Abstract
The effects of a synthetic apoE1 peptide, viz., residues 133–149 (apoE[133–149]), a mimetic that comprises the apoE receptor-binding domain, on N-methyl-D-aspartate (NMDA)/glycine-induced ion flow through NMDA receptor (NMDAR) channels, has been investigated. The activity of apoE[133–149] was found to depend on the low density lipoprotein receptor-related protein (LRP). Competition experiments with receptor associated protein (RAP) and activated α2macroglobulin (α2M*), two proteins that compete for apoE binding to LRP, demonstrate that apoE[133–149] inhibition of NMDAR function is mediated at a locus in LRP that overlaps with the binding sites of RAP and α2M*. A co-receptor of LRP, cell-surface heparin sulfate proteoglycan, did not function in this system. Additional electrophysiology experiments demonstrated that the inhibitory potency of apoE[133–149] was 3-fold greater for NMDAR-transfected wild-type Chinese Hamster ovary (CHO) cells compared with NMDAR-transfected CHO cells deficient in LRP. Studies with truncation and replacement variants of the apoE peptide demonstrated that the NMDAR-inhibitory properties of these peptides correlate with their binding affinities for LRP. These novel results indicate that apoE functions as an inhibitor of NMDAR ion channels indirectly via LRP, and are suggestive of a participatory role for LRP in NMDAR-based neuropathies.
Keywords: conantokins, NMDA receptor subtypes, electrophysiology, LRP, ion channels, apolipoprotein E
1. Introduction
ApoE is a 34 kDa protein that plays a central role in lipid metabolism as a component of chylomicrons, as well as very low-density-, intermediate-density- and high-density lipoproteins (VLDL and IDL, HDL, respectively) and remnants of these particles, by its ability to traffic lipids between various cell types throughout the circulatory system (Weisgraber et al., 1985). Full-length apoE is composed of two independent domains (Wetterau et al., 1988). The N-terminal domain consists of a four-helix 22 kDa N-terminal region (Wilson et al., 1991) that mediates the binding of apoE to LDLR family members, such as LRP (Innerarity et al., 1983), as well as to heparin (Weisgraber et al., 1986) and to HSPGs (Mahley and Ji, 1999) The 10 kDa COOH-terminal domain facilitates the association of apoE with various lipoproteins (Weisgraber and Xi, 1990; Westerlund and Weisgraber, 1993), and is also responsible for the self-association of lipid-free apoE (Aggerbeck et al., 1988) This latter region also contains a second heparin binding site (Weisgraber et al., 1986) With regard to functional consequences of these interactions, it is currently accepted that in order to mediate catabolism of lipoprotein remnants containing apoE, this protein initially interacts with cell surface HSPG, via its heparin binding site(s), and is then transferred to LRP for internalization. The importance of apoE in the pathophysiologic responses of humans is indicated by many studies in which the nature of the distributions of the three common alleles for apoE, viz., E2, E3, and E4 (Zannis and Breslow, 1981), have been associated with risk of central nervous system diseases, e.g., Alzheimer’s disease (AD) (Strittmatter et al., 1993), and coronary artery diseases. Such tendencies are likely due to the altered lipid profiles found in those patients that express these different forms of apoE (Dallongeville et al., 1991).
Molecular mechanistic studies of apoE responses have been greatly aided by the findings that many properties associated with the receptor binding region of apoE are recapitulated by small peptide mimetics of this domain, especially those peptides that are derived from apoE residues 130–155 (apoE[130–155]). As an example, apoE[133–149] and apoE[141–148] inhibit the function of neuronal nicotinic acetylcholine receptor, implying an importance of this region of apoE in AD (Klein and Yakel, 2004). Further, apoE[133–149] initiates signaling in murine macrophages, similar to intact apoE, and functions in this regard via the LRP (Misra et al., 2001). Lastly, apoE[130–149] and apoE[130–155] inhibit-interleukin-2-dependent T-lymphocyte proliferation, similar to intact apoE (Clay et al., 1995).
Functional relationships may also exist between apoE and the glutamate/glycine-gated NMDAR. Functional NMDARs are comprised of several types of subunits, viz., an obligatory NR1 subunit, of which there are eight splice variants (1a/b-4 a/b) (Hollmann et al., 1993; Zukin and Bennett, 1995), and one or more regulatory NR2 (A–D) subunits (Molinoff et al., 1994) and/or NR3 subunits (Das et al., 1998; Chatterton et al., 2002; Madry et al., 2007).NR subunits are expressed temporally and regionally in the central nervous system and the nature of the subunit associations in the functional NMDAR control the properties of this receptor (Paoletti and Neyton, 2007; Prorok and Castellino, 2007). Some functions of apoE have been linked to those of the NMDAR. Specifically, it has been found that an LRP-mediated increase in intracellular calcium in neurons can be abolished with MK-801, a specific NMDAR channel-blocker (Bacskai et al., 2000) It was subsequently found that apoE reduces NMDA-mediated excitotoxicity in neuronal glial cells (Aono et al., 2002), a property mimicked by apoE[133–149] (Aono et al., 2003) While these studies strongly argue for the existence of a functional linkage between the LDLR binding region of apoE and the NMDAR, electrophysiology has not been previously employed in this context and presents a major tool to directly assess excitatory responses. We thus investigated ion current responses in whole cells transfected with defined subunits of the NMDAR, with the aim of establishing relationships between NMDA-mediated currents in cells, as influenced by the LDLR receptor binding region of apoE, the LRP, and cell surface HSPG. This communication provides a summary of these studies.
2. Materials and methods
2.1 Specialized Reagents
The recombinant human RAP and LRP proteins were provided by D. K. Strickland (University of Maryland School of Medicine). Recombinant human α2M (Sigma, St. Louis, MO) was activated in methylamine at 10 mg/ml and stored at −20 °C for no more than 2 wk. NMDA was obtained from Acros Organics (Morris Plains, NJ). Spermine and heparinase-I were purchased from Sigma.
2.2. Peptide synthesis
The amino acid sequences of the apoE peptides that were chemically synthesized for these studies are:
ApoE[133–149]: Ac-LRVRLASHLRKLRKRLL-conh2
ApoE[143–150]: Ac-KLRKRLLR-conh2
ApoE[145–150]: Ac-RKRLLR-conh2
ApoE[133–149/K143,146E]: Ac-LRVRLASHLRELRERLL-conh2
All peptides were acetylated (Ac) at the amino-terminus and amide-capped (CONH2) at thecarboxyl-terminus.
Solid phase peptide synthesis was conducted on a 0.1 mmol scale using an Applied Biosystems (Foster City, CA) model 433A peptide synthesizer, as described previously (Prorok et al., 1996). Peptides were purified using a Sephadex G-15 size exclusion column equilibrated and eluted with 0.1% acetic acid. If additional purification was found to be necessary after this step, a Sephadex CM-25 column was utilized. The peptide was eluted with a linear gradient of 50 mM sodium borate/150 mM NaCl, pH 8.0, as the start solution, and 50 mM sodium borate/500 mM NaCl, pH 8.0, as the limit solution. Fractions corresponding to the major peak were combined and lyophilized. Desalting of the peptide was carried out on a Sephadex G-15 column in the presence of 0.1% acetic acid.
2.3. Expression of recombinant (r) NMDAR subtypes
cDNAs encoding rat NR1a, NR1b, NR2A, and NR2B were provided by D. Lynch (University of Pennsylvania). Plasmid pEGFP-N1, encoding a red-shifted variant of green fluorescent protein (GFP), was purchased from Clontech Laboratories (San Diego, CA).
HEK293 cells (CRL-1573 from ATCC, Manassas, VA) were transiently transfected with NMDAR subunits using calcium phosphate precipitation (Klein et al., 2001) The cells were grown to ~50% confluency in 60 mm poly-D-lysine-coated dishes and transfected (10 µg of DNA/dish), with combinations of NR1a, NR1b, NR2A, and NR2B-containing plasmids, along with pEGFP-N1 for positive selection of transfected cells, at ratios of 1:3:6 for GFP:NR1a/b:NR2A/B (Klein et al., 2001).
WT CHO K1 cells were provided by E. H. Hinchcliffe (University of Notre Dame). Mutant CHO K1 cell lines, namely CHO 14-2-1, that expresses an intracellular nonprocessed LRP, but no surface-expressed protein, and CHO 13-5-1, that displays neither LRP mRNA nor LRP protein (FitzGerald et al., 1995), were obtained from D. K. Strickland. For all CHO cell lines, NMDA receptors were transfected with lipofectamine 2000 (Invitrogen) according to the recommended protocol.
