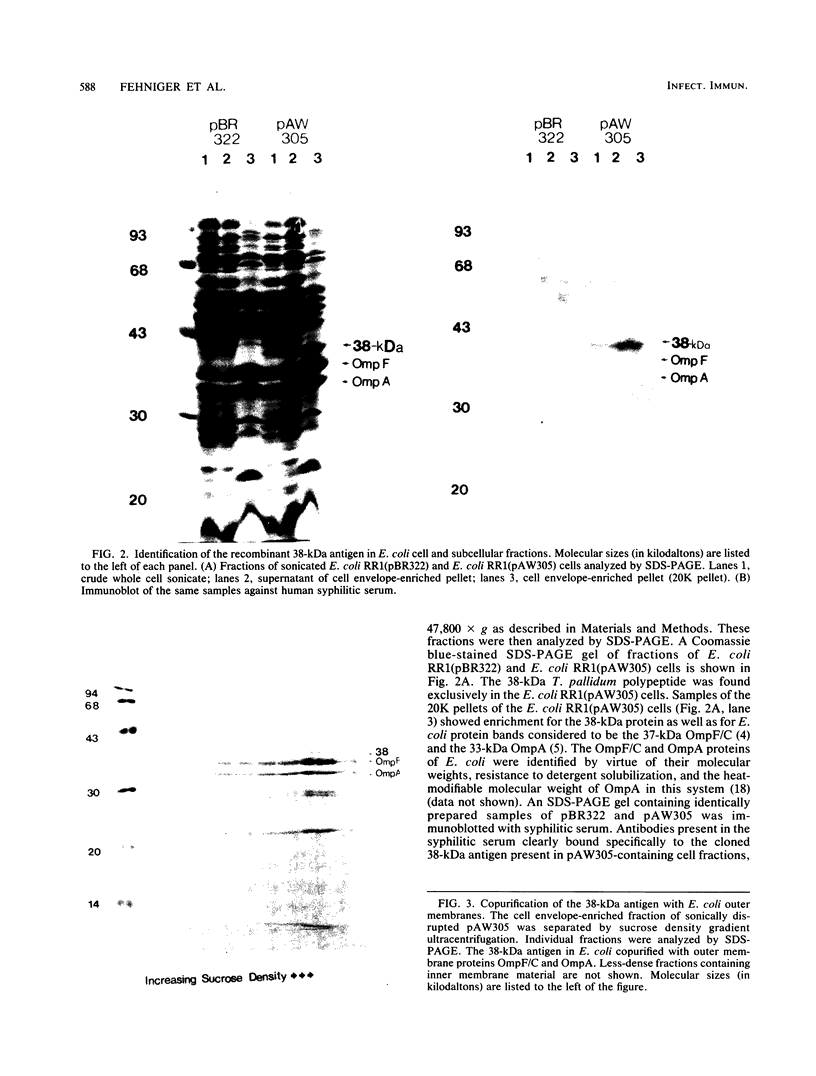
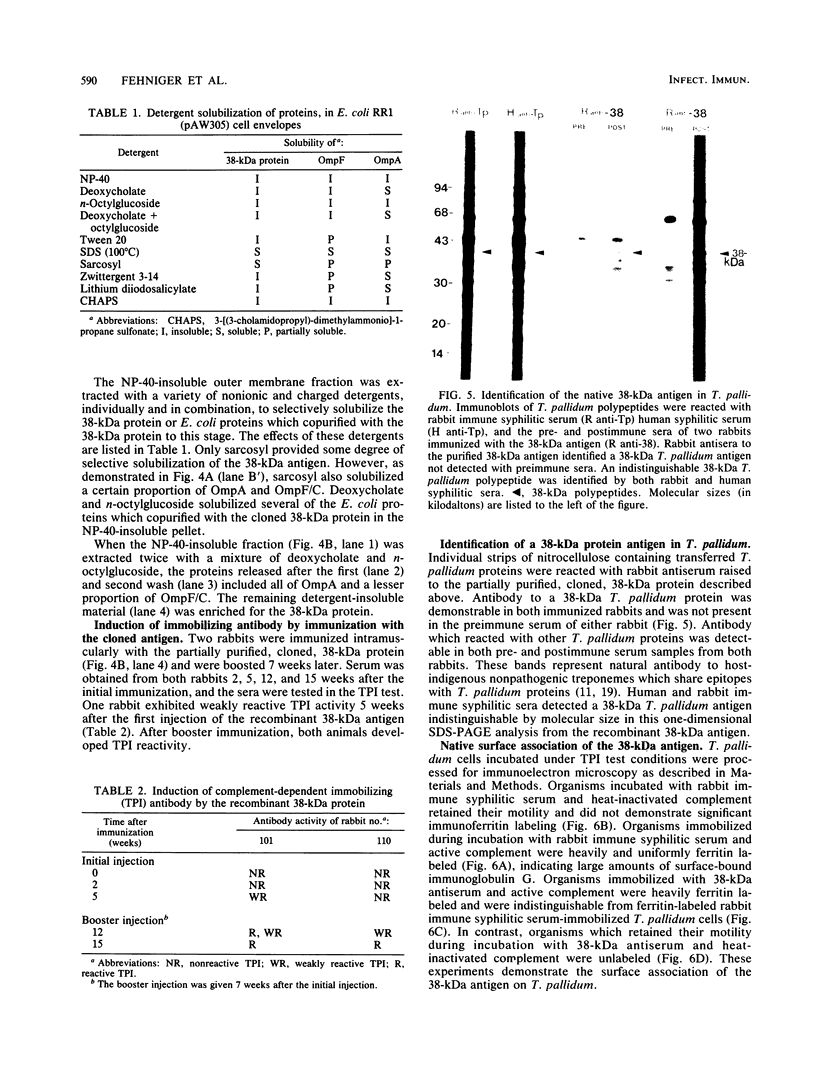
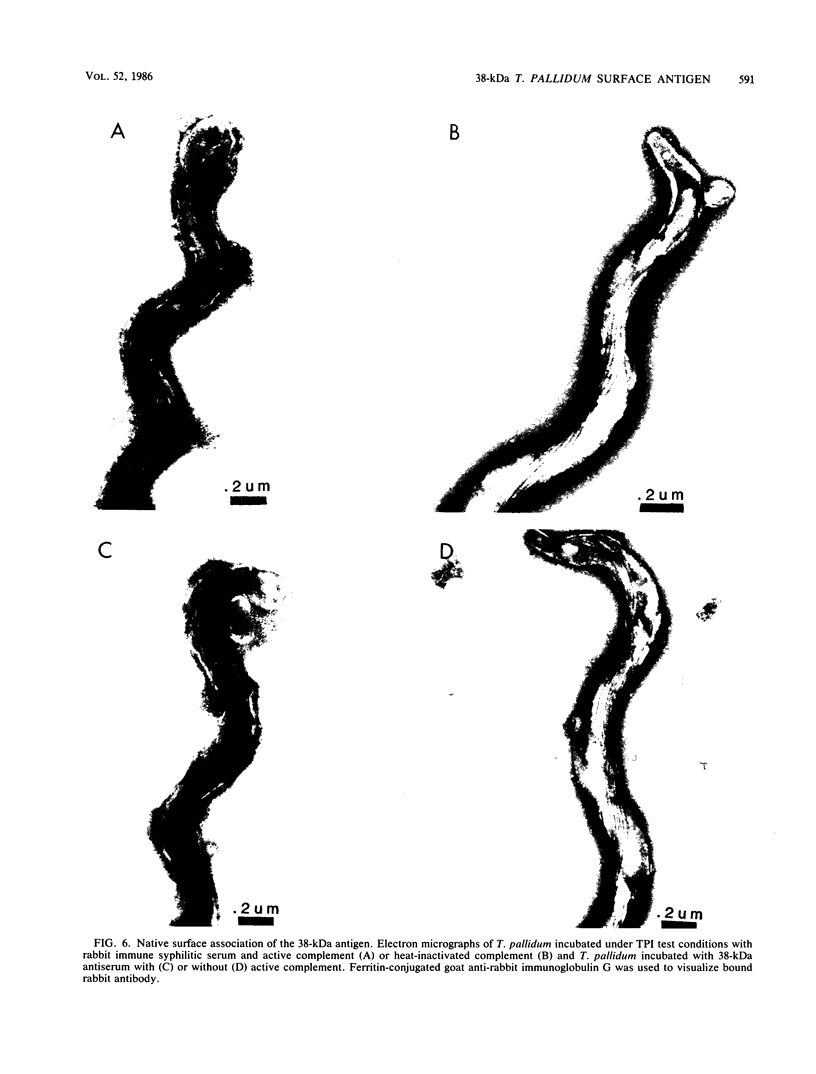
Abstract
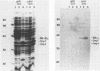
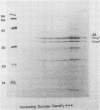
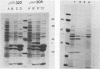
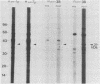
A recombinant plasmid designated pAW305, containing a 6-kilobase insert of Treponema pallidum DNA, directed the expression of a 38-kilodalton (kDa) treponemal antigen in Escherichia coli. The 38-kDa antigen copurified with the outer membrane fraction of the E. coli cell envelope after treatment with nonionic detergents or sucrose density gradient centrifugation. Rabbits immunized with the recombinant 38-kDa antigen developed antibodies which reacted specifically with a 38-kDa T. pallidum antigen on immunoblots, and 38-kDa antisera specifically immobilized T. pallidum in a complement-dependent manner in the T. pallidum immobilization test. Antisera to the 38-kDa recombinant antigen were also used to demonstrate its native surface association on T. pallidum by immunoelectron microscopy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Henning U. Primary structure of major outer membrane protein I of Escherichia coli B/r. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5014–5017. doi: 10.1073/pnas.76.