Abstract
We determined the genome-wide environmental stress response (ESR) expression profile of Candida glabrata, a human pathogen related to Saccharomyces cerevisiae. Despite different habitats, C. glabrata, S. cerevisiae, Schizosaccharomyces pombe and Candida albicans have a qualitatively similar ESR. We investigate the function of the C. glabrata syntenic orthologues to the ESR transcription factor Msn2. The C. glabrata orthologues CgMsn2 and CgMsn4 contain a motif previously referred to as HD1 (homology domain 1) also present in Msn2 orthologues from fungi closely related to S. cerevisiae. We show that regions including this motif confer stress-regulated intracellular localization when expressed in S. cerevisiae. Site-directed mutagenesis confirms that nuclear export of CgMsn2 in C. glabrata requires an intact HD1. Transcript profiles of CgMsn2/4 mutants and CgMsn2 overexpression strains show that they regulate a part of the CgESR. CgMsn2 complements a S. cerevisiae msn2 null mutant and in stressed C. glabrata cells, rapidly translocates from the cytosol to the nucleus. CgMsn2 is required for full resistance against severe osmotic stress and rapid and full induction of trehalose synthesis genes (TPS1, TPS2). Constitutive activation of CgMsn2 is detrimental for C. glabrata. These results establish an Msn2-regulated general stress response in C. glabrata.
Introduction
Adaptation of gene expression through regulation of transcription is a key mechanism in fungal response to fluctuating environmental conditions. Environmental stress causes activation of a variety of signalling mechanisms each responding to the particular situation, such as heat shock or osmotic stress, and in parallel evokes a stereotypic general response. In Saccharomyces cerevisiae, this response was first described and is referred to as general stress response or environmental stress response (ESR) (Gasch et al., 2000; Causton et al., 2001). Comparable ESR patterns have been characterized in Schizosaccharomyces pombe and to a certain extent in Candida albicans (Smith et al., 2004; Enjalbert et al., 2006; Gasch, 2007). Candida glabrata is more closely related to S. cerevisiae than C. albicans and S. pombe (Fitzpatrick et al., 2006), and is the second most common fungal pathogen isolated from humans (Kaur et al., 2005; Pfaller and Diekema, 2007). Infection rates are relatively low but have been constant during the last decade (Sandven et al., 2006). The ESR of C. glabrata is currently relatively unexplored.
For S. cerevisiae, C. albicans and S. pombe, one major mechanism for controlling general stress responses are p38-type SAP kinases (stress-activated mitogen-activated protein kinases). The SAPKs, Hog1, Sty1 and CaHog1 are all activated by hyperosmolarity and oxidative stress, and to a varying degree by other stress agents, such as cadmium (Chen et al., 2003; Smith et al., 2004; Enjalbert et al., 2006). The HOG (high osmolarity glycerol) pathway of C. glabrata functions in a similar manner to S. cerevisiae (Gregori et al., 2007).
In S. cerevisiae, a second general stress-mediating mechanism based on the transcription factor Msn2 and its paralogue Msn4 exists (Martinez-Pastor et al., 1996; Estruch, 2000; Gasch et al., 2000; Causton et al., 2001; Hohmann, 2002). They are activated by a variety of stress conditions and changing nutrient supply situations, such as the exhaustion of the preferred carbon source glucose (Choo and Klug, 1994; Martinez-Pastor et al., 1996; DeRisi et al., 1997; Boy-Marcotte et al., 1998; Gasch et al., 2000; Rep et al., 2000; Causton et al., 2001; Hasan et al., 2002; Cameroni et al., 2004; Schüller et al., 2004; Teixeira et al., 2006). They also have a role in both chronological and replicative ageing (Fabrizio et al., 2001; 2004; Powers et al., 2006). During high-nutrient supply, Msn2 is inactivated by the PKA (protein kinase A) and TOR (target of rapamycin) pathways (Boy-Marcotte et al., 1998; Görner et al., 1998; Garreau et al., 2000; Görner et al., 2002). Activation of Msn2 and Msn4 causes their rapid accumulation in the nucleus and recruitment to chromatin. Msn2 has separate functional domains for nuclear import (nuclear localization signal, NLS), nuclear export (nuclear export signal, NES) and DNA binding. The C2H2 Zn finger DNA binding domain at the C-terminus recognizes the stress response element (STRE). The NLS is found adjacent to the DNA binding domain; it is phosphorylated and inactivated by PKA when glucose is available and rapidly dephosphorylated and activated by glucose starvation (Görner et al., 2002; De Wever et al., 2005). Stress signalling requires a region in the N-terminal part of Msn2 which includes its NES and a short stretch of high similarity to Msn4 designated homology domain 1 (HD1) (Görner et al., 1998; Görner et al., 2002; Durchschlag et al., 2004; Boy-Marcotte et al., 2006). A variety of stress conditions lead to inhibition of nuclear export of Msn2 by an unknown mechanism. The NES and its surrounding region might therefore represent a crucial determinant for the identification of stress-regulated Msn2 orthologues.
Msn2-like factors do not appear to play a role in regulating the stress response in C. albicans and S. pombe. The C. albicans Msn2 orthologue transcription factor designated CaMsn4 (orf 19.4752) is not involved in the ESR (Nicholls et al., 2004). In addition, the Hog1 Map kinase plays a more general role in stress response in C. albicans and S. pombe. These differences point to distinct strategies for regulating the stress response in different fungi.
Here we investigate ESR transcription patterns of C. glabrata and provide evidence that it uses an S. cerevisiae-like Msn2-directed stress response. We identify C. glabrata Msn2 and Msn4 orthologues based on a motif present in the Msn2/4 NES (HD1) and also in putative Msn2 orthologues of Ashbya gossypii and Kluyveromyces lactis. Furthermore, we find that C. glabrata and S. cerevisiae share many common Msn2 target genes. CgMsn2 is required for resistance against severe osmotic stress. In addition, comparison of ESR transcript patterns identifies core similarities and differences between the S. cerevisiae, C. albicans, S. pombe and C. glabrata stress responses.
Results
The ESR pattern of C. glabrata is orthologous to S. cerevisiae
The global immediate transcriptional response of C. glabrata to a set of environmental conditions was determined via microarray analysis. Conditions chosen were acute carbon starvation by removal of glucose from the medium, mild osmotic stress (0.5 M NaCl), heat stress (42°C) and mild oxidative stress (0.4 mM hydrogen peroxide). The treatment times were 20 min at 30°C to avoid indirect transcriptional responses. Transcript profiles were determined by hybridization to genome-wide C. glabrata microarrays. Expression data were filtered and averaged. From the 5063 genes spotted in duplicate, 4166 gave useful data under at least one tested condition. The entire data set was analysed for co-regulated genes by hierarchical clustering (Eisen et al., 1998).
Similar to the common stress response identified in S. cerevisiae, C. glabrata has a set of induced and repressed genes common to several stress conditions (Fig. 1A). To compare the C. glabrata expression pattern with S. cerevisiae data, we used a similarity-based annotation of orthologous genes (Feldmann, 2000) (http://cbi.labri.fr/Genolevures/). Expression data for S. cerevisiae exposed to comparable conditions, such as glucose starvation, 0.32 mM H2O2, heat stress (37°C) and 1 M Sorbitol osmotic stress, were extracted from published ESR data (Gasch et al., 2000). C. glabrata has a optimal growth temperature of 37°C and was therefore heat-stressed at 42°C. Available evidence suggests that NaCl and Sorbitol are comparable in the concentrations used (Hirasawa et al., 2006). Analysis of both ESR data sets highlights clusters corresponding to induced and repressed genes for all environmental conditions (Fig. 1A). This indicates a conserved transcriptional response between C. glabrata and S. cerevisiae. More detailed comparison of individual stress conditions shows induction of expression of orthologous genes (Fig. 1B). For example, heat stress induces expression of conserved HSP genes in both organisms (HSP12, HSP42, HSP78, HSP31, HSP104), whereas oxidative stress affects a smaller set of genes in C. glabrata. The hydrogen peroxide concentration chosen (0.4 mM) is stressful for S. cerevisiae laboratory strains. Furthermore, 0.4 mM H2O2 in vitro corresponds to the in vivo oxidative burden in phagocytic cells as judged by the similar transcriptional response of C. albicans (Enjalbert et al., 2007). However, C. glabrata strains are much more resistant to oxidative stress in vitro and have a less pronounced response to this concentration. Nevertheless, we find a characteristic pattern of induced genes with functions in oxidative stress response. These include core oxidative stress response genes, such as TRR1, TRX1, CTA1, SOD1 and GPX1.
Fig. 1.
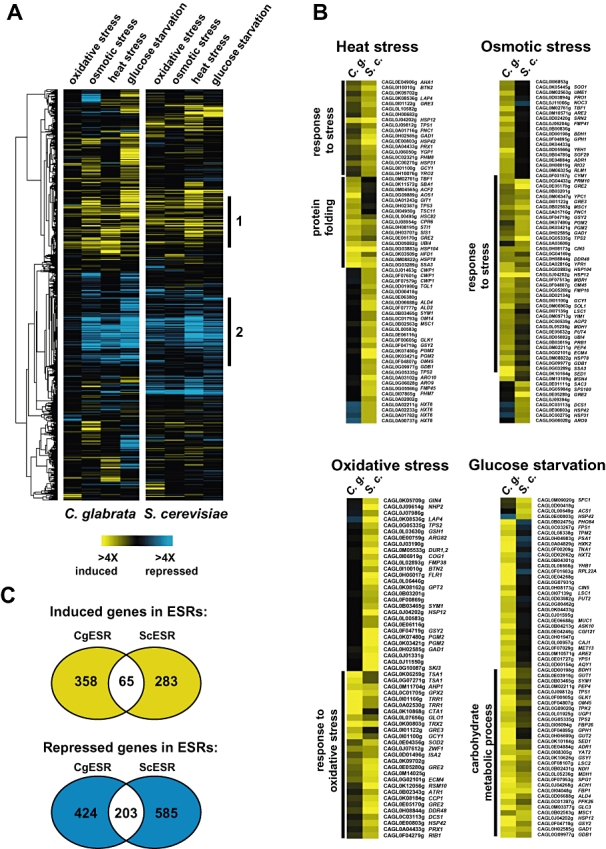
Comparison of genome-wide expression levels in response to environmental changes in C. glabrata and S. cerevisiae.
