Abstract
We recently reported that urinary excretion rates of angiotensinogen provide a specific index of the intrarenal renin-angiotensin system status in angiotensin II-dependent hypertensive rats. Angiotensinogen concentrations in mouse plasma are thought to be much lower than those in rat plasma; however, detailed information is deficient due to lack of direct quantitative measurements of rodent angiotensinogen. To elucidate this issue, we have developed a quantitative method for measurement of rodent angiotensinogen using a sandwich-type ELISA. The standard curve for mouse and rat angiotensinogen exhibited a high linearity at 0.16–10 and 0.08–5 ng/ml, respectively, with correlation coefficients >0.99. While plasma angiotensinogen concentrations of male high serum IgA (HIGA) mice (IgA nephritis model animals, 1,308 ± 47 ng/ml; n = 10) were lower than those of control BALB/c mice (1,620 ± 384; n = 12), urinary angiotensinogen concentrations of HIGA mice (14.6 ± 1.5 ng/ml; n = 34) were higher than those of BALB/c mice (4.6 ± 0.1; n = 2). In a similar manner, while plasma angiotensinogen concentrations of Zucker diabetic fatty (ZDF) obese rats (type 2 diabetic model animals, 1,789 ± 50 ng/ml; n = 5) were lower than those of control ZDF lean rats (2,296 ± 47; n = 5), urinary angiotensinogen concentrations of ZDF obese rats (88.2 ± 11.4 ng/ml; n = 15) were higher than those of ZDF lean rats (31.3 ± 1.9; n = 15). These data indicate that plasma and urinary angiotensinogen concentrations are less in mice than rats. However, these data suggest that urinary angiotensinogen levels are different from plasma angiotensinogen levels in rodents. The development of rodent angiotensinogen ELISA allows quantitative comparisons in mouse and rat angiotensinogen levels in models of hypertension and cardiovascular and kidney diseases.
Keywords: enzyme-linked immunosorbent assay, renin-angiotensin system, plasma, urine, mouse, rat
The Renin-Angiotensin System (RAS) plays an important role in blood pressure regulation and fluid and electrolyte homeostasis (27). In recent years, the focus of interest on the RAS has shifted to a main emphasis on the role of the local/tissue RAS in specific tissues (9). Emerging evidence demonstrates the importance of the tissue RAS in the brain (1), heart (8), adrenal glands (24), vasculature (5, 10), as well as the kidneys (17). There is substantial evidence that the major fraction of ANG II present in renal tissues is generated locally from angiotensinogen delivered to the kidney as well as from angiotensinogen locally produced by proximal tubule cells (6, 11, 26, 31). Renin secreted by the juxtaglomerular apparatus cells into the renal interstitium and vascular compartments also provides a pathway for the local generation of ANG I (25). The ANG-converting enzyme is abundant in the rat kidney and is present in proximal and distal tubules, the collecting ducts, and renal endothelial cells (4). ANG I delivered to the kidney can also be converted to ANG II (22). Therefore, all of the components necessary to generate intrarenal ANG II are present along the nephron (17, 27).
The presence of all the components of the RAS in the kidney provides great flexibility in regulating intrarenal levels of ANG II independent from circulating levels of ANG II (28). Recently, we reported (13–15, 18, 21) that urinary excretion rates of angiotensinogen provide a specific index of intrarenal RAS status in ANG II-dependent hypertensive rats. However, in those studies (13–15, 18, 21), angiotensinogen levels were measured by conversion assay. This conversion assay requires three steps. First, samples are incubated with and without exogenous renin. Then, ANG I concentrations in paired test tubes are measured by RIA. Finally, converted ANG I is calculated as the difference between paired ANG I concentrations. Therefore, this conversion assay needs time-consuming procedures (~2 days), indirect measurements, radioisotopes, and purified renin that is expensive and unobtainable. The direct quantitative assessment of rodent angiotensinogen would be useful for measurements of plasma and urine samples. Angiotensinogen concentrations in mouse plasma have been reported (2, 3, 7, 23) to be much lower than those in rat plasma. However, detailed information is lacking due to the previous unavailability of direct quantitative measurements of rodent angiotensinogen. To elucidate these issues, we have developed a direct quantitative method to measure the plasma and urinary angiotensinogen of mice and rats using a microtiterplate-based sandwich-type ELISA. In contrast to the aforementioned conversion assay, the newly developed ELISA does not need time-consuming procedures (~3 h), indirect measurements, radioisotopes, or purified renin. Moreover, with the use of this ELISA, plasma and urinary angiotensinogen levels were assessed in a couple of experimental animal models to investigate whether urinary angiotensinogen levels are controlled differently from plasma angiotensinogen levels in mice and rats.
