglycolipid Ara2 acceptor.
B. Arabinofuranosyltransferase activity was determined using the synthetic Ara2 acceptor in a cell-free assay with and without EMB (1 mg ml−1) as previously described (Lee et al., 1997). The products of the assay were re-suspended prior to scintillation counting (10%) and the remaining subjected to TLC using silica gel plates (5735 silica gel 60F254, Merck) in CHCl3/CH3OH/H2O/NH4OH (65/25/3.6/0.5, v/v/v/v) with the reaction products visualized by autoradiography. The TLC autoradiogram is representative of several independent experiments.
The lack of α(1→3) arabinofuranosyltransferase activity in the previously reported Ara2 and α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-O-(CH2)7CH3 (Ara3) acceptor-based assays (Lee et al., 1997) required the development of an arabinofuranosyltransferase assay using the Ara-extended synthetic acceptor α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-O-(CH2)8NH2 (Ara5) (Supplementary experimental and Fig. S4) and DP[14C]A as a sugar donor (Lee et al., 1998). TLC/autoradiographic analysis of the products which are only synthesized in the presence of Ara5, when assayed with M. smegmatis membranes resulted in the formation of a single product X (Fig. 8A) through the transfer of a single [14C]Araf residue, with a retardation factor (Rf) consistent with a synthetic Ara6 acceptor (Appelmelk et al., 2008) standard (Fig. 8B). In addition, the synthesis of product X in overexpression studies was enhanced. Consistently from two independent membrane preparations and assays performed in triplicate from M. smegmatis pMV261-Mt-aftC produced product X (6453 cpm) in comparison to membranes from wild-type M. smegmatis (4289 cpm) in the above assays, demonstrating that AftC was functionally involved in the synthesis of product X. The inclusion of EMB in several experiments, even at high concentrations of up to 1 mg ml−1 to the reaction mixture did not inhibit the synthesis of this in vitro synthesized [14C]Araf-Ara5 (Fig. 8A, Product X) illustrating that the Ara5 acceptor was not extended via an EMB-sensitive α(1→5) arabinofuranosyltransferase. Interestingly, membranes prepared from the M. smegmatisΔaftC strain were unable to synthesize the in vitro product to the same level of activity that was observed with wild-type membranes prepared from M. smegmatis (Fig. 8A). This was to be expected, as our earlier in vivo and in vitro studies would have anticipated residual Ara6 product formation, considering that M. smegmatisΔaftC possesses β(1→2) arabinofuranosyltransferase activity. Assays performed with membranes from the M. smegmatisΔaftC pMV261-Mt-aftC complemented strain, gave a similar profile to that of wild-type M. smegmatis (Fig. 8A).
Fig. 8.
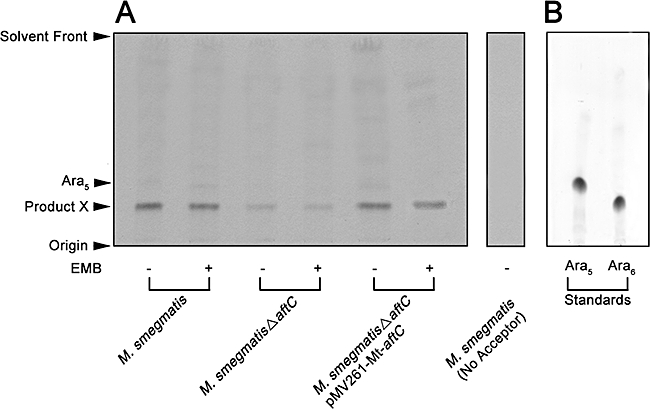
Arabinofuranosyltransferase activity utilizing an Ara5 acceptor and membranes prepared from M. smegmatis, M. smegmatisΔaftC and M. smegmatisΔaftC pMV261-Mt-aftC.
A. Arabinofuranosyltransferase activity was determined using the synthetic Ara5 acceptor in a cell-free assay with and without EMB (1 mg ml−1). The products reflective of three independent enzyme preparations and assays were re-suspended prior to scintillation counting (10%) and the remaining subjected to TLC using silica gel plates (5735 silica gel 60F254, Merck) in isopropanol/acetic acid/water (8/1/1/, v/v/v) with the reaction product X visualized by autoradiography. The TLC autoradiogram is representative of three independent experiments.
B. Ara5 and Ara6 (Appelmelk et al., 2008) acceptor standards were subjected to TLC using silica gel plates (5735 silica gel 60F254, Merck) in isopropanol/acetic acid/water (8/1/1/, v/v/v) with the reaction products visualized by staining with α-naphthol followed by charring.
