Abstract
During embryonic development, the proepicardial organ (PEO) grows out over the heart surface to form the epicardium. Following epithelial-mesenchymal transformation, epicardium-derived cells (EPDCs) migrate into the heart and contribute to the developing coronary arteries, to the valves and to the myocardium. The peripheral Purkinje fiber network develops from differentiating cardiomyocytes in the ventricular myocardium. Intrigued by the close spatial relationship between the final destinations of migrating EPDCs and Purkinje fiber differentiation in the avian heart, viz. surrounding the coronary arteries and at subendocardial sites, we investigated whether inhibition of epicardial outgrowth would disturb cardiomyocyte differentiation into Purkinje fibers.
To this end epicardial development was inhibited mechanically with a membrane, or genetically, by suppressing epicardial epithelial-to-mesenchymal transformation with antisense retroviral vectors affecting Ets transcription factor levels(n=4, HH39-41). In both epicardial inhibition models we evaluated Purkinje fiber development by EAP-300 immunohistochemistry and found that restraints on EPDC development resulted in morphologically aberrant differentiation of Purkinje fibers. Purkinje fiber hyperplasia was observed both periarterially and at subendocardial positions. Furthermore, the cells were morphologically abnormal and not aligned in orderly Purkinje fibers.
We conclude that EPDCs are instrumental in Purkinje fiber differentiation, and we hypothesize that they cooperate directly with endothelial and endocardial cells in the development of the peripheral conduction system.
INTRODUCTION
During heart development, the myocardial heart tube is covered by the epicardial mesothelium, derived from the proepicardial organ (PEO). In the chicken and quail embryo, the PEO develops around day three of incubation as a protrusion of the coelomic wall near the sinus venosus (Virágh et al., 1993). A subset of the epicardial cells undergoes epithelial-mesenchymal transformation (EMT) to generate a mesenchymal cell population occupying the subepicardial layer. These epicardium-derived cells (EPDCs) can migrate into the myocardium and differentiate into interstitial, subendocardial and coronary adventitial fibroblasts, as well as coronary smooth muscle cells (Gittenberger-de Groot et al., 1998; Vrancken Peeters et al., 1999). Several studies have demonstrated crucial roles of EPDCs in formation of the compact myocardium (Yang, Rayburn, and Hynes, 1995;Kwee et al., 1995;Gittenberger-de Groot et al., 2000;Lie-Venema et al., 2003) and coronary development (Eralp et al., 2005), while a morphogenic role for EPDCs in valve development has also been postulated (Gittenberger-de Groot et al., 1998;Gittenberger-de Groot et al., 2003a).
Retroviral tagging experiments showed that the Purkinje fibers of the peripheral conduction system differentiate from cardiomyocytes, in close spatio-temporal relation to the developing coronary vasculature (Gourdie et al., 1995;Hyer et al., 1999), whereas the subendocardial Purkinje fibers develop in the proximity of endocardial cells. These unique differentiation sites suggested an inductive role both for periarterial and subendocardial EPDCs and for a paracrine signal from the endocardium and arterial beds in the recruitment of cardiomyocytes into the Purkinje fiber network (Gittenberger-de Groot et al., 2003b;Gourdie et al., 2003). Recent experimentation showed that Purkinje fiber differentiation is tightly regulated by hemodynamic alterations, while mature endothelin-1 (ET-1) and ET-converting enzyme 1 (ECE1) were identified as inductive molecules (Takebayashi-Suzuki et al., 2000;Reckova et al., 2003;Hall et al., 2004). Concommittant retroviral expression of mature ET-1 and ECE1 was even sufficient for the ectopic conversion of adjacent cardiomyocytes into Purkinje fibers (Takebayashi-Suzuki et al., 2000). Because interstitial EPDCs are present throughout the myocardium (Gittenberger-de Groot et al., 1998), even the ectopically converting cardiomyocytes could have been influenced by a juxtaposed EPDC.
In the present study, we tested the hypothesis that EPDCs play a role in the differentiation of the Purkinje fibers by morphologic analysis of the conductive network after mechanical and genetic disturbance of epicardial development.
MATERIALS AND METHODS
To study the role of EPDCs in the development of Purkinje fibers, we used a mechanical and a genetic experimental model in which EPDC development and differentiation were disturbed. From earlier work we knew that with either model the extent of epicardial disturbance is variable (Lie-Venema et al., 2003;Eralp et al., 2005). For close immunohistochemical examination, we specifically chose the embryos (n=7), without large morphological and coronary aberrations, to ensure that our analysis would focus on the influence of disturbed or delayed epicardial outgrowth rather than on the influence of impaired coronary formation on Purkinje fiber development. Normal controls consisted of untreated Japanese quail embryos (Coturnix coturnix japonica; n=7) from Hamburger and Hamilton stages 39 to 42 (Hamburger and Hamilton, 1951).