A. Hierarchical clustering. Transcript profiles were determined by hybridization to genome-wide C. glabrata microarrays. The sets represent average inductions of replicate profiles of C. glabrata wild-type strain (4166 ORFs) after treatment with 0.4 mM H2O2, upon glucose starvation, heat shock by incubation at 42°C and hyperosmolarity stress treatment with 0.5 M NaCl (left panel). All treatments were done at 30°C for 20 min. The developed profile was compared with corresponding S. cerevisiae expression data (Gasch et al., 2000) (right panel). Major clusters are labelled corresponding to induced and to repressed genes (labelled as 1 and 2), in both C. glabrata and S. cerevisiae.
B. Genes involved in four different stress responses were clustered after specific selection (heat stress > 11-fold, osmotic stress > sixfold, oxidative stress > sixfold and glucose starvation > 10-fold). Identified clusters in both C. glabrata and S. cerevisiae are indicated. Gene names correspond to C. glabrata systematic ORF designations and their corresponding S. cerevisiae orthologues.
C. The overlap between the CgESR and ScESR patterns is depicted as Venn diagram. CgESR was defined as described in Fig. 2A, with genes induced or repressed at least in one tested condition; ScESR data are from Gasch et al. (2000).
Glucose starvation and osmotic stress each induce a set of genes orthologous to S. cerevisiae. These include GPH1, TPS1, TPS2, UGP1, GSY1 and GLK1 for glucose starvation and GRE3, PGM2, HSP12, DDR48 or SSA3 during osmotic stress. The elevated expression of TPS1, TPS2 and also TPS3 is perhaps important, as trehalose has a protective role against environmental stresses (Petter and Kwon-Chung, 1996). Taken together, these patterns suggested that the ESR is conserved between S. cerevisiae and C. glabrata. The overlap between the two ESR patterns is depicted as a Venn diagram of conserved induced and repressed genes (Fig. 1C). The vast majority of repressed genes in both S. cerevisiae and C. glabrata are involved in ribosome biogenesis. Interestingly and in contrast to S. cerevisiae, expression of C. glabrata genes involved in sterol biosynthesis (ERG1, ERG2, ERG3, ERG11, ERG13 and ERG25) was repressed under all conditions tested. ERG3 and ERG11 deletions confer azole resistance (Geber et al., 1995).
Some differences between both ESRs are notable. The detailed data are available as supplementary files. TBF1, encoding a Telobox-containing general regulatory factor (Bilaud et al., 1996), is highly upregulated in C. glabrata, whereas it is mainly downregulated in S. cerevisiae throughout the conditions compared. PHO84, a high-affinity inorganic phosphate transporter and low-affinity manganese transporter (Bun-Ya et al., 1991), is downregulated during all stress responses in S. cerevisiae, but upregulated in C. glabrata during oxidative stress and glucose starvation. The integral membrane protein VPH2, required for vacuolar H+-ATPase function (Jackson and Stevens, 1997), is highly induced in all tested conditions in C. glabrata, whereas it is slightly induced only by oxidative stress in S. cerevisiae.
Part of the ESR is also conserved between C. glabrata, S. pombe, C. albicans and S. cerevisiae. To compare expression patterns of the four fungi, we used reported orthologous genes between S. pombe, C. albicans and S. cerevisiae (Enjalbert et al., 2006). We extended this list by adding the corresponding C. glabrata genes with gene similarity data reported by the Genolevures consortium. Data used to generate these figures are available as supplementary files. Comparison of the specific expression profiles revealed a striking overlap between orthologous genes of the individual species for osmotic stress-induced genes (Fig. S2A) and oxidative stress-induced genes (Fig. S2B).
Orthologues of general stress transcription factors Msn2 and Msn4 in C. glabrata
To explore the regulation of the C. glabrata ESR (referred hereafter as CgESR), we compared transcription patterns with the S. cerevisiae ESR (ScESR). We defined the CgESR by selecting genes from the C. glabrata data set which are induced or repressed more than fourfold in at least one condition. This selection resulted in a set of 760 CgESR genes. Of these, 268 genes overlap with the 868 ScESR genes (Gasch et al., 2000) (Fig. S3A and S3B). Many of the shared stress-induced genes are induced in S. cerevisiae upon overexpression of the general stress transcription factor Msn2 (Chua et al., 2006) (Fig. 2A column MSN2 OE). Furthermore, Msn2 binding sites (STRE; WAGGGG) are present in many CgESR genes. This suggested that the evolutionary conservation of the ESR is not limited to the set of regulated genes, but also extends to the transcriptional control of those genes.
Fig. 2.
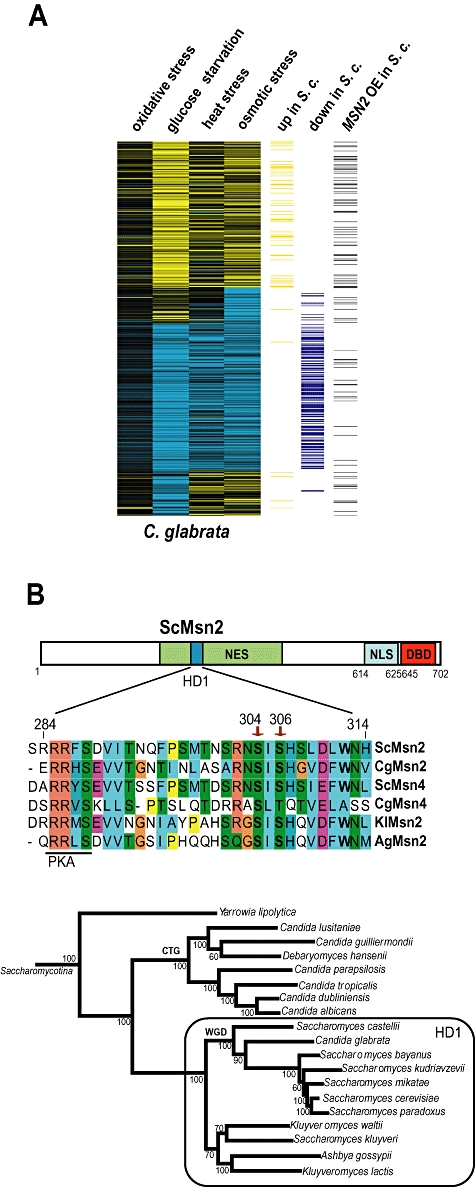
CgESR is similar to ScESR and includes many Msn2-regulated genes.
A. The CgESR shown here includes 760 genes selected by being induced or repressed significantly (fourfold) in one of the tested conditions. Columns 5 (up in S.c.) and 6 (down in S.c.) show the corresponding ScESR genes from S. cerevisiae. Column 7 (MSN2 OE in S.c.) displays induction of the orthologous S. cerevisiae genes by MSN2 overexpression (Chua et al., 2006).
B. Alignment of Msn2 orthologous sequences including the HD1. The shared core motif corresponds to positions 284–314 of ScMsn2. The HD1 signature was detected only in close relatives of S. cerevisiae, circled in the phylogeny (taken from Fitzpatrick et al., 2006). The sequences used in the alignment are: S. cerevisiae (YMR037C, ScMsn2; YKL062W, ScMsn4), A. gossypii (ABR089C, AgMsn2), C. glabrata (CAGL0F05995g, CgMsn2; CAGL0M13189g, CgMsn4) and K. lactis (KLLA0F26961g, KlMsn2).
To identify orthologues of S. cerevisiae Msn2 (ScMsn2) in C. glabrata, we searched for predicted open reading frames (ORF) comprising C2H2 Zn-cluster DNA binding domains in available fungal genomes. One further recognizable feature of ScMsn2 is its central region, which confers regulated nuclear export. This is conserved in the paralogue Msn4, and was previously described as HD1 (Görner et al., 1998). We detected putative Msn2 (CAGL0F05995g) and Msn4 (CAGL0M13189g) orthologues with HD1 domains at synthenic positions in the C. glabrata genome (Fig. 2B). K. lactis and A. gossypii contain a single orthologue of both genes that also contain an HD1 domain, which we have designated Msn2. The HD1 domain is not present in the single Msn2/4 orthologue in C. albicans and related species, nor in similar proteins from Yarrowia lipolytic, nor S. pombe (Fig. 2B).
Stress-regulated nuclear export of both ScMsn2 and ScMsn4 requires an extended region, including the HD1. To pinpoint the HD1 region of the ScMsn2/4 orthologues as a functional component of the NES, we tested if these regions are sufficient to confer stress-regulated intracellular localization. We expressed parts containing the HD1 but not the NLS and the C-terminal Zn-finger DNA binding domains of the K. lactis, A. gossypii and C. glabrata Msn2 orthologues as GFP fusions in S. cerevisiae (Fig. 3A). Sequences were amplified from genomic DNA and fused to an N-terminal nuclear localization signal (SV40NLS) to support constitutive nuclear import. Expression of the fusion genes was driven by the ScADH1 promoter. Cells were grown to early exponential phase, exposed to osmotic stress (0.5 M NaCl) or weak acid stress (10 mM sorbic acid), and the intracellular distribution of the GFP fusion proteins was recorded by fluorescence microscopy. The GFP fusion proteins of the internal regions of Msn2 and Msn4 from S. cerevisiae and C. glabrata, and Msn2 from K. lactis and A. gossypii, accumulated rapidly in the nucleus under stress conditions (Fig. 3A). We also analysed the localization of orf19.4752 (CaMsn4), the C. albicans orthologue of both ScMsn2 and ScMsn4 (Nicholls et al., 2004). CaMsn4 has a similar Zn-finger DNA binding region to the S. cerevisiae and C. glabrata proteins, but lacks a significant similarity to HD1. The GFP fusion protein comprising the CaMsn4 N-terminal 517 amino acids was constitutively enriched in the nucleus in unstressed cells and stress-induced nuclear accumulation was not detectable. As presented below, point mutations in the conserved residues of the HD1 in CgMsn2 also abolish the NES function (Fig. 4C). These data suggest that regulated nuclear export requires the HD1 domain.
Fig. 3.
