Abstract
The purine analog xanthine oxidase (XO) inhibitors (XOIs), allopurinol and oxypurinol, have been reported to protect against heart failure secondary to myocardial infarction or rapid ventricular pacing. Since these agents might influence other aspects of purine metabolism that could influence their effect, this study examined the effect of the non-purine XOI, febuxostat, on pressure overload-induced left ventricular (LV) hypertrophy and dysfunction. Transverse aortic constriction (TAC) in mice caused LV hypertrophy and dysfunction as well as increased myocardial nitrotyrosine at 8 days. TAC also caused increased phosphorylated Akt (p-AktSer473), p42/44 extracellular signal-regulated kinase (p-ErkThr202/Tyr204) and mammalian target of rapamycin (mTOR) (p-mTORSer2488). XO inhibition with febuxostat (5mg/kg/day by gavage for 8 days) beginning ~60 minutes after TAC attenuated the TAC-induced LV hypertrophy and dysfunction. Febuxostat blunted the TAC-induced increases in nitrotyrosine (indicating reduced myocardial oxidative stress), p-ErkThr202/Tyr204 and p-mTORSer2488, with no effect on total Erk or total mTOR. Febuxostat had no effect on myocardial p-AktSer473 or total Akt. The results suggest that XO inhibition with febuxostat reduced oxidative stress in the pressure overloaded LV, thereby diminishing the activation of pathways that result in pathologic hypertrophy and contractile dysfunction.
Keywords: xanthine oxidase inhibition, pressure overload, myocardial hypertrophy, left ventricular dysfunction
Introduction
Xanthine oxidase (XO) is the rate-limiting enzyme in the purine degradation pathway 1 which catalyzes the final two steps in the conversion of adenosine to uric acid:
ATP↔ADP ↔AMP ↔Adenosine ↔ Inosine ↔ Hypoxanthine→ Xanthine→Uric Acid. The final two reactions are irreversible, so their products cannot be reincorporated into ATP by the salvage pathway. XO is activated by ischemia and its expression and activity are increased in hypertrophied and failing hearts 2,3. The XO reaction results in the generation of superoxide anion (O2−), and there is evidence that increased XO activity in congestive heart failure (CHF) contributes to the increased oxidative stress that has been proposed to contribute to the depressed myocardial contractile performance seen in this condition4,5.
Several investigators have reported that XO inhibition with allopurinol or oxypurinol can improve left ventricular (LV) function in the failing heart 4,6,7,8, prevent myocardial infarct-induced LV remodeling 9, 10, 11, and reverse LV remodeling in animals with dilated cardiomyopathy 12. However, allopurinol and oxypurinol are purine analogs that may have other unanticipated effects on purine metabolism. Febuxostat, a new potent selective non-purine XO inhibitor (XOI) 13, has been shown to improve LV function in pacing-induced CHF in dogs 14. The present study was performed to determine whether chronic treatment with febuxostat can attenuate LV oxidative stress, hypertrophy, and CHF induced by transverse aortic constriction (TAC) in mice.
Materials and Methods
Mice
Male C57/BL6 mice at 8–9 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME) for use in the study. Mice were housed in an air-conditioned room with a 12-h:12-h light-dark cycle, received standard mouse chow, and drank tap water. This study was approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Experimental Protocol
This study was designed to determine whether febuxostat treatment, beginning immediately after the onset of acute pressure overload, could protect against TAC-induced LV hypertrophy, dysfunction and mortality. Three treatment groups were included: sham-operated mice (n=20), TAC mice treated with vehicle (0.5% methylcellulose) (n=30), and TAC mice treated with febuxostat (Teijin Limited, Yamaguchi, Japan; suspension in 0.5% methylcellulose) (n=30). To generate LV hypertrophy in a short time period with a significant incidence of cardiac mortality, TAC was created by ligating the transverse aorta against a 27 G needle to create severe LV pressure overload. Since mice tend to take in less water and food during the first 1–3 days after the TAC procedure, febuxostat (5mg/kg/day in 250 μl vehicle) or vehicle (250 μl) was administered daily by oral gavage for a total of 8 days, with the first dose administered on the day of the surgery after the animal awoke from anesthesia (~60 min after the TAC procedure). On the eighth day, LV function was assessed by echocardiography approximately 1 h after the last febuxostat or vehicle dose. Approximately 3 h after echocardiographic evaluation (4 h after the last febuxostat or vehicle dose), the mice were weighed and blood samples collected. The animals were euthanized and the heart and lungs excised; the right and left atria were trimmed away, and the ventricles and lungs were weighed. Tissue from the left ventricle was used for histological evaluation and Western blots.