Mechanical inhibition of epicardial outgrowth
Outgrowth of the PEO in quail embryos (HH15 to HH18; n=15) was inhibited by placing a piece of egg shell membrane between the PEO and the heart tube, as described before (Männer, 1993;Eralp et al., 2005). After re-incubation at 37.5°C (80% humidity), embryos were isolated at stages HH40-HH42.
Genetic inhibition of epicardial development
In earlier work we established that epicardial development could be impaired by antisense down-regulation of the Ets-1 and Ets-2 transcription factors in chicken embryos (Lie-Venema et al., 2003). In this model, Ets-1 and Ets-2 expression were downregulated simultaneously in vivo. In short, white Leghorn chicken embryos (Gallus domesticus) were injected with the retroviral CXasetsIZ construct via the the right anterior vitelline vein at developmental stage HH14/HH15 as described earlier (Lie-Venema et al., 2003). Four embryos of developmental stage HH39-HH41 were analysed.
Immunohistochemistry
Isolation and processing of embryos for immunohistochemistry were done as described earlier (Vrancken Peeters et al., 1997). Serial sections were produced and subjected to standard immunohistochemical procedures (Poelmann et al., 1993; Vrancken Peeters et al., 1997). For analysis of coronary vessel development we used the alpha-actin smooth muscle antibody 1A4 (Sigma, St Louis, 1:3000). For detection of Purkinje fibers, we used the EAP-300 antibody (McCabe et al., 1995) diluted 1:200. The size of the area occupied by fluorescent EAP-positive cells was determined in 5 vessels of comparable diameter in the interventricular septum of the control and experimental groups (3 embryos per group). To maintain and enhance the signal of the secondary FITC-labeled antibody after immunofluorescent data acquisition, sections of interest were incubated with antifluorescein antibody Fab fragment conjugated with peroxidase (Converter-POD, Roche Diagnostics, Mannheim). In paraformaldehyde-fixed tissues this resulted in better signal-to-background ratios than the use of immunofluorescence alone. Apoptosis in the Purkinje fibers was assessed as described earlier (Eralp et al., 2005).
Statistical analysis
Data were represented as average ± standard deviation. A non-paired, two-tailed student’s t-test was used for statistical comparison. A p-value less than 0.05 was considered statistically significant.
RESULTS
To assess the role of EPDCs in Purkinje fiber development, we used two independent avian models in which epicardial development was disturbed. The severity of the cardiac abnormalities in these models is closely related to the degree of epicardial outgrowth inhibition. Especially the procedure for mechanical PEO inhibition yields embryos in which epicardial development is disturbed in variable degrees (Eralp et al., 2005). This results in a broad range of malformations, varying from complete absence of the epicardium and embryonic lethality around stage HH29 (Gittenberger-de Groot et al., 2000) to only a delay in epicardial outgrowth with a mild cardiac phenotype (Eralp et al., 2005) and survival until hatching at stage 46. The peripheral Purkinje fiber conduction system develops rather late in embryonic life, from stage HH36 onwards. Thus, the manipulated embryos surviving beyond stage HH39 that were used for this study, were typically those that were only mildly affected by the inhibition procedure; both in the mechanical and in the genetic inhibition model.
Purkinje fiber development in embryos with a mechanical inhibition of epicardial outgrowth
In the embryos with mechanically inhibited epicardial outgrowth that succeeded to survive beyond developmental stage HH40, severe cardiac malformations were not observed, except for an occasional reduction in heart size. However, immunofluorescent staining with the EAP-300 antibody, specific for avian conduction tissue (McCabe et al., 1995), showed that both the periarterial and the subendocardial Purkinje fibers were affected in these embryos. To quantify the hypoplasia of the periarterial Purkinje fibers (Figure 1), fluorescently stained areas occupied by the periarterially located Purkinje fibers were measured in 15 vessels with a comparable diameter (5 in each of 3 embryos) located in the interventricular septum, at approximately mid-ventricular level in both the experimental and control groups. Because the periarterial occupancy with EAP-300 positive cells may vary between the vessels within the same heart, we analyzed the arteries with the most EAP-300 cells, both in control and experimental embryos. The EAP-300 postitive area was found to be reduced by up to 89% (p<0.05; Figure 1 g–i, and Figure 2). Hypoplasia of the periarterial Purkinje fiber network appeared to be the result of a reduction in the number of EAP-300 positive cells, whereas the amount of EAP-300 signal per cell was not altered significantly in the immunofluorescent stainings.