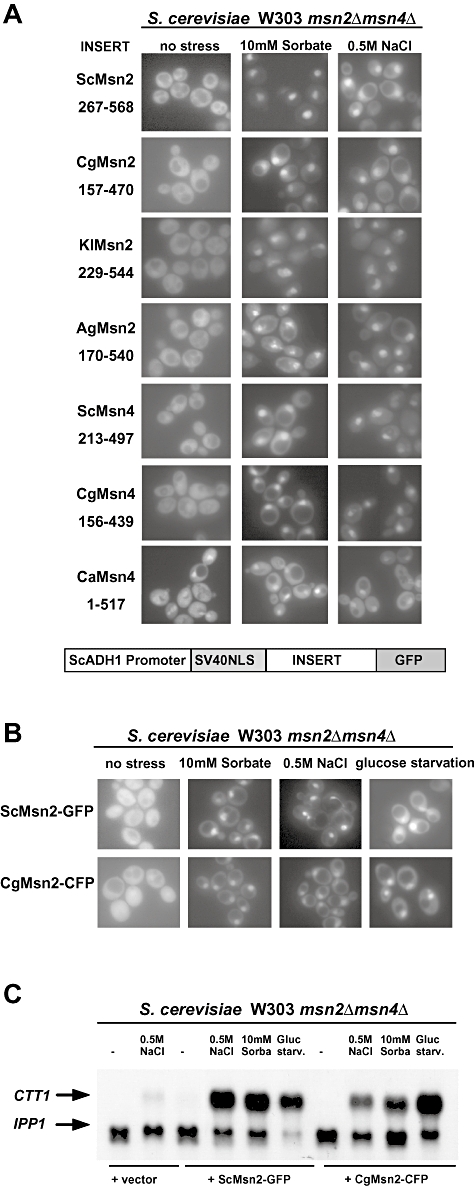
Regulated localization control is functionally conserved in Msn2-like factors.
A. Indicated regions of Msn2 and Msn4 orthologues were fused to GFP and a SV40-NLS. GFP fusion plasmids were expressed in S. cerevisiae strain W303-1A msn2Δ msn4Δ. Localization of GFP fusions was determined by fluorescence microscopy in unstressed cells and 10 min after exposure to weak acid stress (10 mM sorbic acid) and osmotic stress (0.5 M NaCl).
B. S. cerevisiae W303-1A msn2Δ msn4Δ strains containing plasmids expressing either CgMsn2–CFP or ScMsn2–GFP driven by the ScADH1 promoter (pAMG, pACgMC) were grown to exponential phase and exposed to conditions as indicated. Localization was recorded after 10 min by fluorescence microscopy of living cells.
C. mRNA levels of the Msn2-regulated gene CTT1 and the control IPP1 were visualized on Northern blots after 20 min stress treatment.
Fig. 4.
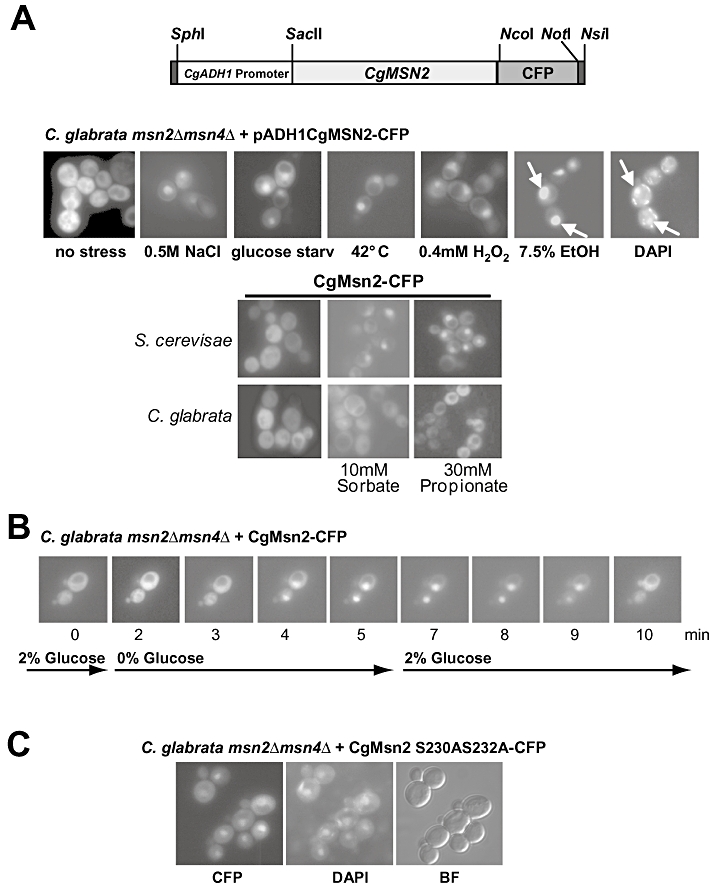
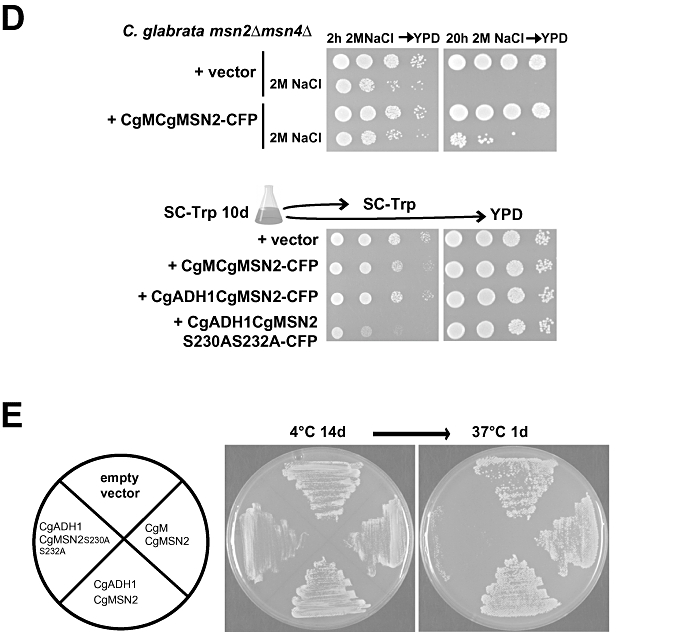
C. glabrata CgMsn2 nuclear localization is stress-regulated.
A. The CgADH1-CgMsn2–CFP construct is illustrated schematically and the positions of used restrictions sites are indicated. Localization of CgMsn2–CFP in the C. glabrata strain Cg msn2Δmsn4Δ was determined by fluorescence microscopy. CFP fluorescence was recorded in exponentially growing cells approximately 10 min after exposure to the indicated stress conditions. Nuclei were stained by addition of 2 μg ml−1 4,6 diamidino-2-phenylindol (DAPI) dye to the cultures 10 min prior to microscopy. In living cells, nucleic acids, such as the mitochondrial DNA, are also stained by DAPI, resulting in background staining. CgMsn2 localization during weak acid stress. CgMsn2–CFP accumulates in S. cerevisiae in the nucleus during weak acid stress (10 mM sorbic acid, 30 mM propionic acid). CgMsn2–CFP does not accumulate in the nucleus in C. glabrata. Arrows indicate stained nuclear DNA.
B. Nucleocytoplasmic shuttling of CgMsn2–CFP during glucose starvation and re-feeding. Localization of CgMsn2–CFP was visualized by fluorescence microscopy of C. glabrata cells fixed to a coverslip with Concanavalin A and localization of the CFP fusion was visualized by fluorescence microscopy.
C. Localization of CgMsn2 S230AS232A–CFP in unstressed cells. Cg msn2Δmsn4Δ cells expressing CgMsn2 S230AS232A–CFP were grown in rich media to early logarithmic phase and localization of the CFP fusion protein was determined by fluorescence microscopy.
D. Viability of Cg msn2Δmsn4Δ mutant cells expressing Msn2 variants. Cultures of Cg msn2Δmsn4Δ transformed with the empty vector as a control, or with a plasmid expressing CgMSN2 under the control of the native promoter, were incubated in selective media containing 2 M NaCl for 2 and 20 h. Cell suspensions were spotted in 10-fold dilutions on YPD plates and incubated at 37°C over night. Cultures of Cg msn2Δmsn4Δ transformed with the empty vector, or with plasmids expressing CgMSN2 under the control of the native or CgADH1 promoter, or expressing CgMSN2 S230AS232A–CFP, were grown in selective media at 30°C for 10 days. Cells were then spotted in 10-fold dilutions on YPD and SC-Trp plates incubated at 37°C over night and growth recorded.
E. High expression of CgMsn2 S230AS232A confers cold sensitivity. Replica-plated patches of Cg msn2Δmsn4Δ transformed with the above vectors were grown on selective plates overnight at 37°C, plates were kept at 4°C and room temperature as a control for 14 days. Plates were replica-plated to fresh and incubated at 37°C over night and growth recorded. Viability was also tested by colony-forming assay showing a threefold reduced colony-forming ability (0.38 of wild type; SD = 0.12; P = 0.05).
To verify if CgMsn2 functions as a stress-responsive transcription factor, we tested whether CgMsn2 can replace and complement the function of ScMsn2 in S. cerevisiae. We expressed full-length CgMSN2 in a S. cerevisiae strain lacking ScMSN2 and ScMSN4. The entire CgMSN2 reading frame was fused to CFP and its expression driven by the constitutive ADH1 promoter. In unstressed cells, CgMsn2–CFP was distributed mainly in the cytoplasm, similar to the analogous ScMsn2–GFP protein. Several environmental stress conditions induced rapid nuclear concentration of CgMsn2 in S. cerevisiae (Fig. 3B). Next we investigated whether CgMsn2 can complement ScMsn2 and confer stress-regulated gene activation in S. cerevisiae. CgMsn2–CFP was expressed in a S. cerevisiae strain lacking both MSN2 and MSN4 genes (W303 msn2Δ msn4Δ) exposed to osmotic, weak acid and glucose starvation stress. mRNA levels of a target gene of ScMsn2, the CTT1 gene coding for catalase T, were analysed by Northern hybridization. As shown in Fig. 3C, CgMsn2 supported the stress induction of CTT1 transcripts in a very similar manner to ScMsn2.
