Summary
Objective
To determine the effects of exercise and weight loss interventions on serum levels of four biomarkers and to examine if changes in biomarker levels correlate with clinical outcome measures in obese and overweight adults with knee OA.
Methods
Serum was obtained at baseline, 6- and 18-months from 193 participants in ADAPT (Arthritis, Diet and Activity Promotion Trial). This was a single-blind 18-month trial with subjects randomized to four groups: healthy-lifestyle (HL), diet (D), exercise (E) and diet plus exercise (D+E). Serum levels of cartilage oligomeric protein (COMP), hyaluronan (HA), antigenic keratan sulfate (AgKS) and transforming growth factor-β1 (TGF-β1) were measured by ELISA.
Results
At baseline there were no significant differences in biomarker levels between intervention groups. When results for all the intervention groups were combined, the levels of HA were found to be negatively correlated with medial joint space width and positively correlated with Kellgren-Lawrence scores (K-L scores) while TGF-β1 levels negatively correlated with K-L scores. When biomarker levels measured at 6 and 18-months were adjusted for baseline values, age, gender, and body mass index (BMI), weak but significant differences between intervention groups were present for mean levels of COMP and TGF-β1. Furthermore, AgKS levels averaged over all groups tended to decrease over time. There were no significant associations of baseline biomarkers and the follow-up outcomes. Weak associations were noted between change in the biomarkers at 18-months and change in outcome measures that included change in weight with AgKS and COMP and change in WOMAC pain with AgKS.
Conclusion
Overall, the exercise and dietary interventions did not show a consistent effect on levels of potential OA biomarkers. The four biomarkers showed differences in correlations with outcome measures suggesting they may measure different aspects of disease activity in OA. The strongest correlations were between serum HA and radiographic measures of OA at baseline.
Keywords: biomarkers, osteoarthritis, clinical trials, hyaluronan, COMP, TGF-β
Introduction
Osteoarthritis (OA) is a progressive, painful and often disabling disease characterized by the loss of articular cartilage, which is accompanied by bony hypertrophy within a diarthrodial joint. Unmet needs in clinical OA studies are the ability to detect early disease prior to radiographic changes, to determine or predict the rate of disease progression of the joint, and to rapidly ascertain whether or not a therapeutic intervention slows down or stops disease progression. These needs must be effectively addressed if the detection and management of OA is to be improved.
At present, plain radiographs are commonly used to classify OA subjects for the purposes of clinical studies and joint space narrowing is often used as a measure of disease progression. Although plain radiography is, at present, the “gold standard” for evaluation of OA progression, it is fraught with problems related to the accurate reproduction of measurements of joint space width, especially in subjects who have knee OA1–3.
Recently, researchers have gained knowledge about the biology of OA and have identified molecular events that lead to the destruction and remodeling of joint tissues, including cartilage and bone4,5. Cytokines, such as interleukin-1 (IL-1) and IL-6 and other inflammatory mediators, are found within the cartilage and are thought to participate locally in cartilage destruction by inhibiting matrix synthesis and stimulating the release of degradative enzymes6,7. Specific degenerative and biosynthetic events, which have been identified and quantified by the use of antibody-based immunoassays, are used to study tissue-specific changes in OA as reflected by molecular biomarkers5,8,9.
Among the most promising of these biomarkers are type II collagen degradation products, antigenic keratan sulfate (AgKS) epitopes, cartilage oligomeric matrix protein (COMP), GP-39/YK-40, type 1 collagen cross-links, several matrix metalloproteinases, and hyaluronan (HA)5,9,10. The biomarkers can be detected principally in the joint tissues where the events occur, but also in body fluids, such as peripheral blood, urine and synovial fluid, into which the biomarkers are released. Three of the most commonly used serum biomarkers associated and/or correlated with OA and joint progression are: AgKS11,12, HA11,13–15, and COMP16–19. Although not as well studied, TGF-β1 levels measured in the serum of OA subjects were found, out of 14 serum and urine biomarkers tested, to be best associated with a change in clinical assessments over a 1-year period20.