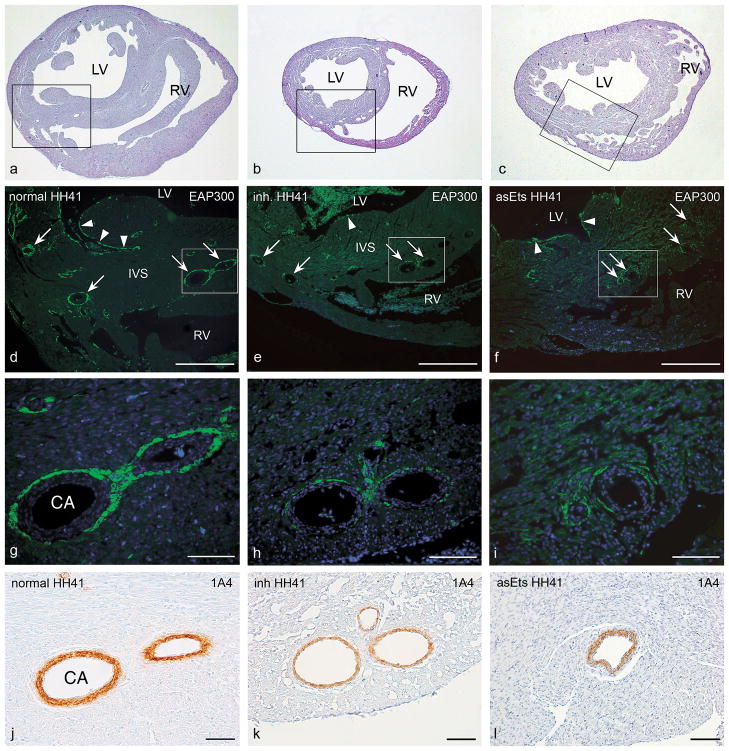
Figure 1.
Photomicrographs of representative transverse sections of a normal control quail heart (HH41; a, d, g, and j ), a quail heart with a mechanical inhibition of epicardial outgrowth (inh; HH41; b, e, h, and k, and a chicken heart with hampered EPDC differentiation due to retrovirally induced down-regulation of Ets-1 and Ets-2 (asEts; HH41; c, f, i, and l). Panels a–c show haematoxylin-eosin stained overviews of the sections, at approximately mid-ventricular level. Boxed areas in a–c delineate the regions depicted in d–f. Serial sections were stained for Purkinje fiber cells with the EAP-300 antibody (in green) and nuclei were stained with DAPI (in blue). In the control heart, (d, g) normal development of the periarterial (arrows) and subendocardial (arrowheads) Purkinje fiber cells was observed. In contrast, in the inhibition embryo (e, h), and in the asEts-1/2 embryo (f, i), we observed a decrease in Purkinje fiber cell numbers. The details (g–i; boxed areas in d–f) show periarterially located Purkinje fiber cells. In embryos with disturbed epicardial contribution EAP-300 positive cell numbers were dramatically reduced compared to normal embryos. Quantification of the area occupied by periarterial Purkinje fibers was performed as described in the text under Materials & Methods, in sections like these, comparing coronary arteries of similar diameter. Panels j–l show the 1A4 (smooth muscle actin) staining in consecutive sections of g– i. The smooth muscle cell layer of the coronary arteries (CA) was slightly thinner in this mechanically PEO-inhibited embryo (k). However, this was not a consistent finding. Also the asEts-1/2 embryos displayed coronary arteries with normal thickness of the smooth muscle cell layer (l). CA, coronary artery; IVS, interventricular septum; LV, left ventricle; RV, right ventricle. Scale bars, 250 μm in d–f; 50 μm in g–l.
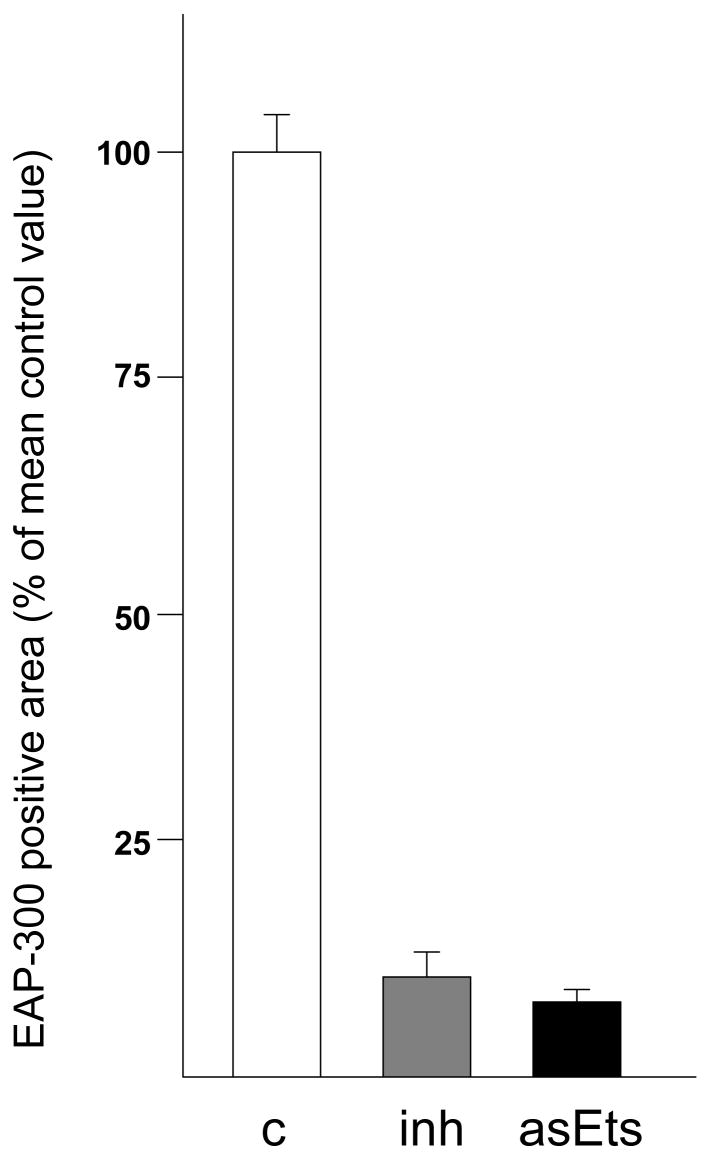
Figure 2.