MATERIALS AND METHODS
Sample collection
The experimental protocol was approved by the Animal Care and Use Committee of Tulane University. Male C57BL/6 mice, Wistar rats, Zucker diabetic fatty (ZDF) obese rats (type 2 diabetic model animals), and the corresponding control ZDF lean rats were purchased from Charles River Laboratories. Male high serum IgA (HIGA) mice (IgA nephritis model animals) and the corresponding control BALB/c mice were purchased from Japan SLC. Trunk blood samples were collected into centrifuge tubes with EDTA after animals were decapitated. Urine samples were collected into centrifuge tubes using metabolic cages. After a centrifugation, plasma and urine samples were transferred and stored at −20°C until assayed. Some kidneys were removed and dropped in zinc-saturated formalin for tissue fixation.
Antibody preparation
We raised two lines of polyclonal antibodies in rabbits against synthetic oligopeptides corresponding to the N terminus of rodent angiotensinogen (AA: 135–150) and to the C terminus of rodent angiotensinogen (AA: 405–420), respectively. Both antibodies were affinity purified.
Western blot
Western blot analysis was performed as described previously (12, 19, 29) using LI-COR Odyssey infrared imaging system.
Immunohistochemistry
Immunohistochemistry was performed as described previously (16, 20, 30) using Dako Autostainer robotic system.
Plate preparation
The ELISA plates were coated with the N-terminal antibody as the amount of 100 μl/well in 100 mmol/l carbonate buffer (pH 9.5) at 4°C for overnight. The plates were washed with PBS and blocked with 200 μl/well of 1% BSA in PBS containing 0.05% NaN3 at 4°C for overnight. The plates were stored at 2–8°C until use.
Expression vectors
The full-length, except for the signal peptide (AA: 1–24), of the mouse angiotensinogen (AA: 25–477) gene (accession number: NM_007428) was amplified by PCR using a high fidelity Pfu DNA polymerase (Promega) with sense primer (5′-CCG CTC GAG gac cgc gta tac atc cac ccc-3′) and antisense primer (3′-AAG AAT GCG GCC GCc acc aca ctc tgg ggg tta ttc-5′) from the mouse liver cDNA library (Clontech). Then, this fragment was inserted into pGEX4T1 expression vectors (Promega) with six consecutive histidine residues (6-His) tag at the 5′-end by restriction enzymes of Xho I and Not I.
In a similar manner, the full-length, except for the signal peptide (AA: 1–24), of the rat angiotensinogen (AA: 25–477) gene (accession number: NM_134432) was amplified by PCR using the Pfu DNA polymerase with sense primer (5′-CGG AAT TCg acc gcg tat aca tcc acc cc-3′) and antisense primer (3′-CCG CTC GAG cac cac att ttg ggg gtt atc c-5′) from the rat liver cDNA library (Clontech). Then, this fragment was inserted into pGEX4T1 expression vectors with glutathione S-transferase (GST) tag at the 5′-end by restriction enzymes of EcoR I and Xho I.
Recombinant proteins
The recombinant constructs were transformed into a high-efficiency expression bacterial strain (Takara). Large-scale bacterial cultures were induced with isopropyl-beta-D-thiogalactopyranoside (Takara) and harvested for protein purification. 6-His-tagged proteins and GST-tagged proteins were purified using purification kits (Upstate) at native conditions.
Development of sandwich ELISA
Highly purified recombinant proteins of rodent angiotensinogen were used as the standards. One hundred microliters per well of standard samples of rodent angiotensinogen (0.16–10 ng/ml for mouse angiotensinogen or 0.08–5.0 ng/ml for rat angiotensinogen diluted in ELISA buffer), plasma (1:2,500 for mice and rats diluted in ELISA buffer), and urine (1:25 for mice and rats diluted in ELISA buffer) samples were added into each well of the plates and incubated at 37°C for 1 h. After the incubation, the plates were washed with a washing buffer (PBS containing Tween-20, 0.05%, pH 7.5) for a total of seven times. In the following step, the plates were incubated with the 100 μl/well of the horseradish peroxidase-labeled C-terminal antibody (1:30 diluted in antibody solution) at 37°C for 30 min. After the incubation, the plates were washed with the washing buffer for a total of nine times. In the next steps, the plates were incubated with the 100 μl/well of 3,3′,5,5′-tetramethylbenzidine solution under light-protected conditions at room temperature for 30 min. Finally, the plates were applied with the 100 μl/well of sulfuric acid (0.5 mol/l) to stop the reaction. The absorbance values were measured at 450 nm.