While most biomarker studies attempt to distinguish patients who have OA from the non-arthritic population, a few studies have used biomarkers to predict progression11,13–18,21,22. Most of these studies are limited by their cross-sectional nature, and those that are longitudinal are observational. Hence, there is little data available on the effects of a therapeutic intervention on changes in biomarker levels.
The objective of the present study was to determine the correlation of selected biomarkers with clinical outcome measures in an intervention study using serum samples collected from the ADAPT (Arthritis, Diet and Activity Promotion Trial) participants. ADAPT was an 18-month single blind randomized clinical trial designed to determine if exercise and dietary weight loss, alone or in combination, were more effective than usual care in improving pain and function in older overweight and obese adults with knee OA23. Both groups receiving the dietary intervention lost on average about 5% body weight and the primary outcome, self-reported physical function ascertained by WOMAC, showed significant improvements of 24% in the diet plus exercise group and 18% in the diet only group. The present study reports data on the effects of the interventions on serum biomarkers, specifically: AgKS, HA, COMP and TGF-β1, and their correlations with outcome measures.
Methods
PARTICIPANTS
Details of the ADAPT study were previously published23,24. Briefly, 316 patients, aged 60 years and older with a body mass index (BMI) of 28 and above, who had clinical evidence of knee OA and who met the study criteria, were assigned to one of the four therapeutic intervention cohorts: healthy lifestyle (HL, control group), diet (D), exercise (E) and diet plus exercise (D+E). The HL intervention consisted of a regular group meeting to provide attention, social interactions and health education. The diet intervention was a behavior modification type intervention designed to produce a group average of 5% weight loss. The exercise intervention consisted of a combination of aerobic (walking) and resistance-training for a total of one hour three times a week. Participants in the D+E group received a combination of the D and E interventions.
For all interventions, self-reported physical function (WOMAC function, pain and stiffness), measures of mobility (stair climb time and six-minute walk distance), weight loss and knee radiographs (medial joint space width and K-L score) were the outcome measures used as previously described in detail23. Outcome measures were determined at baseline, 6-months and 18-months except for the radiographs which were obtained only at baseline and 18-months.
BIOMARKER MEASUREMENTS
After the participants had fasted overnight, blood was collected by venipuncture in the morning (between 07:00 AM and 09:00 AM) at baseline and after 6- and 18-months of intervention. Serum was frozen at −80°C until analyzed. Of the 316 subjects in ADAPT, sufficient serum for biomarker measures was available from all 3 time points in 193 subjects. In the present study, the clinical and radiological outcomes were analyzed for these 193 subjects. Hyaluronan was measured using a well-characterized sandwich enzyme linked immunosorbent assay (ELISA) technique25. Briefly, the procedure utilizes an anti-KS monoclonal antibody (1/20/5-D-4; MP Biomedicals, Irvine, CA) to differentiate between the coated aggregating rat chondrosarcoma proteoglycan, which captures HA, and the AgKS-bearing aggregating proteoglycan added subsequently. Antigenic keratan sulfate was quantified by a previously described ELISA technique26,27 that includes an inhibition step and also utilizes monoclonal antibody 5-D-4.
Cartilage oligomeric matrix protein was measured using the AnaMar COMP ELISA (AnaMar Medical AB Bangardsgatan, Uppsala, Sweden). The COMP ELISA is a solid-phase, two-site enzyme immunoassay, based on a direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the COMP molecule28. The TGF-β1 level was measured using a highly sensitive ELISA (Quantikine Human TGF-β1 kit from R&D Systems Inc., Minneapolis, MN). This assay also employs the quantitative sandwich enzyme immunoassay technique and uses an anti-TGF-β1 monoclonal antibody and a polyclonal antibody against TGF-β 1 conjugated to horseradish peroxidase29.