CgMsn2 is regulated by stress in C. glabrata
These results suggested that CgMsn2 might be the functional orthologue of ScMsn2. To examine the regulation of CgMsn2 in C. glabrata, we first analysed its intracellular localization under stress conditions. CgMsn2 was expressed as CgMsn2–CFP fusion driven by the CgADH1 promoter (Fig. 4A). Live microscopy of CgMsn2–CFP localization revealed a rapid and reversible nuclear accumulation regulated by environmental stress conditions, such as acute glucose starvation, osmotic stress, heat shock, oxidative stress and ethanol stress (Fig. 4A). CgMsn2 activity as a transcriptional activator is not changed by the presence of the C-terminal CFP fusion. In contrast, CgMsn2–CFP failed to accumulate in the nucleus during weak organic acid stress (10 mM sorbic acid, 30 mM propionic acid). Interestingly, CgMsn2–CFP expressed in S. cerevisiae accumulates in the nucleus during weak acid stress. This difference in the response of CgMsn2 during weak acid stress could be either a consequence of the higher resistance of C. glabrata to weak acids compared with S. cerevisiae, which is not the case (Gregori et al., 2007), or the absence of a mechanism signalling weak acid stress to CgMsn2 C. glabrata.
CgMsn2 accumulates in the nucleus within 4 min following acute carbon source starvation, and subsequent addition of glucose (2%) results in rapid nuclear export (Fig. 4B). The kinetics are very similar to those observed in S. cerevisiae (data not shown). To demonstrate that the integrity of the HD1 region is important for NES function, we constructed a mutant derivative by replacing two conserved serine residues S230 and S232 with alanine. The corresponding positions in the ScMsn2 ORF are indicated by arrows in Fig. 2B. As predicted, we find the CgMsn2 S230AS232A-CFP mutant constitutively enriched in the nucleus in unstressed cells, presumably as a result of impaired nuclear export and the basal activity of its nuclear localization signal (Fig. 4C).
We further tested the role of CgMSN2 for C. glabrata survival under extreme osmotic stress. We generated a strain (Cg msn2Δmsn4Δ) lacking both CgMsn2 and CgMsn4 by homologous gene replacement with the CgHIS3 and ScURA3 genes respectively. The correct integrations were confirmed by Southern blot (Fig. S1). The Cg msn2Δmsn4Δ strain has no obvious growth phenotype under laboratory conditions, similar to S. cerevisiae msn2Δmsn4Δ double mutants. Cultures of Cg msn2Δmsn4Δ cells transformed with the empty vector as a control, or with a plasmid expressing CgMsn2–CFP under the control of the native CgMSN2 promoter, were grown to early exponential phase and exposed to a severe hyperosmotic stress (2 M NaCl). Viability was assayed after 2 and 20 h incubation by plating of cells in 10-fold serial dilutions. This assay shows that after 2 h of incubation with 2 M NaCl, viability was similar of both strains. However, after 20 h of exposure to 2 M NaCl, cells lacking Msn2 lost viability while the viability of those expressing CgMSN2 was significantly improved.
Expression of stress genes is tightly regulated and de-regulation has often adverse consequences. We therefore tested if high activity of CgMsn2 compromises long-term viability of C. glabrata. Cg msn2Δmsn4Δ cells transformed with plasmids expressing CgMSN2–CFP or CgMSN2 S230AS232A–CFP driven by the ADH1 promoter were grown for 10 days at 30°C in selective media. Viability was then assayed by spotting 10-fold dilutions. Cells expressing the constitutive nuclear mutant were significantly underrepresented compared with wild type and vector control (about two- to threefold, Fig. 4D, middle panel). The equal cell number on YPD suggests that the CgMSN2 S230AS232A–CFP plasmid, but not the other plasmids, was counter-selected in the culture. Cells carrying the mutant plasmid were also cold-sensitive (Fig. 4E).
Taken together, these data demonstrate that nuclear transport of CgMsn2 is highly regulated and requires the integrity of the HD1 region. CgMsn2 is beneficial under extreme stress conditions and its constitutive expression seems to be detrimental.
The CgMsn2 regulon is related to the ScMsn2 regulon
ScMsn2 has about 100–150 target genes depending on the conditions (Gasch et al., 2000; Schüller et al., 2004). To define the targets of CgMsn2, we compared the expression profiles of C. glabrata wild type and Cg msn2Δmsn4Δ mutant strains during osmotic stress and acute glucose starvation. We also compared in parallel the mRNA profiles of cells carrying plasmids with the CgMsn2 gene under its own or the CgADH1 promoter (construct pCgADH1CgMsn2–CFP). Many stress genes influenced by the absence of Msn2 are also induced by the overexpression of CgMSN2 (Fig. 5A, right panels, supplementary data). Furthermore, we find that C. glabrata and S. cerevisiae share a set of 21 Msn2 targets that are also part of the 65 induced genes shared between the ScESR and CgESR (Fig. 5B). All the genes found in this core set possess at least one STRE site in their promoters. STRE-like sequences were detected among the CgMsn2-regulated genes by an unbiased heuristic search using AlignAce in the upstream regions of the selected CgMsn2-regulated genes (Hughes et al., 2000) and are depicted in the form sequence logo (Fig. 5A, lower panel). The most salient gene functions are connected to stress response (HSP42, HSP12, SML1, DDR48), glycogen and trehalose metabolism (TPS1, NTH1, TPS2, YGP1) or DNA repair (SML1, ribonucleotide reductase) based on GO-terms analysis (P-values < 0.005). Detailed data are available as supplementary files. However, genes regulated only in C. glabrata by CgMsn2 include the glyoxylate cycle enzyme MDH3 (cytoplasmic malate dehydrogenase), the glycolytic enzyme FBP26 (fructose-2,6-bisphosphatase) and PHM8 involved in phosphate metabolism, suggesting that there is different wiring of some metabolic pathways between the two species. In addition, there are changes in expression of signalling components, such as the casein kinase YCK1 involved in cell morphogenesis, which are specific to C. glabrata. Together, these data show that CgMsn2 has a broad set of target genes and that many of them are conserved between S. cerevisiae and C. glabrata. A smaller set of CgMsn2 and ScMsn2 target genes are conserved stress genes also in S. pombe and C. albicans (Fig. S2C). C. glabrata is a nicotinamide adenine dinucleotide (NAD+) auxotroph and its growth is dependent on exogenous supply of NAD+ precursors. A main part of the NAD+ metabolism is the Preiss-Handler pathway, where the nicotinamidase Pnc1 ultimately converts precursors to NAD+ (Ma et al., 2007). PNC1 is upregulated in S. cerevisiae and C. glabrata during oxidative and osmotic stress (Fig. S2C).
Fig. 5.
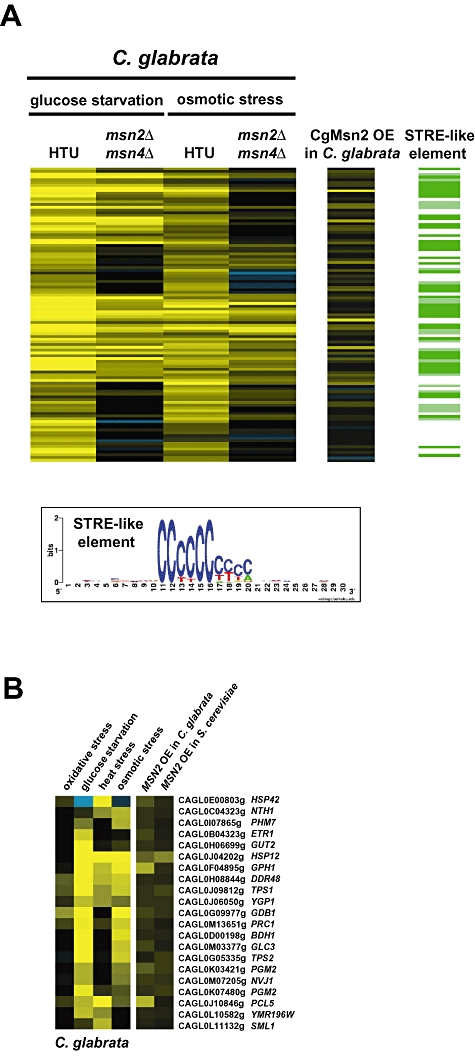
Determination of genes dependent on CgMsn2/CgMsn4.
A. Wild type and Cg msn2Δmsn4Δ cells were exposed to osmotic stress (0.5 M NaCl) and glucose depletion and transcript profiles determined by hybridization to genome-wide C. glabrata microarrays. Hierarchical clustering of genes with a wild type to Cg msn2Δmsn4Δ ratio > 1.5 is shown. The right panel shows the transcript profile of the CgADH1 promoter-driven overexpression (OE) of CgMsn2–CFP compared with CgMSN2 native promoter-driven CgMsn2–CFP expression. Logo representation of a STRE-like sequence pattern found by AlignAce among the CgMsn2-regulated genes (indicated on the right).
B. C. glabrata and S. cerevisiae have orthologous Msn2 target genes. A core set of 21 CgESR genes is induced by overexpression of CgMSN2 in C. glabrata and ScMSN2 in S. cerevisiae.
CgMsn2 is required for rapid induction of osmotic stress-induced transcription of trehalose synthesis genes in C. glabrata
To confirm the function of CgMsn2 as a stress-regulated transcription factor, we measured the expression of CgTPS1, CgTPS2 and CgUBP15 over several time points following exposure to osmotic stress. TPS1 and TPS2, encoding trehalose-6-phosphate synthase and phosphatase respectively, are both required for the synthesis and the storage of the carbohydrate trehalose. UBP15, coding for an ubiquitin-specific protease, is induced by heat and osmotic stress in S. cerevisiae. Northern blot analysis of CgTPS1, CgTPS2 and CgUBP15 showed rapid induction upon treatment with 0.5 M NaCl (30°C and 37°C gave similar results) in the C. glabrata wild type, and also in C. glabrata msn2Δmsn4Δ mutant cells supplemented with CgMsn2 on a plasmid under its own promoter (pCgMCgMSN2) (Fig. 6A). CgADH1 promoter-driven expression of CgMsn2 in this strain (C. glabrata msn2Δmsn4Δ, pCgADH1CgMsn2–CFP) enhanced the signals of all genes in untreated and treated conditions. The CFP tag of CgMsn2 did not disrupt its activity (Fig. S4). In the absence of CgMsn2 and CgMsn4, mRNA levels were severely reduced. Stress-induced expression is not completely abolished in the double mutant cells, suggesting the parallel action of other factors reminiscent of S. cerevisiae (Reiser et al., 1999). These data confirm a direct role for CgMsn2 during osmostress induction. The impact of CgMsn4 remains to be elucidated. We conclude that general stress response mechanisms are at least partially conserved between C. glabrata and S. cerevisiae.