Minimally Invasive TAC Procedure
TAC was performed using the minimally invasive suprasternal approach described by Hu et al 15. Mice were anesthetized with a mixture of 80 mg/kg ketamine and 30 mg/kg xylazine intraperitoneally. A horizontal incision was made at the level of the suprasternal notch to allow direct visualization of the transverse aorta without entering the pleural space. With the aid of a dissecting microscope, aortic constriction was performed by ligating the aorta between the right innominate artery and the left carotid artery over a 27 G needle using 5–0 silk suture. The needle was immediately removed after ligation, leaving the aortic constriction in place. The incision was closed and the animals were allowed to recover. Analgesia was provided before the mice awoke from anesthesia. Sham surgery utilized the same procedure but without aortic ligation.
Echocardiography
Echocardiography was performed on mice anesthetized with 1.5 % isoflurane by inhalation as previously described 16, 17. LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), and LV end-diastolic and end-systolic wall thicknesses (anterior and posterior) were measured using 2-dimensional guided M-mode echocardiography. LV fractional shortening (LVFS, %) was calculated as: LVFS = (LVEDD − LVESD)/LVEDD·100. LV ejection fraction (LVEF, %) was calculated by the cubic method: LVEF = [(LVEDD)3 −(LVESD)3]/(LVEDD)3 × 100%.
Western Analysis
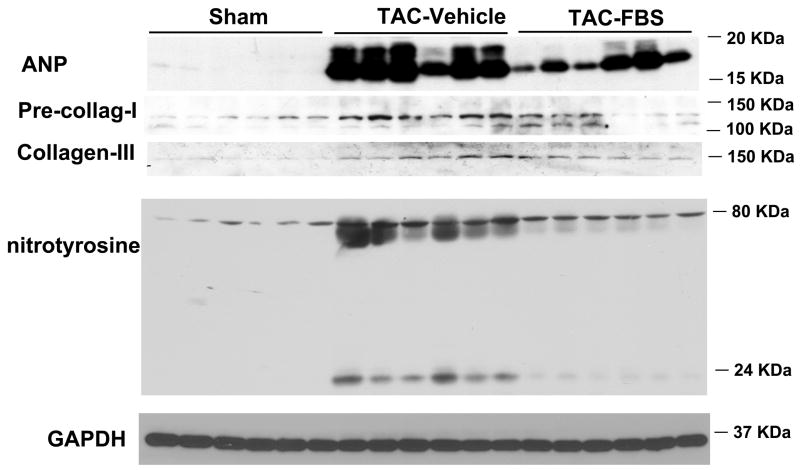
LV homogenates were clarified by centrifugation, and equal amounts of protein were loaded on 8–15 % SDS-polyacrylamide gels and subjected to electrophoresis as described previously 17. The separated protein bands were transferred to a HyBond nitrocellulose membrane, incubated with primary antibodies followed by horseradish peroxidase-labeled secondary antibody, and detected by enhanced chemiluminescent substrate (Amersham, Piscataway, NJ). Light emission was detected by exposure to Fuji RX autoradiography film. Signal intensities were quantified using laser densitometry (Molecular Dynamics, Sunnyvale, CA). Primary antibodies against atrial natriuretic peptide (ANP) (Peninsula Laboratories Inc. San Carlos, CA), nitrotyrosine (Cayman Chemical, Ann Arbor, MI), pre-collagen I (Santa Cruz, Santa Cruz, CA), collagen III (Sigma, St. Louis, MO), total mammalian target of rapamycin (mTOR) (Cell Signaling, Danvers, MA), phosphorylated mTOR (p-mTOR Ser2488) (Cell Signaling, Danvers, MA), Akt [protein kinase B (PKB)] (Santa Cruz, Santa Cruz, CA), phosphorylated Akt (p-Akt Ser473) (Santa Cruz, Santa Cruz, CA), total extracellular signal-regulated kinase (Erk) (Cell Signaling, Danvers, MA), GAPDH (Santa Cruz, Santa Cruz, CA) and phosphorylated Erk (p-ErkThr202/204) (Cell Signaling, Danvers, MA) were used for Western blots.