Statistical analysis
Statistical analysis was performed using a one-way factorial ANOVA with post hoc Scheffé’s F-test. All data are means ± SE. P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Developed antibodies
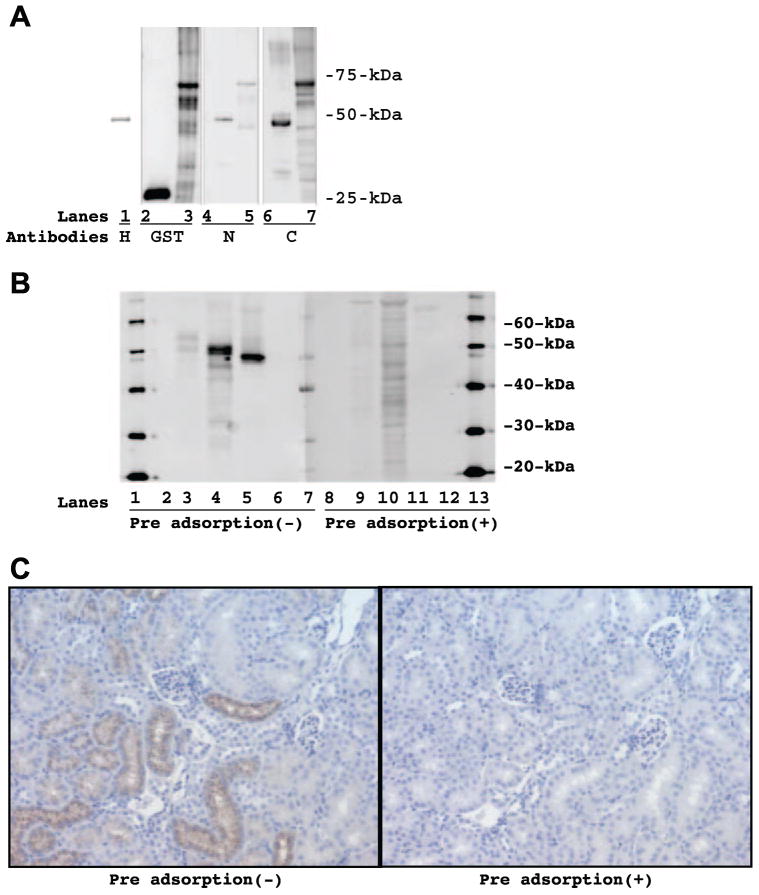
As illustrated in Fig. 1A, an equal amount (10 ng) of various recombinant proteins (lanes 1, 4, and 6: 6-His-tagged mouse angiotensinogen; lane 2: GST; and lanes 3, 5, and 7: GST-tagged rat angiotensinogen) was blotted on a nitrocellulose membrane and Western blot was done with 6-His antibody (lane 1), GST antibody (lanes 2 and 3), rodent angiotensinogen N-terminal antibody (lanes 4 and 5), or rodent angiotensinogen C-terminal antibody (lanes 6 and 7). The 6-His antibody recognized 6-His-tagged recombinant mouse angiotensinogen at 48 kDa (lane 1). The GST antibody recognized recombinant GST at 25 kDa (lane 2) and GST-tagged recombinant rat angiotensinogen at 72 kDa (lane 3). The rodent angiotensinogen N-terminal antibody recognized 6-His-tagged recombinant mouse angiotensinogen at 48 kDa (lane 4) and GST-tagged recombinant rat angiotensinogen at 72 kDa (lane 5). The rodent angiotensinogen C-terminal antibody recognized 6-His-tagged recombinant mouse angiotensinogen at 48 kDa (lane 6) and GST-tagged recombinant rat angiotensinogen at 72 kDa (lane 7). Calculated molecular sizes of 6-His-tag, GST-tag, recombinant mouse angiotensinogen, and recombinant rat angiotensinogen are 1, 25, 47, and 47 kDa, respectively. Therefore, both of developed antibodies recognized recombinant rodent (mouse and rat) angiotensinogen as expected.
Fig. 1.
A: an equal amount (10 ng) of various recombinant proteins [lanes 1, 4, and 6: 6 consecutive histidine residues (6-His)-tagged mouse angiotensinogen; lane 2: glutathione S-transferase (GST); and lanes 3, 5, and 7: GST-tagged rat angiotensinogen] was blotted on a nitrocellulose membrane and Western blot was done with 6-His antibody (lane 1), GST antibody (lanes 2 and 3), rodent angiotensinogen N-terminal antibody (lanes 4 and 5), or rodent angiotensinogen C-terminal antibody (lanes 6 and 7). B, left: extracted protein from kidney of Zucker diabetic fatty (ZDF) obese rat (25 μg on lane 3), extracted protein from kidney of high serum IgA (HIGA) mice (15 μg on lane 4), and 6-His-tagged recombinant mouse angiotensinogen (10 ng on lane 5) were blotted on nitrocellulose membranes and Western blot was done with the C-terminal antibody. B, right: corresponding signals were completely eliminated or reduced to a large extent (lane 9 for ZDF obese rat, lane 10 for HIGA mice, and lane 11 for 6-His-tagged recombinant mouse angiotensinogen) when the C-terminal antibody was preadsorbed with the synthetic oligopeptide corresponding to the C terminus of rodent angiotensinogen (AA: 405–420) for overnight. Lanes 2, 6, 8, and 12 were blank. Lanes 1, 7, and 13 were molecular size markers. C, left: immunohistochemistry demonstrated that angiotensinogen was predominantly localized in proximal tubular cells, especially in the luminal side in kidneys of HIGA mice. C, right: corresponding signals were completely eliminated when the C-terminal antibody was preadsorbed with the synthetic oligopeptide corresponding to the C terminus of rodent angiotensinogen (AA: 405–420) forovernight.