Quantification of periarterial Purkinje fiber hypoplasia in embryos with mechanical and retrovirally induced inhibition of epicardial outgrowth. The size of the area occupied by EAP-300 positive cells was determined as described in the Materials & Methods section. Significant (p<0.05) reduction of the periarterial Purkinje fiber area was observed in both experimental groups (mechanical inhibition: 10.4% ± 2.7%; grey bar; genetic inhibition: 8.3% ± 1.4%; black bar ) compared to control embryos (100% ± 3.8%; white bar). Error bars indicate standard deviations.
To ensure that our analysis would focus on the influence of disturbed or delayed epicardial outgrowth rather than on the influence of impaired coronary formation on Purkinje fiber development, we selected 5 embryos with a normal distribution of the coronary arteries for a more detailed morphological analysis of the periarterial and subendocardial Purkinje fibers. Whereas we could observe hardly any green fluorescein signal above background by immunofluorescence microscopy (not shown), anti-fluorescein signal enhancement and conventional immunohistochemistry revealed a faint EAP-300 signal around the coronary arteries in these embryos (Figure 3 c and d). The coronaries were surrounded by a normal amount of 1A4-positive smooth muscle cells (not shown). In addition, cellular morphology seemed abnormal, especially in the subendocardially located Purkinje fibers. Whereas in normal embryos cells of the subendocardial Purkinje fibers were aligned in continuous fiber-like strands, in the inhibition embryos they were abnormally large, rounded and had failed to differentiate into a coherent Purkinje fiber network (Figure 3).
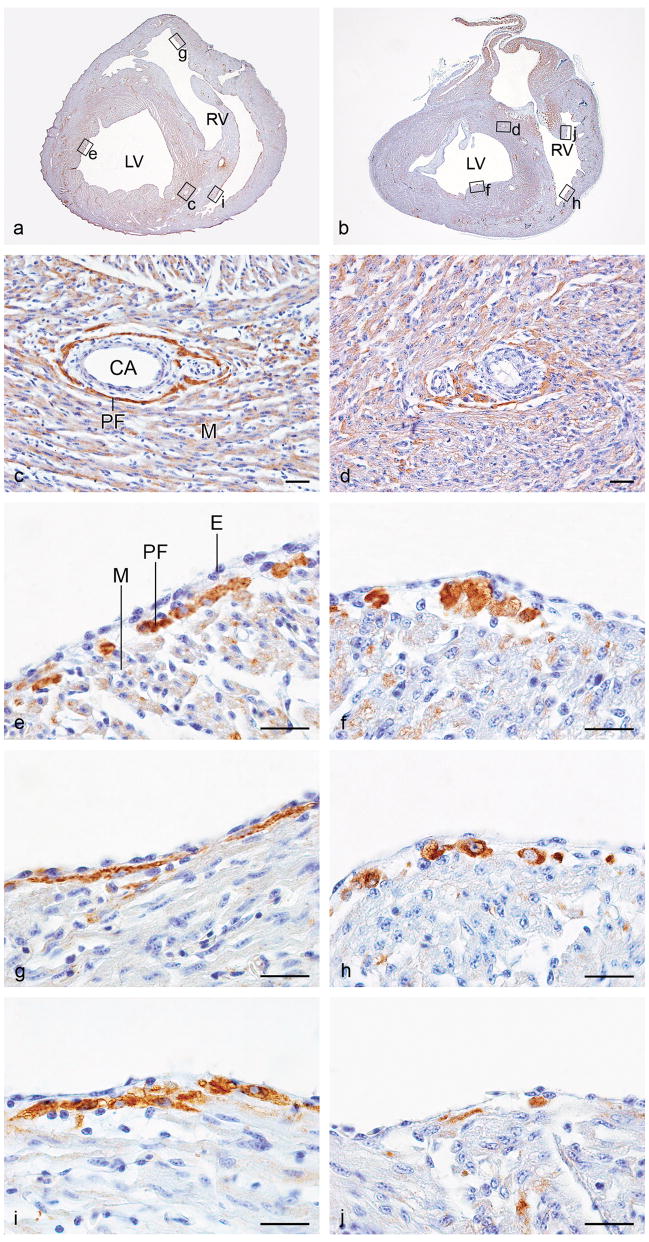
Figure 3.
