Abstract
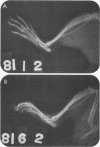
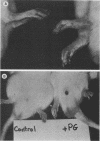
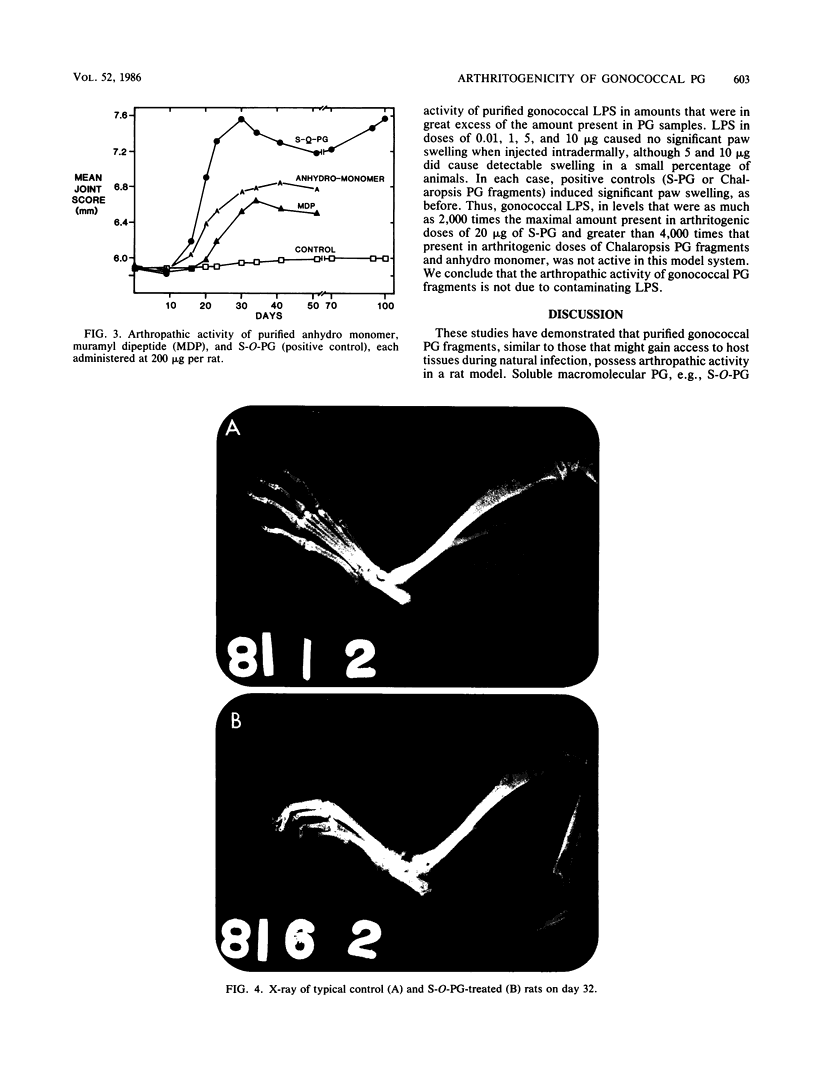
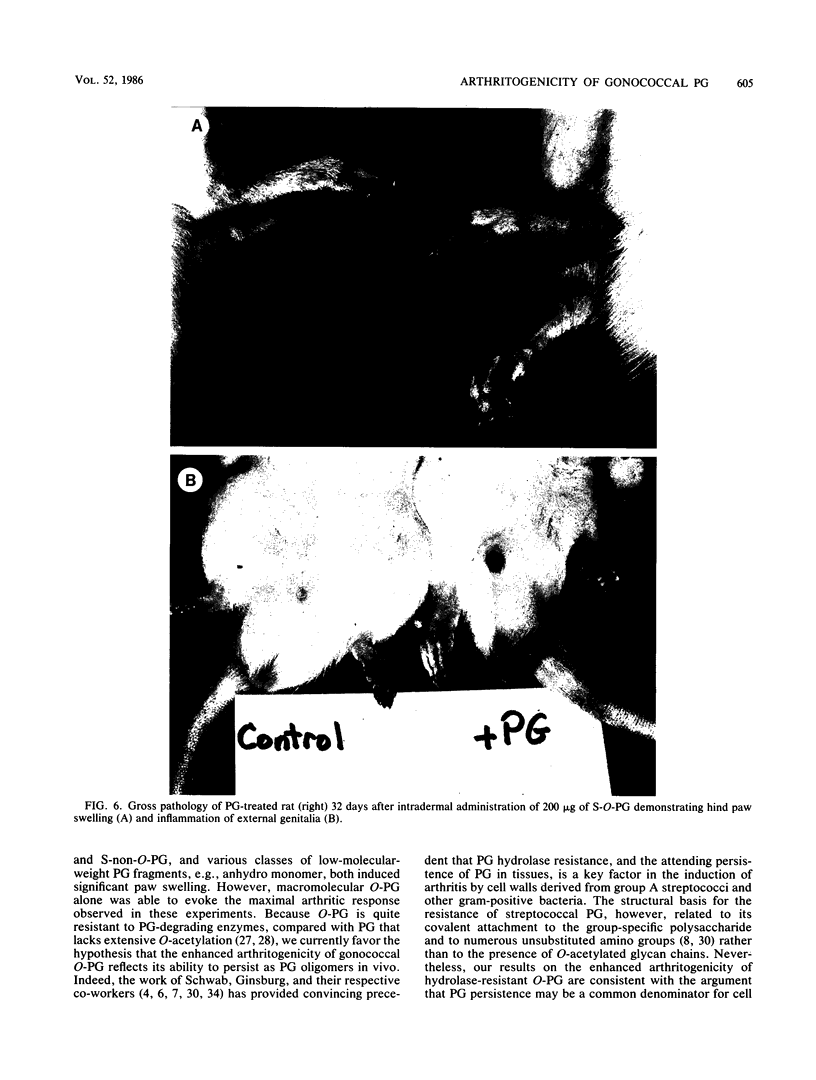
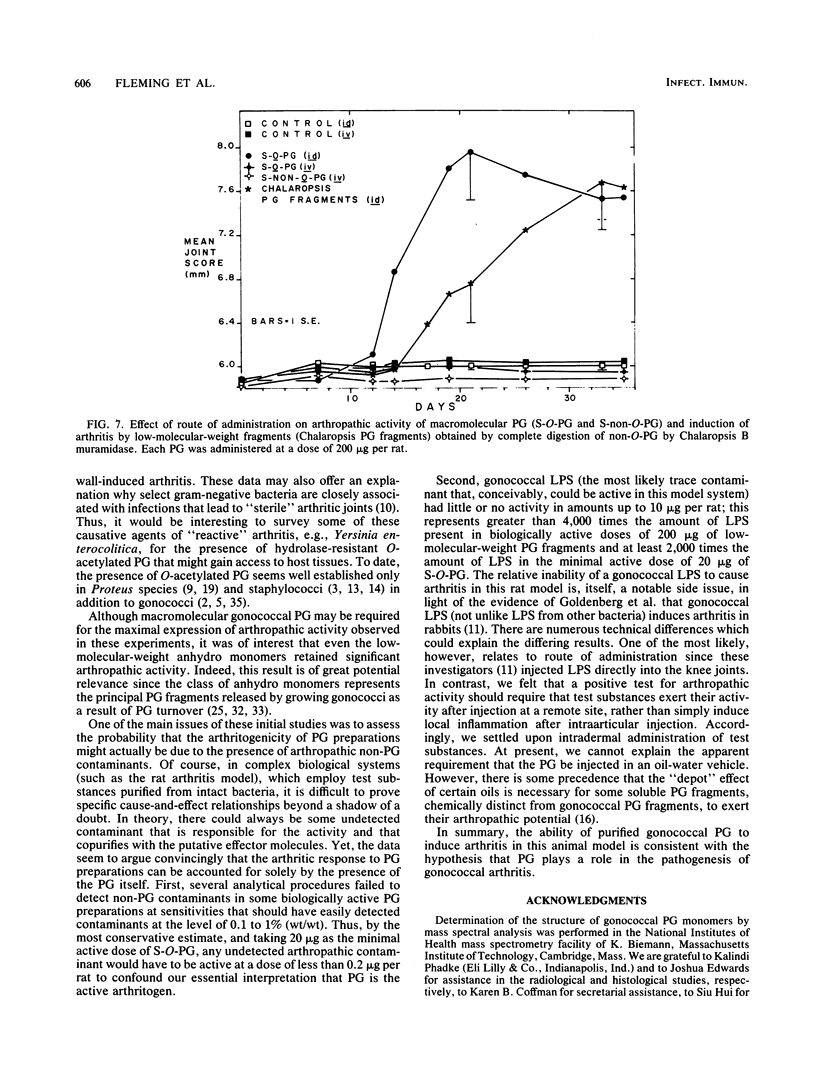
We examined the arthropathic activity of purified peptidoglycan (PG) fragments derived from (i) lysozyme-resistant, extensively O-acetylated PG from Neisseria gonorrhoeae FA19 (O-PG), and (ii) lysozyme-sensitive, O-acetyl-deficient PG from N. gonorrhoeae RD5 (non-O-PG). Male Lewis rats were injected intradermally in the tail with 200 micrograms of PG emulsified in mineral oil and water (1:1) or with the oil and water emulsion alone (controls). Quantitation of hind paw size indicated that macromolecular PG of various chemical and physical forms induced paw swelling (P versus controls, less than 0.01) that was evident at about day 14 and that reached a maximum at about day 24. PG-mediated paw swelling was accompanied by intense synovitis with some cartilage and bone involvement. The minimal arthropathic dose of soluble macromolecular PG was 20 micrograms per rat. Of particular interest was that macromolecular O-PGs from strain FA19 caused considerably more extensive swelling than did either their RD5 non-O-PG counterparts or the homologous FA19 PG that had been de-O-acetylated by mild alkali treatment. This suggested that the persistence of hydrolase-resistant high-molecular-weight fragments, afforded by extensive O-acetylation, may be important for optimal expression of arthropathic activity. However, oligomeric PG was not an absolute requirement, since even low-molecular-weight fragments, including the anhydro-muramyl-containing disaccharide peptide monomer released by growing gonococci, were also arthritogenic. Experiments employing purified gonococcal lipopolysaccharide indicated that the arthropathic activity of PG preparations was not due to contaminating lipopolysaccharide. Based on the arthritogenicity of gonococcal PG in this model system, we suggest that PG may play a role in the pathogenesis of gonococcal arthritis, and that such an activity might be potentiated by the persistence of hydrolase-resistant O-PG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell J. K., Perkins H. R. Effects of beta-lactam antibiotics on peptidoglycan synthesis in growing Neisseria gonorrhoeae, including changes in the degree of O-acetylation. J Bacteriol. 