Abstract
Increased brain-derived neurotrophic factor (BDNF) levels and extracellular-signal regulated kinase (ERK) signaling are associated with reduced brain injury after cerebral ischemia. In particular, mild hypothermia after cardiac arrest increases BDNF and ERK signaling. This study tested whether intracerebroventricular infusions (0.025 µg/ hr × 3 days) of BDNF also improved recovery of rats resuscitated from cardiac arrest and maintained at 37°C. BDNF infusions initiated at the time of cardiac arrest did not alter survival, neurological recovery, or histological injury. Separate experiments confirmed that BDNF infusions increased tissue levels of BDNF. However, these infusions did not increase ERK activation in hippocampus. These data suggest that increased BDNF levels are not sufficient to explain the beneficial effects of mild hypothermia after cardiac arrest, and that exogenous BDNF administration does not increase extracellular ERK signaling.
Keywords: Ischemia, Heart Arrest, Hippocampus, Brain-derived Neurotrophic Factor
Brain derived neurotrophic factor (BDNF) is a eurotrophic factor that binds to the neurotrophin receptor tyrosine kinase receptor B (TrkB). BDNF signaling can increase neuronal survival in some model systems [11]. The beneficial effects of BDNF have been associated with increased activation of p42/p44 mitogen-activated protein kinase (extracellular signal-related kinase, ERK) [2].
In vivo, the effect of exogenous BDNF on ischemic brain injury is unclear. BDNF infusions [3,15,22,27] or pretreatment of brain with BDNF-producing cells [9] decrease neuronal injury after both focal and global cerebral ischemia. Conversely, inhibition of BDNF activity worsens recovery from forebrain ischemia [16]. However, intracranial BDNF infusion does not improve recovery or reduce histological damage in rat brain after resuscitation from ventricular fibrillation cardiac arrest [21].
In other studies of cardiac arrest, BDNF signaling is associated with improved neuronal survival after ischemia. Therapeutic hypothermia (33–34°C) for 12–24 hours improves neurological recovery and survival after resuscitation from cardiac arrest [4,10]. In rats, therapeutic hypothermia increased tissue levels of BDNF [7] and activated ERK [13]. These observations suggested the hypothesis that therapeutic hypothermia produced some of its beneficial effects via BDNF-induced stimulation of intracellular ERK signaling [7, 26]. Therefore, this study tested whether BDNF infusion increases ERK activation in vivo and improves neurological recovery after normothermic cardiac arrest in rats.
METHODS
Asphyxial cardiac arrest and resuscitation in rats were conducted as described previously [7, 8,12,17] according to protocols approved by the University of Pittsburgh IACUC. At 48 hours prior to cardiac arrest, wireless temperature probes (MiniMitter, Sun River, OR, U.S.A.) were implanted into the peritoneal cavity, and stainless-steel injectors (25 gauge) were implanted bilaterally into the lateral ventricles (AP−2.0 mm, ML +2.0 mm, DV −3.0 mm relative to bregma [20]. Injectors were connected by PE-50 tubing to two osmotic minipumps (1003D, Alzet, Cupertino CA), which were implanted subcutaneously in the neck and that delivered fluid at 1 µl/hour. At this first surgery, both pumps were filled with saline. On the day of cardiac arrest, the reservoirs for both osmotic minipumps were exchanged with reservoirs containing the BDNF (0.025 µg/µl) or placebo (saline). The tubing volume from the reservoirs to the cannula allowed BDNF infusion (0.025 µg/hour/side × 72 hours) to commence at the time of cardiac arrest without requiring a separate intracranial procedure.