To establish that the Ara5 acceptor is being utilized by two different arabinofuranosyltransferases, presumably establishing β(1→2) and α(1→3) linkages, assays similar to that used before were scaled up (see Experimental procedures) and product X extracted and purified through preparative TLC for each membrane preparation. GC (Sassaki et al., 2005) and GC/MS (Alderwick et al., 2005) analyses of the partially per-O-methylated, per-O-acetylated alditol acetate derivatives of product X in assays performed with M. smegmatis membranes revealed the addition of β(1→2) [Rt 11.75 min; m/z 129, 130,161,190] and α(1→3) [Rt 12.39 min; m/z 118, 129, 130, 190, 202, 233] linked Araf residues (Fig. 9A and B). Therefore, the product migrating below Ara5 and coincident with the Ara6 acceptor standard on TLC (Fig. 8A and B) is in fact a mixture of two products (Fig. 9B). The addition of β(1→2)-linked Araf residues can be attributed to the function of AftB. The presence of α(1→3)-linked Araf residues in this assay using an Ara5 acceptor clearly highlights the role of a novel arabinofuranosyltransferase(s) capable of functioning in an α(1→3) capacity. Importantly, the level of α(1→3) activity when the Ara5 acceptor is incubated with membranes prepared from M. smegmatisΔaftC is completely abolished (Fig. 9A). However, β(1→2) activity is clearly present in M. smegmatisΔaftC (Fig. 9A). In addition, M. smegmatisΔaftC complemented with pMV261-Mt-aftC restores α(1→3) arabinofuranosyltransferase activity to wild-type M. smegmatis (Fig. 9A). The results clearly establish both from in vivo and in vitro experiments that AftC catalyses the addition of an α(1→3)-Araf unit via an α(1→3) arabinofuranosyltransferase and that this enzyme is also resistant to EMB (Fig. 8A).
Fig. 9.
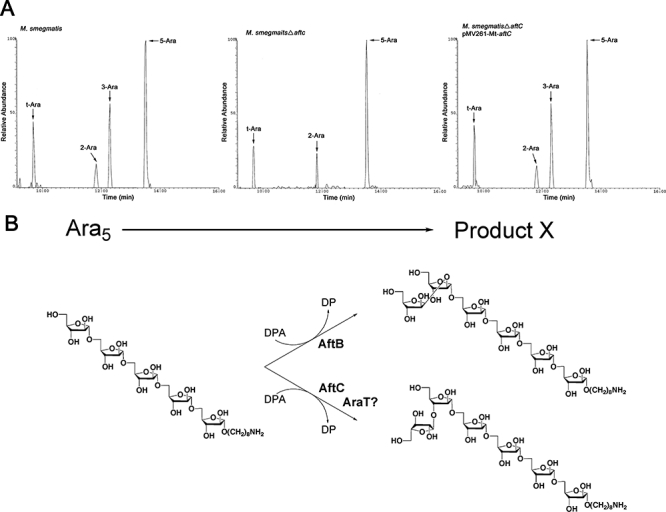
GC characterization of in vitro synthesized product X from the arabinofuranosyltransferase assays utilizing the Ara5 acceptor.
A. GC analysis of the partially per-O-methylated, per-O-acetylated alditol acetate derivative of product X obtained from assays containing membranes prepared from either M. smegmatis, M. smegmatisΔaftC or M. smegmatisΔaftC pMV261-Mt-aftC (Sassaki et al., 2005).
B. Panel illustrates the structure(s) of product X.
Discussion
The mAGP complex represents one of the most important cell wall components of the Corynebacterianeae and is essential for the viability of M. tuberculosis (Vilcheze et al., 2000; Pan et al., 2001; Gande et al., 2004; Mills et al., 2004). It is therefore not surprising that one of the most effective antimycobacterial drugs, EMB, targets its synthesis through inhibition of AG biosynthesis. However, the emergence of MDR-TB and XDR-TB has accelerated the need to discover new drug targets (Brennan and Nikaido, 1995). One of the strategies is to identify genes involved in AG biosynthesis. Based on this strategy we previously identified the presence of a new ‘priming’ enzyme, now termed AftA, which would link the initial Araf unit with the C-5 OH of a β(1→6) linked Galf of a pre-synthesized galactan core (Alderwick et al., 2005), and more recently identified the AftB enzyme responsible for β(1→2) Araf residues.
