Abstract
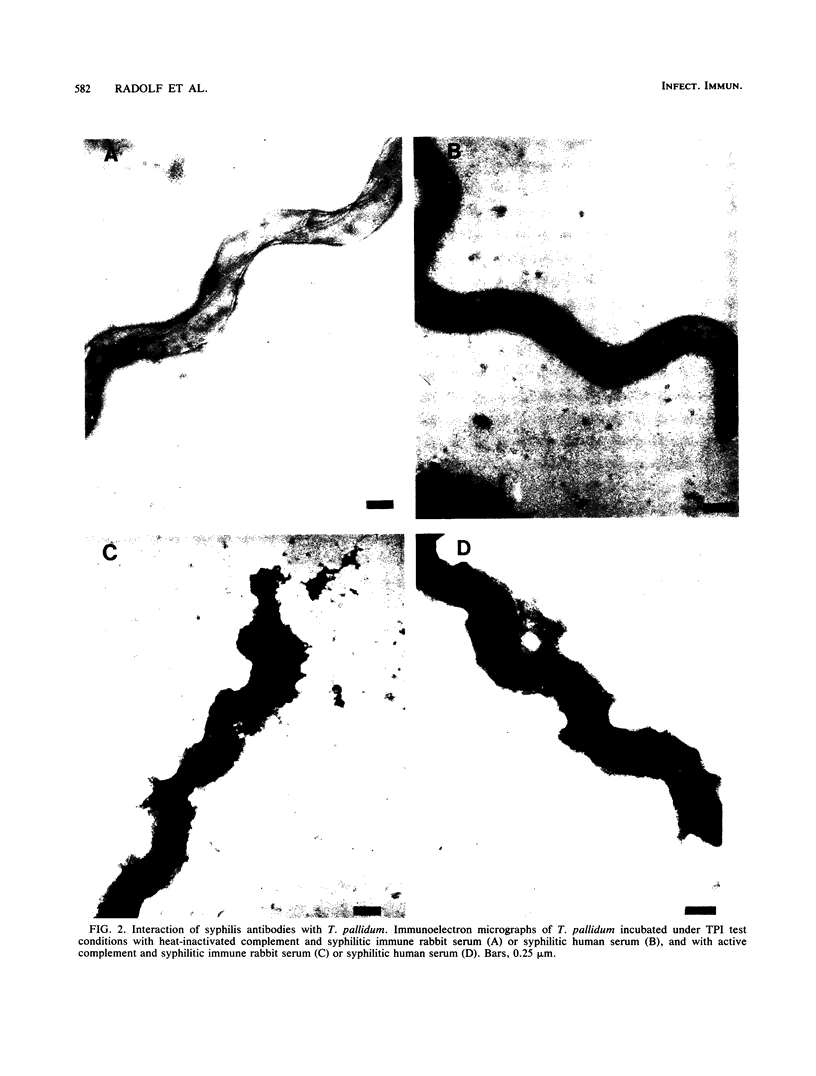
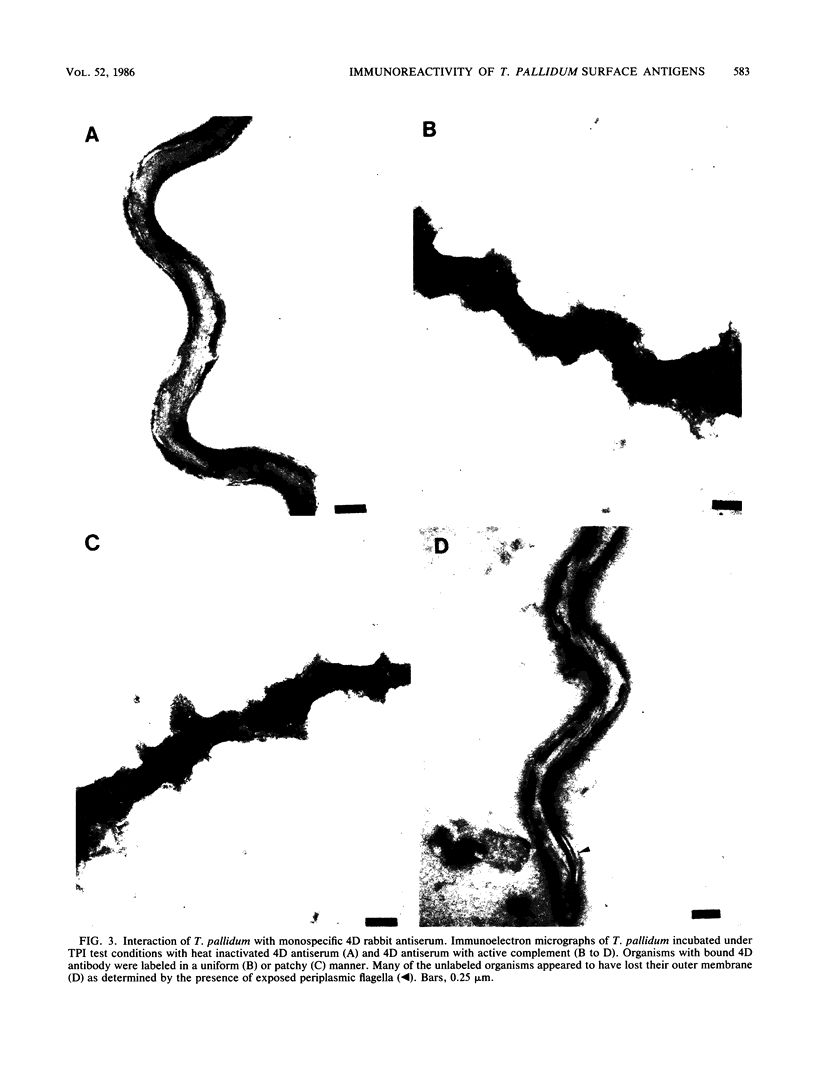
The binding of immunoglobulin G present in syphilitic immune rabbit serum, syphilitic human serum, and rabbit antiserum to purified recombinant Treponema pallidum antigen 4D by T. pallidum, Nichols strain, was studied by immunoelectron microscopy. Treponemes were incubated with antiserum under the conditions of the T. pallidum immobilization test, in which T. pallidum-specific antibody renders the organism nonmotile and avirulent only in the presence of complement after a 16-h incubation period in an anaerobic environment. Antibody was not demonstrable on the surface of T. pallidum incubated with nonimmune rabbit serum or normal human serum in the presence of complement. Similarly, in the absence of complement, little or no antibody was found on the treponemal surface after incubation with syphilitic immune rabbit serum, syphilitic human serum, or rabbit antiserum directed against the recombinant 4D antigen. The addition of complement to syphilitic immune rabbit serum, syphilitic human serum, and anti-4D antibody resulted in immobilization and the deposition of antibody on the entire surface of the immobilized organisms. These results corroborate earlier work by other investigators demonstrating the resistance of freshly isolated T. pallidum to antibody binding in a variety of serological tests. Detection of 4D antigen on the surface of immobilized T. pallidum strongly implies that the use of T. pallidum immobilization test conditions provides a means to demonstrate the association of individual surface antigens on virulent T. pallidum. The resistance of T. pallidum to antibody binding may be relevant to the pathogenesis of syphilis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Blanco D. R., Miller J. N., Hanff P. A. Humoral immunity in experimental syphilis: the demonstration of IgG as a treponemicidal factor in immune rabbit serum. J Immunol. 1984 Nov;133(5):2693–2697. [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Lovett M. A. Properties of an ordered ring structure formed by recombinant Treponema pallidum surface antigen 4D. J Bacteriol. 1986 Mar;165(3):732–739. doi: 10.1128/jb.165.3.732-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Walfield A. M., Cunningham T. M., Miller J. N., Lovett M. A. Native surface association of a recombinant 38-kilodalton Treponema pallidum antigen isolated from the Escherichia coli outer membrane. Infect Immun. 1986 May;52(2):586–593. doi: 10.1128/iai.52.2.586-593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Peacock M. G., Hitchcock P. J., Cole R. L. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985 May;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hovind-Hougen K., Birch-Andersen A., Nielsen H. A. Electron microscopy of treponemes subjected to the Treponema pallidum immobilization (TPI) test. II. Immunoelectron microscopy. Acta Pathol Microbiol Scand C. 1979 Aug;87C(4):263–268. [PubMed] [Google Scholar]