Histological Staining and Measurement of Cardiac Myocyte Hypertrophy
Tissue sections (8 μm thickness) of the central portion of the LV were stained with hematoxylin and eosin (H&E; Sigma, St. Louis, MO) for overall morphology or with Masson’s trichrome (Sigma, St. Louis, MO) for myocardial fibrosis16. FITC-conjugated wheat germ agglutinin (Invitrogen, Carlsbad, CA) was used to stain the cell membranes for determination of cardiac myocyte size. Cardiac myocyte size was assessed by measuring the short axis diameter of cardiomyocytes in cross section. For each heart, more than 100 cells in at least four representative cross sectional areas were measured, and data were averaged from four representative hearts in each treatment group.
Plasma Uric Acid Analysis
The concentration of uric acid in plasma was determined using Uric Acid Reagent (ThermoDMA, Louisville, CO) according to the manufacturer’s protocol.
Data and Statistical Analyses
All values were expressed as mean ± standard error of the mean (SEM). Statistical significance was defined as P < 0.05. One-way analysis of variance (ANOVA) was used to test each variable for differences among the treatment groups with StatView (SAS Institute, Cary, NC). If the ANOVA demonstrated a significant effect, post hoc comparisons were made pairwise using Fisher’s least significant difference test. When the data suggest a trend of difference between the groups, but did not show significance in the above tests, paired Student T test was also used as specially indicated. The Fisher exact test was used to compare mortality data among the treatment groups.
Results
Body and organ weights, mortality, and uric acid
Body weight, ventricular weight, lung weight, the ratio of ventricular weight to body weight, and the ratio of lung weight to body weight are shown in Figure 1 or Table 1. Eight days after TAC, the surviving animals had significant ventricular hypertrophy and pulmonary congestion. The degree of ventricular hypertrophy, assessed by the ratio of ventricular mass to body weight, was reduced by approximately 15% with febuxostat treatment (P<0.05) (Figure 1A). Febuxostat treatment also decreased the degree of pulmonary congestion, assessed by the ratio of lung weight to body weight, by about 18%, although the difference was only statistically significant as compared to the TAC vehicle control group by using a paired Student t test (Figure 1B). TAC resulted in significant mortality (mostly in the first four days after surgery; 12 out of 30 mice died in the TAC vehicle control group), and this was not affected by febuxostat treatment (14 out of 30 mice died) (Figure 1C). TAC significantly increased plasma uric acid. As expected, febuxostat significantly decreased plasma uric acid, and the uric acid level in the febuxostat-treated animals tended to be less than in the untreated sham animals (Figure 1D), indicating that febuxostat treatment caused effective XO inhibition.
Figure 1.
Administration of febuxostat (FBS) for 8 days, beginning ~60 minutes after chronic transverse aortic constriction (TAC), significantly attenuated the TAC-induced left ventricular (LV) hypertrophy (p<0.01) (A) and tended to decrease the TAC-induced pulmonary congestion (B). However, febuxostat had no effect on TAC-induced cardiac sudden death (C). Febuxostat also significantly decreased plasma levels of uric acid (D). *p<0.05 as compared with sham group; #p<0.05 as compared with vehicle group (TAC+VH); †p<0.05 versus sham group and ‡p<0.05 versus TAC+VH group using Student’s paired t test.
Table 1.
Effect of treatment with febuxostat (FBS) beginning ~60 minutes after transverse aortic constriction (TAC) on ventricular and lung weights, left ventricular (LV) dimensions, and LV systolic function.
| Parameter | Sham | TAC + Vehicle | TAC + FBS |
|---|---|---|---|
| Body weight (g) | 23.5 ± 0.6 | 22.8 ± 0.7 | 23.4 ± 0.9 |
| Ventricle weight (mg) | 98 ± 3 | 159 ± 6 * | 138 ± 7.0 *,# |
| Lung weight (mg) | 132 ± 4 | 195 ± 20 * | 163 ± 16 |
| Heart rate (beats/min) | 478 ± 19 | 418 ± 14 * | 415 ± 18 * |
| LV fractional shortening (%) | 39.9 ± 1.6 | 22.1 ± 1.6 * | 31.5 ± 2.2 *,# |
| Anterior wall thickness at end-systole (mm) | 0.98 ± 0.03 | 1.14 ± 0.03 * | 1.10 ± 0.04 * |
| Posterior wall thickness at end-systole (mm) | 0.98 ± 0.03 | 1.13 ± 0.03 * | 1.10 ± 0.03 * |
| Anterior wall thickness at end-diastole (mm) | 0.69 ± 0.02 | 0.90 ± 0.02 * | 0.84 ± 0.02 * |
| Posterior wall thickness at end-diastole (mm) | 0.69 ± 0.02 | 0.90 ± 0.02 * | 0.83 ± 0.02 *,# |
P<0.05 as compared to sham group.