To verify the specificity of the detective antibody, the specificity of the C-terminal antibody was further examined by Western blot and immunohistochemistry.
As illustrated in Fig. 1B, left, extracted protein from kidney of ZDF obese rat (25 μg on lane 3), extracted protein from kidney of HIGA mice (15 μg on lane 4), and 6-His-tagged recombinant mouse angiotensinogen (10 ng on lane 5) were blotted on nitrocellulose membranes and Western blot was done with the C-terminal antibody. The C-terminal antibody recognized rat kidney protein at 52 kDa (lane 3), mouse kidney protein at 50 kDa (lane 4), and 6-His-tagged recombinant mouse angiotensinogen at 48 kDa (lane 5). As previously reported (14), intrarenal angiotensinogen is glycosylated in vivo and the glycosylation may account for this small difference in the molecular size between intrarenal angiotensinogen in vivo and recombinant angiotensinogen. At the same time, the C-terminal antibody was preadsorbed with the synthetic oligopeptide corresponding to the C terminus of rodent angiotensinogen (AA: 405–420) for overnight, and the duplicated membrane was probed with the preadsorbed C-terminal antibody. As illustrated in Fig. 1B, right, the corresponding signals were completely eliminated or reduced to a large extent (lane 9 for ZDF obese rat, lane 10 for HIGA mice, and lane 11 for 6-His-tagged recombinant mouse angiotensinogen). These data assure the specificity of the C-terminal antibody.
Furthermore, as illustrated in Fig. 1C, left, immunohistochemistry demonstrated that angiotensinogen was predominantly localized in proximal tubular cells, especially in the luminal side in kidneys of HIGA mice, which is consistent with a previous work (14) in Sprague-Dawley rats. At the same time, the C-terminal antibody was preadsorbed with the synthetic oligopeptide corresponding to the C terminus of rodent angiotensinogen (AA: 405–420) for overnight, and the consecutive slide was stained with the preadsorbed C-terminal antibody. As illustrated in Fig. 1C, right, the corresponding signals were completely eliminated. These data also assure the specificity of the C-terminal antibody.
Plate stability
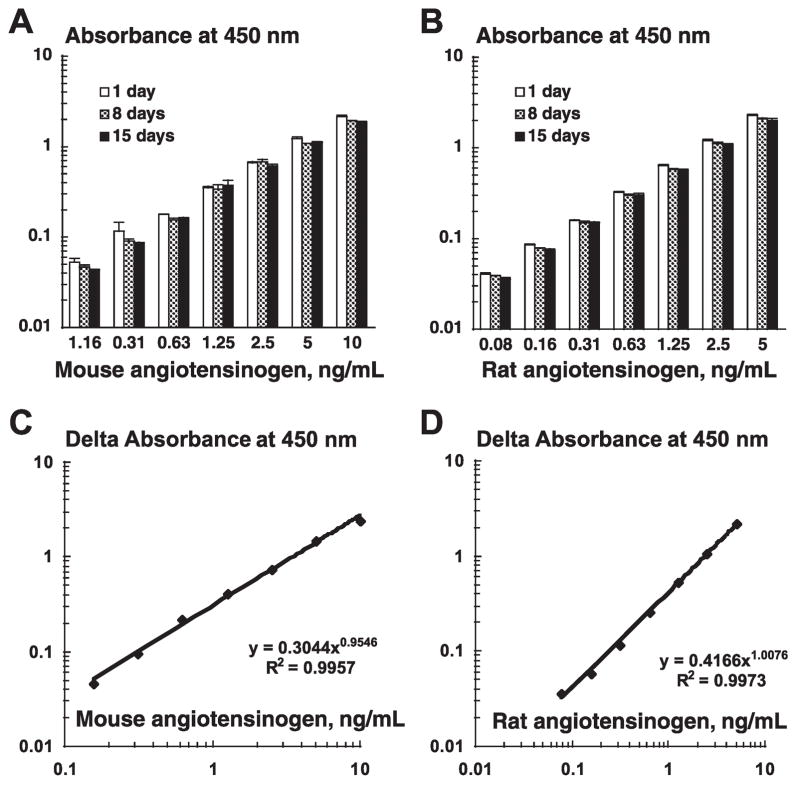
To address stability of plates, the N-terminal antibody-coated ELISA plates were incubated at 37°C for 1, 8, or 15 days. Then, different concentrations of standard samples of rodent angiotensinogen were applied on these plates and processed, and the absorbance values were obtained. As demonstrated in Fig. 2, A and B, each concentration of standard samples of mouse angiotensinogen (A; n = 6) or of rat angiotensinogen (B; n = 6) exhibited similar absorbance values beyond 15 days. These data clearly indicate that the N-terminal antibody-coated ELISA plates are stable at least for 15 days after antibody coating.