The transfected HEK and CHO cells were plated in 35-mm poly-D-lysine-coated dishes for electrophysiology experiments. Following transfection, the cells were maintained in fresh medium containing 500 µM ketamine to prevent glutamate-mediated cell death. Electrophysiological recordings were performed within 24–48 hr post-transfection.
2.4. Expression and purification of the contiguous extracellular domains (NTD-S1-L-S2) of the NR2B subunit
Based on amino acid sequence alignments with the NR2B N-terminal domain (NTD) and ligand binding domains (S1 and S2), three peptides: R27-Y389 (NTD), V390-D557 (S1) and F653-N817 (S2), were isolated by PCR from the cDNA for full-length rat NR2B and joined by a 13-residue polypeptide (STEGEVNAEEEGF) linker (L). The resulting construct, NTD-S1-L-S2, was then inserted into a Puro-pMT vector, with a V5 Tag site and His6 affinity tag at the C-terminus. The coding region of the expression plasmid was confirmed by DNA sequencing. The expression plasmid was purified using a QIAGEN Plasmid Midi kit (QIAGEN), and transfected into Drosophila S2 cells using a calcium phosphate transfection kit (Invitrogen). Stably transfected S2 cells were selected by the addition of 10–15 µg/ml puromycin, and seeded into 1000 ml of EX-CELL 420 serum-free medium (SAFC Biosciences, Lenexa, KS) in a 1-liter spinner flask, at a cell density of 3–6 ×106 cells/ml. Upon reaching a density of 6–8 ×106 cells/ml, the cells were induced with 500 µM copper sulfate for 2–3 days with 5 mg/l aprotinin. The cultured medium was immediately concentrated at 4 °C in an Amicon ultrafiltration cell and loaded onto a 5 ml HiTrap Ni affinity column (GE Healthcare). NTD-S1-L-S2 was eluted with a linear gradient of 0 to 300 mM imidazole in the presence of 6–8 M urea and 15 mM 2-mercaptoethanol, followed by dialysis against PBS buffer (pH, 7.4). Following SDS-PAGE, Western blotting was carried out using rabbit anti-V5 tag (Sigma) as the primary antibody and goat anti-rabbit IgG AP (Sigma) as the secondary antibody. The protein was frozen by flash cooling in liquid nitrogen at 1.5 mg/ml and stored at −80° C.
2.5. Electrophysiology
Whole cell recordings were obtained at room temperature (Klein et al., 2001; Sheng et al., 2007) Ketamine was removed from cells immediately prior to recordings by 3 × 3 mL washes (2 min each) with Mg2+-free extracellular solution, which consisted of 140 mM NaCl/3 mM KCl/2 mM CaCl2/10 mM Na-Hepes/20 mM dextrose, pH 7.35 (in some experiments, the extracellular level of NaCl was varied). Borosilicate glass recording pipettes (Drummond Scientific, Broomall, PA), with a resistance of 2–4 MΩ, were back-filled with a solution containing 140 mM CsF/1 mM CaCl2/2 mM MgCl2/10 mM EGTA/10 mM Cs-Hepes/2 mM tetraethylammonium chloride/4 mM Na2ATP, pH 7.35. Stock solutions of 10 mM apoE[133–149], 10 mM NMDA, and 10 mM glycine were prepared for the experiments.
GFP fluorescence was visualized using a Nikon Eclipse TE200 microscope (Fryer, Chicago, IL) with a fluorescent detection system, illuminated by a Hg light source with a Mercury-100W short arc DC power supply (Chiu Technical Corporation, Kings Park, NY). The application of the agonists was accomplished using a rapid solution changer equipped with a 9 barrel straight-head (RSC-200; Molecular Kinetics, Pullman, WA) positioned 200–300 µm from the cell under study. The whole-cell current was recorded using an Axopatch-200B amplifier (Axon CNS/Molecular Devices, Sunnyvale, CA), low-pass filtered at 5 kHz by a built-in, eight-pole Bessel filter, digitized at 0.5–2 kHz sampling frequency using a Digidata 1322A (Axon). The data were acquired on a personal computer using pCLAMP 8 software (Axon). All measurements, except those in which voltage was varied, were performed with cells voltage-clamped at −70 mV, pH 7.35, at 25 °C. The responses in a particular cell were normalized to maximum current evoked in the absence of peptide.
Unless noted otherwise, each data point is an average of at least three measurements collected from at least three cells. Current measurements of the acquired data were made using pClamp (Axon) and Origin (Microcal, Northampton, MA) software. The data were averaged and are presented as the mean ± S.E.M. Statistical analyses were performed using the two-tailed Student’s t-test, and repetitive measures of analysis of variance (Sigma Stat, Jandel Scientific, San Rafael, CA; and Origin Software). Significance was assigned at P < 0.05. For experiments in which the % inhibition of NMDAR steady-state currents was determined as a function of apoE peptide concentration, the values for the currents obtained at each peptide concentration were normalized to the current obtained with the indicated NMDA and glycine concentrations in the absence of peptide.
2.6. Surface Plasmon Resonance
The binding of apoE[133–149] to different analytes was analyzed by SPR using a BiaCore X (Biacore, Uppsala, Sweden). All experiments were performed at 25 °C employing HBS-P (0.01 M Hepes pH 7.4/0.15 M NaCl/0.005% v/v Surfactant P20) as the running buffer at a flow rate of 20 µl/min. ApoE[133–149] (1 mM in 10 mM sodium acetate, pH 4.0) was coupled to the sensor chip (CM-5; Biacore), using the manufacturer’s amine-coupling kit, at a rate of 5 µl/min for 7 min, resulting in 200–400 RU of immobilized peptide. Between experiments, the chip surface was regenerated by washing (2−3x) with a 30 sec injection of 0.1 M glycine-HCl, pH 1.5/1 M NaCl at a flow rate of 100 µl/min.
Whole cell binding to apoE[133–149] was conducted in a similar manner, except that the peptide was bound to a CM-3 chip and HEK293 cells were applied at a density of 2.5 × 105 cells/ml in the absence and presence of 1 µM RAP.
For assessing the affinity of apoE peptides for LRP, the latter protein was dissolved in 10 mM sodium acetate, pH 4.0, at a concentration of 20 µg/ml and loaded at a rate of 5 µl/min for 7 min to afford ca. 6000 response units (RUs) of immobilized protein. Sensorgrams were obtained with peptide concentrations spanning 0.5–10 µM (3 injections per concentration) in a total injection volume of 50 µL using HBS-P as the running buffer at a flow rate of 20 µl/min. Between experiments, the chip surface was regenerated by washing (1−2x) with a 15 sec injection of 0.1 M phosphoric acid at a flow rate of 100 µl/min. Similar conditions were employed for immobilization of the NTD-S1-L-S2 domain of NR2B and for obtaining sensorgrams of apoE peptide binding to this construct.
All binding curves were analyzed utilizing the BIAevaluation software package version 3.0 (BiaCore).
2.7. Immunofluorescence microscopy
HEK293 cells were grown on coverslips to ~80 % confluence in DMEM containing 10% fetal bovine serum/1% penicillin-streptomycin at 37 °C and 6.5% CO2. The cells were washed 2x with 0.05 M phosphate/1 M NaCl (PBS), pH 7.4, fixed in 2% formaldehyde in PBS, and stained for LRP by immunofluorescence. Staining for LRP was performed by the incubation of the cells with normal goat serum followed by rabbit polyclonal anti-LRP (a gift from D. K. Strickland), which was incubated with the cells for 1 hr at 25 °C. Alexa Fluor 594-labeled goat-anti-rabbit IgG (Invitrogen), was added as the 2° antibody. The images were obtained using a Nikon Eclipse E600 microscope (Nikon) equipped with a set of selective fluorescent filter blocks and a digital SPOT-RT SE camera (Diagnostic Instruments).
For staining of the cells for HSPG, HEK293 cells were plated on 2-chambered slides (Fisher Scientific, Springfield, NJ) and fixed overnight at 4° C with an acetic acid-ethanol fixative. The cells were treated with 15,000 U/ml of bovine testicular hyaluronidase in PBS, pH 7.4, at 25° C for antigen retrieval. After blocking against nonspecific IgG labeling with Serum-Free Protein Block (Dako, Carpinteria, CA), the slides were incubated with a rat anti-HSPG/perlecan antibody (Lab Vision, Fremont, CA). Image-iT (Invitrogen) reagent was applied to the cells to block against background labeling from the conjugated fluorophore. An Alexa Fluor 488-conjugated chicken anti-rat IgG was employed as the 2° antibody. The slides were coverslipped with Prolong Gold with DAPI mounting medium (Invitrogen). Epifluorescent images were acquired using a Photometrics Cascade 512B CCD camera (Roper Scientific, Tucson, AZ) with GFP and DAPI fluorescent filters (Chroma, Rockingham, VT). The images were acquired at a magnification of 400x as .tif files using a Nikon Eclipse TE2000U microscope with MetaMorph 7.0r3 software (Molecular Devices, Sunnyvale, CA). Cell surface HSPG was removed by treating the cells with heparinase-I (Sigma) at a concentration of 10 units/ml of medium for 2 hr at 37 °C.