Photomicrographs of representative transverse sections of a normal control quail heart (HH 41; a, c, e, g, and i) and a quail heart with mechanical inhibition of epicardial outgrowth. (inh; HH 41; b, d, f, h and j). Sections were stained with immunofluorescent EAP-300 antibody and DAB-converted as described in the Materials & Methods section (c–j). Panels a and b show the complete sections at approximately mid-ventricular level, where coronary arteries of similar diameter are present. The location of the enlargements in panels c–j is indicated. In the mechanically inhibited heart, the periarterial EAP-300 signal is faint and diffuse (d) when compared to the signal in the periarterial Purkinje fibers in the normal control heart (c). This is consistent with the finding that EAP-300 immunofluorescence did not surpass background in these embryos (Figure 2). Additionally, EAP-300 staining revealed abnormally large rounded cells in the subendocardial compartment of the peripheral conduction system. These cells were not as neatly organized in fibers as the EAP-300 positive cells in controls (e, f; left ventricular and g, h; right ventricular ventral free walls). Hyperplasia and absence of fiber-like organization of the subendocardial Purkinje fibers was observed throughout, and is illustrated here by EAP-300 staining in the moderator band of the right ventricle (i, j; lateral side of the moderator band). CA, coronary artery, E, endothelium; LV, left ventricle; M, myocardium; PF, Purkinje fiber; RV, right ventricle. Scale bars, 20 μm.
Purkinje fiber development in embryos with genetic inhibition of epicardial outgrowth
To show that the Purkinje fiber hypoplasia observed after PEO inhibition with a piece of eggshell membrane did not depend on this particular inhibition model we confirmed the results in the genetic inhibition model available in our group. Retrovirally delivered antisense Ets-1/2 has been shown to decrease the contribution of EPDCs to heart formation by blocking epicardial epithelial-mesenchymal transformation (Lie-Venema et al., 2003). In the 4 embryos transduced with the CXasEtsIZ retrovirus (antisense-Ets embryos, HH 39–41), there were no gross morphologic abnormalities, coronary architecture and myocardial thickness were within the normal range, and there was some subepicardial mesenchyme present in the atrio-ventricular groove. By this, they were classified as being only mildly affected by the retroviral manipulation of epicardial EMT. Similar to the observations in the mechanically inhibited embryos, a marked reduction (up to 92%, p<0.05) of periarterially located Purkinje fibers was found (Figure 1i and Figure 3). Also resembling those in the mechanically inhibited embryos were the large, rounded subendocardial EAP-300 positive cells that failed to form continuous strands (not shown).
DISCUSSION
Proper timing of the development of the epicardium and sequential transformation of the epicardium into migratory EPDCs is important for cardiac development. It has been shown that EPDCs are essential for formation of the compact myocardium (Gittenberger-de Groot et al., 2000; 2003;Tevosian et al., 2000;Eralp et al., 2005), and that proepicardial organ-derived endothelial precursor cells and EPDCs provide the building blocks of the coronary vascular network (Poelmann et al., 1993; Vrancken Peeters et al., 1999;Lie-Venema et al., 2005). Furthermore, EPDCs are involved in sculpting and remodelling of the outflow tract (Rothenberg et al., 2002;Watanabe et al., 1999) and have a key function in establishing the coronary orifices (Eralp et al., 2005). The experimental results presented in this study provide the first direct evidence that EPDC are also directly involved in the development of the peripheral Purkinje fiber conduction system.
The recruitment of cardiomyocytes into the Purkinje fiber system has been demonstrated to be related to hemodynamic influences derived from the coronary vasculature and endocardial lining of the ventricular lumen (Gourdie et al., 1995;Hyer et al., 1999;Gourdie et al., 2003), with mature ET-1 and the ECE1 metalloprotease as key inductive factors (Takebayashi-Suzuki et al., 2000;Reckova et al., 2003;Hall et al., 2004). Earlier work demonstrated dramatic effects on the development of cardiac architecture and coronary vasculature when epicardial differentiation was disturbed (Gittenberger-de Groot et al., 2000;Tevosian et al., 2000;Lie-Venema et al., 2003;Eralp et al., 2005). However, the developmental effect of epicardial inhibition, either mechanically or genetically, is variable and the late surviving embryos (>HH39) in the present study were specifically included because they were the least affected. This was not only deduced from the fact that they had been able to survive the critical developmental stage of HH35 (when the coronary ostia must have been formed), but also from their normal coronary and myocardial architecture. Accordingly, except for an occasional difference in the size of the hearts, no gross cardiac abnormalities were observed. We therefore advocate a direct effect of EPDCs on the conversion of cardiomyocytes into conduction cells and do not consider hemodynamic alterations to be the cause of the Purkinje fiber network hypoplasia in our experimental embryos. However, we cannot completely rule out this possibility in a minority of the embryos because of the slight concomitant thinning of the coronary smooth muscle layer. Our reasoning that EPDCs have a direct effect on Purkinje fiber formation is further substantiated by the abnormalities in the subendocardial portion of the peripheral conduction system.