Fig. 2.
A–B: to address stability of plates, the N-terminal antibody-coated ELISA plates were incubated at 37°C for 1, 8, or 15 days. Then, different concentrations of standard samples of rodent angiotensinogen were applied on these plates and processed, and the absorbance values were obtained. Each concentration of standard samples of mouse angiotensinogen (A; n = 6) or of rat angiotensinogen (B; n = 6) exhibited similar absorbance values beyond 15 days. C–D: a corresponding standard curve exhibited a high linearity from 0.16 to 10 ng/ml for mouse angiotensinogen (C; n = 6 in each concentration) and from 0.08 to 5 ng/ml for rat angiotensinogen (D; n = 6 in each concentration), respectively. The correlation coefficient was >0.99.
Standard curves
As shown in Fig. 2, C and D, a corresponding standard curve exhibited a high linearity from 0.16 to 10 ng/ml for mouse angiotensinogen (C; n = 6 in each concentration) and from 0.08 to 5 ng/ml for rat angiotensinogen (D; n = 6 in each concentration), respectively. The correlation coefficient was >0.99.
Detection limit
The lowest sensitivity for this system was determined using the guidelines under the National Committee for Clinical Laboratory Standards Evaluation Protocols and was 0.03 ng/ml for mouse angiotensinogen and 0.01 ng/ml for rat angiotensinogen.
Addition and recovery test
Different known concentrations of standard samples of mouse angiotensinogen were added into mouse plasma samples or mouse urine samples, and the added angiotensinogen concentrations were calculated as the difference between angiotensinogen concentrations in samples with and without the added angiotensinogen. Similar procedures were also done in rat samples. As demonstrated in Table 1, recovery rates were >94.3% for mouse plasma, >86.9% for mouse urine, >85.6% for rat plasma, and >85.6% for rat urine.
Table 1.
Addition and recovery test
| Specimen | Theoretical Value, ng/ml | Measured Value, ng/ml | % |
|---|---|---|---|
| Mouse angiotensinogen | |||
| Mouse plasma | 9.45 | 8.91 | 94.3 |
| 6.95 | 7.24 | 104.2 | |
| 5.70 | 5.95 | 104.4 | |
| Mouse urine | 3.08 | 2.83 | 91.9 |
| 1.83 | 1.59 | 86.9 | |
| 1.21 | 1.07 | 88.8 | |
| Rat angiotensinogen | |||
| Rat plasma | 3.82 | 3.27 | 85.6 |
| 2.57 | 2.38 | 92.6 | |
| 1.95 | 1.89 | 97.2 | |
| Rat urine | 4.23 | 3.64 | 86.1 |
| 2.98 | 2.55 | 85.6 | |
| 2.36 | 2.15 | 91.3 | |
Different known concentrations of standard samples of mouse angiotensinogen were added into mouse plasma samples or mouse urine samples, and the added angiotensinogen concentrations were calculated as the difference between angiotensinogen concentrations in samples with and without the added angiotensinogen. Similar procedures were also done in rat samples. Recovery rates were >94.3% for mouse plasma, >86.9% for mouse urine, >85.6% for rat plasma, and >85.6% for rat urine.
