Abstract
Nkx2-5 gene mutations cause cardiac abnormalities including deficits of function in the atrioventricular conduction system (AVCS). In the chick, Nkx2-5 is elevated in Purkinje fiber AVCS cells relative to working cardiomyocytes. Here, we show that Nkx2-5 expression rises to a peak as Purkinje fibers progressively differentiate. To disrupt this pattern, we over-expressed Nkx2-5 from embryonic day 10 as Purkinje fibers are recruited within developing chick hearts. Over-expression of Nkx2-5 caused inhibition of sMHC, a late Purkinje fiber marker but did not affect Cx40 levels. Working cardiomyocytes over-expressing Nkx2-5 in these hearts ectopically up-regulated Cx40, but not sMHC. Isolated embryonic cardiomyocytes over-expressing Nkx2-5 also displayed increased Cx40 and suppressed sMHC. By contrast over-expression of a human NKX2-5 mutant did not effect these markers in vivo or in vitro, suggesting one possible mechanism for clinical phenotypes. We conclude that a prerequisite for normal Purkinje fiber maturation is precise regulation of Nkx2-5 levels.
Keywords: Heart conduction system, Nkx2-5, Purkinje fibers, development, atrioventricular block
INTRODUCTION
Nkx2-5 (Csx/Tinman) is an NK2 class homeodomain transcription factor expressed in diverse species (Evans, 1999). In vertebrate embryos, Nkx2-5 is one of the earliest markers of heart-forming potential. Functional studies have shown that post-gastrulation expression of Nkx2-5 is required for specification of cardiomyogenic cell fate. Absence of Nkx2-5 expression leads to abnormal heart formation in Drosophila, Xenopus and mouse (Grow and Krieg, 1998). Nkx2-5 knockout mice die in utero shortly after looping morphogenesis and display disrupted expression of cardiac genes (Lyons et al., 1995). While these findings establish a function for Nkx2-5 in early cardiogenesis, emerging data suggests this gene has additional developmental roles. Clinical interest in this gene arises from the identification of heterozygous mutations in human NKX2-5, including the Gln170ter NKX2-5 truncation mutant, that cause heart disease (Schott et al., 1998). Although a number of structural and functional cardiac abnormalities are associated with such mutations, one common and salient phenotype is atrioventricular conduction block (Schott et al., 1998). Interestingly, the AV block phenotype emerges during postnatal development and is believed to result from progressive disease of the atrioventricular conduction system (AVCS). This prospect is supported by our recent work in Nkx2-5 haploinsufficent mice, where we reported that loss of a single Nkx2-5 allele is sufficient to cause a severe hyoplasia of the AVCS in the postnatal heart associated with a greater than 50% loss of Cx40-expressing Purkinje fibers in the ventricle (Jay et al., 2004).
The finding that Nkx2-5 expression is elevated in the forming AVCS of higher vertebrates, has provided evidence of a role for this transcription factor in conduction tissue development (Takebayashi-Suzuki et al., 2001; Thomas et al., 2001). Purkinje fiber cells of the AVCS coordinate rapid spread of action potential (AP) in the ventricular myocardium (Pennisi et al.,; Gourdie et al., 2003; Moorman and Christoffels, 2003). To fulfill this role of fast AP conduction, Purkinje fibers express a characteristic set of genes. Such genes may be either uniquely or differentially expressed relative to working cardiomyocytes and encode proteins including ion channels, contractile proteins and signaling molecules. In a process best characterized in the avian embryo e.g., (Harris et al., 2002), the Purkinje fiber-specific pattern of gene expression emerges progressively following septation of the ventricles. Genes up-regulated early during this maturational differentiation include the gap junction proteins Cx40 and Cx45 (Gourdie et al., 1993; Delorme et al., 1995). A well-characterized marker of late phases of Purkinje fiber differentiation in the chick is the slow tonic myosin heavy chain protein sMHC, which initiates expression just prior to hatching. At present, the molecular cues regulating the progressive differentiation of Purkinje fiber phenotype are unknown. Previously, we have shown that Nkx2-5 is first up regulated in prospective Purkinje fibers during inductive recruitment of these cells into the AVCS. In this study, we demonstrate that levels of nuclear-localized Nkx2-5 in Purkinje fiber cells continue to increase throughout development and that disruption of this pattern by prematurely up regulating Nkx2-5 levels has differential effects on genes marking early and late stages of differentiation of conduction cells. Our results suggest that there is a requirement for precise regulation of Nkx2-5 level during differentiation of Purkinje fiber cells.
RESULTS
Elevated Nkx2-5 protein is expressed throughout Purkinje fiber cell differentiation
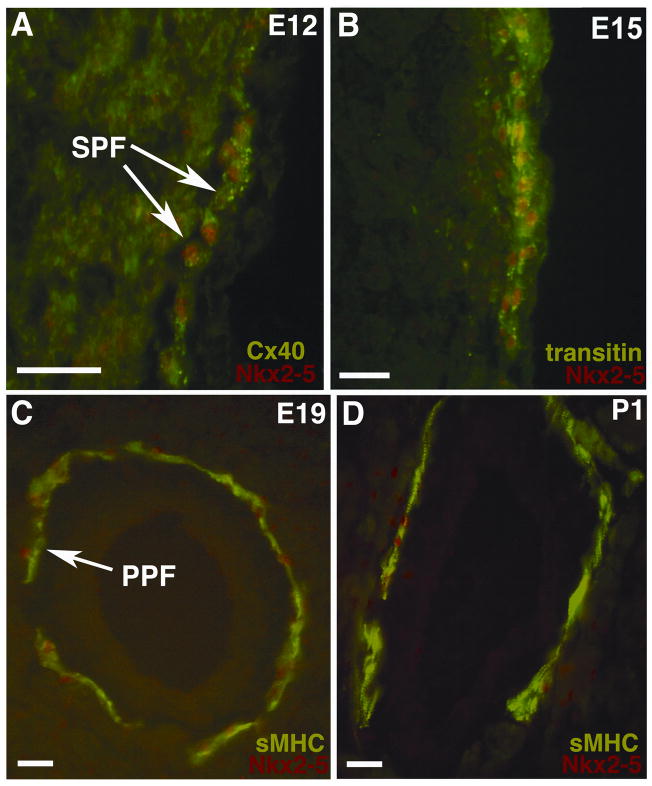
Previously, we have demonstrated that Nkx2-5 is elevated in the developing AVCS of the chick heart relative to working myocardium (Thomas et al., 2001). In the present study, we addressed the mechanistic relevance of this expression pattern by focusing on the role of Nkx2-5 in the differentiation of the peripheral network of sub-endocardial (SPFs) and periarterial Purkinje fibers (PPFs). We first characterized the localization of Nkx2-5 protein expression by multi-label immunohistochemistry using three specific markers that show a progressive sequence of up-regulated expression over the development of Purkinje fiber cells. At embryonic day 12 (E12), the gap junction protein connexin40 (Cx40) provides a marker of the initial stages of Purkinje fiber differentiation (Gourdie et al., 1993). At E12, Nkx2-5 localized to the nuclei of Cx40-positive Purkinje fibers at higher levels relative to working cardiomyocytes (Figure 1A). At E15, subsequent differentiation of Purkinje fibers is characterized by the expression of the intermediate filament protein transitin/EAP-300 (McCabe et al., 1995). We also noted that higher levels of Nkx2-5 labeling were maintained within transitin-positive Purkinje fibers at this stage (Figure 1B). The later stages of conduction cell differentiation in the chick embryo are marked by the onset of expression of the slow tonic myosin heavy chain protein (sMHC) at E17 (Gonzalez-Sanchez and Bader, 1985). At E19, increased levels of nuclear Nkx2-5 immunolabeling were associated with sMHC-positive PPFs (Figure 1C). Elevated Nkx2-5 levels could also be detected in sMHC-positive Purkinje fibers during the post-hatching period (Figure 1D). In summary, these results confirmed that Nkx2-5 is elevated in the nuclei of bona-fide conduction cells, and also demonstrated for the first time that elevation of this Nkx2-5 signal was maintained at early, intermediate and late stages of Purkinje fiber differentiation.