P<0.05 as compared to TAC + vehicle group.
Echocardiographic imaging of the heart
In vehicle-treated animals, at 8 days after TAC, LV ejection fraction (Figure 2A) and fractional shortening (Table 1) were significantly decreased, while LV wall thickness (Table 1) was increased. Febuxostat treatment significantly attenuated the TAC-induced increase of LV end-diastolic wall thickness (Figure 2B) and also attenuated the decreases of LV fractional shortening and ejection fraction following TAC (Figure 2A, Table 1). Moreover, febuxostat treatment showed a trend toward attenuating the TAC-induced increase of LV diameter at end-systole (it was statistically significant by the Student’s t test) (Figure 2C). Febuxostat had no significant effect on LV diameter at end-diastole (Figure 2D). These findings indicate that early treatment with febuxostat protected against the LV hypertrophy and dysfunction that occurred in response to systolic overload.
Figure 2.
Administration of febuxostat (FBS) for 8 days, beginning ~60 minutes after chronic transverse aortic constriction (TAC), significantly attenuated the TAC-induced decrease of left ventricular (LV) ejection fraction (A) and increase of LV end-diastolic posterior wall thickness (B); there was a trend toward attenuation of the increase of LV diameter at end-systole (C). However, LV diameter at end-diastole was not changed (D). *p<0.05 as compared with sham group; #p<0.05 as compared with vehicle group (TAC+VH); †p<0.05 versus sham group and ‡p<0.05 versus TAC+VH group using Student’s paired t test.
Histological staining
TAC significantly increased cardiac myocyte diameter from 13.2 ± 0.2 μm in the sham group to 17.8 ± 0.3 μm in the vehicle-treated TAC group, whereas febuxostat significantly attenuated the TAC-induced increase of cardiomyocyte diameter to 16.3 ± 0.3 μm (p<0.05). This is consistent with the finding of less ventricular hypertrophy in the febuxostat-treated TAC group. TAC significantly increased myocardial fibrosis from 2.6 ± 0.29% in the sham group to 12.2 ± 3.0% in the vehicle-treated TAC group, whereas febuxostat tended to decrease the TAC-induced increase of myocardial fibrosis to 8.3 ± 2.% (p=0.05).
Biochemical analysis
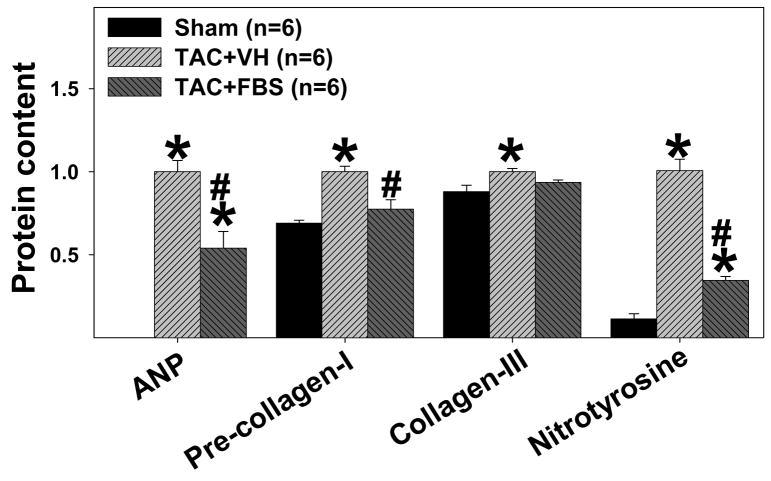
Febuxostat treatment significantly attenuated the TAC-induced increase of myocardial ANP (Figure 3), a biochemical marker of myocardial hypertrophy and CHF 18. In addition, febuxostat significantly attenuated the TAC-induced increases of myocardial pre-collagen I and collagen III content (Figure 3), suggesting that febuxostat treatment limited ventricular collagen synthesis. Furthermore, febuxostat significantly blunted the TAC-induced increase of nitrotyrosine, a biochemical marker of myocardial oxidative stress 19.