All samples were measured in duplicate, and the average of the two values was used for data analyses. Duplicate samples that did not provide a co-efficient of variation <15% were reanalyzed. The intra-assay and inter-assay variation of the tests are as follow: HA (intra: <4% and inter: <6%); KS (intra: <3% and inter: <4%); COMP (intra: 1.7–3.0% and inter: 1.8–4.2%); and TGF-B-1 (intra: 3.7% and inter: 9.8–12.8%).
STATISTICAL ANALYSIS
Statistical analyses were performed using SAS software version 8 (SAS Institute, Cary, NC). A significance level of 0.05 was adopted for all comparisons. Descriptive statistics were calculated for each intervention group (HL, D, E and D+E) at baseline. Values were reported as means ± standard error (SE) unless otherwise indicated. Logarithmic transformations of the biomarker results to satisfy the model assumptions (normally distributed errors and linear relations) were tested, however, the results did not change when compared to non-log transformed values. Hence, non-transformed data were reported. Analysis of variance and chi-square tests were used to determine differences among baseline characteristics between intervention groups.
The effects of diet and or exercise programs on disability, physical function, pain, and measures of mobility measured at 6- and 18-months post-randomization were determined by repeated measures analysis of covariance. All follow-up information was analyzed using SAS PROC MIXED (SAS Institute, Cary, NC). Analyses of group differences were adjusted for the pre-randomization levels of baseline values of the outcome being analyzed and by age, gender and BMI. A random effect of subjects that accounted for the within-subject correlation at the repeated measurements was included. Estimates of intervention effects were obtained at each follow-up observation. To test the consistency of intervention effects during the follow-up period, tests of time of follow-up by intervention effects were conducted. When time-by-intervention interactions were non-significant, average, intervention effects over the follow-up period were estimated and tested for significance.
Pearson’s correlation coefficients were used to examine the relationship between biomarkers and BMI, WOMAC function, WOMAC pain, WOMAC stiffness, six-minute walk distance, stair climb-time, joint space width and the K-L score at baseline. Repeated measures analysis of covariance was also used to investigate the relationship between outcome measures and baseline biomarkers. The same covariates as above were included in the models, except that BMI was not added to the model for weight. The slope for each biomarker is reported.
Results
EFFECTS OF TIME AND GROUP ASSIGNMENT ON BIOMARKER LEVELS
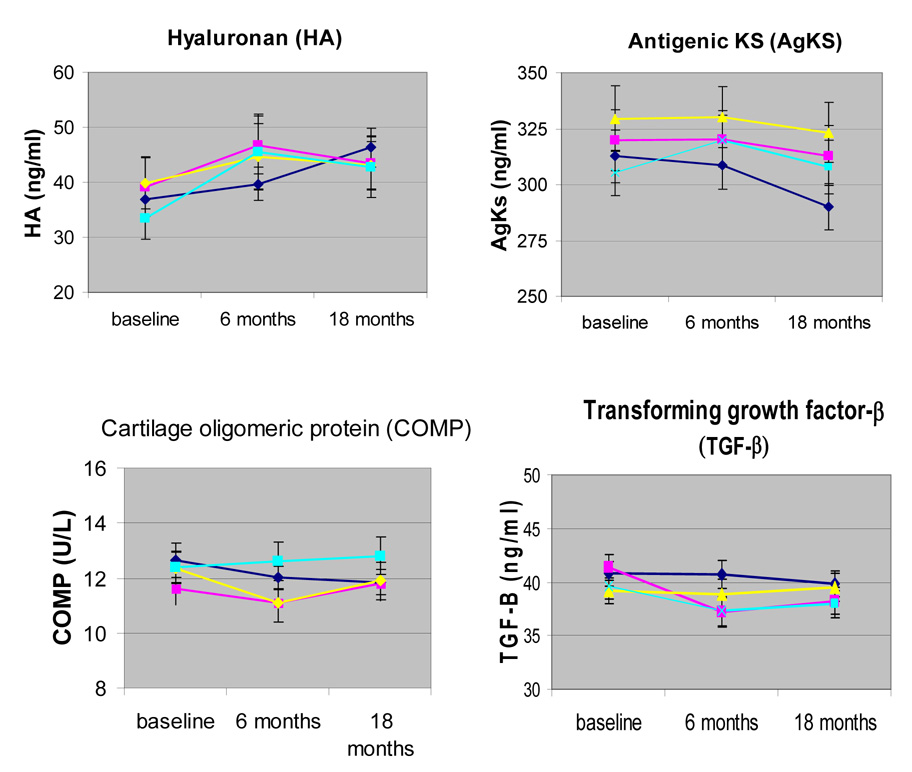
Analyses were conducted on data from 193 ADAPT subjects with a baseline blood sample and any follow-up (6 and 18 month) samples. There were no significant differences in any baseline characteristics of these subjects (Table 1). When examining mean baseline outcome values for all subjects tested, participants in the diet group tended to have lower WOMAC function (p=0.02) pain (p=0.03) and stiffness (p=0.01) (Table 2). All 4 groups showed similar levels of biomarkers at baseline (Figure 1).