Figure 1. Nkx2-5 expression localizes to the AVCS of the embryonic chick heart.
Using double immunohistochemistry with antibodies labeling the AVCS we directly demonstrate that Nkx2-5 protein localizes to Purkinje fibers within sections of chick heart. (A) At embryonic day 12 (E12) the developing conduction system can be specifically immunolabeled for Cx40 (shown in green, FITC) and here nuclear Nkx2-5 protein can be seen at higher levels than the surrounding myocardium (shown in red, TRITC). (B) From E15 onwards the AVCS expresses the intermediate filament protein transitin and nuclear Nkx2-5 protein can again be seen at higher levels than the surrounding myocardium at this stage. (C) At late embryonic stages of AVCS development (E19) sMHC specifically labels specialized conduction cells, arrows indicate sMHC positive PPFs (FITC). At this stage Nkx2-5 shows robust nuclear staining (TRITC) within Purkinje fibers. (D) Nkx2-5 expression patterns are maintained in the post-hatching chick heart and double immunohistochemistry for Nkx2-5 and sMHC protein expression reveals that PPFs at post-hatching day 1 (P1) express Nkx2-5. Scale bars 50 μm.
Nuclear-localized Nkx2-5 increases during Purkinje fiber maturation
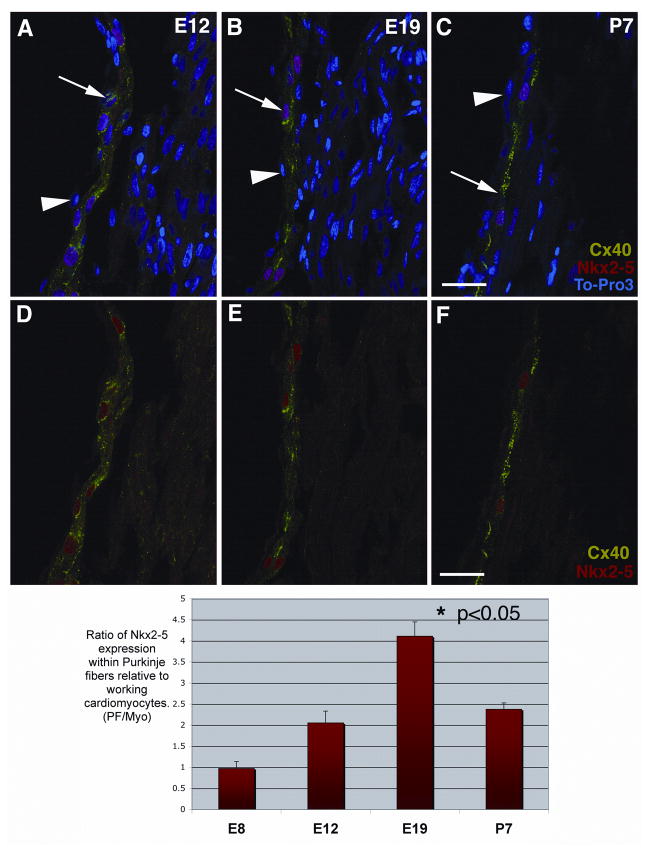
Figure 1 shows that Nkx2-5 protein is elevated in the nuclei of differentiating conduction cells. However, it was our impression that nuclear-localized Nkx2-5 in Purkinje fiber cells continued to increase relative to working cardiomyocytes as development progressed. To quantify this increase, sections from E8, E12, E19 and post-natal day 7 (P7) hearts were multi-labeled with Cx40 and Nkx2-5 antibodies and the nuclear label To-Pro3 and then imaged by confocal optical sectioning (arrows in Figure 2 A–C). In Figure 2 A–C note the preservation of nuclei within the thin endocardial layer (arrowheads) over sub-endocardially located Purkinje fiber cells (i.e., SPFs), indicative of the excellent histological integrity of these heart sections. At each stage analyzed, nuclear To-Pro3 and Cx40 immunolabeling were used to confirm that Nkx2-5 was specifically increased in SPF nuclei. For clarity these images are repeated below without the nuclear To-Pro3 counter stain (Figure 2 D–F). By staining samples at the same time using identical immunolabeling protocols, and maintaining constant settings on the confocal microscope, we were able to compare relative levels of Nkx2-5 signal between developmental stages. Using this rigorously controlled approach it was determined that the mean intensity of Nkx2-5 was at least 2-fold higher in SPF nuclei relative to nuclei of working cardiomyocytes throughout development (Figure 2 G). Moreover, consistent with our qualitative impression, nuclear-localized Nkx2-5 increased significantly (p<0.05) from a 2-fold elevated level at E12 to a 4-fold increase over that of working cardiomyocyte nuclei at E19, before dropping back to 2-fold increased levels one week post hatching (P7).
Figure 2. Increasing levels of nuclear-localized Nkx2-5 defines Purkinje fiber development.
Chick heart sections at four stages; E8, E12 (A, D), E19 (B, E) and P7 (C, F) were immunolabeled for Cx40 (shown in green, FITC), Nkx2-5 protein (shown in red, TRITC) and the nuclear dye, To-Pro3 (shown in blue). These laser scanning confocal images of chick heart sub-endocardium show that Nkx2-5 localizes mainly to the nuclei of Cx40-positive Purkinje fibers and to a lesser extent the nuclei of Cx40-negative cells in the working myocardium at E12, E19 and P7 (A, B, C). For clarity these same images are reproduced, excluding the nuclear stain (D, E, F). These single optical images were used to quantify Nkx2-5 immuno-positive nuclear signal within Purkinje fibers (Cx40-positive) and working cardiomyocytes (Cx40-negative) at the same three stages; E12, E19 and P7. We measured the mean nuclear intensity of the Nkx2-5 immuno-positive signal and expressed it as a ratio, Purkinje fiber/working cardiomyocyte. We found that at E19 the Nkx2-5 immuno-intensity ratio was 100% more than at E12 and also P7 (P< 0.05). The quantitative analysis of E8 cells was undertaken on sub-endocardial cardiomyocytes prior to Cx40 up regulation. This analysis was carried out on multiple images (n=20) from multiple animals (n=3). Scale bars 50μm.