The previously described Emb (Alderwick et al., 2005), AftA (Alderwick et al., 2006a) and AftB proteins (Seidel et al., 2007a) are distinct arabinofuranosyltransferases. Thus, despite some functional relationship, these glycosyltransferases have inherent specific features as evident from the insensitivity of AftA and AftB towards EMB, whereas the single Cg-Emb (Alderwick et al., 2005; Radmacher et al., 2005) and Mt-Emb proteins are sensitive towards EMB (Telenti et al., 1997; Belanger et al., 1996). The number of arabinofuranosyltransferases that are required for mycobacterial arabinan biosynthesis has been a matter of speculation to date depending on how the arabinan chains are assembled. The primary structure of AG (Besra et al., 1995; Daffé et al., 1990) would suggest at least five distinct arabinofuranosyltransferases are required for the complete formation of AG. Interestingly, M. smegmatis embA and embB mutants were found to possess reduced amounts of the non-reducing terminal disaccharide β-d-Araf-(1→2)-α-d-Araf and result in the removal of the dominant terminal non-reducing Ara6 branched motif in the mutant being replaced by a linear Ara4 motif (Escuyer et al., 2001). The authors of this study concluded that the M. smegmatis embA and embB mutants result in a lack of 3-arm branching off the main α(1→5)-arabinan chain proximal to the non-reducing and attachment site of mycolic acids in AG (Escuyer et al., 2001). Initially, it was proposed that the β-d-Araf-(1→2)-α-d-Araf disaccharide was assembled using EmbA and EmbB. However, the recent identification of AftB, the development of specific in vitro assays in combination with mutant strains, and recent structural studies have fuelled speculation that EmbA/B act as α(1→5) arabinofuranosyltransferases (Alderwick et al., 2005; Seidel et al., 2007a; Bhamidi et al., 2008).
In this study, we have identified MSMEG2785 (also Rv2673 and NCgl1822, which we have termed AftC, as a novel branching arabinofuranosyltransferase. More precisely, AftC catalyses the addition of α(1→3) Araf residues as shown through both in vivo and in vitro experiments, ultimately resulting in 3,5-Araf residues after further α(1→5) extension, characteristic of AG. For instance, incubation of membranes prepared from M. smegmatis with DP[14C]A and a linear α(1→5)-Ara5 neoglycolipid acceptor resulted in the synthesis of an Ara6 product. Further chemical characterization of the product by glycosyl linkage analysis established that the α(1→5)-Ara5 acceptor was extended via an EMB-resistant α(1→3) arabinofuranosyltransferase giving rise to 3-linked Araf residues and corroborated our earlier cell wall analysis of the M. smegmatisΔaftC mutant. As it is now established that only α(1→5) arabinofuranosyltransferase(s) is EMB-sensitive it can be further speculated that EmbA and EmbB function in the assembly of the linear α(1→5) arabinan segments as presented in Fig. 10, which is in accordance with previous data and the phenotype of a Cg-Emb mutant (Alderwick et al., 2005). It is clear that further studies are required to establish the precise role of EmbA and EmbB in mycobacteria.
Fig. 10.
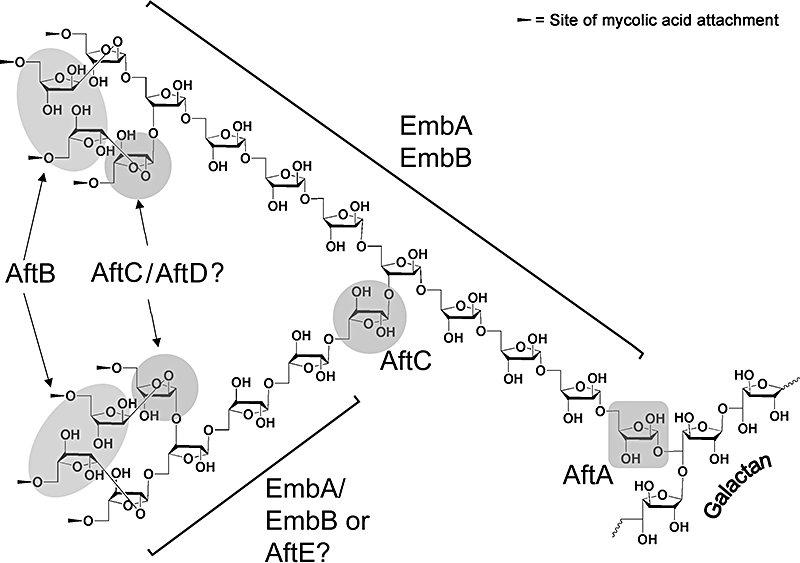
Mycobacterial arabinan biosynthesis and the role of AftC.
The analysis of the M. smegmatisΔaftC mutant to date and based on the Ara : Gal ratio would suggest that the residual arabinan segment in the mutant consists of approximately five Araf residues: β-d-Araf-(1→2)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf located at three branches on the galactan chain (Besra et al., 1995; Alderwick et al., 2005). This is consistent with the recent primary structure of AG (Bhamidi et al., 2008), with a ‘non-variable’ terminal non-reducing Ara17 motif, introduction of a 3,5-Araf residue distal to this non-reducing end by AftC and further extension by a linear α(1→5)Araf domain (Fig. 10). The latter appears to be variable (up to 12/13 residues). However, based on M. smegmatisΔaftC and the subsequent Ara : Gal compositional analysis a dominant Ara22/Ara23 motif would be consistent with recent (Bhamidi et al., 2008) and previous (Besra et al., 1995) structural data on AG and this is represented in terms of biosynthetic considerations in Fig. 10. It is also possible that AftC or a second distinct α(1→3) arabinosfuranosyltransferase (shown as AftD in Fig. 10) may be involved in late stages of AG synthesis, i.e. the non-reducing Ara6 motif and is consistent with our data and the model presented in Fig. 10.