3. Results
3.1. ApoE[133–149] inhibits NMDA- and glycine-evoked currents in NMDAR-transfected HEK293 cells
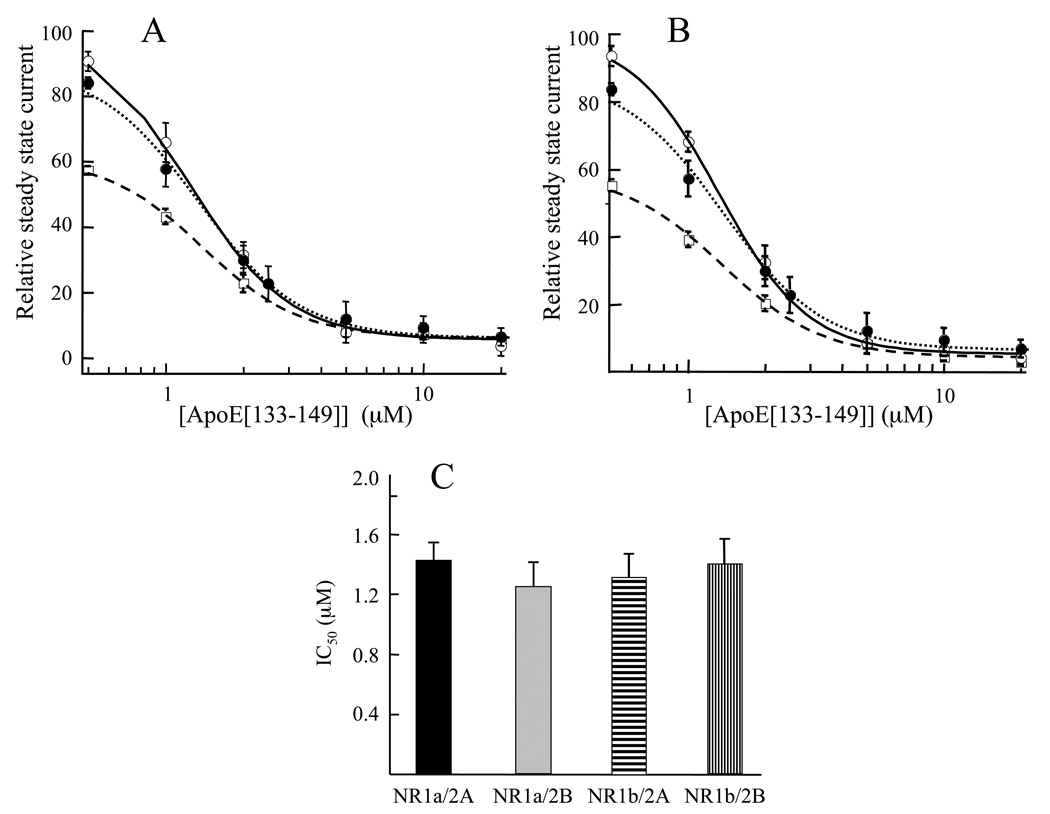
To determine if current flow through the NMDAR could is indeed attenuated by the LDLR binding region of apoE, the electrophysiological responses to various concentrations of apoE[133–149] were measured on NMDA-evoked currents in HEK293 cells transfected with either NR1-1a or NR1-1b, plus either NR2A or NR2B. An example of the data obtained (Fig. 1), in this case with NR1b/2A-transfected cells at a holding voltage of −70 mV, shows a concentration-dependent reduction of the steady state current, elicited with saturating levels of NMDA and glycine, upon addition of various concentrations of apoE[133–149] to the extracellular solution2. At 10 µM apoE[133–149], approximately 90% inhibition was observed. Experiments were then conducted similar in design to those of Fig. 1, to assess the concentration dependency of steady state current inhibition by various levels of apoE[133–149] in extracellular solutions containing 10 µM glycine at different NMDA concentrations, and at 100 µM NMDA at different glycine concentrations. From plots of these concentration-response data, the IC50 values of apoE[133–149] were determined with respect to both NMDA and glycine (a representative plot is shown in Fig 1B). At concentrations of 10 µM glycine, the IC50 values for the apoE peptide at 30 µM, 100 µM, and 500 µM NMDA, were 1.45 ± 0.08 µM, 1.31 ± 0.06 µM, and 1.35 ± 0.05 µM, respectively. Alternatively, at an NMDA concentration 100 µM, the IC50 values associated with apoE[133–149] at 1 µM, 10 µM, and 50 µM glycine were 1.34 ± 0.05 µM, 1.31 ± 0.06 µM, and 1.37 ± 0.05 µM, respectively. The similarity of these values, clustered within a range of 1.31 µM-1.45 µM, show that apoE[133–149] is not a competitive inhibitor of either agonist. From replots of these data (Fig. 2A,B), it also is clear that for each agonist, the same maximal inhibition is achieved by apoE[133–149], regardless of the initial agonist concentration. These observations provide further support that apoE[133–149] is not involved in direct competition with either agonist, but can nonetheless completely inhibit ion flow through the NMDAR by action at a distinct site. Furthermore, the level of inhibition of the steady state currents by apoE[133–149], at 100 µM NMDA/10 µM glycine, were similar for each of the four transfected receptor subunit combinations, i.e., NR1a/2A, NR1b/2B, NR1b/2A, NR1b/2B (Fig. 2C), despite the fact that these subunit combinations display differences in their ligand binding properties (Laurie and Seeburg, 1994; Erreger, et al., 2007) The fact that apoE[133–149] inhibits steady state currents similarly in these receptors is consistent with a mechanism of apoE inhibition that is independent of the nature of the particular NMDAR assembled, supportive of the concept that apoE[133–149] inhibits NMDAR current flow indirectly through effects on other general NMDAR modulators.
Fig. 1.
Inhibition of NMDA/glycine-induced ion flow through recombinant NMDAR channels by apoE[133–149]. (A) The effect of various concentrations (inset) of apoE[133–149] on steady state whole cell currents of NR1b/2A-transfected HEK293 cells. The NMDA concentration was 100 µM and the glycine concentration was 10 µM, which was applied throughout the experiments. ApoE[133–149] was applied as indicated by the horizontal line in the inset. Recordings were obtained with transfected cells voltage-clamped at −70 mV, pH 7.35, at 25° C. (B) A plot of the concentration-response data of Fig 1A. The IC50 value for apoE[133–149] calculated from this plot is 1.31 µM.
Fig. 2.
Inhibition of whole cell steady state currents of NR1b/2A-transfected HEK293 cells by apoE[133–149]. The inhibition of the steady state current produced as a result of addition of different apoE levels was measured at various NMDA and glycine levels. (A) 500 µM NMDA (——), 100 µM NMDA (……), and 30 µM NMDA (-----), at a constant concentration of 10 µM glycine. (B) 50 µM glycine (solid line), 10 µM glycine (……), and 1 µM glycine (------), at a constant concentration of 100 µM NMDA. The values for the currents for each curve in A and B at each apoE[133–149] level are normalized to the current obtained at 100 µM NMDA/10 µM glycine in the absence of apoE[133–149], which is arbitrarily set at 100. (C). IC50 values of apoE[133–149] for whole cell current flow through HEK293 cells transfected with NMDAR of different subunit compositions under constant application of coagonists, NMDA (100 µM) and glycine (10 µM). In all cases in A–C, cells were voltage-clamped at −70 mV, pH 7.35, 25° C.