Adenoviral targeting of developing chick heart in vivo using a shell-less culture model
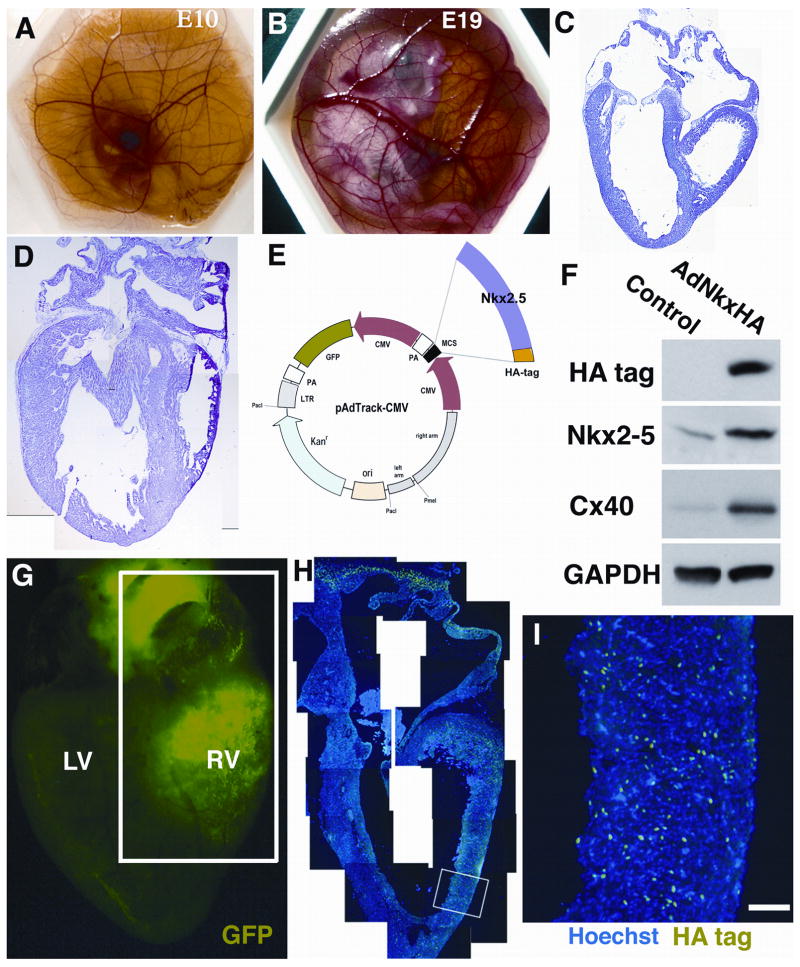
To explore the function of the progressive increase in nuclear-localized Nkx2-5, we devised a strategy to over-express Nkx2-5 in conduction cells from E10 - a timing corresponding to the earliest stages of Purkinje fiber differentiation in advance of the appearance of markers including Cx40, transitin and sMHC (Figure 1 and Harris et al., 2002). To facilitate this strategy, the shell-less culture protocol of (Dunn, 1974) was used in which chick embryos isolated from the egg shell at E2 can be cultured up until E19 (Figure 3A and B). Heart development of shell-less embryos was histologically normal (Figure 3C and D). Of particular utility was that the shell-less protocol facilitated direct visualization of the heart at the E10 time point, enabling high precision microinjection of adenoviral expression vectors at the stage of interest, i.e., the initiation of Purkinje fiber development. The adenoviral vector used, AdNkxHA, has been characterized in earlier studies (Muller et al., 2002). This construct is bicistronic, expressing a HA-tagged Nkx2-5 and GFP under independent CMV promoters (Figure 3E). Western blotting of cultures of chick cardiomyocytes isolated from E3.5 embryos infected by AdNkx2-5 confirmed the ability of the virus to elevate Nkx2-5 levels (Figure 3F). Following microinjection at E10, shell-less embryos were incubated for a further 5–9 days. Survival was good, with 70 % of embryos being viable 7 days after injection. Whole-mount heart preparations were visualized by microscopy and sectors of GFP fluorescence distinguished (Figure 3G). The pattern of viral infection varied depending on the region injected. Typically, sectors were extensive, encompassing the right ventricular myocardium and regions of the left ventricles. HA-immunolabeling of histological sections confirmed that large numbers of cardiomyocytes in such GFP-positive sectors expressed the exogenous HA-tagged Nkx2-5 within nuclei (Figure 3H). Cardiomyocytes over-expressing the exogenous Nkx2-5 were distributed throughout the myocardium, from epicardium to endocardium (Figure 3I). HA-immunolabeling of nuclei was absent in hearts microinjected with AdGFP or vehicle control solution (data not shown).
Figure 3. Shell-less chick preparations facilitate cardiac manipulations in vivo.
(A) A shell-less chick at E10, the stage when direct cardiac micro-injections were performed. (B) A shell-less embryo at E19 prior to harvesting. We analyzed the morphology of shell-less chick hearts at E10 and E17 (C and D) establishing that normal cardiac development occurs. (E) A schematic diagram of the bicistronic adenoviral vector (AdNkxHA) used for these experiments. Nkx2-5HA and Green Fluorescent Protein (GFP) are driven by separate CMV promoter and both GFP acts the HA tag can be used as markers. (F) Protein expression was quantified using embryonic cardiomyocyte cultures by Western blotting. Antibodies to HA and Nkx2-5 demonstrate that AdNkxHA infection markedly increases expression of exogenous Nkx2-5 over the normal endogenous level (at least 3–5 fold). GAPDH confirms equal protein loading. (G) GFP expression, indicating AdNkxHA infection within an experimental heart at E17, 7 days post-injection (LV=Left ventricle, RV=Right ventricle). (H) Montage image of a section through the boxed area in G. The myocardium of the right ventricular free wall and right atrium is stained with HA antibodies (FITC) and counterstained with Hoechst (blue) nuclear dye. (I) Magnified region of (H) showing that HA immunopositive cells are present throughout the myocardium. Scale bar 100 μm.
Constitutive expression of Nkx2-5 disrupts progressive differentiation of Purkinje fibers in vivo
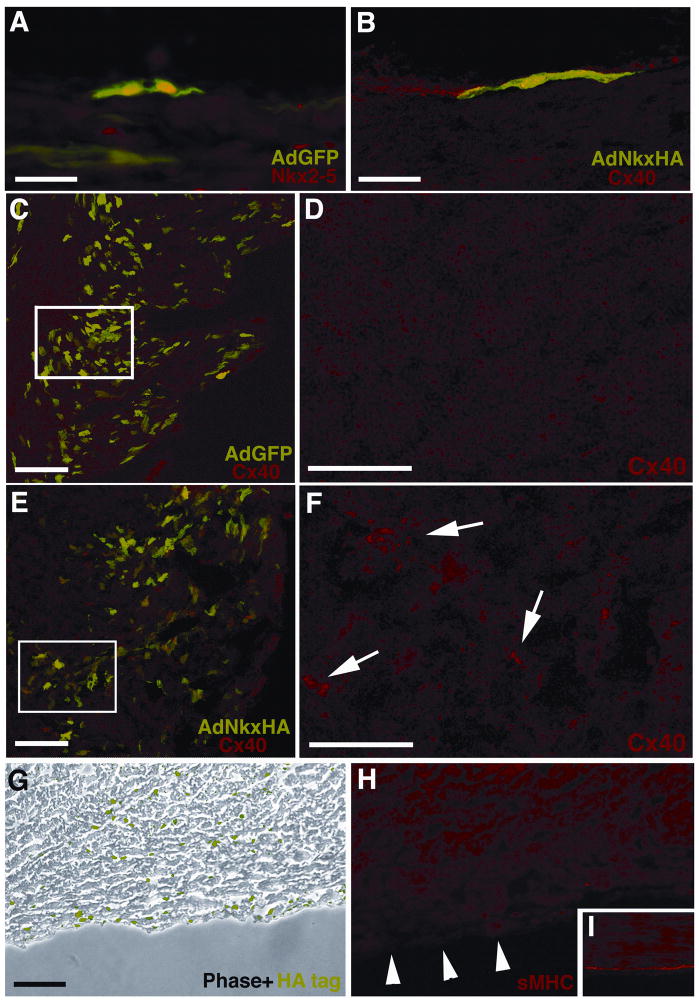
Immunolocalization studies of AVCS markers were carried out on vehicle control, AdGFP and AdNkxHA infected E19 hearts. GFP-positive Purkinje fibers in hearts infected with the control AdGFP virus showed normal patterns of AVCS development and Nkx2-5 expression (Figure 4A). Interestingly, in hearts targeted with the AdNkxHA virus, Cx40 immunolabeling patterns of HA-positive sectors of the sub-endocardium were not altered (figure 4B). Thus, maintenance of over-expressed Nkx2-5 at higher than endogenous levels for a protracted period starting at E10 did not appear to affect Cx40 expression by Purkinje fibers. However, analysis of AdNkxHA-positive domains within regions of the working myocardium in the same hearts revealed a different response. The working ventricular myocardial tissues of the developing chick normally express low or negligible levels of Cx40 (Gourdie et al., 1993). Consistent with this, working ventricular myocardium infected with control AdGFP adenovirus revealed no evidence of Cx40 expression. However, similar regions of ventricle infected with AdNkxHA adenovirus showed a striking up-regulation of Cx40 (compare Figure 4C and D to 4E and F). These ectopic foci of bright punctate Cx40 immuno-positive gap junctions were co-localized within cells expressing GFP, mediated by AdNkxHA infection. These data were reproducible in multiple experimental animals. Thus, Nkx2-5 over-expression was sufficient to up-regulate Cx40 ectopically in working cardiomyocytes in vivo.