Sample stability
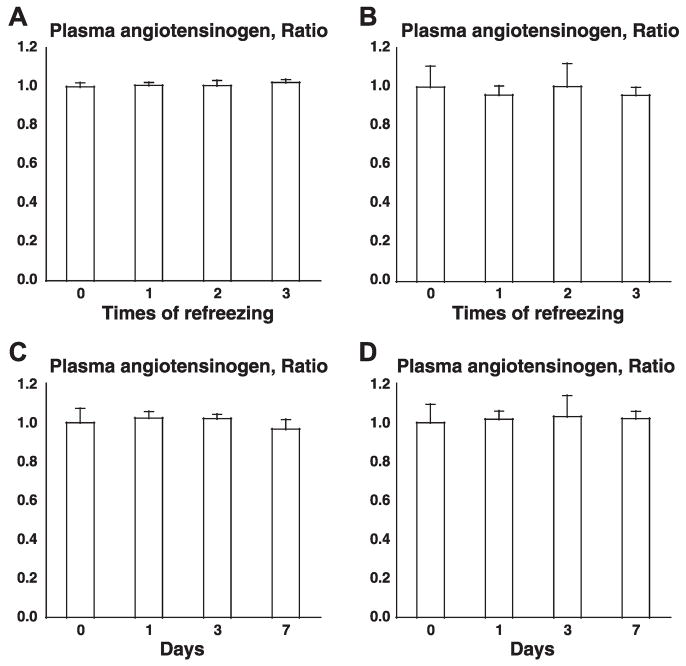
To address stability of samples, two tests were performed: one was a freeze and thaw test and the other was an accelerated test. In a freeze and thaw test, one mouse plasma sample and one rat plasma sample were frozen and thawed zero, one, two, and three times, and plasma angiotensinogen concentrations were measured by the present ELISA system. As described in Fig. 3, A (mouse) and B (rat), significant differences were not observed. In an accelerated test, one mouse plasma sample and one rat plasma sample were incubated at 37°C for 0, 1, 3, and 7 days and plasma angiotensinogen concentrations were measured by the present ELISA system. As shown in Fig. 3, C (mouse) and D (rat), significant differences were not observed. These data clearly indicate that angiotensinogen in plasma samples is stable without any preservatives or proteinase inhibitors except EDTA.
Fig. 3.
A–B: freeze and thaw test. One mouse plasma sample and one rat plasma sample were frozen and thawed 0, 1, 2, and 3 times, and plasma angiotensinogen concentrations were measured by the present ELISA system. Significant differences were not observed in mouse (A) and in rat (B). C–D: accelerated test. One mouse plasma sample and one rat plasma sample were incubated at 37°C for 0, 1, 3, and 7 days, and plasma angiotensinogen concentrations were measured by the present ELISA system. Significant differences were not observed in mouse (C) and in rat (D). To focus on the changes in freeze and thaw test (A–B) or the changes in accelerated test (C–D), the data were presented as the ratios to angiotensinogen concentrations without freeze and thaw (A–B) or the ratios to angiotensinogen concentrations without acceleration (C–D).
Intra-assay test
Intra-assay tests were performed using three different concentrations of mouse plasma samples, mouse urine samples, rat plasma samples, and rat urine samples. As summarized in Table 2, the percentages of the coefficient of variation among wells are from 3.1 to 10.0%.
Table 2.
Intra-assay test
| Specimen | Measured Value, ng/ml | SD | %CV | n |
|---|---|---|---|---|
| Mouse angiotensinogen | ||||
| Mouse plasma | 4.36 | 0.43 | 9.8 | 16 |
| 1.34 | 0.10 | 7.3 | 16 | |
| 0.40 | 0.02 | 6.1 | 16 | |
| Mouse urine | 1.05 | 0.08 | 7.8 | 16 |
| 0.65 | 0.05 | 8.0 | 16 | |
| 0.34 | 0.03 | 9.2 | 16 | |
| Rat angiotensinogen | ||||
| Rat plasma | 1.70 | 0.17 | 10.0 | 16 |
| 0.60 | 0.02 | 3.1 | 16 | |
| 0.21 | 0.01 | 4.7 | 16 | |
| Rat urine | 0.37 | 0.01 | 3.5 | 16 |
| 0.26 | 0.01 | 5.0 | 16 | |
| 0.14 | 0.01 | 6.5 | 16 | |
Intra-assay tests were performed using 3 different concentrations of mouse plasma samples, mouse urine samples, rat plasma samples, and rat urine samples. Percentages of the coefficient of variation (%CVs) among wells are from 3.1 to 10.0%.
Inter-assay test
Inter-assay tests were performed using three different concentrations of mouse plasma samples, mouse urine samples, rat plasma samples, and rat urine samples. As summarized in Table 3, the percentages of the coefficient of variation among plates are from 7.7 to 16.8%.
Table 3.