One series of rats (N=32) was subjected to cardiac arrest with BDNF or placebo infusions. On the day of cardiac arrest, temperature was monitored telemetrically every 3 seconds by a computer using commercial software (Vital View; MiniMitter, Sun River, OR), Temperature was regulated via software-driven relays connected to a 100-W heating lamp and a cooling fan, which keeps temperature within 0.1°C of 37°C [5,12,17]. Anesthesia was induced with 4.0% halothane in oxygen, which was reduced to 1.5% halothane during surgery and intraperitoneal ampicillin (50 mg/kg) was administered. Rats were orotracheally intubated with a 14-gauge catheter, and mechanically ventilated (tidal volume of 9 mL/kg, 40 respirations/min, positive end-expiratory pressure of 5 cm H2O) (Harvard Rodent Ventilator; Harvard Apparatus, South Natick, MA, U.S.A.). The left femoral vein and artery were exposed via an incision and cannulated with catheters (PE-50 tubing; Harvard Apparatus, South Natick, MA) for continuous arterial blood pressure recording, blood gas analysis (I-STAT, Heska, Waukesha, WI) and drug administration. Ventilation was adjusted to maintain eucapnia (PCO2= 35–45 mm Hg) and normal pH before asphyxia.
After chemical paralysis with intravenous vecuronium (2 mg/kg), halothane was discontinued and rats were ventilated with room air for two minutes. This interval is too brief for rats to regain consciousness but reduces residual halothane. Asphyxial cardiac arrest was induced by disconnecting the ventilator at end-expiration for 8 minutes, resulting in complete circulatory arrest lasts for 5 minutes [12,17]. After 8 minutes, the ventilator was reconnected, with 100% oxygen at a rate of 60 respirations/min. Intravenous epinephrine (0.005 mg/kg) and bicarbonate (1.0 mEq/kg) were administered, and external chest compressions were performed at a rate of 200 compressions/min until spontaneous pulses returned (~60 seconds). After stabilization for at least 60 minutes and confirmation of adequate spontaneous respirations, rats were extubated and weaned from oxygen to room air. Hemodynamic and blood gas values were recorded at 10, 30, and 60 minutes after reuscitation. Rats were maintained at 37°C for 24 hours and received additional doses of ampicillin (50 mg/kg) at 12 and 24 hours. Animals were supported with subcutaneous fluids (50% D5W and normal saline at 40 ml/kg/d) and hand-fed oral fluids until they were able to feed independently.
Rats were weighed and evaluated daily using a standardized neurological score described previously [8,12,17]. Rats losing greater than 20% of baseline body weight were sacrificed. Neurological scores measured sensorimotor function (grooming, forepaw grasp, limb movement, and sensation for each limb), axial body tone, righting, locomotion, and balance. Scores range from 0 to 26, with a lower number corresponding to a more severe insult and a score of 26 representing a normal rat.
After 14 days, halothane-anesthetized rats were transcardially perfused with 100 mL of PBS, followed by 100 mL of 10% formalin. Brains were removed into formalin at 4°C for at least 48 hours and transferred to 20% sucrose in PBS at least 24 hours prior to sectioning on a freezing microtome. Serial 20 µm sections were stored at −20°C in a cryopreservative solution (30% ethylene glycol, 30% sucrose, 1% polyvinyl-pyrrolidone, 0.1M PBS, pH 7.4) prior to further processing. Free-floating sections were labeled using anti-neuronal nuclear antigen antibodies (anti-NeuN, Chemicon, Temecula, CA, USA). Sections were washed at room temperature in PBS for 15 minutes, followed by 1% horse serum (in 0.01M PBS with 0.1% Tween-20, pH 7.4) for 30 minutes. Sections were then incubated overnight at 4°C in 1:1000 dilutions of primary antibody and washed with PBS-T. Immune complexes were visualized using the Vectastain Elite ABC kit with a 3, 5-diaminobenzamide chromagen (Vector Laboratories Inc, Burlingame, CA, USA). Sections were mounted on slides, dehydrated in ethanols, cleared in xylenes and covered. Photomicrographs of the anterior hippocampus (corresponding to −3.60 mm from bregma [20] were acquired with a digital imaging system (Kodak MDS290, Kodak, Rochester, NY, USA) and a Nikon E400 microscope (Nikon Corporation, Tokyo, Japan). Labeled neurons in the CA1 region of the hippocampus were counted in separate sections at the same level (0.7 mm strip centered above the tip of the upper-blade of the dentate gyrus) by observers blinded to treatment group. This region has reproducible injury in this same model [8,13,17].