Figure 4. Over-expression of Nkx2-5 in vivo perturbs AVCS development.
Hearts microinjected at E10 with various constructs were harvested at E18. GFP fluorescence and immunohistochemistry was used to analyze heart sections for AVCS markers. (A) Control vector GFP expression alone does not disrupt the normal nuclear Nkx2-5 expression (TRITC) of SPFs at E18. (B) In this single GFP positive SPF over-expression of Nkx2-5 by AdNkxHA does not disrupt Cx40 expression (TRITC). (C) Low magnification image of RV myocardium labeled with GFP and immunostained with Cx40, which only localizes to SPFs. (D) Enlargement of boxed area in (C) revealing little Cx40 immunolabeling of the myocardium. (E) Low magnification image of RV myocardium labeled with GFP (indicating AdNkxHA infection) and immunostained with Cx40 showing that ectopic Cx40 localizes with GFP expression in the myocardium. (F) In contrast to D ectopic Cx40 immunolabeling is present (arrows) within this enlarged image of boxed area in E. (G) Immunolabeling for HA-tag reveals abundant Nkx2-5 over-expression within AdNkxHA infected tissue (phase contrast, HA-tag in green). (H) A sister section to G shows reduced sMHC immunostaining in the sub-endocardium (arrowheads). (I) Control tissue shows typical sMHC staining of sub-endocardial Purkinje fibers. Scale bars 50 μm (A, B, D, F), 100 μm (C, E, G, H, I).
More significantly with respect to specialized myocardial differentiation, was the effect of Nkx2-5 over-expression on the late-expressed marker of Purkinje fiber phenotype, sMHC. Figure 4G shows a section through the right ventricular free-wall of an E19 heart that had been targeted with AdNkxHA. Prominent HA immunolabeling can be seen sub-endocardially within this region, as well as scattered nuclei within the myocardium, indicating the presence of cardiomyocytes over-expressing exogenous Nkx2-5 (Figure 4G). In the sub-endocardium, normally highly immuno-positive for sMHC at E19, there is a complete absence of signal in the sister section of that shown in Figure 4G (Figure 4H). No such loss of sMHC-immunopositive cells was observed in uninfected control hearts incubated normally and in control hearts targeted ex-ovo with the AdGFP virus (Figure 4I). This change in the expression of sMHC was consistent with an inhibition of the normal progression of Purkinje fiber differentiation in hearts injected with the AdNkxHA virus. Interestingly, although working myocardial cells infected by AdNkxHA showed increased levels of Cx40 immunolabeling (Figure 4E), these same cells showed no detectable up regulation of sMHC (data not shown).
Over-expression of Nkx2-5 within embryonic cardiomyocytes in vitro differentially affects AVCS markers
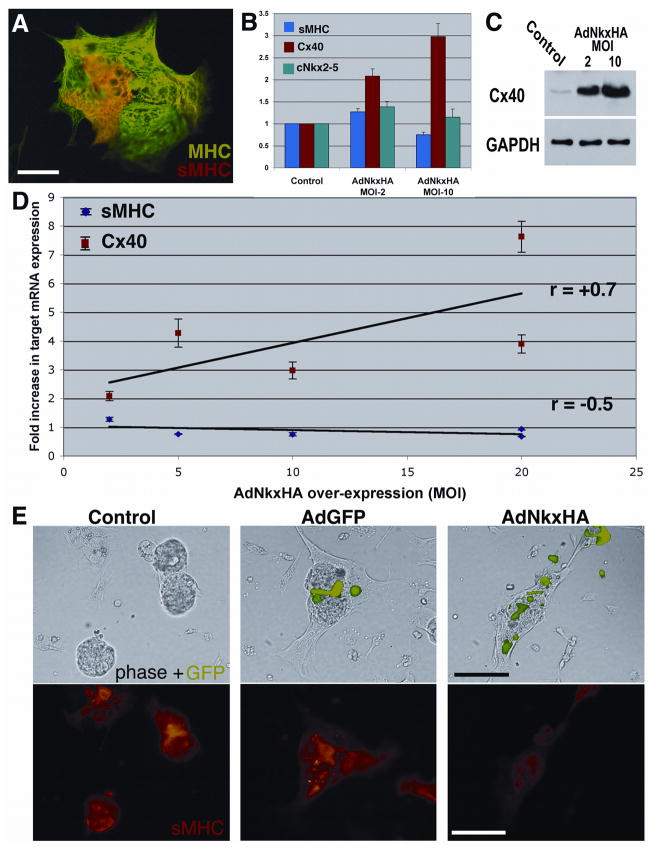
At embryonic day 3.5 (E3.5), neither Cx40 nor sMHC are expressed at detectable levels in the ventricular myocardium of the chick embryo. However, after 3 days in culture, a proportion (~5%) of cardiomyocytes derived from E3.5 hearts immunolabel for sMHC (Gourdie et al., 1998). Consistent with these findings, we confirmed that when embryonic chick cardiomyocytes from E3.5 hearts were cultured for a period of 5 days, a subpopulation of MF20-positive cells immunolabeled for sMHC (Figure 5A). The frequency of sMHC immunolabeled cells in these cultures was increased by treatment with endothelin-1, which was also consistent with our previous results (data not shown). Using quantitative Real-Time RT-PCR, Western blotting and immunocytochemistry we sought to investigate the effect of exogenous Nkx2-5 over-expression on levels of Cx40, sMHC and endogenous Nkx2-5 in this in vitro model of conduction cell differentiation (Figure 5).
Figure 5. In vitro, AdNkxHA but not AdGFP perturbs AVCS markers.
Chick cardiomyocytes isolated at E3 were cultured for five days and double immuno-labeled. (A) In this semi-aggregated culture the cardiomyocytes express Myosin Heavy Chain (MHC) (FITC), while a subpopulation also express sMHC (stained red, TRITC) indicating AVCS cells. (B) Identical cultures were infected with AdNkxHA and the results quantified at the mRNA level by Real-Time RT-PCR for sMHC, Cx40 and endogenous cNkx2-5. (C) Protein expression was quantified on parallel cultures by Western blotting. showin that increasing MOIs of AdNkxHA infection increases expression of Cx40 in vitro proportionally. GAPDH was used to ensure equal protein loading. (D) Multiple experiments were performed using 4 different AdNkxHA MOIs on identical cultures and Real-Time RT-PCR. Increasing Nkx2-5 gene dosage induces Cx40 but suppresses sMHC mRNA (E) Immunocytochemistry of identical cultures infected with the indicated constructs 12 hours after isolation and then cultured for four days. GFP fluorescence over-layered onto phase images shows transduced cells. Immunocytochemistry was used to detect the expression of sMHC in the same cells (TRITC) (bottom panels). Note that sMHC expression is reduced when the culture is infected with AdNkx2-5HA, while both control cultures display sMHC-positivity. Scale bars 50 μm (B), 100 μm (C–H).
While exogenous Nkx2-5 expression had no effect on levels of endogenous chick Nkx2-5 mRNA, Cx40 mRNA level showed proportional increases at multiplicities of AdNkx2-5HA infection (i.e., MOIs) of 2 and 10 (Figure 5B). Western blotting of protein from the same samples confirmed that Cx40 protein was increased in response to over-expression of Nkx2-5 (Figure 5C). In contrast to the effect of Nkx2-5 over-expression on Cx40, sMHC mRNA was reduced in cultures of E3.5 cardiomyocytes at an AdNkx2-5HA MOI of 10 (Figure 5B). To explore this further, we infected cultures with increasing MOIs of 2, 5, 10 and 20 and used Real-Time RT-PCR to assay Cx40 and sMHC mRNAs (Figure 5D). Cx40 mRNA levels showed a linear increase in relation to AdNkxHA MOI. By contrast, sMHC mRNA levels showed a steady downward trend as viral MOI increased.