Fig. 6.
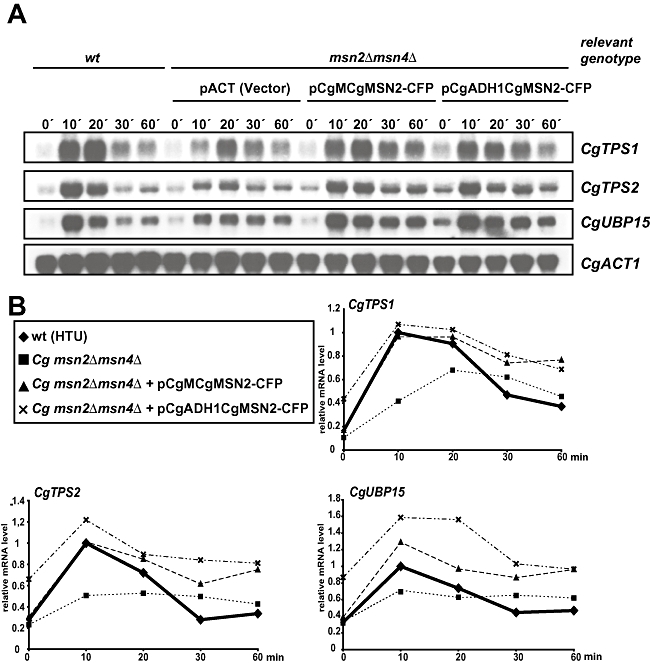
CgMsn2 is required for rapid induction of transcription after osmotic stress.
A. Northern blot analysis of CgTPS1, CgTPS2 and CgUBP15 transcripts during 0.5 M NaCl induced osmotic stress. C. glabrata wild type, Cg msn2Δmsn4Δ and Cg msn2Δmsn4Δ transformed with pCgMCgMsn2–CFP or pCgADH1CgMsn2–CFP were grown to exponential phase before 0.5 M NaCl was added. Samples for RNA extraction were taken at indicated time points. mRNA levels were visualized by hybridization of radio-labelled probes and autoradiography. CgACT1 mRNA was used as loading control.
B. Quantification of mRNA levels of CgTPS1, CgTPS2 and CgUBP15 normalized to CgACT1 and expressed relative to the highest wild-type level.
CgMsn2 is not required for virulence in a Drosophila melanogaster infection model
Candida glabrata is a human opportunistic pathogen. To test for a possible role of CgMsn2 in virulence, we tested the C. glabrata msn2Δmsn4Δ mutant in a D. melanogaster model of infection. Wild type flies are resistant to injection of 7500 C. glabrata cells (ΔHTU) (Fig. 7). In contrast, MyD88 mutant flies, in which Toll signalling, and thus the humoral arm of the antifungal response, are blocked, succumb in a few days to the same dose of cells. C. glabrata mutants lacking a functional Hog1 pathway have reduced virulence in this infection model, demonstrating its applicability for stress response mutants (not shown). However, we find that there is no difference in the survival of wild-type or immunosuppressed flies when injected either with wild type (ΔHTU) or Cg msn2Δmsn4Δ mutants. Similarly, we find no difference between the virulence of strains expressing CgMsn2–CFP under the control of the endogenous promoter or the strong constitutive CgADH1 promoter, and the empty control plasmid. These findings are in line with a role of Msn2 during extreme stress situation probably not encountered in the fly hemocoel.
Fig. 7.
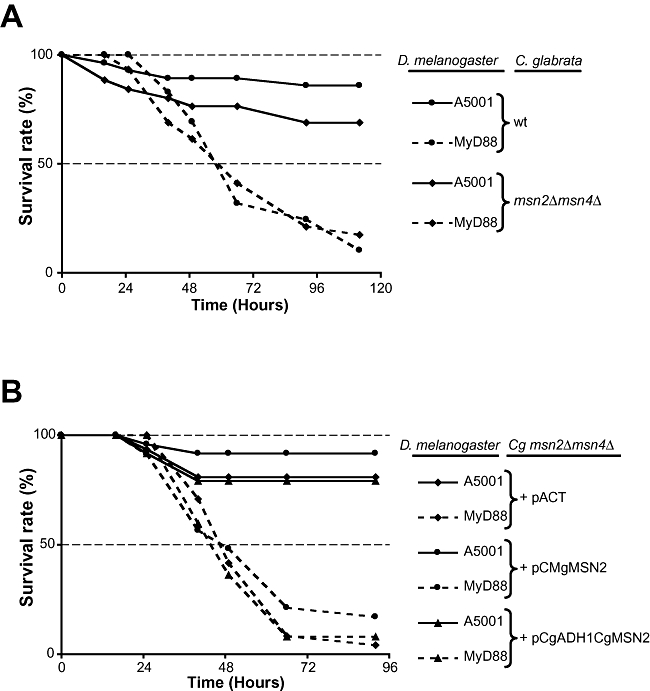
Survival of D. melanogaster to wild type or msn2Δmsn4Δ mutant C. glabrata infection.
A. Flies were injected with 7500 C. glabrata cells. MyD88 mutant flies succumbed rapidly to a challenge with either wild-type C. glabrata or Cg msn2Δmsn4Δ mutant strain. The genotypes of the infected flies are indicated (A5001: wild-type).
B. Immunosuppressed flies succumb rapidly when challenged with Cg msn2Δmsn4Δ transformed with either the empty pACT plasmid or plasmids expressing CgMsn2 (pCgMCgMSN2 or pCgADH1CgMSN2). Survival was monitored at 29°C. The survival rate is expressed as a percentage. These survival experiments are representative of at least three independent experiments for each panel. The slight difference observed between wild-type flies injected with wild-type or mutant yeasts is not reproducible. Similar results were observed with a lower dose of injected C. glabrata (5000).
Discussion
Despite their very different environmental niches and several hundred million years of phylogenetic distance, the transcriptional responses of S. cerevisiae, C. albicans and S. pombe to diverse environmental conditions (ESR) share significant similarities (Enjalbert et al., 2006; Gasch, 2007). C. glabrata is phylogenetically related to S. cerevisiae, but it is adapted to a mammalian host environment. This is reflected by adhesin-mediated adherence to surfaces, the absence of certain biosynthetic pathways (Domergue et al., 2005; Kaur et al., 2005) and different physiology, for example, optimal growth at 37°C. We report an analysis of part of the CgESR. The CgESR is similar to the ScESR and we identify a conserved role for the general stress transcription factor Msn2.
In many organisms, p38 MAP kinases are central to the control of environmental responses. The S. pombe Sty1, C. albicans CaHog1 and S. cerevisiae Hog1 SAPK mediate the response to a variety of environmental conditions (Toone and Jones, 1998; Wysong et al., 1998; Alonso-Monge et al., 1999; Hwang et al., 2002; Enjalbert et al., 2006). S. pombe Sty1 is required for most SpESR regulation (Chen et al., 2003). S. cerevisiae Hog1 is most strongly induced by osmotic stress and activated to lower levels by other stresses, including oxidative stress and weak acid stress (Bilsland et al., 2004; Lawrence et al., 2004; Sotelo and Rodriguez-Gabriel, 2006). However, many ScESR genes are not exclusively dependent on Hog1, but rely also on the general stress factors Msn2 and Msn4. The C. albicans ESR is influenced by CaHog1 and in parallel also by other pathways, but Msn2 does not play any role (Enjalbert et al., 2006). This raises the question as to whether S. cerevisiae has developed a unique stress response mechanism involving Msn2 and Msn4.
Do functional Msn2 orthologues exist in other ascomycete fungi apart from S. cerevisiae? One characteristic feature of Msn2 is its DNA binding domain recognizing the corresponding recognition element STRE. Many fungal genome sequences contain ORFs with high similarity to the Msn2 DNA binding domain. However, these genes usually have very little other sequence conservation to ScMsn2. Zn-finger proteins binding to STRE-like sequences were reported from S. pombe and Trichoderma atroviridae (Kunitomo et al., 2000; Seidl et al., 2004), although these factors are not mediating stress responses. The most significant similarity between ScMsn2 orthologues apart from the DNA binding domain (Fig. 2B) is a region designated earlier as Msn2 HD1 (Görner et al., 1998). This motif is not present in the Msn2 orthologue from C. albicans or its close relatives, including Candida parapsilosis, Candida tropicalis, Candida lusitaniae, Candida guilliermondii, Lodderomyces elongisporus, Debaryomyces hansenii and Pichia stipitis.
The intracellular localization of ScMsn2 rapidly changes from the cytoplasm to the nucleus in response to nutrient and stress conditions. (Görner et al., 1998; Beck and Hall, 1999; Görner et al., 2002). This switch is the result of two independent activities within ScMsn2. The NLS near the C-terminus drives nuclear import. Nuclear export of Msn2 requires the exportin Msn5 which also transports other transcription factors, such as Mig1, Crz1 and Pho4 (Kaffman et al., 1998; DeVit, 1999; Chi et al., 2001; Boustany and Cyert, 2002). Activation of Msn2 by stress and nutrient starvation requires HD1 which overlaps with the NES function (Görner et al., 2002; Boy-Marcotte et al., 2006). PKA and TOR inhibit Msn2 activity through this region. Interestingly, all close Msn2 orthologues have an embedded conserved PKA phosphorylation site (Fig. 2B). However, the function of PKA has not been explored in C. glabrata.
We have shown that the region including the HD1 motif of Msn2/4 from K. lactis, A. gossypii, S. cerevisiae and C. glabrata is sufficient for nuclear export. We provide direct evidence by mutation of two conserved serine residues to alanine in the CgMsn2 HD1, which leads to constitutive nuclear enrichment. Similar changes in the ScMsn2 abolish its NES function (W. Reiter, G. Ammerer and C. Schüller, unpubl. obs.). In contrast, a large part of CaMsn4 (1–517) which is devoid only of its NLS and Zn-finger region at the C-terminus does not support regulated nuclear export.