Figure 3.
Administration of febuxostat (FBS) for 8 days, beginning ~60 minutes after transverse aortic constriction (TAC), significantly attenuated the TAC-induced increase of ANP, pre-collagen-I, collagen-III, and myocardial nitrotyrosine. *p<0.05 as compared with sham group; #p<0.05 as compared with vehicle group (TAC+VH). n indicates the number of mice.
Myocardial p-mTOR and p-Erk Thr202/204
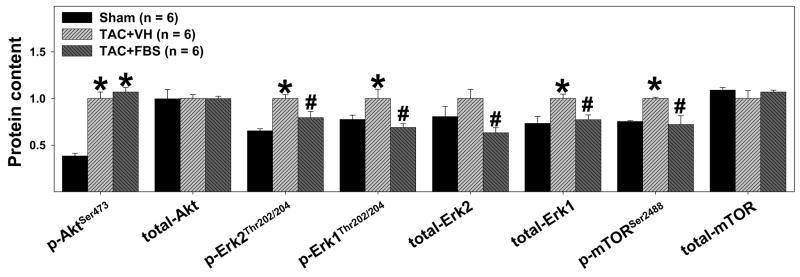
Systolic overload-induced myocardial hypertrophy is associated with activation of the PI3 kinase-Akt/PKB and mitogen-activated protein kinase signaling pathways, which play crucial roles in control of cell growth. Therefore, myocardial levels of total Akt, p-AktSer473, total mTOR, p-mTORSer2488 (a downstream target of Akt), total Erk, and p-ErkThr202/204 were determined by Western blots. TAC caused significant increases of p-Akt Ser473, p-mTOR Ser2488, t-Erk, and p-Erk Thr202/204, but had no effect on t-Akt and t-mTOR (Figure 4). Treatment with febuxostat significantly attenuated the TAC-induced increases of p-mTOR Ser2488, t-Erk, and p-Erk Thr202/204, but not of t-Akt and p-Akt Ser473 (Figure 4), indicating that febuxostat blunted the TAC-induced activation of the mTOR and Erk signaling pathways.
Figure 4.
Administration of febuxostat (FBS) for 8 days, beginning ~60 minutes after transverse aortic constriction (TAC), significantly attenuated the TAC-induced increase of phosphorylation of p-Erk1 (44 KDa), p-Erk2 (42 KDa), total Erk1 (t-Erk1), total Erk2 (t-Erk2), and p-mTORSer2488. Total Akt (t-Akt) and total mTOR (t-mTOR) were not changed after TAC. *p<0.05 as compared with sham group; #p<0.05 as compared with vehicle group (TAC+VH).
Discussion
In the present study, systolic overload produced by TAC resulted in LV hypertrophy with impaired systolic function that was associated with evidence of increased oxidative stress. Febuxostat attenuated the TAC-induced increase in myocardial oxidative stress, as well as the ventricular hypertrophy, collagen content and contractile dysfunction. Febuxostat also attenuated the TAC-induced phosphorylation of mTOR and Erk, events that are often associated with myocardial hypertrophy. The findings suggest that febuxostat caused a decrease of oxidative stress that blunted activation of the Erk-mTOR signaling pathway following TAC, with a consequent decrease in myocardial hypertrophy and LV dysfunction. The implications of these findings will be discussed below.
Although no previous data are available regarding the effect of a XOI on the LV response to chronic TAC, several investigators have reported the effects of XO inhibition with allopurinol on LV function in other murine models of cardiac failure 9, 10, 11. Stull et al 9 administered allopurinol in drinking water to mice beginning at the time of left anterior descending coronary artery occlusion that produced a massive myocardial infarction. Four weeks after coronary ligation, LV systolic fractional shortening was markedly better in animals receiving allopurinol than in untreated mice. A similar protective effect of XO inhibition on infarction-induced LV remodeling in mice was reported by two other groups 10, 11. This beneficial effect of allopurinol was associated with decreases of myocardial superoxide anion production 10, and infarction-induced abnormalities of myocardial high energy phosphates 11. The significant improvement of LV function in febuxostat-treated mice in the present study is consistent with a recent report that febuxostat attenuated myocardial infarction-induced ventricular remodeling in rabbits 20. The finding of increased myocardial nitrotyrosine in our mice in response to pressure overload is consistent with previous reports from our laboratory 16, 17, 21 and others 22, demonstrating that oxidative stress is increased in the failing heart 16, 17, 22, 19. Our finding that administration of febuxostat shortly after TAC attenuated myocardial oxidative stress, hypertrophy, and LV dysfunction is consistent with previous reports using purine-derived XOIs in other models of heart failure 14, 9, 10, 11, 23.