The discovery of AftC has now shed new light on the key arabinofuranosyltransferases to build an arabinan domain typical for Corynebacterianae. In this context, the genomic organization in the genomes of the Corynebacterianae sequenced is intriguing, revealing high synteny of the M. tuberculosis aftC locus to the maps of all other Mycobacterium and Corynebacterium species. The identification of new cell wall biosynthetic drug targets is of great importance, especially with the emergence of MDR-TB. This newly discovered DPA-dependent arabinofuranosyl transferase represents, along with a straightforward in vitro enzyme assay, a promising candidate for further exploitation as a potential drug target.
Experimental procedures
Bacterial strains and growth conditions
Corynebacterium glutamicum ATCC 13 032 (referred to the remainder of the text as C. glutamicum) and Escherichia coli DH5αmcr were grown in Luria–Bertani broth (LB, Difco) at 30°C and 37°C respectively. The recombinant strains generated in this study were grown on complex brain–heart infusion medium (BHI, Difco), and the salt medium CGXII used for C. glutamicum as described (Eggeling and Reyes, 2005). Kanamycin and ampicillin were used at a concentration of 50 μg ml−1. M. smegmatis strains were grown in tryptic soy broth (TSB; Difco), containing 0.005% Tween80 (TSBT). Solid media were made by adding 1.5% agar to the above-mentioned broths. The concentrations of antibiotics used for M. smegmatis were 100 μg ml−1 for hygromycin and 20 μg ml−1 for kanamycin. Minimal inhibitory concentrations were determined by plating cells on solid media supplemented with various concentrations of EMB, rifampicin and chloramphenicol. The minimal inhibitory concentration was defined as the first concentration of drug that would inhibit 100% of growth after 5 days of incubation (Belanger et al., 1996). M. tuberculosis H37Rv DNA was obtained from the NIH Tuberculosis Research Materials and Vaccine Testing Contract at Colorado State University. All other chemicals were of reagent grade and obtained from Sigma-Aldrich.
Construction of plasmids and strains
Approximately 1 kb of upstream and downstream flanking sequences of MSMEG2785 were PCR amplified from M. smegmatis mc2155 genomic DNA using the primer pairs MS2785LL (TTTTTTTTCCATAAATTGGATCCGCTGACCGACCTCATC) and MS2785LR (TTTTTTTTCCATTTCTTGGCGAGCCCGAGCTTGAAGTTG), and MS2785RL (TTTTTTTTCCATAGATTGGTTCCTGCTGCTGTCCCTTGG) and MS2785RR (TTTTTTTTCCATCTTTTGGCGAACTCAGCGGCGATTCAC) respectively (all primers are given in 5′ to 3′ direction). Following restriction digestion of the primer incorporated Van91I sites, the PCR fragments were cloned into Van91I-digested p0004S to yield the knockout plasmid pΔMSMEG2785 which was then packaged into the temperature-sensitive mycobacteriophage phAE159 as described previously (Bardarov et al., 2002) to yield phasmid DNA of the knockout phage phΔMSMEG2785. Generation of high titre phage particles and specialized transduction were performed as described earlier (Stover et al., 1991; Bardarov et al., 2002). Deletion of MSMEG2785 in one hygromycin-resistant transductant was confirmed by Southern blot. To enable expression of MSMEG2785 and Rv2673, in the deletion mutant, these were amplified using primer pairs designed for subsequent cloning into the mycobacterial-shuttle vector pMV261 (Stover et al., 1991). All cloned fragments were verified by sequencing.
To construct the deletion vector pK19mobsacBΔaftC (NCgl 1822), cross-over PCR was applied with primer pairs AB (A, CGTTAAGCTTCGATCTTGTTGATGTGTGGCATCACACG; B, CCCATCCACTAAACTTAAACAGCGCCATCAACAACATGG) and CD (C, TGTTTAAGTTTAGTGGATGGGTGATCCAACGCACGACCATC; D, GCATGGATCCACGCATACCGAGGGAAAGATCTTC) and C. glutamicum genomic DNA as template. Both amplified products were used in a second PCR with primer pairs AD to generate a 656 bp fragment consisting of sequences adjacent to Cg-aftC, which was ligated with BamHI–HindIII-cleaved pK19mobsacB. All plasmids were confirmed by sequencing. The chromosomal deletion of Cg-aftC was performed as described previously using two rounds of positive selection (Schafer et al., 1994), and its successful deletion was verified by use of two different primer pairs.