A separate series of rats (N=28) were implanted with osmotic minipumps containing saline or BDNF in an identical fashion. These rats were sacrificed at 12, 24, 48 or 72 hours after starting infusions (n=3–4 per group), and the brains collected for measurement of tissue BDNF and activated ERK levels. A final series of rats (N=6) were injected with boluses of BDNF (2.5 µg) into the right lateral ventricle via the guide cannula, and sacrificed at 2, 6 or 12 hours after injection.
Immunoblotting was performed as in previous studies (Hicks 2000b, D’Cruz 2002). Brains were hand-dissected into cold PBS. After 1 minute, the right and left hippocampus were separated and frozen at −70°C. Frozen tissue was sonicated for 5 seconds in 50 mmol/L Tris-Cl, 100 mmol/L NaCl, 5 mmol/L EDTA, (pH 7.4) containing 1% Nonidet P-40, protease inhibitors (100 mmol/L phenylmethylsulfonyl fluoride, 1 µg/mL aprotinin, 5 µg/mL leupeptin, 1 µg/mL pepstatin) and phosphatase inhibitors (1 mmol/L Na3VO4 and 1 mmol/L NaF). Supernatant was separated by centrifugation at 12,000g for 15 minutes and stored at −70°C. Protein (5–20 µg according to Bradford method, Bio-Rad) was denatured by 5 minutes of boiling in sodium dodecyl sulfate sample buffer. separated by electrophoresis in denaturing 10% polyacrylamide gels, and transferred to polyvinyl difluoride membranes (Immobilon-P; Millipore Corp., Bedford, MA). Membranes were blocked for 1 hour with 5% dry milk in PBS with 0.5% Tween-20 (PBS-T) before overnight incubation at 4°C with primary antibodies in 1:2,000 dilution. Primary antibodies included total and phospho-p44/42 MAP kinase (Thr202/Tyr204) (Cell Signaling, Beverly, MA, USA) and BDNF (N-20, Santa Cruz Biotechnology, Santa Cruz, CA). Blots were visualized using1:5000 dilution horseradish peroxidase–conjugated species-specific anti-IgG secondary antibody (Bio-Rad Laboratories, Hercules, CA, USA) in 5% dry milk / PBS-T followed by enhanced chemiluminescence using a commercial kit (Renaissance, New England Nuclear, Boston, MA, U.S.A.). Membranes were exposed to x-ray film (X-OMAT; Kodak, Rochester, NY, USA) (30 seconds - 20 minutes) and images were scanned and quantified using NIH Image software.
Protein molecular weights were determined by comparison with protein molecular weight markers (Full Range Rainbow; Amersham Life Science, Little Chalfont, Buckinghamshire, U.K.). Equal loading of lanes and transfer of protein was confirmed by stripping each membrane and reprobing with antibody against total p44/p42. In all instances, total protein levels did not differ significantly between lanes, and uncorrected densitometry for the original antibody was used for data analysis. Exposure times and concentrations were adjusted empirically to achieve a linear relation between protein concentration and densitometry within gels, as confirmed by loading different volumes of the same sample in separate lanes. Samples were run in duplicate, and results were confirmed by separate duplicate experiments.
Survival was compared between groups using Kaplan-Meier curves with log-rank test. Neurological scores were compared using nonparametric Kruskal-Wallis or Wilcoxon tests. Other continuous variables were compared using ANOVA or t-test.
RESULTS
32 rats were resuscitated from cardiac arrest and received continuous infusions of saline (n=17) or BDNF (n=15). Physiological measurements were similar to prior studies, with rats exhibiting an initial metabolic acidosis that resolved over 30 minutes. Groups did not differ in baseline or post-resuscitation physiological measurements (TABLE 1). Baseline characteristics of rats in other experiments also were not different.