Several potential sources for increased oxidative stress have been identified in the failing heart, including the mitochondrial respiratory chain 24, uncoupled iNOS or eNOS 12, 16, 22,25, NADPH oxidase 19, and xanthine oxidase2, as well as a decrease of extracellular superoxide dismutase 17, 21. Administration of tetrahydrobiopterin (BH4) to prevent NOS uncoupling 22, or selective inhibition of xanthine oxidase or NADPH oxidase 19, have been reported to attenuate oxidative stress and ventricular dysfunction in this model of cardiac overload. These findings suggest that superoxides generated at multiple sites contribute to increased oxidative stress in the failing heart.
The mechanism for the beneficial effects of XOI treatment may be multi-factorial, including calcium-sensitizing effects on the failing myocardium 26, improved myocardial energy metabolism 11, 11, and reduced levels of reactive oxygen species (ROS) 3, 9. In the normal heart, xanthine oxidoreductase exists predominately as xanthine dehydrogenase, but can be converted to the oxidase form of the enzyme during ischemia or oxidative stress 27, 25. Xanthine dehydrogenase preferentially transfers electrons released during oxidation to NADH+ to produce NADH 28. In contrast, XO can transfer electrons to molecular oxygen, thereby generating superoxide (O2−). The conversion of xanthine dehydrogenase to XO can be mediated by either proteolytic conversion or can be secondary to oxidation of the thiol groups of Cys535 and Cys992 28. Thus, perfusion of rat hearts with oxidizing agents such as hydrogen peroxide or diamide caused conversion of xanthine dehydrogenase to xanthine oxidase 27. It is possible that increased oxidative stress in systolic overload could mediate conversion of xanthine dehydrogenase to XO, an event that would further augment O2− generation.
In mice with LV failure secondary to myocardial infarction, the increased XO activity was associated with evidence of increased lipid peroxidation of myocardial proteins, and treatment with allopurinol suppressed oxidative protein modifications, implying that XO contributed to the oxidative stress in the failing heart 9. Stull et al 9 proposed that the increased ROS in the failing heart resulted in oxidation of myofilament proteins to impair cross-bridge cycling, leading to progression of heart failure. This was supported by the finding that failing myocardium exhibited higher XO activity than non-failing myocardium, and the magnitude of the functional improvement with XO inhibition was dependent on the initial level of XO activity. The XO reaction may have increased importance in CHF, since myocardial adenosine levels are increased in the failing heart, providing additional substrate for the purine degradation pathway 18. Although XO activity was not measured in the present study, the finding that plasma uric acid levels were increased in the vehicle-treated TAC animals is consistent with increased XO activity in the overloaded hearts. Furthermore, the finding that plasma uric acid was markedly decreased in animals treated with febuxostat indicates that a high degree of XO blockade was achieved.
Oxidative stress is known to activate the Erk signaling pathway. Constitutive activation of Erk1,2 by cardiac-specific overexpression of their upstream regulator MAPK kinase 1 29 or an increase of oxidative stress 21, 30, 31 results in myocardial hypertrophy or congestive heart failure, while a decrease of oxidative stress is associated with decreased Erk activation 16, suggesting that activation of the Erk signaling pathway can mediate myocardial hypertrophy and heart failure. The finding of increased p-ErkThr202/Tyr204 in response to pressure overload in the present study is consistent with our previous reports 16, 21, while the decrease of p-ErkThr202/Tyr204 in response to treatment with febuxostat is consistent with the decreased oxidative stress and attenuated myocardial hypertrophy. Akt phosphorylation can produce myocardial hypertrophy 32, 33, and oxidative stress has been reported to activate Akt 34. The increased p-Akt Ser473 after TAC is consistent with previous reports16, 21, while it is not clear why febuxostat depressed myocardial nitrotyrosine content but had no effect on myocardial p-Akt Ser473. Previous reports have demonstrated that inhibition of mTOR signaling with rapamycin attenuated or reversed TAC-induced LV hypertrophy produced by TAC 34, 35. Interestingly, a recent study reported that activation of mTOR and NFκB contribute to the development of myocardial hypertrophy, while the antioxidant pyrrolidine dithiocarbamate attenuated NFκB and p70S6K activation and reduced TAC-induced LV hypertrophy 34. The finding that febuxostat attenuated TAC-induced oxidative stress and LV hypertrophy, and blunted the TAC-induced increases of p-ErkThr202/Tyr204 and p-mTORSer2488, suggests that the beneficial effect of febuxostat might involve attenuation of the Erk-mTOR signaling pathway as the result of decreased superoxide anion produced by XO.