We have not found evidence that increased apoptosis was the cause of the Purkinje fiber hypoplasia. Pruning of conduction tissue development by apoptotic events occurs specifically between stage HH29-32 in the future His bundle and bundle branches, and similar apoptotic pruning was suggested but not shown for the developing peripheral Purkinje fibers (Cheng et al., 2002). It can not be excluded that the apoptosis in the central conduction system is neural crest cell related (Poelmann, Mikawa, and Gittenberger-de Groot, 1998), whereas the peripheral Purkinje network has a specific relation with EPDCs (Gittenberger-de Groot et al., 2003b). In embryos with epicardial outgrowth inhibition we observed decreased, not increased, levels of apoptosis in the ventricular myocardium at stage HH30 (Eralp et al., 2005). Additionally, in the present study we did not find apoptotic cells in the Purkinje fiber network of both the normal and the PEO-inhibited hearts at stage HH40 (not shown).
We can only speculate on the molecular mechanism by which EPDCs promote Purkinje fiber differentiation. We favour the idea of a direct paracrine effect of EPDCs on the cardiomyocytes to differentiate into Purkinje fibers, supported by the abnormalities of the subendocardial Purkinje fibers in our embryos. We propose a model in which EPDCs cooperate with endothelial cells in the differentiation of conductive tissue from cardiomyocytes (see Figure 4). In our favourite hypothesis, EPDCs cause cardiomyocytes to change into more primitive cardiomyocytes. The fate of these EPDC-induced primitive cardiomyocytes (PCMs) is then determined by local factors, like the ET-1 cleavage product of ECE1, which could as a second ‘hit’ be delivered by endothelial or endocardial cells, and cause the PCM to finally differentiate into a conductive cell of the Purkinje fiber network. Absence of or a decrease in either the first EPDC-derived, or the second, endothelial/endocardial cell-derived inductive event leads to hypoplasia of the Purkinje fiber network. In earlier studies demonstrating the inductive capacities of endothelin and ECE-1 in Purkinje fiber conversion, the requirement for the initial EPDC-derived signal was fulfilled by the presence of interstitial EPDCs (fibroblasts) in either the cardiomyocyte culture in vitro (Gourdie et al., 1998), or in vivo, in the myocardium (Takebayashi-Suzuki et al., 2000). It can also be hypothesized that the endocardial or endothelial cell would signal to the EPDC, which in turn, would impose a directive signal on the neighbouring cardiomyocyte. Alternatively, we can explain our findings with a model in which the levels of mature ET-1 are increased, either indirectly, by a cascade in which the EPDC instructs the endocardial/endothelial cell to enhance its ET-1 and/or ECE1 production, or directly, by the EPDCs. It has been shown that cultured EPDCs have ET-1 in their secretion repertoire (Eid et al., 1994); it remains to be investigated whether this also applies for EPDCs in vivo at the time of Purkinje fiber development.
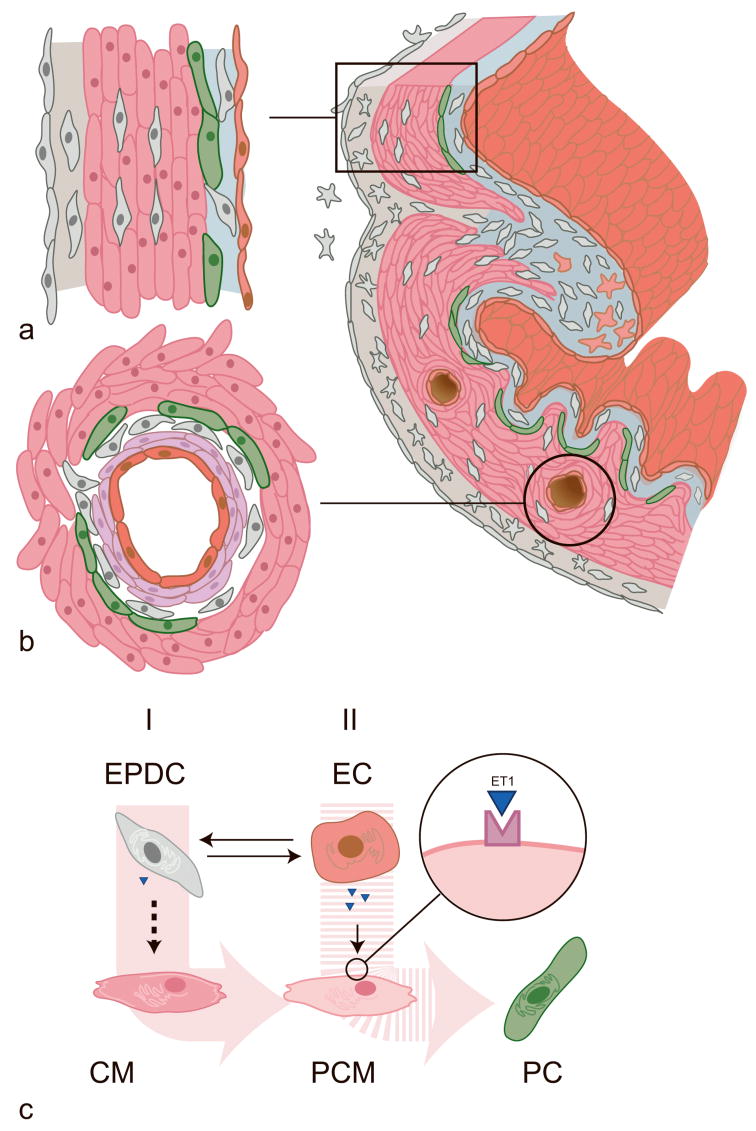
Figure 4.