3.2. The characteristics of apoE[133–149] and spermine attenuation of NMDAR current responses are distinct
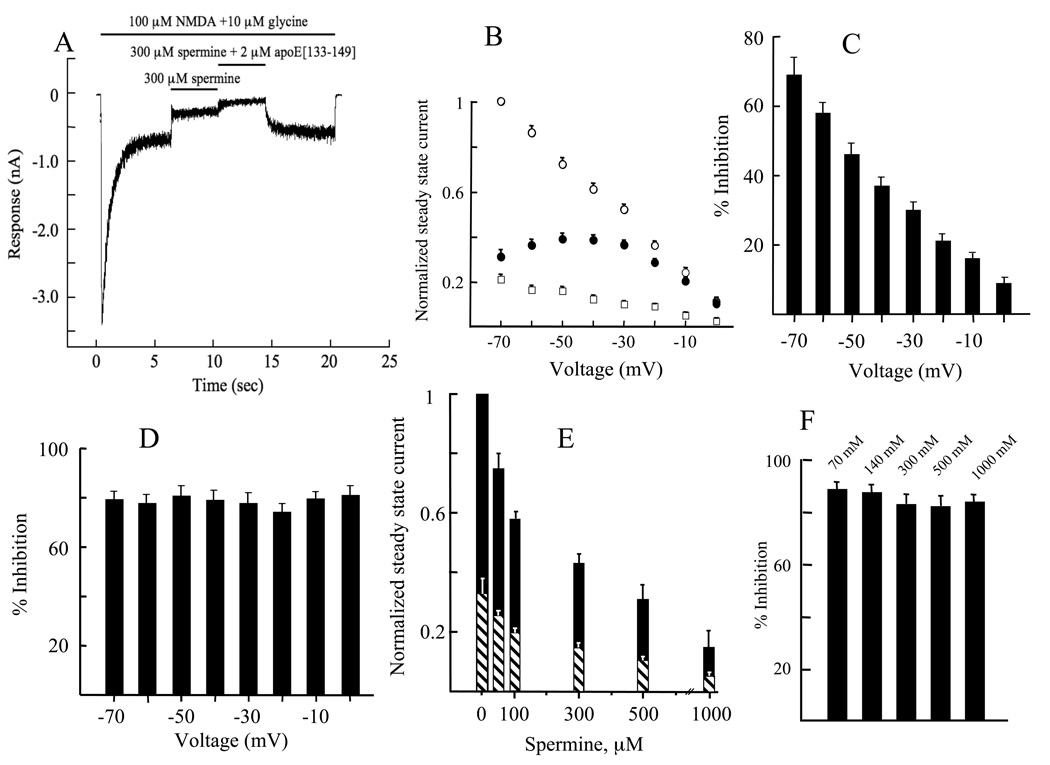
Spermine is a positively charged allosteric regulator of the NMDAR channel, acting as a stimulator at low concentrations and an inhibitor at higher concentrations (Benveniste and Mayer, 1993). Thus, we examined whether apoE[133–149], by virtue of its highly cationic nature, produced its inhibitory effects via competition with spermine. In our experimental system of NR1b/2A-transfected HEK293 cells, and under the conditions used, spermine exerted only a voltage-dependent inhibition of steady state currents evoked by NMDA (100 µM)/glycine (10 µM) when added to the cells after the steady state current was attained (Fig. 3A). After the new steady state current level was established with spermine, addition of apoE[133–149] to the cells resulted in further inhibition of the current. Fig. 3B illustrates the voltage dependency of the whole-cell steady state current with NMDA/glycine, NMDA/glycine/spermine, and NMDA/ glycine/apoE[133–149]. The inhibition of the steady state currents by spermine was voltage dependent (Fig. 3C), decreasing in value as the voltage differential decreases between the inside and outside of the cells. However, the level of inhibition by apoE[133–149] did not display voltage dependency (Fig. 3D), suggesting that apoE[133–149] does not inhibit NMDAR responses via the same mode as spermine. Also, because the relative inhibition exerted by apoE[133–149] is voltage independent (at holding potentials from −70 to 20 mV), an open channel block mechanism can be excluded as a mode of inhibition. The effect of 3 µM apoE[133–149] on the inhibition by spermine of steady state currents is shown on Fig. 3E. The change in percent inhibition attending each incremental spermine addition is the same in both the presence and absence of apoE[133–149]. This would not be the outcome if some fraction of spermine binding sites were already occupied by apoE[133–149] and provides further evidence that the spermine and apoE[133–149] binding sites are distinct and independent of each other. The possibility that the high electropositive character of apoE[133–149] was contributing to a reduction in cation flux through the NMDAR by exerting a “charge-screening” effect (Araneda et al., 1999) was also considered. However, this phenomenon was ruled out on the basis of both the voltage-independent nature of apoE[133–149] inhibition (Fig. 3D) and the insensitivity of apoE[133–149] potency to the ionic strength of the extracellular solution (Fig. 3F).
Fig. 3.
Effects of apoE[133–149] on the antagonism of NMDAR ion channels by spermine. (A). An example of electrophysiological recordings of whole cell steady state currents of HEK293 cells transfected with NR1b/2A by spermine (300 µM) in the absence and presence of apoE[133–149] (2 µM). In this example, the cells were voltage clamped at −70 mV, pH 7.35, 25° C. The bars above the traces denote the intervals during which the indicated solutions were applied to the cells. (B). The effect of the holding voltage on: (○), the agonist activities of NMDA (100 µM)/glycine (10 µM). The steady state current (SSC) obtained at −70 mV was set at 1.0 for normalization of currents at all clamp voltages; (●), the inhibition by 500 µM spermine of the agonist activities of NMDA (100 µM)/glycine (10 µM); (□), the inhibition by 3 µM apoE[133–149] of the agonist activities of NMDA (100 µM)/glycine (10 µM). In each case, NR1b/2A-transfected HEK293 cells were used at pH 7.35, 25° C. (C). Holding voltage dependency of spermine inhibition of current flow through the ion channels of NR1b/2A-transfected HEK293 cells at 25° C. The plot was generated from the data in (B) where, at each indicated voltage, % inhibition = [((normalized SSCNMDA/glycine - normalized SSCNMDA/glycine/spermine)/normalized SSCNMDA/glycine) × 100. (D). Holding voltage dependency of apoE[133–149] inhibition of current flow through the ion channels of NR1b/2A-transfected HEK293 cells at 25° C. The plot was generated from the data in (B) where, at each indicated voltage, % inhibition = [((normalized SSCNMDA/glycine - normalized SSCNMDA/glycine/apoe[133–149])/normalized SSCNMDA/glycine) × 100. (E). The effect of a constant apoE[133–149] concentration (3 µM) on inhibition of steady state current flow by various concentrations of spermine through the ion channels of NR1b/2A-transfected HEK293 cells at −70 mV, pH 7.35, 25° C. The concentrations of NMDA and glycine were 100 µM and 10 µM, respectively. Normalized values for the steady state currents were calculated by arbitrarily setting at 1.0 the value of the current obtained at 100 µM NMDA/10 µM glycine in the absence of spermine and apoE[133–149]. (Black bar) 500 µM spermine and (hatched black and white bar) 500 µM spermine/3 µM apoE[133–149], each added to the extracellular solution after the initial steady state current was established with NMDA (100 µM)/glycine (10 µM). (F). Inhibition by 10 µM apoE[133–149] of steady state whole cell currents induced by 100 µM NMDA/10 µM glycine in NR1b/2A-transfected HEK293 cells when perfused with extracellular solution containing the indicated concentrations of NaCl. The concentrations of NaCl are presented above each bar. A holding voltage of −70 mV, pH 7.35, 25° C, was used.
3.3. The LRP ligands, RAP and α2M*, alter the effects of apoE[133–149] on NMDAR activity
Since the inhibition by apoE[133–149] of NMDAR ion channel currents is consistent with an indirect effect of this peptide on the NMDAR, we explored the possibility that this could result from peptide interaction with LRP, since LRP stimulation has been shown to increase NMDAR-mediated Ca2+ fluxes into neurons (Bacskai et al., 2000). First, we established, from LRP-specific immunofluorescence analyses, that NR1b/2A-transfected HEK293 cells contain LRP (Fig. 4A). Next, the likelihood that the LRP is involved in apoE[133–149]-based attenuation of NMDAR responses is suggested by the results of Fig. 4B, in which the apoE[133–149]-mediated inhibition of NMDAR function is relieved by heparin, a known antagonist of LRP function. Separate studies (data not shown) have demonstrated that heparin did not affect whole cell currents in NR1b/2A-transfected HEK293 cells, when present alone. We considered that the effects of heparin in the presence of apoE[133–149] are due to its interaction with apoE[133–149] (Croy et al., 2004; Weisgraber et al., 1986). This was confirmed by SPR, as shown in the inset of Fig. 4B. This binding of heparin and apoE[133–149] likely inhibits binding of apoE[133–149] to LRP (Croy et al., 2004), similar to that which is observed with protein ligands for LRP that contain known heparin binding sites, e.g., thrombospondin-1 (Godyna et al., 1995) and C4 binding protein (Westein et al., 2002). RAP is another inhibitor of LRP function and was also tested for possible effects on the apoE[133–149] suppression of NMDAR activity. We first sought to establish that RAP competes with apoE[133–149] for binding to LRP. This was achieved by an SPR-monitored experiment in which the affinity of NR1b/2A-transfected HEK293 cells for immobilized apoE peptide was evaluated in the presence and absence of RAP. From the SPR results of Fig. 4C, it is seen that RAP effectively blocked the interaction of apoE[133–149] with these cells. Separate from the case with heparin, RAP did not interact with apoE[133–149]. These results support competition between apoE[133–149] and RAP for binding to LRP. Non-transfected HEK293 cells displayed a similar series of sensorgrams, in agreement with our hypothesis that apoE[133–149] exerts its inhibitory effects on NMDAR activity via an interaction with LRP rather than the NMDAR (data not shown). In light of this apparent competition for LRP binding, we examined whether RAP could reverse the antagonism of NMDAR-mediated currents exerted by apoE[133–149]. As shown in Fig. 4D, a pre-incubation of RAP (250 nM) with NR1b/2A-transfected HEK293 cells attenuated the apoE[133–149]-mediated inhibition of agonist-evoked steady state currents in a time-dependent fashion, reaching equilibrium at 30 min.