TABLE 1. Physiological Variables in Outcome Experiment.
Values are mean (SD). All variables were similar between groups.
| Saline (N=17) |
BDNF (N=15) |
|
|---|---|---|
| Baseline | ||
| Weight (g) | 320 (20) | 317 (24) |
| Anesthesia Duration (min) | 44 (6) | 38 (14) |
| Heart Rate (beats / min) | 334 (40) | 341 (20) |
| Mean Blood Pressure (mmHg) | 107 (8) | 112 (8) |
| PH | 7.44 (0.03) | 7.44 (0.02) |
| PCO2 (torr) | 45 (3) | 45 (3) |
| PO2 (torr) | 152 (38) | 167 (36) |
| Glucose (mg/dl) | 137 (26) | 126 (18) |
| CPR Duration (s) | 49 (7) | 56 (15) |
| 10 minutes after cardiac arrest | ||
| Heart Rate (beats / min) | 444 (33) | 438 (26) |
| Mean Blood Pressure (mmHg) | 164 (23) | 170 (18) |
| PH | 7.34 (0.05) | 7.34 (0.05) |
| PCO2 (torr) | 41 (5) | 41 (5) |
| PO2 (torr) | 261 (33) | 275 (66) |
| Glucose (mg/dl) | 141 (54) | 142 (52) |
| Lactate (mEq/l) | 4.1 (1.3) | 4.3 (0.7) |
| 30 minutes after cardiac arrest | ||
| Heart Rate (beats / min) | 437 (43) | 432 (32) |
| Mean Blood Pressure (mmHg) | 88 (27) | 95 (19) |
| PH | 7.43 (0.04) | 7.41 (0.04) |
| PCO2 (torr) | 42 (5) | 45 (5) |
| PO2 (torr) | 374 (39) | 387 (45) |
| Glucose (mg/dl) | 96 (19) | 93 (13) |
| Lactate (mEq/l) | 1.3 (0.6) | 1.7 (1.1) |
| 60 minutes after cardiac arrest | ||
| Heart Rate (beats / min) | 355 (30) | 351 (50) |
| Mean Blood Pressure (mmHg) | 81 (28) | 89 (25) |
| PH | 7.42 (0.05) | 7.40 (0.05) |
| PCO2 (torr) | 44 (7) | 47 (6) |
| PO2 (torr) | 411 (24) | 442 (43) |
| Glucose (mg/dl) | 96 (17) | 107 (9) |
| Lactate (mEq/l) | 0.8 (0.3) | 0.9 (0.4) |
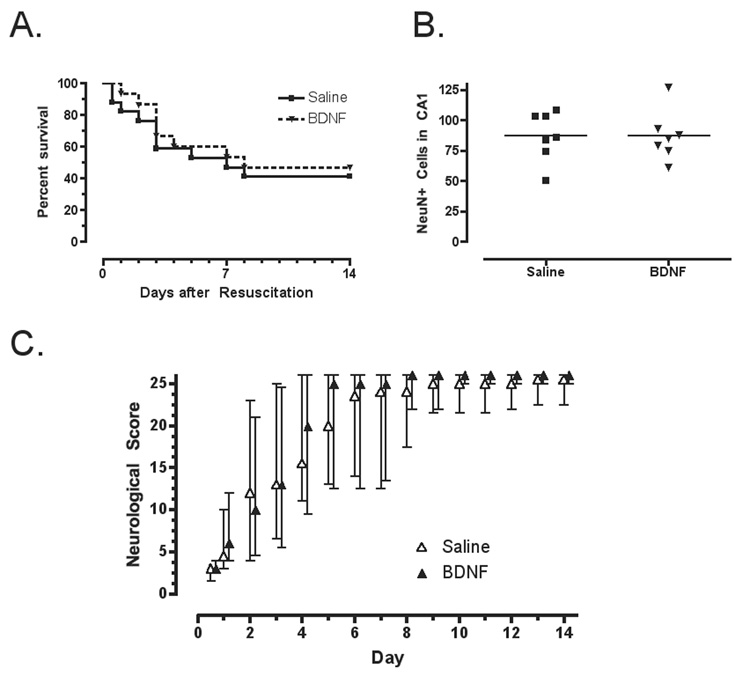
Cardiac arrest produced neurological injury and mortality that did not differ between treatment groups. Rats with the greatest neurological deficits died or were sacrificed between 1 and 7 days after resuscitation (FIGURE 1 A). Survival did not differ between groups (log-rank = 0.20; p = 0.65). Histology from surviving rats revealed decreased numbers of surviving neurons in the CA1 region of the hippocampus, but the number of surviving neurons did not differ between groups (FIGURE 1B) (mean difference=0; 95% CI: −24, 24). Neurological scores changed over time (Kruskal-Wallis =159.5; df=14; p=0.0001), but were not different between BDNF-treated and saline-treated groups at any time point (FIGURE 1 C and D).