Inter-assay test
| Specimen | Measured Value, ng/ml | SD | %CV | n |
|---|---|---|---|---|
| Mouse angiotensinogen | ||||
| Mouse plasma | 4.72 | 0.58 | 12.3 | 27 |
| 1.37 | 0.14 | 10.3 | 27 | |
| 0.39 | 0.03 | 7.7 | 27 | |
| Mouse urine | 1.02 | 0.10 | 10.0 | 24 |
| 0.63 | 0.08 | 12.6 | 24 | |
| 0.32 | 0.05 | 14.8 | 24 | |
| Rat angiotensinogen | ||||
| Rat plasma | 1.85 | 0.25 | 13.5 | 28 |
| 0.57 | 0.05 | 8.8 | 28 | |
| 0.18 | 0.03 | 16.8 | 28 | |
| Rat urine | 0.40 | 0.04 | 9.8 | 24 |
| 0.24 | 0.03 | 10.4 | 24 | |
| 0.13 | 0.02 | 12.1 | 24 | |
Inter-assay tests were performed using 3 different concentrations of mouse plasma samples, mouse urine samples, rat plasma samples, and rat urine samples. %CVs among plates are from 7.7 to 16.8%.
Specificity
To confirm the cross-reactivity with other proteins, several numbers of proteins (20 μg/ml) were applied on the present ELISA system and processed. As shown in Table 4, the cross-reactivity for other proteins in this ELISA system was negligible. In addition, downstream products of angiotensinogen (20 μg/ml) were also applied to the present ELISA system. As demonstrated in Table 4, the cross-reactivity for ANG peptides in this ELISA system was also negligible.
Table 4.
Specificity
| Mouse/Rat Protein or Angiotensin Peptide | Cross-Reactivity |
|---|---|
| Mouse angiotensinogen | 100% |
| Rat angiotensinogen | 100% |
| Mouse immunoglobulin | <0.1% |
| Mouse albumin | <0.1% |
| Mouse transferring | <0.1% |
| Mouse osteopontin | <0.1% |
| Mouse leptin | <0.1% |
| Rat immunoglobulin | <0.1% |
| Rat TNF-α | <0.1% |
| Rat IL-1β | <0.1% |
| Rat IL-8 | <0.1% |
| Rat growth-regulated oncogene-β | <0.1% |
| ANG I | <0.1% |
| ANG II | <0.1% |
| ANG III | <0.1% |
| ANG IV | <0.1% |
| ANG 1–7 | <0.1% |
| ANG 1–9 | <0.1% |
To confirm the cross-reactivity with other proteins, several numbers of proteins (20 μg/ml) were applied on the present ELISA system and processed. The cross-reactivity for other proteins in this ELISA system was negligible. In addition, downstream products of angiotensinogen (20 μg/ml) were also applied on the present ELISA system. The cross-reactivity for angiotensin peptides in this ELISA system was also negligible.
Plasma and urinary angiotensinogen concentrations in mice and rats
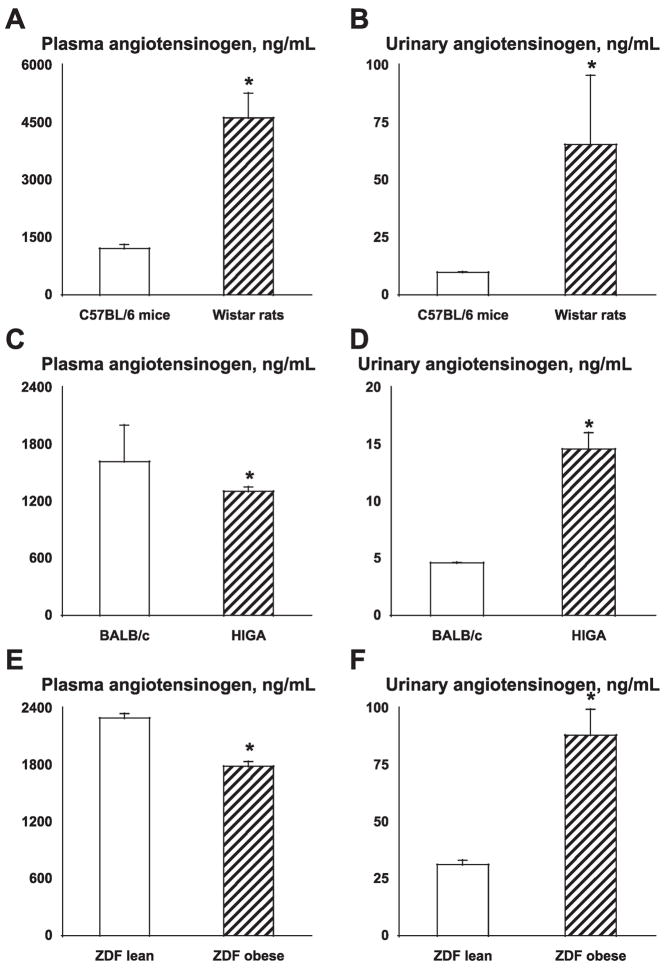
As demonstrated in Fig. 4A, plasma angiotensinogen concentrations were significantly higher in Wistar rats (4,626 ± 645 ng/ml; n = 2) compared with C57BL/6 mice (1,216 ± 101 ng/ml; n = 7). As demonstrated in Fig. 4B, urinary angiotensinogen concentrations were also significantly higher in Wistar rats (65.4 ± 30.2 ng/ml; n = 2) compared with C57BL/6 mice (9.8 ± 0.3 ng/ml; n = 3). Angiotensinogen concentrations in mouse plasma are thought to be much lower than those in rat plasma (2, 3, 7, 23). In agreement, the present data also indicate that plasma and urinary angiotensinogen concentrations in mice are lower that in rats. The plasma levels are about one-fourth while urinary levels are about one-seventh compared with values in rats.