To further characterize of the effect of Nkx2-5 over-expression in vitro, we examined sMHC and Cx40 protein expression by immunocytochemically following AdNkx2-5HA infection. Consistent with PCR and Western blot data, cultures infected with AdNkxHA consistently showed increases in Cx40 immunolabeling and suppression of sMHC relative to AdGFP and non-infected controls (Figure 5E). The inhibition of sMHC immunolabeling by Nkx2-5 was particularly striking an observation that was reproduced in at least 10 independent experiments. In summary, the results derived from this in vitro model of Purkinje fiber cell differentiation were similar to those obtained in vivo. On the one hand, over-expression of Nkx2-5 caused up-regulated Cx40 expression in these cultures. In contrast, the same treatment inhibited sMHC, quantifiably suppressing the expression of this late marker of Purkinje fiber development.
Mutant NKX2-5 associated with AV block has no effect on Cx40 in vivo or in vitro
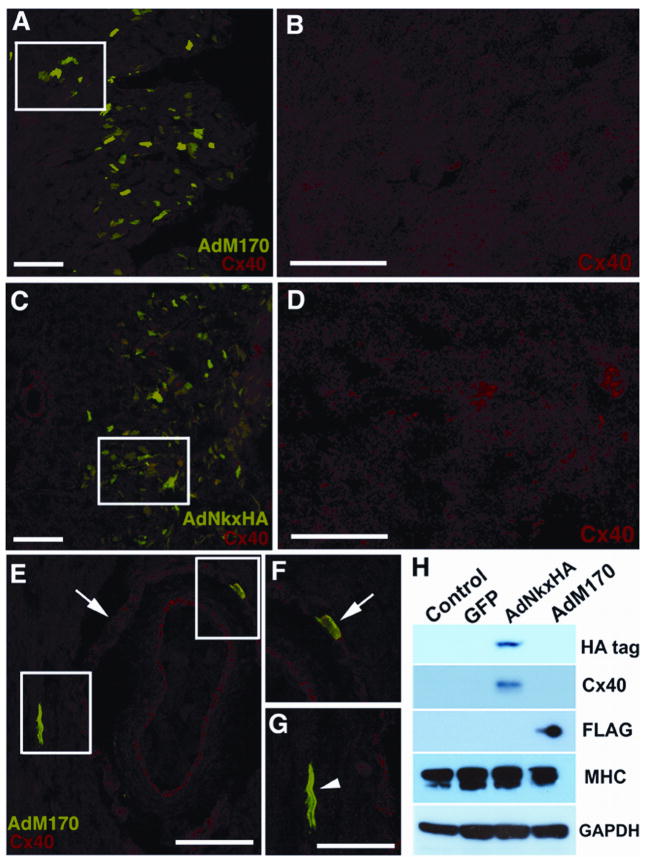
In the final part of this study we used our in vivo and in vitro models of Nkx2-5 function to probe the Gln170ter NKX2-5 mutant, which was amongst the first NKX2-5 mutations to be described in humans (Schott et al., 1998). This mutation occurs at nucleotide position 618, within the DNA binding homeodomain and is predicted to result in a truncated NKX2-5. We cloned this mutant into an adenoviral vector, termed AdM170, and using an identical strategy as above, we targeted cardiomyocytes in vivo and in vitro. Immuno-analysis of the working myocardium within AdM170 targeted sectors of shell-less cultured chick hearts revealed that Cx40 levels remained unaltered (Figure 6A and B). In fact, the low levels of Cx40 immunostaining remained identical to controls (see Figure 3E and F). This was in striking contrast to similar regions of ventricle infected with the AdNkxHA virus, which as we outline above, displayed ectopic foci of Cx40 up-regulation (Figure 6C and D, see also Figure 3C and D). We also investigated what affect this truncated NKX2-5 mutant had on Purkinje fibers. As detailed above, over-expression of wildtype Nkx2-5 did not alter Cx40 immunolabeling patterns within Purkinje fibers. Infection with AdM170 also failed to effect the expression of Cx40 within any of the Purkinje fibers analyzed (Figure 6E and F). Thus, over-expression of the truncated NKX2-5 mutant in developing conduction cells did not appear to disrupt normal levels of Cx40, suggesting that this mutant may not exert a dominant negative affect. We next investigated how the function of this Nkx2-5 mutant differed from wildtype Nkx2-5 using the in vitro model. Western blotting of these embryonic cardiomyocyte cultures confirmed the expression of Nkx2-5 protein following infection with constructs, AdNkx2-5HA (HA-tag immunoblot) and AdM170 (FLAG-tag immunoblot) (Figure 6H). Consistent with earlier results, AdNkx2-5HA induced up-regulation of Cx40 in comparison to control cultures in this experiment. By contrast, cells infected with AdM170, did not elicit a comparable effect, Cx40 remained low and similar to control levels. These data indicated that unlike wildtype Nkx2-5, the NKX2-5 M170 mutant was unable to increase Cx40 ectopically in cardiomyocytes in vivo or in vitro.
Figure 6. The Nkx2-5 M170 mutant does not transactivate Cx40.
GFP fluorescence and immunohistochemistry was used to analyze sections of chick myocardium infected with the NKX2-5 mutant M170 (AdM170). (A) Low magnification image of RV myocardium labeled with GFP and immunostained with Cx40 (TRITC). (B) Enlargement of boxed area in (A) revealing low level Cx40 expression. (C) Low magnification image of RV myocardium infected with AdNkxHA and immunostained with Cx40. (D) Enlargement of boxed area in (C) showing ectopic punctate Cx40 immunolabeling absent in the AdM170 infected myocardium above. Over expression of AdM170 does not disrupt Cx40 expression within PPFs. (E) This single PPF surrounding an artery is GFP positive (indicating mutant M170 NKX2-5 over-expression) while remaining Cx40 positive (TRITC) similar to neighboring PPFs, shown enlarged in F (arrow). A single cardiomyocyte within the myocardium is an over-expressor and does not display ectopic Cx40 immuno-positivity, enlarged in G (arrowhead). (H) Western blotting of cultured embryonic cardiomyocytes demonstrates that the NKX2-5 mutant M170 does not induce ectopic Cx40 expression, unlike AdNkxHA. HA and FLAG antibodies specifically detect tagged wildtype and mutant Nkx2-5 respectively. An antibody to MHC demonstrates cardiomyocytes are the protein source. GAPDH ensures equal protein loading. Scale bars 100 μm (A, C, E,) 50 μm (B, D, F, G).
DISCUSSION
In this study, we show that cardiac Purkinje fibers express at least 2-fold more Nkx2-5 in cell nuclei relative to adjacent working cardiomyocytes throughout differentiation. Moreover, using genes marking initial, intermediate and later stages of Purkinje fiber development it is demonstrated that this nuclear-localized Nkx2-5 signal increases progressively up until hatching. Constitutive over-expression of Nkx2-5 at the initiation of Purkinje fiber development disrupts this maturational sequence of gene expression, in particular inhibiting sMHC, a protein up-regulated at later phases of conduction cell differentiation. Finally, we show a mutant Nkx2-5 form, M170, known to cause cardiac disease in humans does not reciprocate the effects of over-expression of wildtype Nkx2-5, indicating that M170 may disrupt AVCS development by acting as a non-functional allele, reducing gene dosage via haploinsufficiency. Based on these data, we propose that elevated Nkx2-5 defines a population of cardiomyocytes actively differentiating into Purkinje fibers. Furthermore, we suggest that precise temporal regulation of Nkx2-5 level is necessary for normal differentiation of Purkinje fiber cells.