Table 1.
Demographics of ADAPT Subjects Utilized for Biomarker Studies, by Intervention Arm
| Diet+Exercise N=46 |
Diet N=48 |
Exercise N=46 |
Control N=53 |
P-Value | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Male | 9 (19) | 14 (30) | 15 (32) | 19 (38) | .24 |
| White | 35 (74) | 39 (81) | 38 (81) | 45 (85) | .62 |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Age (years) | 67.80 (0.95) | 68.51 (0.83) | 68.67 (0.86) | 68.94 (0.83) | .82 |
| Weight (lbs) | 194.8 (4.6) | 208.4 (5.0) | 196.2 (4.3) | 207.8 (5.3) | .08 |
| BMI (kg/m2) | 33.10 (0.67) | 33.64 (0.59) | 33.65 (0.86) | 34.26 (0.73) | .72 |
SE = Standard Error
Table 2.
Outcome Measures at Baseline of ADAPT Subjects Utilized for Biomarker Studies, by Intervention Arm
| Diet+Exercise N=46 |
Diet N=48 |
Exercise N=46 |
Control N=53 |
P-Value | |
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| KL score | 2.52 (0.14) | 2.54 (0.11) | 2.50 (0.13) | 2.51 (0.13) | .99 |
| WOMAC Function | 23.38 (1.76) | 17.76 (1.74) | 25.33 (1.74) | 22.42 (1.67) | .02 |
| WOMAC Pain | 6.33 (0.53) | 5.11 (0.53) | 7.39 (0.53) | 6.42 (0.51) | .03 |
| WOMAC Stiffness | 3.53 (0.22) | 2.78 (0.22) | 3.87 (0.22) | 3.36 (0.21) | .01 |
| Distance walked (ft) | 1472 (46.9) | 1392 (38.7) | 1535 (48.9) | 1485 (44.6) | .17 |
| Stair climb time (s) | 9.14 (0.64) | 9.12 (0.84) | 9.90 (1.55) | 9.85 (0.82) | .91 |
| JSW_LA (mm) | 4.89 (0.20) | 4.70 (0.17) | 4.63 (0.22) | 4.72 (0.18) | .81 |
| JSW_MED (mm) | 3.34 0.24) | 3.17 (0.21) | 3.47 (0.22) | 3.23 (0.24) | .80 |
KL= Kellgren-Lawrence; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; ft = feet; s= seconds; JSW = joint space width; LA= lateral; MED=medial; mm=millimeters
Fig. 1.
Biomarker levels by group assignment over the course of the study. The levels of the indicated biomarkers were measured by ELISA assays in serum samples obtained from ADAPT subjects at baseline, 6-months, and 18–months.  = healthy life-style control;
= healthy life-style control;  = diet;
= diet;  = diet + exercise;
= diet + exercise;  = exercise.
= exercise.