1981 Aug;147(2):633–641. doi: 10.1128/jb.147.2.633-641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Peptidoglycan biosynthesis in Neisseria gonorrhoeae strains sensitive and intrinsically resistant to beta-lactam antibiotics. J Bacteriol. 1983 Jan;153(1):429–435. doi: 10.1128/jb.153.1.429-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Kroll H. P. Murein biosynthesis and O-acetylation of N-acetylmuramic acid during the cell-division cycle of Proteus mirabilis. Eur J Biochem. 1981 Jun;117(1):171–177. doi: 10.1111/j.1432-1033.1981.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L. "Postinfectious" arthritis. New look at an old concept with particular attention to disseminated gonococcal infection. Am J Med. 1983 Jun;74(6):925–928. doi: 10.1016/0002-9343(83)90782-9. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Reed J. I., Rice P. A. Arthritis in rabbits induced by killed Neisseria gonorrhoeae and gonococcal lipopolysaccharide. J Rheumatol. 1984 Feb;11(1):3–8. [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Watanabe Y., Kotani S., Koga T. Structural requirements for arthritogenicity of peptidoglycans from Staphylococcus aureus and Lactobacillus plant arum and analogous synthetic compounds. J Immunol. 1976 Jun;116(6):1635–1639. [PubMed] [Google Scholar]
- Kohashi O., Tanaka A., Kotani S., Shiba T., Kusumoto S., Yokogawa K., Kawata S., Ozawa A. Arthritis-inducing ability of a synthetic adjuvant, N-acetylmuramyl peptides, and bacterial disaccharide peptides related to different oil vehicles and their composition. Infect Immun. 1980 Jul;29(1):70–75. doi: 10.1128/iai.29.1.70-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M., Karnovsky M. L., Martin S. A., Pappenheimer J. R., Walter J., Biemann K. Peptidoglycans as promoters of slow-wave sleep. II. Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. J Biol Chem. 1984 Oct 25;259(20):12659–12662. [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Karnovsky M. L., Krueger J. M., Pappenheimer J. R., Biemann K. Peptidoglycans as promoters of slow-wave sleep. I. Structure of the sleep-promoting factor isolated from human urine. J Biol Chem. 1984 Oct 25;259(20):12652–12658. [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Oken M. M., Peterson P. K., Wilkinson B. J. Endogenous pyrogen production by human blood monocytes stimulated by staphylococcal cell wall components. Infect Immun. 