We, and others, have reported that Nkx2-5 is expressed at elevated levels in conduction tissues of the bird (Takebayashi-Suzuki et al., 2001; Thomas et al., 2001). Our earlier study also noted that the timing of increased Nkx2-5 in central and peripheral conduction cells corresponded with the sequence of cellular recruitment to these different AVCS tissues established by retroviral clonal analyses and birthdating studies (Gourdie et al., 1995; Cheng et al., 1999; Sedmera et al., 2003). Thus, in the present study an important advance is the ability to correlate temporal variation in Nkx2-5 immunolabeling to a sequence of protein markers defining stages of Purkinje fiber maturation. As we show here, by precociously up regulating Nkx2-5 levels we were able to modify this sequence of gene expression, thereby implicating the importance of strict temporal regulation of Nkx2-5 dosage to the progressive maturation of Purkinje fiber phenotype.
The initial induction of Purkinje fibers has been shown to be associated with factors secreted by endothelial cells, including endothelin and neuregulin, and may involve input by epigenetic mechanical factors such as shear stress and tissue strain (Gourdie et al., 1998; Rentschler et al., 2002; Sedmera et al., 2003; Patel and Kos, 2005). For peripheral Purkinje fibers in vivo, the timing of this inductive recruitment corresponds to the initiation of Cx40 expression at E12. We show here that the onset of Cx40 expression in Purkinje fibers, is marked by the coincident up-regulated expression of Nkx2-5 (quantified as a 2 fold induction). Given that Purkinje fibers subsequently undergo a progressive sequence of differentiation, apparently determined at least to some degree by increasing levels of nuclear-localized Nkx2-5, an interesting question is how is this process orchestrated. On the one hand, regulation of Nkx2-5 levels may be determined genetically. Certainly, the Cx40 promoter contains binding sites for Nkx2-5, Tbx5 and members of the GATA transcription factor family: the latter two being known binding partners of Nkx2-5 (Lee et al., 1998; Sepulveda et al., 1998; Hiroi et al., 2001; Sedmera et al., 2003). Nkx2-5 may transactivate Cx40 either alone or in association with Tbx5, another transcription critical to AVCS development (Hatcher et al., 2003; Moskowitz et al., 2004). However, what of the later-expressed markers of Purkinje fibers such as sMHC? As we demonstrate in our in vivo and in vitro models, sMHC is particularly sensitive to increase in exogenous Nkx2-5, and the maintenance of expression of sMHC in vivo appears to be associated with decreases in levels of nuclear-localized Nkx2-5. Ongoing work is necessary to determine whether Nkx2-5 down-regulation and sMHC up regulation in vivo occurs as a consequence of a predetermined genetic program or whether these changes occur in response to paracrine cues, as is probably the case in the initial induction of Purkinje fibers. Most likely a mechanism involving interplay between both genetically determined and inductive signaling processes orchestrate this differentiation.
While sMHC was sensitive to Nkx2-5 dosage, Cx40 levels in Purkinje fibers were not noticeably perturbed by over-expression of Nkx2-5 in vivo. The results furthermore indicate that although Nkx2-5 is sufficient to increase Cx40 in vitro in E3.5 cardiomyocytes, further elevation of Nkx2-5 after E10 in vivo will not drive Cx40 expression yet higher. These observations parallel results from our recent study in the Nkx2-5 heterozygous null mouse. While a reduction in Nkx2-5 dosage via haploinsufficiency resulted in a greater than 50% loss of Purkinje fibers in this mouse model, Cx40 within the residual conduction cells remained at quantifiably normal levels (Jay et al., 2004). Together, the data in chick and mouse suggest that once selection of Purkinje fiber fate has been initiated, incipient conduction cells become refractory to subsequent alterations in Nkx2-5 dosage, at least with respect to Cx40 expression. We would emphasize that the same paradigm does not appear to hold for Cx40-negative cardiomyocytes, which can be induced to up regulate Cx40 by Nkx2-5 over-expression. Whether the up regulation of Cx40 represents an initial step down the Purkinje fiber differentiation pathway or a transactivation of a single gene with no particular implications for the potential of cardiomyocytes to undergo subsequent differentiation into a conduction cell remains to be determined.
It is noteworthy that our observation of up regulated Cx40 in working cardiomyocytes in response to exogenous Nkx2-5 over-expression differs from the results of Kasahara and colleagues (Kasahara et al., 2001). These workers reported that over-expression of wildtype Nkx2-5 in adult mouse cardiomyocytes caused decreased expression of Cx43, and no detectable change in Cx40. This apparent disparity may reflect interspecies variation and/or changes in the competence of different-staged cardiomyocytes to respond to Nkx2-5. It is of pertinence here that Nkx2-5 has been shown to be rapidly up-regulated in working myocardial tissues in a mammalian model of hypertrophy (Thompson et al., 1998). Elevated levels of Nkx2-5 have also been detected in the ventricle following stimulation of hypertrophy by adrenergic agonists (Saadane et al., 1999). As such, the ectopic up-regulation of Nkx2-5 in the working myocardium that we model in the embryonic chick may be an aspect of certain pathologies of the mature heart. Interestingly, Cx40 up regulation has also been observed within the myocardium of humans with congestive heart failure (Dupont et al., 2001b) and increased Cx40 levels are associated with predisposition to atrial fibrillation (Dupont et al., 2001a). Although these studies did not assay Nkx2-5, our data showing that over-expression of this transcription factor is sufficient to up regulate Cx40 expression in working cardiomyocytes in vivo, suggests the possibility of relationships between these two proteins in the diseased myocardium.
A second aspect of this study with implications for cardiac disease processes relates to the use of our in vitro and in vivo models to assay the function of known NKX2-5 mutants. Over thirty NKX2-5 mutations have been identified in humans that result in functional and structural cardiac abnormalities including AV block (Schott et al., 1998; Benson et al., 1999; Goldmuntz et al., 2001; Gutierrez-Roelens et al., 2002; Ikeda et al., 2002; Watanabe et al., 2002; McElhinney et al., 2003). The presence of AV block is especially associated with mutations involving the homeodomain of Nkx2-5 (Benson et al., 1999; Gutierrez-Roelens et al., 2002). One such mutant is the M170 variant in which the mutated NKX2-5 locus expresses a carboxy-terminal truncation at the level of homeodomain. Unlike wildtype Nkx2-5, over-expression of the M170 truncation mutant in our in vitro and in vivo models of Purkinje fiber differentiation did not appear to disrupt Cx40 or sMHC expression patterns. In both models, endogenous wildtype Nkx2-5 alleles were present, indicating that the M170 mutant is not exerting a dominant negative affect. As such, we would conclude that the Nkx2-5 M170 protein is probably a null mutation and results in loss of Nkx2-5 function via haploinsufficiency. Other recent studies in mice have pointed to the importance of Nkx2-5 gene dosage in AVCS development (Jay et al., 2004; Pashmforoush et al., 2004). Here, further novel insight is provided with the demonstration that elevation and subsequent controlled increases in Nkx2-5 level are required for normal Purkinje fiber differentiation. This requirement for precise spatiotemporal regulation of Nkx2-5 is likely to have implications in the development of therapeutic strategies designed to ameliorate the effects of Nkx2-5 mutation on AVCS function.
EXPERIMENTAL PROCEDURES
Adenovirus vector construction and infection of isolated cardiomyocytes
The pAd-Track shuttle using the method of Vogelstein and the pAd Easy-1 adenoviral vector system (Stratagene, USA) was used to clone human wildtype Nkx2-5 and a known Gln170ter Nkx2-5 truncation mutant cDNA, termed AdM170 as previously described (He et al., 1998). Nkx2-5 M170 was cloned in a similar fashion (Schott et al., 1998). AdGFP, an adenovirus expressing GFP driven by the CMV promoter, was a kind gift of Larry Rothblum’s laboratory.