The NES of ScMsn2 is much larger than the generic exportin 1-driven signal (Wen et al., 1995; Kutay and Guttinger, 2005). Importantly, nuclear export of CgMsn2 expressed in S. cerevisiae also requires Msn5 and PKA (A. Roetzer and C. Schüller, unpubl. results). These data support our hypothesis that the HD1 is the target site for the exportin Msn5, and that the conserved PKA phosphorylation site within the HD1 site has a regulatory role. Our results suggest that the Saccharomycotina can be divided into two groups according to their stress response mechanisms. The HD1 motif and the stress-mediating role of Msn2 orthologues appear after the separation of C. albicans from the lineage leading to S. cerevisiae (Fig. 2B). The functional conservation of ScMsn2 and CgMsn2 highlights the important regulatory motifs of both proteins and will be used as a guide for further structure–function analysis.
Candida glabrata as a human commensal and occasional pathogen exists in very different environmental niches compared with S. cerevisiae. Our global transcript analysis of C. glabrata cells exposed to the generic stress types carbon starvation, heat, osmotic and oxidative stress reveals a transcription pattern related to C. albicans, S. pombe and S. cerevisiae. The most conserved component of the ESRs is dependent on Msn2-like factors in C. glabrata and S. cerevisiae. Several lines of evidence presented here suggest that CgMsn2 is an orthologue of ScMsn2. It can functionally substitute for ScMsn2 in S. cerevisiae for stress-dependent induction of an Msn2 target gene. Intracellular localization of a CgMsn2–CFP fusion protein is stress-regulated in both S. cerevisiae and C. glabrata. CgMsn2 is required for rapid and full induction of several target genes tested CgTPS1, CgTPS2 and CgUBP15 which all contain putative STRE sequences in their promoter region. Conserved Msn2-dependent genes, such as TPS1, NTH1, TPS2, HSP42, HSP12, SML1, DDR48 and YGP1, are involved in stress response, glycogen and trehalose metabolism or DNA repair (SML1, ribonucleotide reductase) based on GO-terms analysis (P-values < 0.005). C. glabrata stress genes were also found in a global analysis of the CgPdr1-regulated drug response (Vermitsky et al., 2006).
CgMSN2 and CgMSN4 are not required for virulence in a Drosophila infection model. This may reflect an absence of extreme stressful conditions for C. glabrata in the fly hemocoel. Alternatively, other pathways may allow C. glabrata to adapt to the host environment. Finally, the fly model might not reflect all host niches C. glabrata is adapted to. Adhesion is essential for C. glabrata virulence (Cormack et al., 1999). We found no difference of adhesion to a plastic surface (Iraqui et al., 2005) in C. glabrata Msn2/4 deletion and Msn2 overexpression strains (data not shown). The adhesins encoded by EPA genes are regulated differently during environmental stress conditions. Two genes EPA3/CAGL0E06688g and CAGL0I00220g are highly induced by osmotic stress and glucose starvation; however, with a minor role for CgMsn2.
Our results highlight the conserved regulation of Msn2 between S. cerevisiae and C. glabrata, but we also find differences that require further investigation. Stress regulation of many Msn2 target genes is conserved up to S. pombe, indicating a selective advantage for such regulation regardless of the particular transcription factors. It will be of particular interest to analyse the role of Msn2 orthologues which we suspect to be stress-regulated factors in the pre-whole-genome duplication clade, such as Kluyveromyces species and A. gossypii. We suggest that C. glabrata Msn2 functions to improve survival under severe stress conditions.
Experimental procedures
Yeast strains and plasmids
Yeast strains used in this study are listed in Table 1. Rich medium (YPD) and synthetic medium (SC) supplemented with appropriate auxotrophic components were prepared as described elsewhere (Current Protocols in Molecular Biology; Wiley). Unless otherwise indicated, all strains were grown at 30°C.
Table 1.
Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| S. cerevisiae | ||
| W303-1A | a ura3 leu2 his3 trp1 ade2 can1 | Nasmyth K. |
| W303 msn2Δmsn4Δ | a msn2Δ::TRP1 msn4Δ::HIS3 | Görner et al. (1998) |
| C. glabrata | ||
| ΔHTU | his3Δ trp1Δ ura3Δ | Kitada et al. (1995) |
| Cg msn2Δ | msn2Δ::CgHIS3 | This study |
| Cg msn2Δmsn4Δ | msn2Δ::CgHIS3 msn4Δ::ScURA3 | This study |
| K. lactis | − | Nasmyth K; Oxford |
| C. albicans SC5314 | − | Gillum et al. (1984) |
Plasmids and oligonucleotides used in this study are listed in Table 2 and Table S1 respectively. Cg msn2Δmsn4Δ strain was obtained by genomic integration. PCR products of ScURA3 for CgMSN4 and CgHIS3 for CgMSN4 were amplified from the plasmids pRS316 (Sikorski and Hieter, 1989) and pTW23 (Kamran et al., 2004) using fusion PCR (CgMsn4, primer series MSN4; CgMsn2, primer series MSN2) (Noble and Johnson, 2005). Correct genomic integration of all fragments was verified by genomic PCR (primer series Ctrl) followed by Southern analysis using labelled probes generated by amplification with primers MSN2-5′3′/MSN2-5′5′ and MSN4-1/MSN4-3 from genomic DNA.
Table 2.
Plasmids used in this study.
| Plasmids | Relevant Inserts | Source |
|---|---|---|
| pAMG | ScADH1-ScMsn2–GFP (pAMG) | Görner et al. (1998) |
| pSK + CTT1 | ScCTT1 PCR fragment | This study |
| pACgMC | ScADH1-CgMsn2–CFP (SalI/NcoI and NotI/NotI); ScLEU2 marker. | This study |
| pCgADH1CgMsn2–CFP | CgADH1-Promoter CgMsn2–CFP (SphI/SacII and SacII/NsiI); CgTRP1 marker. | This study |
| pCgADH1CgMSN2 | CgADH1-Promoter CgMSN2 (SphI/SacII and SacII/NsiI); CgTRP1 marker. | This study |
| pCgMCgMsn2–CFP | CgMSN2-Promoter CgMsn2–CFP (SphI/SacII); CgTRP1 marker. | This study |
| pCgMCgMSN2 | CgMSN2 Promoter CgMSN2 (SphI/SacII); CgTRP1 marker. | This study |
| pCgMSCgMSN2 S230AS232A–CFP | CgMSN2 Promoter-CgMsn2–CFP (SphI/SacII); .S230A and S232A, CgTRP1 marker | This study |
| pASMG1 | ScADH-SV40NLS-ScMsn2–GFP | Görner et al. (1998) |
| pASNScM2G | ScADH1, SV40-NLS, ScMSN2 (267–568), GFP; SalI site between SV40 and MSN2 in pASMG1 | This study |
| pASNAgM2G | ScADH1, SV40-NLS, AgMSN2 (170–450), GFP | This study |
| pASNKlM2G | ScADH1, SV40-NLS, KlMSN2 (229–544), GFP | This study |
| pASNCgM2G | ScADH1, SV40-NLS, CgMSN2 (157–470), GFP | This study |
| pASNCgM4G | ScADH1, SV40-NLS, CgMSN4 (156–439), GFP | This study |
| pASNScM4G | ScADH1, SV40-NLS, ScMSN4 (213–497), GFP | This study |
| pASNCaM4G | ScADH1, SV40-Nls, CaMsn3 (1–517), GFP | This study |
| pTW23 | pSK+ with CgHIS3 | Haynes K; London |
| pRS316 | CEN6, ARSH4, ScURA3 | Sikorski and Hieter (1989) |
| pAMC | ScADH1-ScMSN2-CFP, ScLEU2 | This study |
| pAG1334 | MSN2 from A. gossypii | Philippsen P; Basel |
| pACT14 | ARS, CEN and TRP1 marker from C. glabrata | Kitada et al. (1996) |
| pGEM-ACT | ARS, CEN and TRP1 marker from C. glabrata | Gregori et al. (2007) |
Cloned PCR fragments used in this study were confirmed by sequencing. Plasmid pASMG1, which was described in Görner et al., (1998), is based on vector YCpLac111 (Gietz and Sugino, 1988) containing the S. cerevisiae ADH1 promoter followed by the SV40 NLS (PKKKRKV), and a part of MSN2 coding for amino acid position 267–568 and GFP. To create a unique SalI site after the SV40 NLS, the plasmid was modified by site-directed mutagenesis using a Quick Change Site-directed Mutagenesis Kit (Stratagene) and primers Re-SalI-5/Re-SalI-3 resulting in plasmid pASNScM2G. Sequence fragments from Msn2 orthologues were exchanged with the ScMsn2 sequences by excision with SalI/NcoI. Plasmid pASNKlM2G was created by a SalI/NcoI digest of pASNScM2G and integration of a SalI/NcoI cut fragment obtained by PCR using primers KlM2SalI and KlM2NcoI from genomic K. lactis DNA as a template. Plasmid pASNAgM2G was created by using an XhoI/BsmBI fragment of a PCR product generated with primers AgM2XhoI and AgM2NcoI and plasmid pAG1334 as a template. The C. glabrata Msn2 and Msn4 orthologues (CAGL0F05995g and CAGL0M13189g) sequences were amplified by PCR from genomic DNA from strain ΔHTU. The CgMsn2 PCR fragment obtained with primers CgM2XhoI and CgM2NcoI was cut with XhoI/NcoI, the CgMsn4 fragment (primers CgM4SalI and CgM4NcoI) was cut with SalI/NcoI and both were inserted into the SalI/NcoI-cut pASNScM2G. pASNScM4G was created by insertion of a SalI/NcoI-digested PCR fragment obtained using primers ScM4SalI and ScM4NcoI and genomic DNA from W303-1A. The CaMsn4 fragment was amplified via PCR (primers CaMsn4-5 and CaMsn4-3) from genomic C. albicans DNA and cut with SalI and NcoI. pACgMC was cloned by integration of a SalI/NcoI-cut PCR fragment containing the CgMsn2 ORF into pAMC. pCgACgMsn2–CFP is a derivative of pGEM-ACT (Gregori et al., 2007). Nine hundred base pairs of the CgADH1 promoter were inserted as a SphI/SacII PCR product obtained with primers CgAdhPro-SphI and CgAdhPro-SacII. The coding sequence for CgMsn2–CFP was amplified from pACgMC using primers CgMsn2Cfp-SacII and CgMsn2Cfp-NsiI, and inserted as a SacII/NsiI-cut fragment. The native promoter (860 base pairs) was inserted as a SphI/SacII PCR product obtained with primers SphI-msn2nat and SacII-msn2nat. Exchange of single amino acids in CgMsn2 was done via site-directed mutagenesis using a Quick Change Site-directed Mutagenesis Kit (Stratagene) using the primers 5-SASA and 3-SASA. Probes for Northern and Southern analysis were amplified by PCR from genomic DNA: IPP1 from S. cerevisiae, CgACT1, CgTPS1, CgTPS2 and CgUBP15 from C. glabrata. For ScCTT1, a KpnI/SacI fragment from the plasmid pKSCTT1 was used.