Fig. 4.
LRP mediates the inhibitory effects of apoE[133–149] on the NMDAR. (A). Immunofluorescence images for HEK293 cells show the expression of LRP. Left - negative control without anti-LRP antibody; right -NR1b/2A-transfected HEK293 cells incubated with anti-LRP, followed by the secondary antibody, labeled with Alexa Fluor 594 (red stain). (B). Effect of heparin on recovery of NR1b/2A-transfected HEK293 cells from inhibition by apoE[133–149], as measured by whole cell current flow in the presence of 10 µM apoE[133–149]. The IC50 for heparin was 8 µg/ml. Currents were measured at a holding voltage of −70 mV, pH 7.35, 25° C. Inset: SPR sensorgram of the binding of 0.5 µM heparin to apoE[133–149] immobilized on a CM-5 chip. (C). SPR measurements of the effect of RAP on the binding of HEK293 cells to apoE[133–149] immobilized on a CM-3 sensor chip. NR1b/2A-transfected HEK293 cells (2.5 × 105 cells/ml) were passed over immobilized apoE[133–149] in the absence (a) and presence (b) of 1 µM RAP. Injection of RAP alone (c), did not yield a response. (D). Inhibitory effect of 2 µM apoE[133–149] on steady state whole cell currents in NR1b/2A-transfected HEK293 cells in the presence of 250 nM RAP at different preincubation times. The holding voltage was −70 mV, at pH 7.35, 25° C.
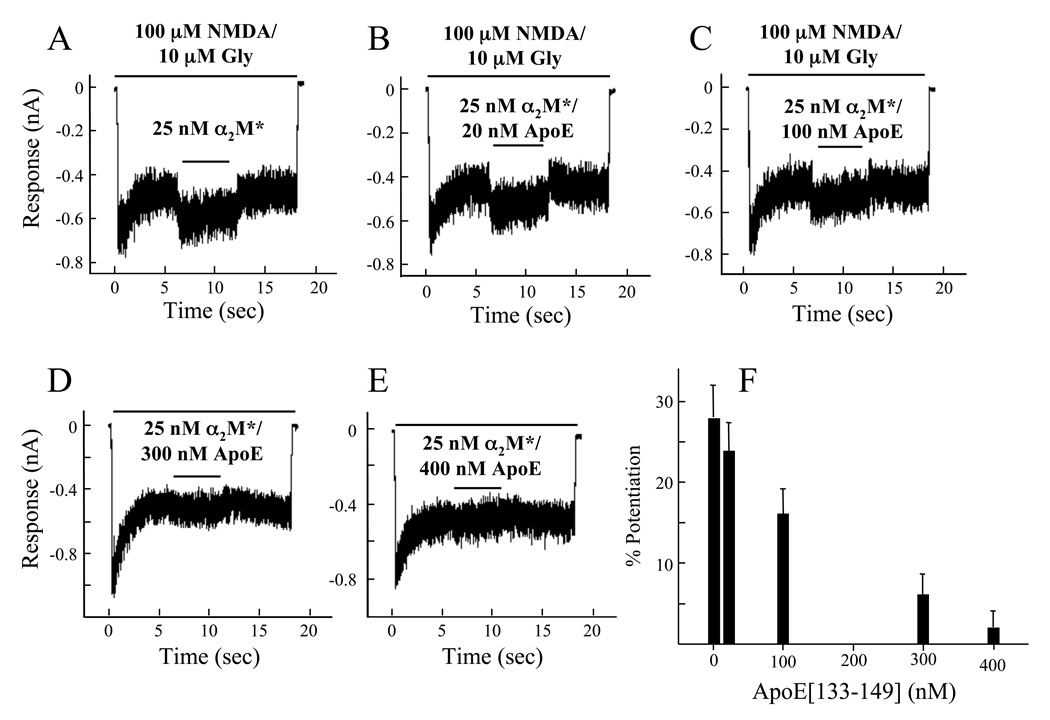
LRP is also a receptor for binding of α2M* (Strickland et al., 1990), and this binding leads to functional consequences, e.g., enhancement of TGF-β1-induced growth responses of smooth muscle cells (Stouffer et al., 1993). We, thus examined whether α2M* would affect current flow in NR1b/2A-transfected cells. Unactivated α2M displayed no effect on steady state currents of these cells, but methylamine-activated α2M (α2M*) at a concentration of 25 nM potentiated the steady state current obtained with 100 µM NMDA/10 µM glycine (Fig. 5A). We next evaluated whether apoE[133–149] would influence this α2M*-induced potentiation of NMDAR whole cell current flow. For these experiments, the highest concentration of apoE[133–149] employed was 400 nM, a concentration at which the inhibitory effect of apoE[133–149] (<5%) is negligible. Figs. 5A–F show that as the concentrations of apoE[133–149] were increased from 20 nM to 400 nM, potentiation of the whole cell currents by 25 nM α2M* was diminished, and reached full recovery at the highest concentration of apoE[133–149]. Using SPR, we found that α2M* did not measurably interact with apoE[133–149] (data not shown). Thus, the abrogation of α2M*-induced NMDAR current potentiation by apoE[133–149] is most likely due to competition with α2M* for the same binding site on the LRP. These data provide further proof that apoE affects NMDAR ion channel activity via the LRP pathway.
Fig. 5.
Effect of α2M* on ion channel steady state currents of the NMDAR. (A). Potentiation of whole cell steady state currents of HEK293 cells transfected with NR1b/2A that are produced with 100 µM NMDA/10 µM glycine during a 10 sec application of 25 nM α2M* (black bar above each trace). In further experiments, the following concentrations of apoE[133–149] were co-applied with α2M*: (B) 20 nM apoE[133–149]; (C) 100 nM apoE[133–149]; (D) 300 nM apoE[133–149]; (E) 400 nM apoE[133–149]. (F). The potentiation values of 25 nM α2M*, obtained from changes in the whole cell steady state currents, are plotted against the concentrations of apoE[133–149], illustrating the attenuation of α2M* stimulation by apoE[133–149]. In all experiments, the holding voltage was −70 mV, at pH 7.35, 25° C.
It has been shown that HSPG cofunctions with LRP in metabolism of apoE-rich lipid remnants (Ji et al., 1993) by initially sequestering apoE-rich particles prior to uptake of apoE by the LRP pathway. This step is also believed to be important in delivering apoE to neurons (Mahley, 1996). Thus, the role of the HSPG as a coreceptor for LRP in this NMDAR ion channel inhibition by apoE[133–149] was examined. The immunofluorescence micrographs of Fig. 6A show that NR1b/2A transfected HEK293 cells possess HSPG on their cell surfaces and that our protocol for treatment of the cells with heparinase-I does, in fact, remove HSPG from the HEK293 cell surface. Despite this, inhibition of NR1b/2A-transfected HEK293 cells by 5 µM apoE[133–149] is very similar without (Fig. 6B) and with (Fig. 6C) heparinase-I treatment. Thus, HSPG does not cofunction with LRP in this case, even though apoE[133–149] can interact with HSPG (Ji et al., 1993; Saito et al., 2003).
Fig. 6.