Isolated embryonic cardiomyocytes received AdNkxHA, AdM170 or AdGFP at a multiplicity of infection (MOI) of 10. GFP was the marker of infection and expression was confirmed using immunocytochemistry and Western blotting (HA tag and FLAG). Data shown is representative of multiple independent experimental preparations of primary cardiomyocytes.
Shell-less chick culture preparations and microinjections
Chick eggs were incubated to E2 and then cracked into polystyrene dishes (Fisher Scientific, USA), (Dunn, 1974). These were placed into Petri dishes containing sterile water to maintain humidity. Chicks were maintained at 38.5°C until E10. The hearts of these embryos were visualized and targeted by direct trans-thoracic microinjection using a Leica dissection microscope and Pico Pulser apparatus inside a glove box. Injection volumes were 5 and 10 μl of vehicle control (PBS) or high titer adenoviral vectors (1 × 106 PFU). Following microinjection, shell-less embryos received an eggshell supplement to the albumin to sustain developmental calcium requirements. Shell-less chick preparations were further cultured up to and including E19. Finally the embryos were euthanized by decapitation. The chest cavities were opened and the chicks placed into 4% paraformaldehyde for 2 hours. Following 3 × 30 minute PBS washes the chicks were examined with a fluorescent dissection microscope for GFP expression in the heart and whole mount images captured. Adenoviral infected hearts were then cryoprotected and processed for frozen sectioning.
Embryonic chick cardiomyocyte cultures
Cardiomyocytes were isolated from the ventricles of E3 chicken embryos as detailed previously (Gourdie et al., 1998). Cardiomyocytes were dissociated using a low concentration of trypsin (0.1%) for 10 minutes prior to plating on slides or Petri dishes. Cultures were maintained in medium M199 (Gibco, Grand Island, NY) supplemented with penicillin and streptomycin, 1% chick serum, and 1:100 of insulin, transferrin and selenium. Cells were allowed to attach for 12 hours, before the addition of media containing adenoviral constructs. After a further 12 hours, this media was exchanged for fresh media. Cultures were incubated for up to 5 days at 37°C with 5% CO2 and were terminated by fixing with 4% paraformaldehyde (immunocytochemistry) or by scraping for Western blotting.
Protein extraction and Western blotting
Isolated cardiomyocytes were homogenized ultrasonically in protein extraction buffer as previously described (Muller et al., 2002). Normally triplicate samples were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions. Separated proteins were transferred to nitrocellulose membrane, Hybond-N, in gel transfer tanks (Biorad, CA, USA). Non-specific binding sites were blocked for 1 hour using 10% fat-free milk powder and 1% BSA in TBST (10mM Tris, 100mM NaCl, 0.1% Tween 20) at 4°C. Antibodies included anti-HA, anti-FLAG (Sigma, USA) and anti-GAPDH (Research Diagnostics Inc, USA). Antibodies were incubated overnight at 4°C in 1% fat free milk powder TBST, at appropriate concentrations. Blots were washed in TBST, 6 × 5 minutes at room temperature. Secondary antibodies, directed against primary immunoglobulins, conjugated to horseradish peroxidase (HRP) were used at, 1:2000 in TBST, for 1 hour at room temperature. Blots were finally washed in TBST 6 × 5 minutes at room temperature, before being developed for 1 minute using the substrate Western Lightning Chemiluminescence Reagent Plus (NEN Life Science Products, USA) as per manufacturer’s protocol. Blots were covered in cling film and visualized by exposure to Kodak BioMax Mr-1 film developed from 45 seconds to 10 minutes later in an X-Ograph Compact X2 developer.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (Real Time RT-PCR)
Total RNA was extracted from embryonic cardiomyocyte cultures using Ultraspec RNA total RNA isolation agent. Following RNA isolation, the RNA was resuspended in a 1mM concentration of MgCl2. RQ1 DNase was added to the samples, which were incubated at 37°C for 20–30 minutes to remove DNA from the reactions. The DNase was then heat inactivated for 5 minutes at 90°C. RNA concentration was determined by spectroscopy and analyzed for integrity of the RNA by agarose gel electrophoresis under denaturing conditions. Equal amounts of RNA, equivalent to 250ng total RNA from the various samples were used for RT-PCR together with a water control with oligonucleotide primer pairs. PCR was performed in 25μl reaction volumes containing 20mM Tris-HCl, pH 8.4, 50mM KCl, 1.5mM MgCl2, 0.2mM dNTPs, 25pmoles of each primer and 2.5 units Taq DNA-polymerase. The reaction conditions for each primer pair were optimized with respect to MgCl2 concentration, annealing temperature, extension time and cycle number. The primers used were as follows: Cx40 For- GGAGGAGAAGAGAAAGATGAAG, Rev- TCCCAGCAAGACAACTCAG, sMHC For- GCGGGAAGAGCAGGCAGAG Rev- TCACACGAGGATGGAGCAAGC, 18S For- TATGGTTCCTTTGGTCGCTC Rev- GGTTGGTTTTGATCTGATCTGATAAAT.
To quantify mRNA levels PCR reactions were performed in a Biorad Icycler in combination with a Quantitect SYBR Green RT-PCR detection kit (Qiagen, USA). PCR conditions were as follows: 1 RT-PCR step of 45 mins, 1 Polymerase activation step of 15 mins, followed by 35 cycles of a 15 sec at 94°C (denaturation), b 30 sec at 60°C (annealing) and c 30 sec at 72°C (elongation). A melt curve analysis was used to determine specific target amplification. Upon completion gel loading buffer (10x) was added to each sample and 10μl of each reaction was electrophoresed on 2% agarose gels including ethidium bromide in 1x Tris Acetate EDTA (TAE) buffer in a Horizon 58 gel apparatus (BRL-Life Technologies, Gaithersburg, MD). Loading buffer consisted of 0.5M EDTA, pH 7.5, 10% SDS, 50% Glycerol and 0.25% bromophenol blue. Electrophoresed bands were visualized on a UVP dual intensity transilluminator and documented using Polaroid photography. Data was first normalized to 18S levels and then the DDCT method used to analyze samples, where control values were set to 1. All experiments were repeated in triplicate and carried out on multiple experiments as detailed in figure legends.
Multilabel immunoconfocal microscopy
Frozen sections of 8 micron thickness were blocked for 1 hour at room temperature and incubated with antibodies overnight at 4°C at concentrations determined to give the best signal to noise ratio. Primary antibodies; anti-mouse Connexin 40 (Chemicon, USA), anti-human Nkx2-5 and anti-GATA4 (Santa-Cruz Biotechnology Inc, CA, USA), anti-sMHC (Ald-58)(Gonzalez-Sanchez and Bader, 1985), anti-MHC (MF20) (Bader et al., 1982), anti-transitin (EAP300) (McCabe et al., 1995, Gourdie et al., 1995), and anti-HA. Following 3 × 5 minute washes in PBS, specific fluorochrome conjugated secondary antibodies were applied. These included anti-rabbit Alexa 488, Alexa 555 (Molecular Probes, USA), anti-rabbit Cy5, anti-mouse FITC, anti-mouse TRITC (Chemicon, USA), anti-goat TRITC (Jackson ImmunoResearch inc, USA). Nuclei counter stains were Hoechst 33258 (Sigma, USA), DRAQ5 (Biostatus LTD, UK) and To-Pro-3 (Molecular Probes, USA).
Immunolabeled slides were viewed using either a Zeiss Axioskop epi-fluorescence light microscope or a TCS Laser-Scanning Confocal microscope (Leica, Germany). Images were imported into NIH image, and Photoshop 7.0 using an Apple Macintosh G4. For image quantitations all settings were standardized on the confocal microscope and in NIH image.