1981 Jan;31(1):208–213. doi: 10.1128/iai.31.1.208-213.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Rosenthal R. S. Complement consumption gonococcal peptidoglycan. Infect Immun. 1982 Feb;35(2):442–448. doi: 10.1128/iai.35.2.442-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Blundell J. K., Perkins H. R. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1982 Aug;37(2):826–829. doi: 10.1128/iai.37.2.826-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Folkening W. J., Miller D. R., Swim S. C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect Immun. 1983 Jun;40(3):903–911. doi: 10.1128/iai.40.3.903-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Gfell M. A., Folkening W. J. Influence of protein synthesis inhibitors on regulation of extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1985 Jul;49(1):7–13. doi: 10.1128/iai.49.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Cromartie W. J., Ohanian S. H., Craddock J. G. Association of experimental chronic arthritis with the persistence of group A streptococcal cell walls in the articular tissue. J Bacteriol. 1967 Nov;94(5):1728–1735. doi: 10.1128/jb.94.5.1728-1735.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Effect of penicillin G on release of peptidoglycan fragments by Neisseria gonorrhoeae: characterization of extracellular products. Antimicrob Agents Chemother. 1981 Jul;20(1):98–103. doi: 10.1128/aac.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swim S. C., Gfell M. A., Wilde C. E., 3rd, Rosenthal R. S. Strain distribution in extents of lysozyme resistance and O-acetylation of gonococcal peptidoglycan determined by high-performance liquid chromatography. Infect Immun. 1983 Nov;42(2):446–452. doi: 10.1128/iai.42.2.446-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. C., Ahlin T. D., Tung K. S., Williams R. C., Jr Circulating immune complexes in disseminated gonorrheal infection. Ann Intern Med. 1978 Jul;89(1):28–33. doi: 10.7326/0003-4819-89-1-28. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K. Factors affecting complement activation by Staphylococcus aureus cell walls, their components, and mutants altered in teichoic acid. Infect Immun. 1981 Apr;32(1):216–224. doi: 10.1128/iai.32.1.216-224.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]