Involvement of HSPG in the inhibition by apoE[133–149] of ion flow in the NMDAR. (A). Immunofluorescence images of NR1b/2A-transfected HEK293 without (left) and with (right) heparinase-I (10 units/µl for 2 hr). The cells were labeled with rat-anti-heparan sulphate proteoglycan/perlecan antibody, followed by Alexa Fluor 488-conjugated chicken-anti-rat IgG for fluorescence development (green stain). The nuclei were labeled with prolong gold with DAPI (blue stain). (B, C). Inhibition of whole cell current flow induced in NR1b/2A-transfected HEK293 cells by 5 µM apoE without (B) and with (C) heparinase-I treatment, showing similar levels of inhibition. The holding voltage was −70 mV, at pH 7.35, 25° C.
3.4. The LRP pathway is the predominant mediator of NMDAR current inhibition by apoE[133–149]
It has been established that receptors for apoE other than LRP, most notably apoE receptor-2 (apoER2) and VLDL receptor (VLDLR), are also present in NR1-expressing neurons (Sinagra et al., 2005). Also, in the case of apoER2, evidence has emerged suggesting its participation in a multiprotein complex that includes NMDAR subunits (Hoe, et al., 2006). Unlike LRP, neither apoER2 nor VLDLR are present in CHO cells (Tacken et al., 2000). To assess the contribution of LRP to the regulation of apoE-mediated inhibition of NMDAR function, we performed electrophysiology experiments on NR1b/2A-transfected CHO K1 cells, along with 2 mutant CHO K1 cell lines, similarly transfected, that do not express cell-surface LRP. Here, IC50 values for the inhibition of ion current flow, as a function of the concentration of apoE[133–149], were determined with WT CHO cells, mutant CHO 14-2-1 cells expressing only intracellular LRP, and CHO 13-5-1 cells, which do not express any LRP (Fig. 7). IC50 values for apoE[133-149] were increased from 2.8 ± 0.4 µM in WT CHO cells to 8.7 ± 1.1 µM and 8.7 ± 0.7 µM in 14-2-1 CHO cells and 13-5-2 CHO cells, respectively. These results imply that LRP is not the sole mediator of apoE[133–149] inhibition of NMDAR-associated current in NR1b/2A-transfected CHO K1 cells. However, based on the 3-fold greater NMDAR inhibitory potency of the apoE peptide observed with these cells, it appears that the LRP pathway plays a predominant role in this regard.
Fig. 7.
Concentration-response curves of apoE[133–149] antagonism of NMDA/glycine-evoked currents in NR1b/2A-transfected WT and LRP-deficient CHO cells. Currents were elicited with 100 µM NMDA and 10 µM, glycine. Cells were voltage clamped at −70 mV, pH 7.35, at 25° C. The graph depicts the inhibitory effects of increasing apoE[133–149] concentration on current flow through the NR1b/2A ion channel of transfected (■) WT CHO K1 cells, (●) mutant 14-2-1 CHO K1 cells, and (○) mutant 13-5-1 CHO K1 cells. The IC50 values for apoE[133–149]-mediated NMDAR inhibition were 2.8 ± 0.4 mM in WT CHO cells, 8.7 ± 1.1 mM in 14-2-1 CHO cells and 8.7 ± 0.7 mM in 13-5-2 CHO cells.
3.5. The NMDAR inhibitory potency and LRP affinity of apoE-derived peptides are correlated
Results thus far accrued with apoE[133–149] point towards a mode for modulation of NMDAR current responses that is contingent upon a direct peptide-LRP interaction. If operant, this mechanism would predict a correspondence between the LRP binding affinity of apoE-derived peptides and their NMDAR inhibitory potency. To explore this hypothesis, three variants of the LDLR binding region of apoE were synthesized and compared to apoE[133–149] with respect to both LRP affinity and effect on NMDAR current responses. These include the truncation peptides, apoE[143–150] and apoE[145–150], and the replacement variant, apoE[133–149/K143,146E]. Based on previous mutational studies, these peptides were expected to display compromised LRP binding compared to the peptide spanning residues 133–149 (Lalazar, et al., 1988; Zaiou et al., 2000). Binding of these peptides to the LRP was evaluated by SPR, as depicted in Fig. 8A for apoE[133–149] and apoE[133–149/K143,146E]. The Kd values determined in this manner for the four peptides are shown in Table 1, accompanied by their IC50 values for the inhibition of currents in HEK293 expressing the NR1b/2A combination of NMDAR subunits. Concentration-response data (from which the IC50 values were calculated) for apoE[133–149] and apoE[133–149/K143,146E] are plotted in Fig. 8B. All three peptide variants display a decrease in LRP affinity, as well as diminished NMDAR inhibitory activity. These trends reveal that the ability of these peptides to inhibit NMDAR-associated currents is consonant with the strength of their interaction with LRP. This concept is further supported by the SPR results of Fig. 8C, which depict sensorgrams obtained for the binding of apoE[133–149] and apoE[133–149/K143,146E] to a contiguous version of the soluble, extracellular portion of the NR2B receptor. This construct, termed NTD-S1-L-S2, contains the N-terminal domain (NTD) and S1 domain of the ligand binding site joined by a 13-residue linker to the S2 loop of the ligand binding core. By SPR, we have determined that NTD-S1-L-S2 binds glutamate-site agonists and antagonists with affinities comparable to those obtained by electrophysiology on functional NR2B-containing NMDARs, confirming the viability of this polypeptide as a structural mimic of the extracellular portion of NR2B (unpublished data). In marked contrast to their affinities for LRP, the two apoE peptides bind NTD-S1-L-S2 with similar Kd values, i.e., 4.2 ± 1.2 µM for apoE[133–149] and 6.4 ± 1.5 µM for apoE[133–149/K143,146E]. The 73-fold increase in binding affinity that apoE[133–149] displays for LRP versus NTD-S1-L-S2, when considered in tandem with the ability of this peptide to effectively inhibit NMDAR function, points toward a mechanism whereby the interaction of apoE[133–149] with LRP subsequently modulates NMDAR ion channel function. An involvement model in which apoE peptides attenuate NMDAR-associated current through direct binding to the NMDAR would predict similar NMDAR inhibitory activities for apoE[133–149] and apoE[133–149/K143,146E], commensurate with their comparable affinities for NTD-S1-L-S2.
Fig. 8.
The interaction of apoE peptides with LRP and the NMDAR. (A) SPR sensorgrams obtained for the binding of apoE[133–149] (——) and apoE[133–149/K143,146E] (………) to immobilized LRP. Each peptide was injected at a concentration of 4 µM. (B) Concentration-response data for the inhibition of antagonism NMDA/glycine-evoked currents by (○) apoE[133–149] and (●) apoE[133–149/K143,146E] in NR1b/2A-transfected HEK293 cells. The concentrations of NMDA and glycine were 100 µM and 10 µM, respectively. (C) SPR sensorgrams obtained for the binding of apoE[133–149] (——) and apoE[133–149/K143,146E] (------) to NTD-S1-L-S2 of NR2B. Each peptide was injected at a concentration of 4 µM.
Table 1.
LRP binding and NMDAR inhibitory potency of apoE peptides.
| Peptide | Kd (µM)a | IC50 (µM)b |
|---|---|---|
| apoE[133–149] | 0.057 ± 0.002 | 1.3 ± 0.1 |
| apoE[143–150] | 0.72 ± 0.21 | 3.3 ± 0.1 |
| apoE[145–150] | 15.0 ± 6.0 | 17.5 ± 0.6 |
| apoE[133–149/K143,146E] | 6.7 ± 1.7 | >100 |
Values were obtained from SPR-monitored binding of peptides to immobilized LRP.
Concentration of peptide needed to achieve 50% inhibition of NMDA/glycine-evoked currents in NR1b/2A-transfected HEK293 cells.
4. Discussion
The cholesterol transport protein, apoE, is also an isoform-dependent risk factor for neurological diseases. At least four polymorphic forms of apoE exist, based on amino acid substitutions at positions 112 and 158 in apoE helices 3 and 4, respectively. One isoform in particular, apoE-4 (Arg112, Arg158), is a genetic determinant for AD (Tsai et al., 1994). Many neuropathies are also linked to disruption of the NMDAR ion channel, which may play an important role in the synaptic basis of neocortical plasticity deficit in AD (Battaglia et al., 2007; Morimoto et al., 1991) Excessive Ca2+ influx in neuronal cells via the NMDAR ion channel is known to contribute to excitotoxic cell death. The glutamatergic properties of the NMDAR are dependent on its particular subunit composition, in both health and disease, and alterations in the temporal and spatial gene expression of NMDAR subunits can lead to various neuropathologies. For example, in postmortem brain samples of AD patients, reduced expression levels of NR2A and NR2B (Bi and Sze, 2002), as well as NR1 (Ulas and Cotman, 1997), but not NR2C and NR2D (Hynd et al., 2004), were found in the hippocampus region of the brain, an area linked with certain types of learning, cognition and memory.