Fig. 4.
A–B: plasma and urinary angiotensinogen concentrations in mice and rats. Plasma angiotensinogen concentrations were significantly higher in Wistar rats compared with C57BL/6 mice (A). Urinary angiotensinogen concentrations were also significantly higher in Wistar rats compared with C57BL/6 mice (B). C–D: plasma and urinary angiotensinogen concentrations in high serum IgA (HIGA) mice (IgA nephritis model). Plasma angiotensinogen concentrations of HIGA mice were significantly lower than those of the corresponding control BALB/c mice (C). However, urinary angiotensinogen concentrations of HIGA mice were significantly higher than those of BALB/c mice (D). E–F: plasma and urinary angiotensinogen concentrations in Zucker diabetic fatty (ZDF) obese rats (type 2 diabetic model). Plasma angiotensinogen concentrations of ZDF obese rats were significantly lower than those of the corresponding control ZDF lean rats (E).
Plasma and urinary angiotensinogen concentrations in IgA nephritis model mice
As demonstrated in Fig. 4C, plasma angiotensinogen concentrations of HIGA mice (1,308 ± 47 ng/ml; n = 10) were significantly lower than those of the corresponding control BALB/c mice (1,620 ± 384 ng/ml; n = 12). However, as demonstrated in Fig. 4D, urinary angiotensinogen concentrations of HIGA mice (14.6 ± 1.5 ng/ml; However, urinary angiotensinogen concentrations of ZDF obese rats were significantly higher than those of ZDF lean rats (E). n = 34) were significantly higher than those of BALB/c mice (4.6 ± 0.1 ng/ml; n = 2).
Plasma and urinary angiotensinogen concentrations in type 2 diabetic model rats
As demonstrated in Fig. 4E, plasma angiotensinogen concentrations of ZDF obese rats (1,789 ± 50 ng/ml; n = 5) were significantly lower than those of the corresponding control ZDF lean rats (2,296 ± 47 ng/ml; n = 5). However, as demonstrated in Fig. 4F, urinary angiotensinogen concentrations of ZDF obese rats (88.2 ± 11.4 ng/ml; n = 15) were significantly higher than those of ZDF lean rats (31.3 ± 1.9 ng/ml; n = 15).
In conclusion, we have developed antibodies and a sensitive and specific quantification system for mouse and rat angiotensinogen using sandwich ELISA. The present data suggest that urinary angiotensinogen levels are controlled differently from plasma angiotensinogen levels in rodents. The development of rodent angiotensinogen ELISA allows quantitative comparisons in mouse and rat angiotensinogen levels in models of hypertension and cardiovascular and kidney diseases.
Acknowledgments
GRANTS
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-072408); the National Center for Research Resources (P20-RR-017659); the National Heart, Lung, and Blood Institute (R01-HL-026371); and the Health Excellence Fund from Louisiana Board of Regents.
Footnotes
DISCLOSURES
Y. Hagiwara and K. Miyashita are presently employees at Immuno-Biological Laboratories.
References
- 1.Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Bohlender J, Menard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance: implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 4.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 5.Danser AH, Admiraal PJ, Derkx FH, Schalekamp MA. Angiotensin I-to-II conversion in the human renal vascular bed. J Hypertens. 1998;16:2051–2056. doi: 10.1097/00004872-199816121-00029. [DOI] [PubMed] [Google Scholar]
- 6.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 7.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, Durand J, Hankes GH, Oparil S. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 10.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 11.Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986;78:1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 18.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 23.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 24.Mazzocchi G, Malendowicz LK, Markowska A, Albertin G, Nussdorfer GG. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab. 2000;278:E1027–E1030. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 25.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris BJ, Johnston CI. Renin substrate in granules from rat kidney cortex. Biochem J. 1976;154:625–637. doi: 10.1042/bj1540625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navar LG, Prieto-Carrasquero MC, Kobori H. Chapter 170: Renal renin-angiotensin system. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. 1. Burlington, MA: Elservier; 2006. pp. 1235–1242. [Google Scholar]
- 29.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-κB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]