FIGURE 1.
A. Survival curves did not differ for groups of rats treated with intracerebral saline or BDNF. B. Total number of neurons (NeuN-positive cells per microscope field) in the CA1 region of the hippocampus did not differ between saline and BDNF treated rats surviving to 14 days. For comparison, sham-operated rats without cardiac arrest have ~100–110 cells per field [8, 17]. C. The neurological scores (median and interquartile range) for rats treated with saline or BDNF improved over the first few days. Scores range from 0 (brain dead) to 26 (normal). Rats with persistently low neurological scores did not survive for 14 days. Scores did not differ between groups.
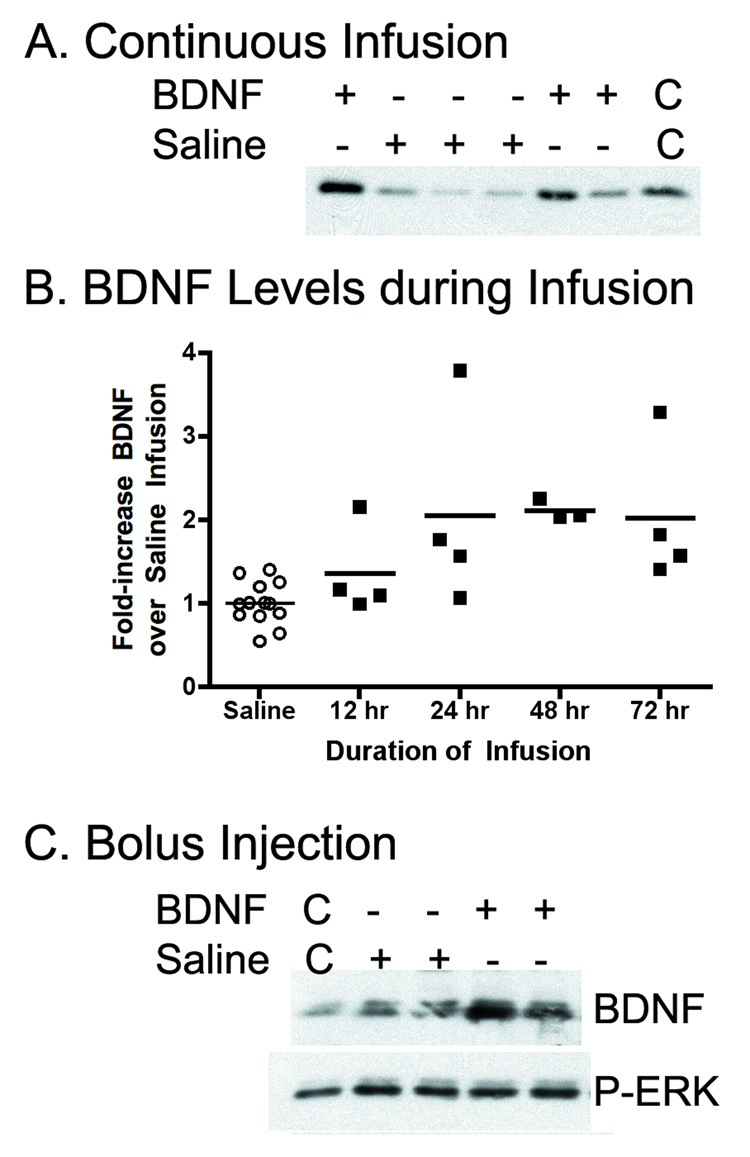
BDNF infusions in separate rats (n=15) increased hippocampal BDNF levels (FIGURE 2A) with mean levels increasing 2-fold over levels in rats with saline infusions (n=13) (FIGURE 2B) ((F[4,23]=4.69, p=0.006). This increase in tissue BDNF immunoreactivity is similar in time-course and magnitude to that observed after cardiac arrest and hypothermia treatment in the same model [7]. Immunoblotting of the tissue from brains after BDNF infusions did not reveal any increase in P-ERK levels. To exclude the possibility that the levels of BDNF after infusions was inadequate to detect an effect, unilateral bolus injections of BDNF (2.5 µg) were also conducted in separate rats (n=6). These brains also did not show any increase in ERK activation in the hippocampus on the BDNF-injected side at 2, 6 or 12 hours after injection, despite clear increases in BDNF immuoreactivity (FIGURE 2C).
FIGURE 2.
A. Infusion of BDNF (2.5 µg per side over 72 hours) into the brain increased tissue levels of BDNF in the hippocampus. Linearity control lanes (C) contain double-volumes of one of the samples. B. Levels of BDNF in the hippocampi of individual rats during infusions of saline or BDNF reveal two-fold increases during BDNF infusions that was different from controls at 24, 48 and 72 hours (p<0.05). C. After bolus injections of BDNF, hippocampal BDNF levels increase, but active ERK (P-ERK) do not increase. Linearity controls (C) contain half-volumes of one of the samples.