Fly strains and survival experiment
Stocks were raised on standard cornmeal-agar medium at 25°C. wA5001 flies were used as wild type throughout the experiments because the MyD88 mutant was generated in this background. Batches of 25–30 wild type and mutant strains were challenged with 7500 cells of wild type or mutant C. glabrata using a Nanoject II apparatus (Drummond Scientific). Overnight cultures of C. glabrata were collected by centrifugation and washed with PBS 0.01% Tween 20. The solutions were adjusted after counting so that 7500 cells of C. glabrata in 13.8 nl were injected into each fly. The quantity of cells injected was checked on a culture plate. After infection, the vials were put in an incubator at 29°C and the surviving flies counted as required. Flies were usually placed into new vials every 2 days. Each infection was carried out using three independent replicates.
Northern and Southern blot analysis
RNA extraction and analysis followed essentially the protocol as described (Current Protocols In Molecular Biology; Wiley). Cells were grown over night and diluted to an OD600 of 0.1 in fresh medium, grown to an OD600 of 1 and treated as described. Cells were harvested by centrifugation and frozen immediately. For each RNA extraction, 25 ml of yeast cells was collected. Frozen pellets were re-suspended in RNA isolation buffer (50 mM Tris pH 7.5/5 mM EDTA/5% SDS/130 mM NaCl), 200 μl PCI solution (Roth) and glass beads (2/3 of total volume) were added and total RNA was extracted using FastPrep (2 × 12′′, speed 6, Thermo Savant). RNA samples (20 μg) were separated on a 1.1% agarose gel in FGRB (5×: 0.1 M MOPS/40 mM NaAc pH 7/5 mM EDTA) containing 2.2 M formaldehyde. After transfer to nylon membranes and UV cross-linking, the quality and amount of RNA were determined by staining with Methylene Blue. Hybridization of [32P-α]-ATP-labelled probes occurred over night in hybridization buffer (0.5 M sodium phosphate buffer pH 7.2/7% SDS/1 mM EDTA) at 65°C. After washing, the membrane was exposed to an X-ray film. For DNA extraction, yeast cells were grown to an OD600 of 6 (10 ml), collected and washed once; cell pellets were re-suspended in Lysis buffer (2% Triton X-100/1% SDS/100 mM NaCl/10 mM Tris pH 8/1 mM EDTA). Genomic DNA was isolated by PCI extraction. Digestion of 10 μg of genomic DNA was done over night with ScaI and BglII (5 U μg−1 DNA). The digests were separated and blotted. After cross-linking, the radioactive labelled probes were hybridized over night at 65°C. Bands were detected by X-ray film and PhosphoImager.
Microscopy
Fluorescence microscopy was performed as described previously (Görner et al., 1998). GFP and CFP were visualized in live cells without fixation. Nuclei were stained by addition of 2 μg ml−1 4,6-diamidino-2-phenylindol dye to the cultures 10 min prior to microscopy. All cells were viewed using a Zeiss Axioplan 2 fluorescence microscope. Images were captured with a Spot Pursuit (Sony) CCD camera using MetaVue (Molecular Devices) and Spotbasic software. Time-lapse imaging of life cell was done by adhering the cells to a coverslip with Concanavalin A (Sigma) fixed above a chamber allowing continuous flow of medium driven by a pump. Incoming medium could be switched between two sources containing rich medium without glucose and with 2% glucose. Medium flow rate was about 500 μl min−1.
Candida glabrata long-term viability
Cg msn2Δmsn4Δ transformed with the above plasmids were grown on selective plates, replica-plated to YDP grown over night and then stored for 14 days at 4°C and on room temperature as a control. Plates were then replica-plated and incubated at 37°C over night and growth recorded. Cell patches expressing CgMSN2 S230AS232A–CFP failed to grow, suggesting loss of viability. Viability was also verified by colony-forming assay by plating showing a threefold reduced colony-forming ability (0.38 of wild type; SD = 0.12; P = 0.05).
Microarray analysis
Microarrays were produced (G. Butler lab) by spotting 5908 69- or 70-mer oligonucleotides synthesized at the Pasteur Institute to Corning UltraGAPS slides. Slides were pre-incubated in 0.5% NaBH4 (in 75% PBS and 25% EtOH) solution, washed three times with water and dipped in isopropanol. cDNA was synthesized using 15–20 μg of RNA and Superscript III kit (Invitrogen), including either Cy3-dCTP or Cy5-dCTP (Amersham Biosciences). RNA was hydrolysed in 50 mM NaOH at 65°C for 15 min, the solution was neutralized with acetic acid and cDNA was purified using a GFX purification kit (GE Healthcare). Labelled cDNAs were concentrated, pooled and hybridized in 60 μl in DigEasyHyb solution (Roche Diagnostics) with 0.1 mg ml−1 salmon sperm DNA (Sigma) at 37°C for 14–16 h. Microarrays were disassembled in 1× SSC, washed two times in 1× SSC, 0.1% SDS at 50°C for 20 min, followed by a 1 min wash in 1× SSC at room temperature. Slides were spun dry for 5 min at 700 r.p.m. Slides were scanned immediately on an Axon4000B scanner (Axon Instruments) and analysed using GenePix Pro4.1 software (Axon Instruments). All standard protocols were provided by the Ontario Cancer Institute (Toronto; http://www.microarrays.ca/). Each experiment was repeated twice.
Analysis of microarray data
The raw data set of this study is available as supplemental material and has been deposited at array express (http://www.ebi.ac.uk/arrayexpress/; accession number: E-MEXP-1427). Microarrays were analysed with GenePixPro4.1. Values of not found features were excluded from further analysis. Mean ratios were calculated for features with at least four data points and their quality was approximated by their coefficient of variation (CV) values and subsequent exclusion of values with CV smaller than 1. Only genes with at least four data points and a CV > 1 were included in subsequent analysis. Genes assigned as dubious ORFs by the Genolevures consortium analysis were removed from analysis. The filtered median of ratio values were normalized. The normalized values used for further analysis are available as supplementary file Cg_array_data.xls. Cluster analysis (Eisen et al., 1998) was performed with cluster3 and TreeView (see http://bonsai.ims.u-tokyo.ac.jp/~mdehoon/software/cluster/index.html). Association to GO terms was analysed with the T-Profiler (Boorsma et al., 2005) by using the orthologous S. cerevisiae genes. Values of genes associated with the most significant terms were visualized by Cluster analysis using complete linkage and correlation as similarity metric. The cluster results were confirmed by K means and SOM clustering. P-values of overlapping gene sets were calculated by hypergeometric distribution. Of the 5063 gene features spotted in duplicate on our arrays, 4166 gave useful data under at least one tested condition. Systematic C. glabrata IDs were used from the Genolevures resource (5215 ORFs) and linked to systematic names of S. cerevisiae. The ESR was defined as the set of genes with values above a fourfold induction and below a fourfold repression after applying different stresses. To estimate the contribution of the transcription factors CgMsn2/CgMsn4, the ratio of the wild type versus double mutant and induced genes was calculated. In addition, data from an all-against-all blastp search of protein sequences from S. cerevisiae, C. albicans and S. pombe (Enjalbert et al., 2006) were compared with C. glabrata data for osmotic and oxidative stress. Normalized values with canonical gene names are available in plain text format as supplementary data as well as Cluster3 output files corresponding to the figures. Sequence patterns were found by AlignAce (Hughes et al., 2000) using the C. glabrata genomic DNA. The most recent annotation as of 09.Nov.2007 was used to define ORF and promoter regions. Sequence logo was generated at http://weblogo.berkeley.edu/(Crooks et al., 2004).
Acknowledgments
We thank members of the Ammerer lab for discussions and advice, Elisabeth Brown for providing the Msn2–CFP plasmid, Peter Phillipsen for A. gossypii clones, Ken Haynes for the C. glabrata‘starter kit’ and Steffen Rupp for a crucial advice. C.S. was supported by the Herzfelder Foundation and the FWF Grant P19966-B12, and the Vienna Hochschuljubiläumsstiftung. This work was also supported by FWF Grant F34567 and by the WWTF (HOPI-WWTF-LS133), the FP6 project EURESFUN (FP6-2004-PL518199) (to KK), and by the Wellcome Trust (072420 to G.B.) and from QUASI, a grant from the European FP6 to G.A. D.F.'s laboratory is supported by the CNRS and Fondation Recherche Médicale (Equipe FRM). Marie-Céline Lafarge's technical expertise is gratefully acknowledged. We acknowledge the support by the ERA-Net Pathogenomics programme (FWF-I125-B09 to KK and CS).