Isolation of the mAGP complex, glycosyl composition and linkage analysis of alditol acetates by GC and GC/MS
The thawed cells were re-suspended in phosphate-buffered saline containing 2% Triton X-100 (pH 7.2), disrupted by sonicaton and centrifuged at 27 000 g (Besra et al., 1995; Alderwick et al., 2005). The pelleted material was extracted three times with 2% SDS in phosphate-buffered saline at 95°C for 1 h, washed with water, 80% (v/v) acetone in water and acetone, and finally lyophilized to yield a highly purified cell wall preparation (Besra et al., 1995; Alderwick et al., 2005). Cell wall or per-O-methylated cell wall preparations (Alderwick et al., 2005) were hydrolysed in 2 M TFA, reduced with NaB2H4 and the resultant alditols per-O-acetylated and examined by GC and GC/MS as described previously (Besra et al., 1995; Alderwick et al., 2005).
Extraction and analysis of cell wall-bound mycolic acids
In terms of M. smegmatis strains equivalent amounts of freeze-dried bacilli (100 mg) were processed as described previously (Seidel et al., 2007a), following two consecutive CHCl3/CH3OH/H2O (10:10:3, v/v/v) extractions for 4 h at 50°C in the analysis of cell wall-associated lipids, and cell wall-bound MAMEs. Alternatively, M. smegmatis and C. glutamicum cultures (5 ml) were grown and metabolically labelled at mid-logarithmic phase of growth using 1 μCi ml−1 [1,2-14C]acetate (50–62 mCi mmol−1, GE Healthcare, Amersham Bioscience) for 4 h at either 30°C or 37°C with gentle shaking, harvested, washed and freeze-dried. Cells were then extracted by two consecutive extractions with 2 ml of CHCl3/CH3OH/H2O (10:10:3, v/v/v) for 4 h at 50°C to provide cell wall-associated lipids and analysed as described previously (Seidel et al., 2007a). The crude lipid extracts were re-suspended in CHCl3/CH3OH (2:1) and equivalent aliquots (50 000 cpm) analysed by TLC using silica gel plates (5735 silica gel 60F254, Merck) developed in CHCl3/CH3OH/NH4OH (80:20:2, v/v/v) to separate [14C]-labelled TDM and phospholipids (Mikusova et al., 1995). Lipids were visualized by autoradiography by overnight exposure of Kodak X-Omat AR film to the TLC plates to reveal labelled lipids, quantified by phosphorimaging and compared with know standards (Mikusova et al., 1995). The bound MAMEs/CMAMEs from the above de-lipidated extracts were released by the addition of 2 ml of 5% aqueous solution of tetra-butyl ammonium hydroxide followed by overnight incubation at 100°C. After cooling, water (2 ml), CH2Cl2 (4 ml) and CH3I (500 μl) were added and mixed thoroughly for 30 min. The lower organic phase was recovered following centrifugation and washed three times with water (4 ml), dried and re-suspended in diethyl ether (4 ml). After centrifugation the clear supernatant was again dried and re-suspended in CH2Cl2 (100 μl). An aliquot (5 μl) from each strain was subjected to scintillation counting and an equivalent (5 μl) aliquot analysed by TLC using silica gel plates (5735 silica gel 60F254, Merck), developed in petroleum ether/acetone (95:5, v/v) and either visualized by autoradiography by exposure of Kodak X-Omat AR film to the TLC plates to reveal [14C]-labelled MAMEs/CMAMEs, or charred following spraying with 5% molybdophosphoric acid in ethanol at 100°C and compared with know standards.