The possible association of apoE with reduction of glutamate neurotoxicity in a cell culture model of cerebral ischemia (Aono et al., 2002), and the possible role of the LDLR in this activity (Aono et al., 2003; Lee et al., 2004), provided the impetus for the current study. It has become increasingly attractive to speculate that the links between apoE, NMDAR and LDLR should focus on LRP, since ligands of LRP, e.g., activated (*) α2M, stimulate Ca2+ influx into neuronal cells via the NMDAR (Bacskai et al., 2000; Harris-White and Frautschy, 2005; Qiu et al., 2002) In the current report, we especially focused on assessing mechanisms of the relationships the NMDAR activity, the LDLR-binding region of apoE, and LRP. This was accomplished by synthesizing peptides with homology to the LDLR-binding region of apoE and, by employing a HEK293 cell system that was transfected with specific NMDAR subunits, assessing their influence on current flow through the NMDAR by electrophysiology.
The data obtained from the current studies on the nature of the inhibition, by apoE[133–149], in the presence of NMDAR coagonists, NMDA and glycine, and of an independent NMDAR modulator, spermine, suggest that apoE[133–149] does not function directly with the NMDAR. This supposition is further supported by the finding that apoE[133–149] inhibition of ion flow through NMDAR channels is not influenced by the subunit nature of the NMDAR, indicating that the effects of apoE[133–149] on NMDAR activity are of an indirect nature. Because apoE[133–149] was derived from the LDLR binding region of apoE, we hypothesized that it exerts its NMDAR inhibitory effects primarily through direct interaction with LRP. This hypothesis is supported by work on a model system of neuroAIDS, in which it was shown that the HIV-encoded gene product, tat, mediates the neural pathology via a macromolecular complex involving LRP and NMDAR, suggesting a link between LRP and NMDAR function in whole cell systems (Eugenin et al., 2007) Indeed, our immunofluorescence studies with NMDAR-transfected HEK293 cells shows the presence of LRP on these cells, a necessary feature of our hypothesis. The attenuation of the apoE[133–149]-based inhibition of ion currents that develops in NMDAR-transfected HEK cells upon addition of RAP and α2M* provides strong direct support for the involvement of LRP in the electrophysiological response of the NMDAR. Confirmation of this conclusion is offered by the experiments presented that show that NMDAR-transfected mutant CHO cell lines, devoid of surface LRP, display 3-fold higher IC50 values for apoE[133–149] inhibition of NMDAR-associated currents compared to WT CHO cells, which do contain cell surface LRP. Hence, we conclude that LRP is the primary binding locus for mediating apoE[133–149] inhibition of the NMDAR, with residual activity attributable to other receptor-linked pathways. Furthermore, our results with additional apoE-based peptides reveal that the NMDAR inhibitory potency of these derivatives is commensurate with their LRP binding affinity. Along these lines, one member of this apoE-derived peptide panel, apoE[133–149/K143,146E], binds the extracellular ligand binding domain of the NMDAR with a Kd value comparable to that obtained for apoE[133–149], but manifests greatly reduced LRP affinity (~70-fold), as well as compromised NMDAR inhibitory activity (at least 75-fold) compared with the parent apoE peptide. Hence, the agreement between NMDAR inhibition and LRP affinity coupled with the incongruity between NMDAR inhibition and NMDAR affinity strongly suggests that the direct binding of apoE-based peptides to the NMDAR does not contribute to observed modulation of the receptor.
It has been shown previously that heparin can interact with a variety of functional modulators of the NMDAR, and, consequently, abrogate their effects in this system (Charriaut-Marlangue et al., 1991; Pittaluga et al., 2000) Since we show that heparin, alone, does not effect ion currents through the NMDAR in our system, we conclude that heparin exerts its effects via interaction with apoE[133–149], an interaction that is known to occur (Croy et al., 2004; Weisgraber et al., 1986) 9and this study). Heparin also interacts with ligands of LRP that contain heparin binding domains, and inhibits the interaction of these ligands with LRP. Thus, it is likely that heparin binding to apoE[133–149] limits binding of apoE to LRP, thus attenuatingthe function of apoE[133–149]. We also explored the possibility that apoE[133–149] first interacts with HSPG, also shown herein to be present on the cell surface of NMDAR-transfected HEK293 cells, and is then shuttled to LRP in a process that is inhibited by heparin. However, we show that the heparinase-treated cells, which are rendered essentially devoid of HSPG, do not affect the inhibitory properties of apoE[133–149] toward NMDAR ion flow, strongly suggesting that HSPG does not function as a necessary coreceptor for LRP in this system.
In summary, we have used electrophysiology to assess the functional mechanisms involved in the inhibition by the LDLR domain of apoE of NMDAR ion currents. The results show that the effects of apoE in this regard are indirect, and require LRP for this activity to be expressed in whole cell systems. These findings have important implications for assessing molecular drug target potential in the many neuropathies involving altered NMDAR function.
Acknowledgments
This work was supported by grant HL019982 (to FJC) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nonstandard abbreviations: α2M, α2-macroglobulin; *α2M, activated α2-macroglobulin; apoE, apolipoprotein E; CHO, Chinese hamster ovary cells; DMEM, Dulbecco’s modified Eagle’s medium; GFP, green fluorescent protein; HEK293, human embryonic kidney 293 cells; HSPG, heparin sulphate proteoglycans; LDLR, low-density lipoprotein receptor; LRP, low density lipoprotein receptor-related protein; NMDA, N-methyl-D-aspartate; NMDAR, N-methyl-D-aspartate receptor; RAP, receptor-associated protein; NR1, N-methyl-D-aspartate receptor subunit 1, and its 8 splice variants (1a/b-4a/b); NR2, N-methyl-D-aspartate receptor subunit 2, with its 4 gene products (A–D); SPR, surface plasmon resonance; RU, response units; WT, wild-type.
All electrophysiology traces depicted in the figures correspond to NR1b/2A-transfected HEK293 or CHO cells. In general, the extent of desensitization (ratio of peak to steady-state currents) and rate of deactivation (decay to the steady-state current) were greater for NR2A-versus NR2B-containing cells, as has been noted in previous studies (Vicini et al, 1998; Kendrick et al., 1998). However, significant variability in the degree and rate of desensitization was observed among cells expressing each of the four possible NR1(a or b)/NR2(A or B) combinations. This can be seen in the figures for the NR1b/2A-containing transfectants. Such variability within discrete subunit combinations is not uncommon and may reflect such factors as cell-to-cell differences in post-translational modification of the NMDAR and/or subunit copy number (Vicini et al., 1998).