DISCUSSION
This study demonstrated that exogenous infusions of BDNF that were sufficient to increase tissue levels of BDNF did not alter brain injury after cardiac arrest. The sample size for this study was about twice that required to demonstrate the beneficial effects of hypothermia in the same model [7,8,12,17]. In addition, direct infusion of BDNF into the brain did not increase ERK signaling. These data contradict the original hypothesis that BDNF infusions would promote neuronal survival by increasing intracellular ERK signaling [7], and differ from the results with other models of brain ischemia [3,15,22]. These data suggest that increased levels of BDNF are not sufficient to reproduce the beneficial effects of post-resuscitation mild hypothermia on brain injury after cardiac arrest. In addition, this experiment points out that the effects of BDNF may vary between different models of brain injury.
The neurological outcomes confirm other observations that BDNF (6–168µg) does not alter outcome after cardiac arrest [21] but differ from data derived from arterial occlusion models of brain ischemia in which BDNF (0.8 – 20 µg) does improve outcome [3,15, 22]. It is possible that the ischemic brain injury created by cardiac arrest and resuscitation differs qualitatively from the injury produced by the arterial occlusion models. For example, qualitative histological differences have been noted between asphyxial and ventricular-fibrillation-induced cardiac arrest in dogs, suggesting that systemic factors also contribute to brain injury [25]. Furthermore, after resuscitation from cardiac arrest, there is a significant systemic cytokine release that may result in vascular or glial responses in brain, which may not occur in arterial occlusion models [1].