Arabinofuranosyltransferase activity with membrane preparations of M. smegmatis, M. smegmatis pMV261-Mt-aftC, M. smegmatisΔaftC and M. smegmatisΔaftC pMV261-Mt-aftC
Membranes were prepared as described previously (Lee et al., 1997; Alderwick et al., 2006a) and re-suspended in 50 mM MOPS (pH 7.9), containing 5 mM β-mercaptoethanol and 10 mM MgCl2 (buffer A) to a final concentration of 15–10 mg ml−1. The neoglycolipid acceptors used in this study were α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-(1→5)-α-d-Araf-O-(CH2)8NH2 (Ara5, see Supplementary material) and α-d-Araf-(1→5)-α-d-Araf-O-(CH2)7CH3 (Ara2) (Lee et al., 1995; 1998). The acceptors (either Ara2 or Ara5) and DP[14C]A (Lee et al., 1995; 1998) (stored in CHCl3/CH3OH, 2:1, v/v) were aliquoted into 1.5 ml eppendorf tubes to a final concentration of 2 mM and 200 000 cpm (90 μM), respectively, and dried under nitrogen. The arabinofuranosyltransferase assay was carried out as described previously (Lee et al., 1997) with modifications. IgePal™ (Sigma-Aldrich) was added (0.1%, v/v) with the appropriate amount of buffer A (final volume 80 μl). Tubes were sonicated for 15 min to re-suspend lipid linked substrates and then mixed with the remaining assay components, which included membrane protein from either M. smegmatis, M. smegmatis pMV261-Mt-aftC M. smegmatisΔaftC or M. smegmatisΔaftC pMV261-Mt-aftC (1 mg), 1 mM ATP, 1 mM NADP and in some cases EMB (0–1 mg ml−1). Assays were incubated for 1 h at 37°C and quenched by the addition of 533 μl CHCl3/CH3OH (1:1, v/v). After mixing and centrifugation at 27 000 g for 15 min at 4°C, the supernatant was removed and dried under nitrogen. The residue was then re-suspended in 700 μl of CH3CH2OH/H2O (1:1, v/v) and loaded onto a 1 ml SepPak strong anion exchange cartridge (Supelco), pre-equilibrated with CH3CH2OH/H2O (1:1, v/v). The column was washed with 2 ml CH3CH2OH and the eluate collected, dried and partitioned between the two phases arising from a mixture of n-butanol (3 ml) and water (3 ml). The resulting organic phase was recovered following centrifugation at 3500 g and the aqueous phase again extracted twice with 3 ml of water-saturated n-butanol. The pooled extracts were back-washed twice with n-butanol-saturated water (3 ml). The n-butanol fraction was dried and re-suspended in 200 μl butanol. The extracted radiolabelled material was quantified by liquid scintillation counting using 10% of the labelled material and 5 ml of EcoScintA (National Diagnostics, Atlanta). The incorporation of [14C]Araf was determined by subtracting counts present in control assays (incubations in the absence of acceptor). The remaining labelled material was subjected to thin-layer chromatography (TLC) using either isopropanol/acetic acid/water (8:1:1, v/v/v) for the assays utilizing the Ara5 acceptor or CHCl3/CH2OH/H2O/NH4OH (65:25:3.6:0.5, v/v/v/v) in the case of the Ara2 acceptor on aluminum-backed Silica Gel 60 F254 plates (Merck, Darmstadt, Germany). Autoradiograms were obtained by exposing TLCs to X-ray film (Kodak X-Omat) for 3 days.
Characterization of α(1→3)-arabinofuranosyltransferase activity with membranes prepared from M. smegmatis, M. smegmatisΔaftC and M. smegmatisΔaftC pMV261-Mt-aftC
Large-scale reaction mixtures containing cold DPA (200 μg, 0.75 mM) (Lee et al., 1997) and 50 mM of the acceptor Ara5 were mixed and given an initial incubation at 37°C with membranes prepared from either M. smegmatis, M. smegmatisΔaftC or M. smegmatisΔaftC pMV261-Mt-aftC for 1 h. The assays were replenished with fresh membranes (1 mg) and re-incubated for 1 h at 37°C with the entire process repeated thrice. Products were extracted from reaction mixtures by n-butanol/water phase separation as described earlier to extract products. Products were applied to preparative TLC plates, developed in isopropanol/acetic acid/water (8:1:1, v/v/v) and sprayed with 0.01% 1,6-diphenylhexatriene in petroleum-ether/acetone (9:1, v/v), and the products localized under long-wave (366 nm) UV light (Lee et al., 1997). The plate was then re-developed in toluene to remove the reagent and the bands recovered from the plates by extraction with n-butanol. The butanol phases were washed with water saturated with n-butanol and the dried products subjected to GC (Sassaki et al., 2005) and GC/MS as described (Lee et al., 1997; Alderwick et al., 2006a).
Acknowledgments
H.L.B. is a Medical Research Council Quota Student. G.S.B. acknowledges support in the form of a Personal Research Chair from Mr James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council and The Wellcome Trust (081569/Z/06/Z). A.B. is supported by a Career Development Award from the Medical Research Council. The contributions of T.L.L. and Y.B. to this work were supported by the Alberta Ingenuity Centre for Carbohydrate Science and the Natural Sciences and Engineering Research Council of Canada. The authors would also like to thank W.R. Jacobs Jr. and Tsungda Hsu (Albert Einstein College of Medicine, New York) for the gift of plasmid p0004S.