Hypothetical model for the role of EPDCs in the differentiation of Purkinje fibers. EPDCs derive from the epicardial mesothelium (grey outer layer) by epithelial-mesenchymal transition, to form the subepicardial mesenchyme (stellate cells, grey). Following migration, these cells differentiate into periarterial smooth muscle cells (SMCs, in purple) and fibroblasts (spindle-shaped, in grey), myocardial interstitial fibroblasts (grey) and subendocardial fibroblasts (grey). Purkinje fiber cells (green) develop at a) subendocardial and b) periarterial sites, where both EPDCs (smooth muscle cells and/or fibroblasts) and endocardial/endothelial cells (red) are in close proximity of the cardiomyocytes (pink) that convert into conduction tissue. c) Purkinje fiber cell (PC) differentiation occurs after two subsequent inductive ‘hits’. Firstly, an EPDC-derived factor causes the cardiomyocyte (CM) to change into a more primitive cardiomyocyte (PCM). Thereafter, hemodynamically determined expression of ET-1 and ECE1 by endocardial/endothelial cells (EC) brings about the final signal that allows the primitive cardiomyocyte to convert into a conduction cell. Alternatively, the endothelial or endocardial cell instructs the EPDC to express one or more inductive factors. Among these, ET-1 is a candidate since it has been shown that cultured EPDCs can express this molecule (Eid et al., 1994).
Whatever mechanism it may turn out to be, the present study indicates that Purkinje fiber development is influenced by EPDCs within a narrow time window. We observed aberrant Purkinje fiber development in embryos with practically no morphological defects. The smooth muscle layer of the coronary arteries is comprised of differentiated EPDCs and because this layer is almost as good as normal in the inhibition embryos used in this study, it can be deduced that a drastic decrease of periarterial EPDCs does not account for the Purkinje fiber hypoplasia. During the procedure of mechanical inhibition of proepicardial outgrowth, we may well have induced only a delay in the time of arrival of the subendocardial and periarterial EPDCs, by destroying the extracellular matrix bridges connecting the PEO to the heart (Nahirney, Mikawa, and Fischman, 2003) while inserting a piece of eggshell membrane. When subsequently the piece of membrane failed to stay in place, PEO cells may have grown around the membrane and “rescued” the normal phenotype, except for an epicardial outgrowth delay of approximately one day with less noticeable developmental defects as a consequence. A similar phenomenon has been noted in PEO-inhibited chicken embryos rescued with a quail PEO inserted into the pericardial cavity. In these embryos coronary ostia formation was disturbed (Eralp et al., 2005). Preliminary electrographical experiments (not shown) indicating a concommitant decrease in action potential propagation from endocardium to epicardium, implicate that such minor changes in EPDC behaviour may have functional consequences as well, which is of particular interest from a clinical point of view.
In summary, we found that inhibition of epicardial outgrowth leads to morphological abnormalities of the Purkinje fiber network. We postulate that EPDCs, in close collaboration with endothelial and endocardial cells, play an essential role in the development of the peripheral conduction system of the heart. Our preliminary indications for electrocardiographical defects due to Purkinje fiber hypoplasia provide a basis for further research on the role of EPDCs in the development of congenital conduction deficits.
Acknowledgments
Grant information
Grant sponsor: The Netherlands Heart Foundation; grant numbers: 2001B015 (to H.L.-V.) and 2001B057 (to N.M.S.vdA.)
Grant sponsor: NIH; grant numbers: HD39946-05 and HL56728-09 (to R.G.G).
We gratefully acknowledge Jan H. Lens and Ron Slagter for photographical assistance and digital artwork. This study was supported financially by grants NHS 2001B015 and 2001B057 from the Netherlands Heart Foundation (to H.L.-V. and N.M.S. vd A., respectively) and by NIH grants HD39946-05 and HL56728-09 (to R.G.G).