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon W. E., Lucas J. B., Price E. V. Fluorescent treponemal antibody-absorption (FTA-ABS) test for syphilis. JAMA. 1966 Nov 7;198(6):624–628. [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Lovett M. A. Properties of an ordered ring structure formed by recombinant Treponema pallidum surface antigen 4D. J Bacteriol. 1986 Mar;165(3):732–739. doi: 10.1128/jb.165.3.732-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Law J. H., Tsukagoshi N., Wilson G. A density label for membranes. Proc Natl Acad Sci U S A. 1970 Oct;67(2):598–605. doi: 10.1073/pnas.67.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Miller J. N., Lovett M. A. Molecular characterization of common treponemal antigens. Infect Immun. 1983 May;40(2):825–828. doi: 10.1128/iai.40.2.825-828.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Pedersen P. E., Schouls L. M., Severin E., van Embden J. D. Genetic characterization and partial sequence determination of a Treponema pallidum operon expressing two immunogenic membrane proteins in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1227–1237. doi: 10.1128/jb.162.3.1227-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
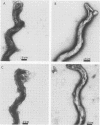
- Hovind-Hougen K., Birch-Andersen A., Nielsen H. A. Electron microscopy of treponemes subjected to the Treponema pallidum immobilization (TPI) test. II. Immunoelectron microscopy. Acta Pathol Microbiol Scand C. 1979 Aug;87C(4):263–268. [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985 Jan;134(1):585–592. [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMPSON F. A., Jr, McLEOD C. P. Relationship between treponemal immobilizing antibodies and acquired immunity in experimental syphilis. J Immunol. 1951 Jul;67(1):41–48. [PubMed] [Google Scholar]
- METZGER M., RUCZKOWSKA J. INFLUENCE OF LYSOZYME UPON THE REACTIVITY OF TREPONEMA PALLIDUM IN THE FLUORESCENT ANTIBODY REACTION. Arch Immunol Ther Exp (Warsz) 1964;12:702–708. [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Sell S. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J Immunol. 1984 Nov;133(5):2686–2692. [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Fehniger T. E., Silverblatt F. J., Miller J. N., Lovett M. A. The surface of virulent Treponema pallidum: resistance to antibody binding in the absence of complement and surface association of recombinant antigen 4D. Infect Immun. 1986 May;52(2):579–585. doi: 10.1128/iai.52.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Bassford P. J., Jr Cellular and extracellular protein antigens of Treponema pallidum synthesized during in vitro incubation of freshly extracted organisms. Infect Immun. 1985 Mar;47(3):799–807. doi: 10.1128/iai.47.3.799-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER T. B., NELSON R. A., Jr The relationship of treponemal immobilizing antibody to immunity in syphilis. Trans Assoc Am Physicians. 1950;63:112–117. [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]