- Hovind-Hougen K., Nielsen H. A., Birch-Andersen A. Electron microscopy of treponemes subjected to the Treponema pallidum immobilization (TPI) test. I. Comparison of immunoimmobilized cells and control cells. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):217–222. [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER M., RUCZKOWSKA J. INFLUENCE OF LYSOZYME UPON THE REACTIVITY OF TREPONEMA PALLIDUM IN THE FLUORESCENT ANTIBODY REACTION. Arch Immunol Ther Exp (Warsz) 1964;12:702–708. [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Michalska E., Podwińska J., Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969 Dec;45(4):299–304. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr, MAYER M. M. Immobilization of Treponema pallidum in vitro by antibody produced in syphilitic infection. J Exp Med. 1949 Apr 1;89(4):369–393. doi: 10.1084/jem.89.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J. In vitro cultivation of Treponema pallidum: independent confirmation. Infect Immun. 1982 Apr;36(1):437–439. doi: 10.1128/iai.36.1.437-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Sell S. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J Immunol. 1984 Nov;133(5):2686–2692. [PubMed] [Google Scholar]
- Penn C. W. Avoidance of host defences by Treponema pallidum in situ and on extraction from infected rabbit testes. J Gen Microbiol. 1981 Sep;126(1):69–75. doi: 10.1099/00221287-126-1-69. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Rhodes J. G. Surface-associated antigens of Treponema pallidum concealed by an inert outer layer. Immunology. 1982 May;46(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale J. D., Kellogg D. S., Jr, Miller C., Schammel P., Thayer J. D. Separation of Treponema pallidum from tissue debris through continuous-particle electrophoresis. Appl Microbiol. 1970 Feb;19(2):287–289. doi: 10.1128/am.19.2.287-289.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Folds J. D., Bassford P. J., Jr Expression of Treponema pallidum antigens in Escherichia coli K-12. Infect Immun. 1982 Jun;36(3):1238–1241. doi: 10.1128/iai.36.3.1238-1241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Ultrastructural studies of treponemes: location of axial filaments and some dimensions of Treponema pallidum (Nichols strain), Treponema denticola, and Treponema reiteri. Infect Immun. 1973 Jan;7(1):100–110. doi: 10.1128/iai.7.1.100-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]