In summary, systolic overload produced by TAC in mice caused LV hypertrophy, dysfunction, and fibrosis, as well as increased levels of plasma uric acid and myocardial nitrotyrosine, a marker of myocardial oxidative stress; all of these parameters were significantly reduced or attenuated by eight days of treatment with the non-purine XOI, febuxostat, starting shortly after TAC. Moreover, febuxostat also attenuated the TAC-induced phosphorylation of mTOR and Erk, events that are thought to mediate myocardial hypertrophy. These results suggest that the beneficial effect of febuxostat seen in this mouse model might involve reduction of oxidative stress in the pressure-overloaded LV, thereby diminishing the activation of pathways that result in pathologic hypertrophy and contractile dysfunction.
Acknowledgments
This study was supported by a research grant awarded by TAP Pharmaceutical Products Inc. and by NIH grants HL71790 and HL21872. PZ received a Scientist Development Award from American Heart Association, National Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 2.de Jong JW, van der Meer P, Nieukoop AS, Huizer T, Stroeve RJ, Bos E. Xanthine oxidoreductase activity in perfused hearts of various species, including humans. Circulation research. 1990;3:770–773. doi: 10.1161/01.res.67.3.770. [DOI] [PubMed] [Google Scholar]
- 3.Kittleson MM, Hare JM. Xanthine oxidase inhibitors: an emerging class of drugs for heart failure. Eur Heart J. 2005;15:1458–1460. doi: 10.1093/eurheartj/ehi321. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;5:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 5.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;3:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 6.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;20:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 7.Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation. 2001;5:750–755. doi: 10.1161/01.cir.103.5.750. [DOI] [PubMed] [Google Scholar]
- 8.Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, Hare JM. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J Mol Cell Cardiol. 2005;3:531–536. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;10:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 10.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;15:2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 11.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;2:H837–843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 12.Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;2:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 13.Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci. 2005;16:1835–1847. doi: 10.1016/j.lfs.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Hou M, Hu Q, Chen Y, Zhao L, Zhang J, Bache RJ. Acute effects of febuxostat, a nonpurine selective inhibitor of xanthine oxidase, in pacing induced heart failure. J Cardiovasc Pharmacol. 2006;5:255–263. doi: 10.1097/01.fjc.0000249961.61451.da. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;3:H1261–1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;7:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;1:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;6:749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;3:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Roche BM, Wessale JL, Kijtawornrat A, Lolly JL, Shemanski D, Hamlin RL. Chronic xanthine oxidase inhibition following myocardial infarction in rabbits: Effects of early versus delayed treatment. Life Sci. 2008;9–10:495–502. doi: 10.1016/j.lfs.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 21.van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, Bache RJ, Duncker DJ, Chen Y. Extracellular superoxide dimutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;7:1305–1313. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;5:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi K, Kimata H, Obata K, Matsushita A, Fukata A, Hashimoto K, Noda A, Iwase M, Koike Y, Yokota M, Nagata K. Xanthine oxidase inhibition improves left ventricular dysfunction in dilated cardiomyopathic hamsters. Journal of cardiac failure. 2008;3:238–244. doi: 10.1016/j.cardfail.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;4:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 25.McKelvey TG, Hollwarth ME, Granger DN, Engerson TD, Landler U, Jones HP. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988;(5 Pt 1):G753–760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- 26.Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;4:423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- 27.Bindoli A, Cavallini L, Rigobello MP, Coassin M, Di Lisa F. Modification of the xanthine-converting enzyme of perfused rat heart during ischemia and oxidative stress. Free Radic Biol Med. 1988;3:163–167. doi: 10.1016/0891-5849(88)90024-x. [DOI] [PubMed] [Google Scholar]
- 28.Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. Faseb J. 1995;11:995–1003. [PubMed] [Google Scholar]
- 29.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;9:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 30.Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;8:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;2:676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;3:220–223. [PubMed] [Google Scholar]
- 33.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;24:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 34.Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med. 2005;12:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;12:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]