Supplementary material
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/
j.1365-2958.2008.06301.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, et al. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Bilaud T, Koering CE, Binet-Brasselet E, Ancelin K, Pollice A, Gasser SM, Gilson E. The telobox, a Myb-related telomeric DNA binding motif found in proteins from yeast, plants and human. Nucleic Acids Res. 1996;24:1294–1303. doi: 10.1093/nar/24.7.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland E, Molin C, Swaminathan S, Ramne A, Sunnerhagen P. Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol Microbiol. 2004;53:1743–1756. doi: 10.1111/j.1365-2958.2004.04238.x. [DOI] [PubMed] [Google Scholar]
- Boorsma A, Foat BC, Vis D, Klis F, Bussemaker HJ. T-profiler: scoring the activity of predefined groups of genes using gene expression data. Nucleic Acids Res. 2005;33:W592–W595. doi: 10.1093/nar/gki484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustany LM, Cyert MS. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 2002;16:608–619. doi: 10.1101/gad.967602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy-Marcotte E, Garmendia C, Garreau H, Lallet S, Mallet L, Jacquet M. The transcriptional activation region of Msn2p. Saccharomyces cerevisiae, is regulated by stress but is insensitive to the cAMP signalling pathway. Mol Genet Genomics. 2006;275:277–287. doi: 10.1007/s00438-005-0017-4. [DOI] [PubMed] [Google Scholar]
- Boy-Marcotte E, Perrot M, Bussereau F, Boucherie H, Jacquet M. Msn2p and Msn4p control a large number of genes induced at the diauxic transition which are repressed by cyclic AMP in Saccharomyces cerevisiae. J Bacteriol. 1998;180:1044–1052. doi: 10.1128/jb.180.5.1044-1052.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya M, Nishimura M, Harashima S, Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991;11:3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameroni E, Hulo N, Roosen J, Winderickx J, De Virgilio C. The novel yeast PAS kinase Rim15 orchestrates g(0)-associated antioxidant defense mechanisms. Cell Cycle. 2004;3:462–468. [PubMed] [Google Scholar]
- Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, et al. Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, et al. Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell. 2003;14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001;15:1078–1092. doi: 10.1101/gad.867501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Y, Klug A. Selection of DNA binding sites for zinc fingers using rationally randomized DNA reveals coded interactions. Proc Natl Acad Sci USA. 1994;91:11168–11172. doi: 10.1073/pnas.91.23.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua G, Morris QD, Sopko R, Robinson MD, Ryan O, Chan ET, et al. Identifying transcription factor functions and targets by phenotypic activation. Proc Natl Acad Sci USA. 2006;103:12045–12050. doi: 10.1073/pnas.0605140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Ghori N, Falkow S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science. 1999;285:578–582. doi: 10.1126/science.285.5427.578. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVit MJ, Johnston M. The nuclear exportin Msn5 is required for nuclear export of the Mig1 glucose repressor of Saccharomyces cerevisiae. Curr Biol. 1999;9:1231–1241. doi: 10.1016/s0960-9822(99)80503-x. [DOI] [PubMed] [Google Scholar]
- De Wever V, Reiter W, Ballarini A, Ammerer G, Brocard C. A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J. 2005;24:4115–4123. doi: 10.1038/sj.emboj.7600871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- Domergue R, Castano I, De Las Penas A, Zupancic M, Lockatell V, Hebel JR, et al. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science. 2005;308:866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- Durchschlag E, Reiter W, Ammerer G, Schüller C. Nuclear localization destabilizes the stress-regulated transcription factor Msn2. J Biol Chem. 2004;279:55425–55432. doi: 10.1074/jbc.M407264200. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJ, Quinn J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell. 2006;17:1018–1032. doi: 10.1091/mbc.E05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, MacCallum DM, Odds FC, Brown AJ. Niche-specific activation of the oxidative stress response by the pathogenic fungus Candida albicans. Infect Immun. 2007;75:2143–2151. doi: 10.1128/IAI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol Rev. 2000;24:469–486. doi: 10.1111/j.1574-6976.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pletcher SD, Minois N, Vaupel JW, Longo VD. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004;557:136–142. doi: 10.1016/s0014-5793(03)01462-5. [DOI] [PubMed] [Google Scholar]
- Feldmann H. Genolevures – a novel approach to ‘evolutionary genomics’. FEBS Lett. 2000;487:1–2. doi: 10.1016/s0014-5793(00)02304-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DA, Logue ME, Stajich JE, Butler G. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol. 2006;6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreau H, Hasan RN, Renault G, Estruch F, Boy-Marcotte E, Jacquet M. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology. 2000;146:2113–2120. doi: 10.1099/00221287-146-9-2113. [DOI] [PubMed] [Google Scholar]
- Gasch AP. Comparative genomics of the environmental stress response in ascomycete fungi. Yeast. 2007 doi: 10.1002/yea.1512. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber A, Hitchcock CA, Swartz JE, Pullen FS, Marsden KE, Kwon-Chung KJ, Bennett JE. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob Agents Chemother. 1995;39:2708–2717. doi: 10.1128/aac.39.12.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schüller C. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 2002;21:135–144. doi: 10.1093/emboj/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori C, Schüller C, Roetzer A, Schwarzmuller T, Ammerer G, Kuchler K. The high-osmolarity glycerol response pathway in the human fungal pathogen Candida glabrata strain ATCC 2001 lacks a signaling branch that operates in baker's yeast. Eukaryot Cell. 2007;6:1635–1645. doi: 10.1128/EC.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan R, Leroy C, Isnard AD, Labarre J, Boy-Marcotte E, Toledano MB. The control of the yeast H2O2 response by the Msn2/4 transcription factors. Mol Microbiol. 2002;45:233–241. doi: 10.1046/j.1365-2958.2002.03011.x. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Ashitani K, Yoshikawa K, Nagahisa K, Furusawa C, Katakura Y, Shimizu H, Shioya S. Comparison of transcriptional responses to osmotic stresses induced by NaCl and sorbitol additions in Saccharomyces cerevisiae using DNA microarray. J Biosci Bioeng. 2006;102:568–571. doi: 10.1263/jbb.102.568. [DOI] [PubMed] [Google Scholar]
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JD, Estep PW, Tavazoie S, Church GM. Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J Mol Biol. 2000;296:1205–1214. doi: 10.1006/jmbi.2000.3519. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology. 2002;148:3705–3713. doi: 10.1099/00221287-148-11-3705. [DOI] [PubMed] [Google Scholar]
- Iraqui I, Garcia-Sanchez S, Aubert S, Dromer F, Ghigo JM, d'Enfert C, Janbon G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol Microbiol. 2005;55:1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x. [DOI] [PubMed] [Google Scholar]
- Jackson DD, Stevens TH. VMA12 encodes a yeast endoplasmic reticulum protein required for vacuolar H+-ATPase assembly. J Biol Chem. 1997;272:25928–25934. doi: 10.1074/jbc.272.41.25928. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Rank NM, O'Neill EM, Huang LS, O'Shea EK. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- Kamran M, Calcagno AM, Findon H, Bignell E, Jones MD, Warn P, et al. Inactivation of transcription factor gene ACE2 in the fungal pathogen Candida glabrata results in hypervirulence. Eukaryot Cell. 2004;3:546–552. doi: 10.1128/EC.3.2.546-552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Domergue R, Zupancic ML, Cormack BP. A yeast by any other name: Candida glabrata and its interaction with the host. Curr Opin Microbiol. 2005;8:378–384. doi: 10.1016/j.mib.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kitada K, Yamaguchi E, Arisawa M. Cloning of the Candida glabrata TRP1 and HIS3 genes, and construction of their disruptant strains by sequential integrative transformation. Gene. 1995;165:203–206. doi: 10.1016/0378-1119(95)00552-h. [DOI] [PubMed] [Google Scholar]
- Kitada K, Yamaguchi E, Arisawa M. Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene. 1996;175:105–108. doi: 10.1016/0378-1119(96)00132-1. [DOI] [PubMed] [Google Scholar]
- Kunitomo H, Higuchi T, Iino Y, Yamamoto M. A zinc-finger protein, Rst2p, regulates transcription of the fission yeast ste11(+) gene, which encodes a pivotal transcription factor for sexual development. Mol Biol Cell. 2000;11:3205–3217. doi: 10.1091/mbc.11.9.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Guttinger S. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 2005;15:121–124. doi: 10.1016/j.tcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Botting CH, Antrobus R, Coote PJ. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol Cell Biol. 2004;24:3307–3323. doi: 10.1128/MCB.24.8.3307-3323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Pan SJ, Zupancic ML, Cormack BP. Assimilation of NAD(+) precursors in Candida glabrata. Mol Microbiol. 2007;66:14–25. doi: 10.1111/j.1365-2958.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Pastor MT, Marchler G, Schüller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Nicholls S, Straffon M, Enjalbert B, Nantel A, Macaskill S, Whiteway M, Brown AJ. Msn2- and Msn4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen Candida albicans. Eukaryot Cell. 2004;3:1111–1123. doi: 10.1128/EC.3.5.1111-1123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petter R, Kwon-Chung KJ. Disruption of the SNF1 gene abolishes trehalose utilization in the pathogenic yeast Candida glabrata. Infect Immun. 1996;64:5269–5273. doi: 10.1128/iai.64.12.5269-5273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RW, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V, Ruis H, Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep M, Krantz M, Thevelein JM, Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- Sandven P, Bevanger L, Digranes A, Haukland HH, Mannsaker T, Gaustad P. Candidemia in Norway (1991–2003): results from a nationwide study. J Clin Microbiol. 2006;44:1977–1981. doi: 10.1128/JCM.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller C, Mamnun YM, Mollapour M, Krapf G, Schuster M, Bauer BE, et al. Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:706–720. doi: 10.1091/mbc.E03-05-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl V, Seiboth B, Karaffa L, Kubicek CP. The fungal STRE-element-binding protein Seb1 is involved but not essential for glycerol dehydrogenase (gld1) gene expression and glycerol accumulation in Trichoderma atroviride during osmotic stress. Fungal Genet Biol. 2004;41:1132–1140. doi: 10.1016/j.fgb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo J, Rodriguez-Gabriel MA. Mitogen-activated protein kinase Hog1 is essential for the response to arsenite in Saccharomyces cerevisiae. Eukaryot Cell. 2006;5:1826–1830. doi: 10.1128/EC.00225-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MC, Fernandes AR, Mira NP, Becker JD, Sa-Correia I. Early transcriptional response of Saccharomyces cerevisiae to stress imposed by the herbicide 2,4-dichlorophenoxyacetic acid. FEMS Yeast Res. 2006;6:230–248. doi: 10.1111/j.1567-1364.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Toone WM, Jones N. Stress-activated signalling pathways in yeast. Genes Cells. 1998;3:485–498. doi: 10.1046/j.1365-2443.1998.00211.x. [DOI] [PubMed] [Google Scholar]
- Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol. 2006;61:704–722. doi: 10.1111/j.1365-2958.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wysong DR, Christin L, Sugar AM, Robbins PW, Diamond RD. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect Immun. 1998;66:1953–1961. doi: 10.1128/iai.66.5.1953-1961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