References
- Cheng G, Wessels A, Gourdie RG, Thompson RP. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev Dyn. 2002;223:119–133. doi: 10.1002/dvdy.1244. [DOI] [PubMed] [Google Scholar]
- Eid H, de Bold K, Chen JH, de Bold AJ. Epicardial mesothelial cells synthesize and release endothelin. J Cardiovasc Pharmacol. 1994;24:715–720. doi: 10.1097/00005344-199424050-00005. [DOI] [PubMed] [Google Scholar]
- Eralp I, Lie-Venema H, DeRuiter MC, Van Den Akker NM, Bogers AJ, Mentink MM, Poelmann RE, Gittenberger-de Groot AC. Coronary Artery and Orifice Development Is Associated With Proper Timing of Epicardial Outgrowth and Correlated Fas Ligand Associated Apoptosis Patterns. Circ Res. 2005;96:526–534. doi: 10.1161/01.RES.0000158965.34647.4e. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Bartram U, Oosthoek PW, Bartelings MM, Hogers B, Poelmann RE, Jongewaard IN, Klewer SE. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat Rec. 2003a;275A:1109–1116. doi: 10.1002/ar.a.10126. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Blom NM, Aoyama N, Sucov H, Wenink AC, Poelmann RE. The role of neural crest and epicardium-derived cells in conduction system formation. Novartis Found Symp. 2003b;250:125–134. doi: 10.1002/0470868066.ch8. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters M-PFM, Bergwerff M, Mentink MMT, Poelmann RE. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters M-PFM, Mentink MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, Edmondson AM, Cheng G, Sedmera D, O’Brien TX, Mikawa T, Thompson RP. His-Purkinje lineages and development. Novartis Found Symp. 2003;250:110–122. [PubMed] [Google Scholar]
- Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates purkinje fibers of the cardiac conduction system. Development. 1995:121. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Wei Y, Kim D, Klatt SC, Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting purkinje fibers. Proc Natl Acad Sci U S A. 1998;95:6815–6818. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CE, Hurtado R, Hewett KW, Shulimovich M, Poma CP, Reckova M, Justus C, Pennisi DJ, Tobita K, Sedmera D, Gourdie RG, Mikawa T. Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart. Development. 2004;131:581–592. doi: 10.1242/dev.00947. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hyer J, Johansen M, Prasad A, Wessels A, Kirby ML, Gourdie RG, Mikawa T. Induction of purkinje fiber differentiation by coronary arterialization. Proc Natl Acad Sci U S A. 1999;96:13214–13218. doi: 10.1073/pnas.96.23.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee L, Baldwin HS, Min Shen H, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- Lie-Venema H, Eralp I, Maas S, Gittenberger-de Groot A, Poelmann R, DeRuiter M. Myocardial heterogeneity in permissiveness for epicardium-derived cells and endothelial precursor cells along the developing heart tube at the onset of coronary vascularization. Anat Rec. 2005 doi: 10.1002/ar.a.20154. [DOI] [PubMed] [Google Scholar]
- Lie-Venema H, Gittenberger-de Groot AC, van Empel LJP, Boot MJ, Kerkdijk H, de Kant E, DeRuiter MC. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ Res. 2003;92:749–756. doi: 10.1161/01.RES.0000066662.70010.DB. [DOI] [PubMed] [Google Scholar]
- Männer J. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol. 1993;187:281–289. doi: 10.1007/BF00195766. [DOI] [PubMed] [Google Scholar]
- McCabe CF, Gourdie RG, Thompson RP, Cole GJ. Developmentally regulated neural protein EAP-300 is expressed by myocardium and cardiac neural crest during chick embryogenesis. Dev Dyn. 1995;203:51–60. doi: 10.1002/aja.1002030106. [DOI] [PubMed] [Google Scholar]
- Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev Dyn. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC, Mentink MMT, Bökenkamp R, Hogers B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res. 1993;73:559–568. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Mikawa T, Gittenberger-de Groot AC. Neural crest cells in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Dev Dyn. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Reckova M, Rosengarten C, Dealmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93:77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- Rothenberg F, Hitomi M, Fisher SA, Watanabe M. Initiation of apoptosis in the developing avian outflow tract myocardium. Dev Dyn. 2002;223:469–482. doi: 10.1002/dvdy.10077. [DOI] [PubMed] [Google Scholar]
- Takebayashi-Suzuki K, Yanagisawa M, Gourdie RG, Kanzawa N, Mikawa T. In vivo induction of cardiac Purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development. 2000;127:3523–3532. doi: 10.1242/dev.127.16.3523. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Virágh Sz, Gittenberger-de Groot AC, Poelmann RE, Kálmán F. Early development of quail heart epicardium and associated vascular and glandular structures. Anat Embryol. 1993;188:381–393. doi: 10.1007/BF00185947. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters M-PFM, Gittenberger-de Groot AC, Mentink MMT, Hungerford JE, Little CD, Poelmann RE. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn. 1997;208:338–348. doi: 10.1002/(SICI)1097-0177(199703)208:3<338::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters M-PFM, Gittenberger-de Groot AC, Mentink MMT, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial-mesenchymal transformation of the epicardium. Anat Embryol. 1999;199:367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Choudhry A, Berlan M, Singal A, Siwik E, Mohr S, Fisher SA. Developmental remodelling and shortening of the cardiac outflow tract involves myocyte programmed cell death. Development. 1999;125:3809–3820. doi: 10.1242/dev.125.19.3809. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]