Acknowledgments
We thank Dr. David Sedmera, Dr. Tom Trusk, and Mr. T. Gallien for their insight and assistance.
This work is supported by NIH Grants HL56728 (RGG), HL36059 (RGG), and HD39946 (RGG, TXO, DWB), by Merit and Reap awards from the Research and Development Service of the Department of Veteran Affairs (TXO), by NIH Grant P20RR016434 from the National Center for Research Resources (BSH) and by American Heart Association Grant 0425546U (BSH).
References
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson MC, Watson MS, Seidman JG, Seidman CE, Plowden J, Kugler JD. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104:1567–1573. doi: 10.1172/JCI8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- Delorme B, Dahl E, Jarry-Guichard T, Marics I, Briand JP, Willecke K, Gros D, Theveniau-Ruissy M. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev Dyn. 1995;204:358–371. doi: 10.1002/aja.1002040403. [DOI] [PubMed] [Google Scholar]
- Dunn BE. Technique of shell-less culture of the 72-hour avian embryo. Poult Sci. 1974;53:409–412. doi: 10.3382/ps.0530409. [DOI] [PubMed] [Google Scholar]
- Dupont E, Ko Y, Rothery S, Coppen SR, Baghai M, Haw M, Severs NJ. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001a;103:842–849. doi: 10.1161/01.cir.103.6.842. [DOI] [PubMed] [Google Scholar]
- Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001b;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- Evans SM. Vertebrate tinman homologues and cardiac differentiation. Semin Cell Dev Biol. 1999;10:73–83. doi: 10.1006/scdb.1999.0282. [DOI] [PubMed] [Google Scholar]
- Goldmuntz E, Geiger E, Benson DW. NKX2.5 mutations in patients with tetralogy of fallot. Circulation. 2001;104:2565–2568. doi: 10.1161/hc4601.098427. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sanchez A, Bader D. Characterization of a myosin heavy chain in the conductive system of the adult and developing chicken heart. J Cell Biol. 1985;100:270–275. doi: 10.1083/jcb.100.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res. 1993;72:278–289. doi: 10.1161/01.res.72.2.278. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, Justus C, Hewett KW, O’Brien TX, Thompson RP, Sedmera D. Development of the cardiac pacemaking and conduction system. Birth Defects Res Part C Embryo Today. 2003;69:46–57. doi: 10.1002/bdrc.10008. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Wei Y, Kim D, Klatt SC, Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc Natl Acad Sci U S A. 1998;95:6815–6818. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grow MW, Krieg PA. Tinman function is essential for vertebrate heart development: elimination of cardiac differentiation by dominant inhibitory mutants of the tinman-related genes, XNkx2-3 and XNkx2-5. Dev Biol. 1998;204:187–196. doi: 10.1006/dbio.1998.9080. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Roelens I, Sluysmans T, Gewillig M, Devriendt K, Vikkula M. Progressive AV-block and anomalous venous return among cardiac anomalies associated with two novel missense mutations in the CSX/NKX2-5 gene. Hum Mutat. 2002;20:75–76. doi: 10.1002/humu.9041. [DOI] [PubMed] [Google Scholar]
- Harris BS, O’Brien TX, Gourdie RG. Coronary arteriogenesis and differentiation of periarterial Purkinje fibers in the chick heart: is there a link? Tex Heart Inst J. 2002;29:262–270. [PMC free article] [PubMed] [Google Scholar]
- Hatcher CJ, Diman NY, McDermott DA, Basson CT. Transcription factor cascades in congenital heart malformation. Trends Mol Med. 2003;9:512–515. doi: 10.1016/j.molmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R, Komuro I. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat Genet. 2001;28:276–280. doi: 10.1038/90123. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Hiroi Y, Hosoda T, Utsunomiya T, Matsuo S, Ito T, Inoue J, Sumiyoshi T, Takano H, Nagai R, Komuro I. Novel point mutation in the cardiac transcription factor CSX/NKX2.5 associated with congenital heart disease. Circ J. 2002;66:561–563. doi: 10.1253/circj.66.561. [DOI] [PubMed] [Google Scholar]
- Jay PY, Harris BS, Maguire CT, Buerger A, Wakimoto H, Tanaka M, Kupershmidt S, Roden DM, Schultheiss TM, O’Brien TX, Gourdie RG, Berul CI, Izumo S. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130–1137. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Wakimoto H, Liu M, Maguire CT, Converso KL, Shioi T, Huang WY, Manning WJ, Paul D, Lawitts J, Berul CI, Izumo S. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J Clin Invest. 2001;108:189–201. doi: 10.1172/JCI12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese RJ, Markham BE, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- McCabe CF, Gourdie RG, Thompson RP, Cole GJ. Developmentally regulated neural protein EAP-300 is expressed by myocardium and cardiac neural crest during chick embryogenesis. Dev Dyn. 1995;203:51–60. doi: 10.1002/aja.1002030106. [DOI] [PubMed] [Google Scholar]
- McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol. 2003;42:1650–1655. doi: 10.1016/j.jacc.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Development of the cardiac conduction system: a matter of chamber development. Novartis Found Symp. 2003;250:25–34. discussion 34–43, 276–279. [PubMed] [Google Scholar]
- Moskowitz IP, Pizard A, Patel VV, Bruneau BG, Kim JB, Kupershmidt S, Roden D, Berul CI, Seidman CE, Seidman JG. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107–4116. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- Muller JG, Thompson JT, Edmonson AM, Rackley MS, Kasahara H, Izumo S, McQuinn TC, Menick DR, O’Brien TX. Differential regulation of the cardiac sodium calcium exchanger promoter in adult and neonatal cardiomyocytes by Nkx2.5 and serum response factor. J Mol Cell Cardiol. 2002;34:807–821. doi: 10.1006/jmcc.2002.2019. [DOI] [PubMed] [Google Scholar]
- Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- Patel R, Kos L. Endothelin-1 and Neuregulin-1 convert embryonic cardiomyocytes into cells of the conduction system in the mouse. Dev Dyn. 2005;233:20–28. doi: 10.1002/dvdy.20284. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Rentschler S, Gourdie RG, Fishman GI, Mikawa T. Induction and patterning of the cardiac conduction system. Int J Dev Biol. 2002;46:765–775. [PubMed] [Google Scholar]
- Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadane N, Alpert L, Chalifour LE. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol. 1999;127:1165–1176. doi: 10.1038/sj.bjp.0702676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, Thompson RP. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec. 2003;274A:773–777. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- Sepulveda JL, Belaguli N, Nigam V, Chen CY, Nemer M, Schwartz RJ. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Mol Cell Biol. 1998;18:3405–3415. doi: 10.1128/mcb.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi-Suzuki K, Pauliks LB, Eltsefon Y, Mikawa T. Purkinje fibers of the avian heart express a myogenic transcription factor program distinct from cardiac and skeletal muscle. Dev Biol. 2001;234:390–401. doi: 10.1006/dbio.2001.0270. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Kasahara H, Edmonson AM, Izumo S, Yacoub MH, Barton PJ, Gourdie RG. Elevated expression of Nkx-2.5 in developing myocardial conduction cells. Anat Rec. 2001;263:307–313. doi: 10.1002/ar.1106. [DOI] [PubMed] [Google Scholar]
- Thompson JT, Rackley MS, O’Brien TX. Upregulation of the cardiac homeobox gene Nkx2-5 (CSX) in feline right ventricular pressure overload. Am J Physiol. 1998;274:H1569–1573. doi: 10.1152/ajpheart.1998.274.5.H1569. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Benson DW, Yano S, Akagi T, Yoshino M, Murray JC. Two novel frameshift mutations in NKX2.5 result in novel features including visceral inversus and sinus venosus type ASD. J Med Genet. 2002;39:807–811. doi: 10.1136/jmg.39.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]