References
- Aggerbeck LP, Wetterau JR, Weisgraber KH, Wu CS, Lindgren FT. Human apolipoprotein E3 in aqueous solution. II. Properties of the amino- and carboxyl-terminal domains. J Biol Chem. 1988;263:6249–6258. [PubMed] [Google Scholar]
- Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- Aono M, Lee Y, Grant ER, Zivin RA, Pearlstein RD, Warner DS, Bennett ER, Laskowitz DT. Apolipoprotein E protects against NMDA excitotoxicity. Neurobiol Dis. 2002;11:214–220. doi: 10.1006/nbdi.2002.0541. [DOI] [PubMed] [Google Scholar]
- Araneda RC, Lan JY, Zheng X, Zukin RS, Bennett MVL. Spermine and arcaine block and permeate N-methyl-D-aspartate receptor channels. Biophys J. 1999;76:2899–2911. doi: 10.1016/S0006-3495(99)77445-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, Ghilardix MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer's Disease in humans and rodents. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.02.027. in press. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Multiple effects of spermine on N-methyl-D-aspartaic acid responses of rat cultured hippocampal neurones. J Physiol. 1993;161:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Sze CI. N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer's disease. J Neurol Sci. 2002;200:11–18. doi: 10.1016/s0022-510x(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Ciu J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Otani S, Creuzet C, Ben-Ari Y, Loeb J. Rapid activation of hippocampal casein kinase II during long-term potentiation. Proc Natl Acad Sci USA. 1991;88:10232–10236. doi: 10.1073/pnas.88.22.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MA, Anantharamaiah GM, Mistry MJ, Balasubramaniam A, Harmony JA. Localization of a domain in apolipoprotein E with both cytostatic and cytotoxic activity. Biochemistry. 1995;34:11142–11151. doi: 10.1021/bi00035a020. [DOI] [PubMed] [Google Scholar]
- Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, ApoE(130–149) and ApoE(141–155)2, bind to LRP1. Biochemistry. 2004;43:7328–7335. doi: 10.1021/bi036208p. [DOI] [PubMed] [Google Scholar]
- Dallongeville J, Roy M, Leboeuf N, Xhignesse M, Davignon J, Lussier-Cacan S. Apolipoprotein E polymorphism association with lipoprotein profile in endogenous hypertriglyceridemia and familial hypercholesterolemia. Arterioscler Thromb. 1991;11:272–278. doi: 10.1161/01.atv.11.2.272. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jørgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol Pharmacol. 2007;72:907–920. doi: 10.1124/mol.107.037333. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald DJ, Fryling CM, Zdanovsky A, Saelinger CB, Kounnas M, Winkles JA, Strickland D, Leppla S. Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein. J Cell Biol. 1995;129:1533–1541. doi: 10.1083/jcb.129.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer's and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4:489–460. doi: 10.2174/156800705774322102. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, Ehlers MD, Rebeck GW. Apolipoprotein E receptor 2 interactions with N-methyl-D-aspartate receptor. J Biol Chem. 2006;281:3425–3431. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonis-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Differential expression of N-methyl-D-aspartate receptor NR2 isoforms in Alzheimer's disease. J Neurochem. 2004;90:913–919. doi: 10.1111/j.1471-4159.2004.02548.x. [DOI] [PubMed] [Google Scholar]
- Innerarity TL, Friedlander E, Rall SC, Weisgraber KH, Mahley RW. The receptor-binding domain of human apolipoprotein E. Binding of apolipoprotein E fragments. J Biol Chem. 1983;258:12341–12347. [PubMed] [Google Scholar]
- Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TL, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–10167. [PubMed] [Google Scholar]
- Kendrick SJ, Dichter MA, Wilcox KS. Characterization of desensitization in recombinant N-methyl-D-aspartate receptors: comparison with native receptors in cultured hippocampal neurons. Brain Res Mol Brain Res. 1998;57:10–20. doi: 10.1016/s0169-328x(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Klein RC, Prorok M, Galdzicki Z, Castellino FJ. The amino acid residue at sequence position 5 in the conantokin peptides partially governs subunit-selective antagonism of recombinant N-methyl-D-aspartate receptors. J Biol Chem. 2001;276(29):26860–26867. doi: 10.1074/jbc.M102428200. [DOI] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Inhibition of nicotinic acetylcholine receptors by apolipoprotein E-derived peptides in rat hippocampal slices. Neuroscience. 2004;127:563–567. doi: 10.1016/j.neuroscience.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Lalazar A, Weisgraber KH, Rall SC, Jr, Giladi H, Innerarity TL, Levanon AZ, Boyles JK, Amit B, Gorecki M, Mahley RW, Vogel T. Site-specific mutagenesis of human apolipoprotein E. Receptor binding activity of variants with single amino acid substitutions. J Biol Chem. 1988;263:3542–3545. [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lee Y, Aono M, Laskowitz D, Warner DS, Pearlstein RD. Apolipoprotein E protects against oxidative stress in mixed neuronal-glial cell cultures by reducing glutamate toxicity. Neurochem Int. 2004;44:107–118. doi: 10.1016/s0197-0186(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Madry C, Mesic I, Bartholomäus I, Nicke A, Betz H, Laube B. Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function. Biochem Biophys Res Commun. 2007;354:102–108. doi: 10.1016/j.bbrc.2006.12.153. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Heparan sulfate proteoglycan/low density lipoprotein receptor-related protein pathway involved in type III hyperlipoproteinemia and Alzheimer's disease. Isr J Med Sci. 1996;32:414–429. [PubMed] [Google Scholar]
- Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukoc Biol. 2001;70:677–683. [PubMed] [Google Scholar]
- Molinoff PB, Williams K, Pritchett DB, Zhong J. Molecular pharmacology of NMDA receptors: modulatory role of NR2 subunits. Prog Brain Res. 1994;100:39–45. doi: 10.1016/s0079-6123(08)60766-9. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Katayama K, Inoue K, Sato K. Effects of competitive and noncompetitive NMDA receptor antagonists on kindling and LTP. Pharmacol Biochem Behav. 1991;40:893–899. doi: 10.1016/0091-3057(91)90103-9. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Bonfanti A, Raiteri M. Somatostatin potentiates NMDA receptor function via activation of InsP(3) receptors and PKC leading to removal of the Mg(2+) block without depolarization. Br J Pharmacol. 2000;130:557–566. doi: 10.1038/sj.bjp.0703346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok M, Castellino FJ. The molecular basis of conantokin antagonism of NMDA receptor function. Curr Drug Targets. 2007;8:633–642. doi: 10.2174/138945007780618481. [DOI] [PubMed] [Google Scholar]
- Prorok M, Warder SE, Blandl T, Castellino FJ. Calcium binding properties of synthetic γ-carboxyglutamic acid containing marine cone snail "sleeper" peptides, conantokin-G and conantokin-T. Biochemistry. 1996;35:16528–16534. doi: 10.1021/bi9621122. [DOI] [PubMed] [Google Scholar]
- Qiu ZH, Strickland DK, Hyman BT, Rebeck GW. alpha 2-macroglobulin exposure reduces calcium responses to N-methyl-D-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J Biol Chem. 2002;277:14458–14466. doi: 10.1074/jbc.M112066200. [DOI] [PubMed] [Google Scholar]
- Saito H, Dhanasekaran P, Nguyen D, Baldwin F, Weisgraber KH, Wehrli S, Phillips MC, Lund-Katz S. Characterization of the heparin binding sites in human apolipoprotein E. J Biol Chem. 2003;278:14782–14787. doi: 10.1074/jbc.M213207200. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Dai Q, Prorok M, Castellino FJ. Subtype-selective antagonism of N-methyl-d-aspartate receptor ion channels by synthetic conantokin peptides. Neuropharmacology. 2007;53:145–156. doi: 10.1016/j.neuropharm.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci. 2005;25:6127–6136. doi: 10.1523/JNEUROSCI.1757-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer GA, LaMarre J, Gonias SL, Owens GK. Activated alpha 2-macroglobulin and transforming growth factor-beta 1 induce a synergistic smooth muscle cell proliferative response. J Biol Chem. 1993;268:18340–18344. [PubMed] [Google Scholar]
- Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken PJ, Beer FD, Vark LC, Havekes LM, Hofker V, Willems Van Dijk K. Very-low-density lipoprotein binding to the apolipoprotein E receptor 2 is enhanced by lipoprotein lipase, and does not require apolipoprotein E. Biochem J. 2000;347:357–361. doi: 10.1042/0264-6021:3470357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Tangalos EG, Petersen RC, Smith GE, Schaid DJ, Kokmen E, Ivnik RJ, Thibodeau SN. Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- Ulas J, Cotman CW. Decreased expression of N-methyl-d-aspartate receptor 1 messenger RNA in select regions of Alzheimer brain. Neuroscience. 1997;79:973–982. doi: 10.1016/s0306-4522(97)00023-7. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH, Innerarity TL, Rall SC, Mahley RW. Receptor interactions controlling lipoprotein metabolism. Can J Biochem Cell Biol. 1985;63:898–905. doi: 10.1139/o85-111. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH, Rall SC, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein E. Determination of the heparin binding sites of apolipoprotein E3. J Biol Chem. 1986;261:2068–2076. [PubMed] [Google Scholar]
- Weisgraber KH, Xi Q. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
- Westein E, Denis CV, Bouma BN, Lenting PJ. The alpha -chains of C4b-binding protein mediate complex formation with low density lipoprotein receptor-related protein. J Biol Chem. 2002;277:2511–2516. doi: 10.1074/jbc.M102293200. [DOI] [PubMed] [Google Scholar]
- Westerlund JA, Weisgraber KH. Discrete carboxyl-terminal segments of apolipoprotein E mediate lipoprotein association and protein oligomerization. J Biol Chem. 1993;268:15745–15750. [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Rall SC, Weisgraber KH. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J Biol Chem. 1988;263:6240–6248. [PubMed] [Google Scholar]
- Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Breslow JL. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981;20:1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- Zaiou M, Arnold KS, Newhouse YM, Innerarity TL, Weisgraber KH, Segall ML, Phillips MC, Lund-Katz S. Apolipoprotein E;-low density lipoprotein receptor interaction. Influences of basic residue and amphipathic alpha-helix organization in the ligand. J Lipid Res. 2000;42:1087–1095. [PubMed] [Google Scholar]