One limitation of this study is that the neurological score measures very global functions and is not specific for any particular brain region. Recovery of these global behaviors reflect the emergence from coma and vegetative function, and this level of recovery is most important for survival after cardiac arrest in humans [1,4,10]. Deficits in spatial memory or learning do occur after cardiac arrest, and might be more specifically associated with histological damage in the hippocampus [13]. However, hippocampal cell loss is measured simply as a convenient and reliable marker of histological brain injury after ischemia. The analysis is limited by the fact that histology is not available for rats that did not survive for the entire 14 days. The goal of this study was not to draw links between histological injury and behavioral recovery, but to screen for any behavioral or histological improvement after BDNF infusion. Given that there was no improvement in global behaviors (neurological scores) or in hippocampal neuronal survival (NeuN-positive CA1 cells), it is unlikely that more detailed behavioral testing would have revealed BDNF effects on spatial memory or learning.
Furthermore, BDNF signaling may have beneficial effects on some types of brain injury that are not sufficient to protect ischemic brain. For example, BDNF-induced ERK signaling may reduce apoptotic cell death, but be ineffective at reducing necrotic cell death [2,14]. Cerebral ischemia in vivo results in a pattern of cell death that has features of both apoptosis and necrosis [6]. It is possible that successful inhibition of apoptosis merely allows damaged neurons to succumb to necrosis. Thus, the exogenous BDNF in this study may have reduced one possible mechanism of cell death without affecting the other.
Other hypotheses may explain the failure of BDNF to improve recovery. First, injury produced in this study may have been too severe to be improved by BDNF. This hypothesis is unikely, because hypothermia improves neurological recovery in this model [7,12,17], and only partial loss of neurons was observed in the hippocampus (Figure 1B). Even if the insult to the brain was too global to be affected by infusions into the ventricles, some effect on the histological damage to periventricular structure like the hippocampus should be apparent.
Another possibility is that higher concentrations of BDNF were required for neuronal preservation. However, during hypothermia treatment, BDNF levels increase over a similar 24-hour time-course. An additional possibility is that exogenous BDNF was unable to reach or affect its receptors. Cerebral ischemia may increase expression and change the subcellular localization of TrkB receptors [9,18]. The techniques used in this study are too gross to determine whether receptor localization or receptor coupling to intracellular effectors was altered in these rats, which might be explored in future experiments.
The present data do not support a relationship between BDNF and ERK in hippocampus [2]. In this same model, elevated BDNF levels parallel activation of ERK, and levels of both BDNF and active ERK are increased by hypothermia [7]. In another study, pretreatment with an inhibitor of ERK activation, U0126, did not alter neurological recovery after cardiac arrest [8]. Taken together, these data indicate that ERK activation is not necessary for the beneficial effects of hypothermia, and BDNF activation is not sufficient to mimic hypothermia. Future experiments with pharmacological inhibition of BDNF or knockdown of BDNF expression might test whether BDNF is necessary for the effects of post-ischemic hypothermia.
It is possible that increased ERK activation and BDNF levels are epiphenomena of recovering brains. Both ERK and BDNF are increased by activity, and therapies that accelerate behavioral recovery might increase these signaling pathways [19,23]. Cerebral ischemia, including this model, increases BDNF levels by specific regulation of gene transcription [24, 26]. Future experiments might determine what upstream effectors of BDNF and ERK activation have a more fundamental relationship to the therapeutic interventions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a sepsis-like syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 2.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 3.Beck T, Lindholm D, Castren E, Wree A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J. Cereb. Blood Flow Metab. 1994;14:689–692. doi: 10.1038/jcbfm.1994.86. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Colbourne F, Sutherland GR, Auer RN. An automated system for regulating brain temperature in awake and freely moving rodents. J Neurosci Methods. 1996;67:185–190. [PubMed] [Google Scholar]