Supplementary material
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/
j.1365-2958.2008.06354.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alderwick LJ, Radmacher E, Seidel M, Gande R, Hitchen PG, Morris HR, et al. Deletion of Cg-emb in corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J Biol Chem. 2005;280:32362–32371. doi: 10.1074/jbc.M506339200. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. Identification of a novel arabinofuranosyltransferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2006a;281:15653–15661. doi: 10.1074/jbc.M600045200. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Dover LG, Seidel M, Gande R, Sahm H, Eggeling L, Besra GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-d-arabinose metabolism. Glycobiology. 2006b;16:1073–1081. doi: 10.1093/glycob/cwl030. [DOI] [PubMed] [Google Scholar]
- Amin AG, Goude R, Shi L, Zhang J, Chatterjee D, Parish T. EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology. 2008;154:240–248. doi: 10.1099/mic.0.2007/012153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Bardarov S, Bardarov S, Pavelka MS, Sambandamurthy V, Larsen M, Tufariello J, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- Belanger AE, Besra GS, Ford ME, Mikusova K, Belisle JT, Brennan PJ, Inamine JM. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. A new interpretation of the structure of the mycolyl-arabinogalactan complex of Mycobacterium tuberculosis as revealed through characterization of oligoglycosylalditol fragments by fast-atom bombardment mass spectrometry and 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:4257–4266. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]
- Bhamidi S, Scherman MS, Rithner CD, Prenni JE, Chatterjee D, Khoo KH, McNeil MR. The identification and location of succinyl residues and the characterization of the interior arabinan region allows for a model of the compete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J Biol Chem. 2008;283:12992–13000. doi: 10.1074/jbc.M800222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A, Kremer L, Dai AZ, Sacchettini JC, Jacobs WR., Jr Conditional depletion of KasA, a key enzyme of mycolic acid biosynthesis, leads to mycobacterial cell lysis. J Bacteriol. 2005;187:7596–7606. doi: 10.1128/JB.187.22.7596-7606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Brudno M, Chapman M, Gottgens B, Batzoglou S, Morgenstern B. Fast and sensitive multiple alignment of large genomic sequences. BMC Bioinformatics. 2003;4:66. doi: 10.1186/1471-2105-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffé M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990;265:6734–6743. [PubMed] [Google Scholar]
- Dover LG, Cerdeno-Tarraga AM, Pallen MJ, Parkhill J, Besra GS. Comparative cell wall core biosynthesis in the mycolated pathogens, Mycobacterium tuberculosis and Corynebacterium diphtheriae. FEMS Microbiol Rev. 2004;28:225–250. doi: 10.1016/j.femsre.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- Eggeling L, Reyes O. Experiment. In: Eggeling L, Bott M, editors. Handbook of Corynebacterium glutamicum. Boca Raton, FL: CRC Press, Taylor Francis Group; 2005. pp. 535–566. [Google Scholar]
- Escuyer VE, Lety MA, Torrelles JB, Khoo KH, Tang JB, Rithner CD, et al. The role of the embA and embB gene products in the biosynthesis of the terminal hexaarabinofuranosyl motif of Mycobacterium smegmatis arabinogalactan. J Biol Chem. 2001;276:48854–48862. doi: 10.1074/jbc.M102272200. [DOI] [PubMed] [Google Scholar]
- Gande R, Gibson KJ, Brown AK, Krumbach K, Dover LG, Sahm H, et al. Acyl-CoA carboxylases (accD2 and accD3), together with a unique polyketide synthase (Cg-pks), are key to mycolic acid biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J Biol Chem. 2004;279:44847–44857. doi: 10.1074/jbc.M408648200. [DOI] [PubMed] [Google Scholar]
- Guvener ZT, McCarter LL. Multiple regulators control capsular polysaccharide production in Vibrio parahaemolyticus. J Bacteriol. 2003;185:5431–5441. doi: 10.1128/JB.185.18.5431-5441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye K, Frieden TR. Tuberculosis control: the relevance of classic principles in an era of acquired immunodeficiency syndrome and multidrug resistance. Epidemiol Rev. 1996;18:52–63. doi: 10.1093/oxfordjournals.epirev.a017916. [DOI] [PubMed] [Google Scholar]
- Lee RE, Mikusova K, Brennan PJ, Besra GS. Synthesis of the arabinose aonor β-d-arabinofuranosyl-1-monophosphoryldecaprenol, development of a basic arabinosyl-transferase assay, and identification of ethambutol as an arabinosyltransferase inhibitor. J Am Chem Soc. 1995;117:11829–11832. [Google Scholar]
- Lee RE, Brennan PJ, Besra GS. Mycobacterial arabinan biosynthesis: the use of synthetic arabinoside acceptors in the development of an arabinosyl transfer assay. Glycobiology. 1997;7:1121–1128. doi: 10.1093/glycob/7.8.1121. [DOI] [PubMed] [Google Scholar]