- 6.Colbourne F, Sutherland GR, Auer RN. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci. 1999;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Cruz BJ, Fertig KC, Filiano AJ, Hicks SD, DeFranco DB, Callaway CW. Hypothermic reperfusion after cardiac arrest augments brain-derived neurotrophic factor activation. J Cereb Blood Flow Metab. 2002;22:843–851. doi: 10.1097/00004647-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 8.D'Cruz BJ, Logue ES, Falke E, DeFranco DB, Callaway CW. Hypothermia and ERK activation after cardiac arrest. Brain Res. 2005;1064:108–118. doi: 10.1016/j.brainres.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer I, Ballabriga J, Martí E, Pérez E, Alberch J, Arenas E. BDNF up-regulates TrkB protein and prevents the death of CA1 neurons following transient forebrain ischemia. Brain Pathol. 1998;8:253–261. doi: 10.1111/j.1750-3639.1998.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HACA - Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 11.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion improves functional recovery and selectively alters stress-induced protein expression after global cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:520–530. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Hicks SD, Parmele KT, DeFranco DB, Klann E, Callaway CW. Hypothermia differentially increases ERK and JNK/SAPK activation in hippocampus after asphyxial cardiac arrest. Neuroscience. 2000;98:677–685. doi: 10.1016/s0306-4522(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Zhao X, Tu CH, Casaccia-Bonnefil P, Chao MV. Prevention of apoptotic but not necrotic cell death following neuronal injury by neurotrophins signaling through the tyrosine kinase receptor. J Neurosurg. 2004;100:79–87. doi: 10.3171/jns.2004.100.1.0079. [DOI] [PubMed] [Google Scholar]
- 15.Kiprianova I, Sandkuhler J, Schwab S, Hoyer S, Spranger M. Brain-derived neurotrophic facor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Exp Neurol. 1999;159:511–519. doi: 10.1006/exnr.1999.7109. [DOI] [PubMed] [Google Scholar]
- 16.Larsson E, Nanobashvili A, Kokaia Z, Lindvall O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1220–1228. doi: 10.1097/00004647-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Logue ES, McMichael MJ, Callaway CW. Comparison of hypothermia at 33°C or 35°C after cardiac arrest in rats. Acad Emerg Med. 2007;14:293–300. doi: 10.1197/j.aem.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 18.Merlio JP, Ernfors P, Kokaia Z, Middlemas DS, Bengzon J, Kokaia M, Smith ML, Siesjö BK, Hunter T, Lindvall O, et al. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- 19.Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 21.Popp E, Padosch SA, Vogel P, Schabitz WR, Schwab S, Bottiger BW. Effects of intracerebroventricular application of brain-derived neurotrophic factor on cerebral recovery after cardiac arrest in rats. Crit Care Med. 2004;32(9 Suppl):S359–S365. doi: 10.1097/01.ccm.0000134223.09056.fc. [DOI] [PubMed] [Google Scholar]
- 22.Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsukahara T, Iihara K, Hashimoto N, Nishijima T, Taniguchi T. Increases in levels of brain-derived neurotrophic factor mRNA and its promoters after transient forebrain ischemia in the rat brain. Neurochem Int. 1998;33:201–207. doi: 10.1016/s0197-0186(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 25.Vaagenes P, Safar P, Moossy J, Rao G, Diven W, Ravi C, Arfors K. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs. Differences in cerebral resuscitation effects--a preliminary study. Resuscitation. 1997;35:41–52. doi: 10.1016/s0300-9572(97)01108-8. [DOI] [PubMed] [Google Scholar]
- 26.Vosler PS, Logue ES, Repine MJ, C.W. Callaway CW. Delayed hypothermia preferentially increases expression of brain-derived neurotrophic factor exon III in rat hippocampus after asphyxial cardiac arrest. Mol Brain Research. 2005;135:21–29. doi: 10.1016/j.molbrainres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Yanamoto H, Nagata I, Sakata M, Zhang Z, Tohnai N, Sakai H, Kikuchi H. Infarct tolerance induced by intra-cerebral infusion of recombinant brain-derived neurotrophic factor. Brain Res. 2000;859:240–224. doi: 10.1016/s0006-8993(00)01966-1. [DOI] [PubMed] [Google Scholar]