- Lee RE, Brennan PJ, Besra GS. Synthesis of β-d-arabinofuranosyl-1-monophosphoryl polyprenols: examination of their function as mycobacterial arabinosyl transferase donors. Bioorg Med Chem Lett. 1998;8:951–954. doi: 10.1016/s0960-894x(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Mushegian A. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 2003;12:1418–1431. doi: 10.1110/ps.0302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M, Daffe M, Brennan PJ. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990;265:18200–18206. [PubMed] [Google Scholar]
- McNeil M, Daffe M, Brennan PJ. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J Biol Chem. 1991;266:13217–13223. [PubMed] [Google Scholar]
- Mikusova K, Slayden RA, Besra GS, Brennan PJ. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39:2484–2489. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JA, Motichka K, Jucker M, Wu HP, Uhlik BC, Stern RJ, et al. Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J Biol Chem. 2004;279:43540–43546. doi: 10.1074/jbc.M407782200. [DOI] [PubMed] [Google Scholar]
- Minnikin DE. Lipids: complex lipids, their chemistry, biosynthesis and roles. In: Ratledge C, Stanford J, editors. The Biology of the Mycobacteria. London: Academic Press; 1982. pp. 95–184. [Google Scholar]
- Mishra AK, Alderwick LJ, Rittmann D, Tatituri RV, Nigou J, Gilleron M, et al. Identification of an α(1→6) mannopyranosyltransferase (MptA), involved in Corynebacterium glutamicum lipomanann biosynthesis, and identification of its orthologue in Mycobacterium tuberculosis. Mol Microbiol. 2007;65:1503–1517. doi: 10.1111/j.1365-2958.2007.05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Alderwick LJ, Rittmann D, Wang C, Bhatt A, Jacobs WR, Jr, et al. Identification of a novel α(1→6) mannopyranosyltransferase (MptB) from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomanann- and lipoarabinomann-deficient mutant. Mol Microbiol. 2008;68:1595–1613. doi: 10.1111/j.1365-2958.2008.06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Jackson M, Ma Y, McNeil M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. J Bacteriol. 2001;183:3991–3998. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolo WF, Jr, Nosanchuk JD. Tuberculosis in New York city: recent lessons and a look ahead. Lancet Infect Dis. 2004;4:287–293. doi: 10.1016/S1473-3099(04)01004-7. [DOI] [PubMed] [Google Scholar]
- Parish T, Roberts G, Laval F, Schaeffer M, Daffe M, Duncan K. Functional complementation of the essential gene fabG1 of Mycobacterium tuberculosis by Mycobacterium smegmatis fabG but not Escherichia coli fabG. J Bacteriol. 2007;189:3721–3728. doi: 10.1128/JB.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portevin D, De Sousa-D'Auria C, Houssin C, Grimaldi C, Chami M, Daffe M, Guilhot C. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc Natl Acad Sci USA. 2004;101:314–319. doi: 10.1073/pnas.0305439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmacher E, Stansen KC, Besra GS, Alderwick LJ, Maughan WN, Hollweg G, et al. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits 1-glutamate efflux of Corynebacterium glutamicum. Microbiology. 2005;151:1359–1368. doi: 10.1099/mic.0.27804-0. [DOI] [PubMed] [Google Scholar]
- Sassaki GL, Iacaomini M, Gorin PAJ. Methylation-GC-MS analysis of arabinofuranose-and galactofuranose-containing structures; rapid synthesis of partially O-methylated alditol acetates. An Acad Bras Cienc. 2005;77:223–234. [Google Scholar]
- Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Seidel M, Alderwick LJ, Birch HL, Sahm H, Eggeling L, Besra GS. Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J Biol Chem. 2007a;282:14729–14740. doi: 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- Seidel M, Alderwick LJ, Sahm H, Besra GS, Eggeling L. Topology and mutational analysis of the single Emb arabinofuranosyltransferase of Corynebacterium glutamicum as a model of Emb proteins of Mycobacterium tuberculosis. Glycobiology. 2007b;17:210–219. doi: 10.1093/glycob/cwl066. [DOI] [PubMed] [Google Scholar]
- Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martin-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–387. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Takayama K, Kilburn JO. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989;33:1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Philipp WJ, Sreevatsan S, Bernasconi C, Stockbauer KE, Wieles B, et al. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat Med. 1997;3:567–570. doi: 10.1038/nm0597-567. [DOI] [PubMed] [Google Scholar]
- Vilcheze C, Morbidoni HR, Weisbrod TR, Iwamoto H, Kuo M, Sacchettini JC, Jacobs WR., Jr Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J Bacteriol. 2000;182:4059–4067. doi: 10.1128/jb.182.14.4059-4067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolucka BA, McNeil MR, de Hoffmann E, Chojnacki T, Brennan PJ. Recognition of the lipid intermediate for arabinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J Biol Chem. 1994;269:23328–23335. [PubMed] [Google Scholar]
- Zhang N, Torrelles JB, McNeil MR, Escuyer VE, Khoo KH, Brennan PJ, Chatterjee D. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol Microbiol. 2003;50:69–76. doi: 10.1046/j.1365-2958.2003.03681.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