Biomarker levels remained relatively stable during the 18-months of the study (Figure 1 and Table 3), except for AgKS levels which, averaged over all groups, decreased significantly over time (p=0.02). Statistically significant differences between groups were present for mean levels of COMP (p=0.04) and TGF-β1 (p=0.02) (Table 3). Pair-wise comparisons revealed that participants in the exercise group had significantly higher levels of COMP than those in the diet and exercise group (p=0.01) and in the control group (p=.03). TGF-β1 levels in the diet group were significantly lower than in the control group (p=0.003) and in the diet and exercise group (p=.04). These group differences did not vary significantly over time.
Table 3.
Biomarker levels measured at 6 months and 18 months, by intervention arms, adjusted for baseline values, age, gender and BMI, mean (SE)
| Outcomes | Diet+Exercise | Diet | Exercise | Control | P-value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6-Month N=46 |
18-Month N=46 |
6-Month N=48 |
18-Month N=48 |
6-Month N=45 |
18-Month N=45 |
6-Month N=52 |
18-Month N=53 |
Grp | Time | |
| HA (ng/ml) | 42.28 (3.79) | 45.33 (3.63) | 46.95 (3.64) | 44.21 (3.44) | 45.68 (3.67) | 43.45 (3.54) | 40.46 (3.58) | 47.67 (3.35) | .99 | .50 |
| AgKS (ng/ml) | 310.22 (7.62) | 310.93 (7.32) | 312.61 (7.29) | 306.38 (6.90) | 322.79 (7.35) | 314.11 (7.09) | 308.67 (7.17) | 286.66 (6.71) | .07 | .02 |
| COMP (U/L) | 10.80 (0.49) | 11.81 (0.46) | 11.58 (0.47) | 12.21 (0.44) | 12.77 (0.46) | 12.84 (0.44) | 11.75 (0.45) | 11.72 (0.42) | .04 | .10 |
| TGFβ1 (ng/ml) | 38.89 (1.14) | 39.06 (1.07) | 36.40 (1.08) | 36.65 (1.01) | 37.63 (1.08) | 38.46 (1.04) | 40.93 (1.04) | 39.41 (0.98) | .02 | .87 |
HA = hyaluronan, AgKS = antigenic keratan sulfate, COMP = cartilage oligomeric protein, TGF-β1 = transforming growth factor-B1.
Interactions for Group and time not statistically significant.
OUTCOME MEASURES AND LEVELS OF BIOMARKERS
There were no statistically significant correlations between baseline outcome measures and baseline levels of biomarkers (Table 4), except for KL-scores which were positively associated with HA (r=0.35, p=0.0001) and negatively correlated with TGF-β1 (r=−0.19, p=0.01). In addition, medial joint space width (JSW) was also associated with HA (r=−0.23, p=0.004). Slopes for the associations between follow-up outcomes and baseline levels of biomarkers tended to be small and statistically non-significant (Table 5). Only weight was significantly associated with AgKS (slope =0.02, p= 0.01).
Table 4.
Correlation of baseline biomarker levels with baseline outcome measures, r (p value)
| Outcomes | HA | AgKS | COMP | TGF-β1 |
|---|---|---|---|---|
| WEIGHT | 0.05 (.49) | 0.04 (.60) | −0.08 (.29) | 0.02 (.78) |
| WOMC function | 0.12 (.11) | −0.03 (.67) | −0.07 (.36) | −0.14 (.07) |
| WOMC pain | 0.09 (.21) | −0.12 (.10) | −0.04 (.62) | −0.09 (.22) |
| WOMC stiffness | 0.11 (.12) | −0.07 (.36) | −0.08 (.31) | −0.08 (.30) |
| Distance walked | −0.02 (.78) | 0.09 (.27) | 0.15 (.06) | −0.03 (.71) |
| Stair climb time | 0.05 (.54) | −0.13 (.07) | −0.11 (.14) | −0.09 (.22) |
| JSW lateral | −0.08 (.32) | 0.11 (.14) | −0.13 (.10) | −0.13 (.10) |
| JSW medial | −0.23 (.004) | 0.02 (.83) | −0.06 (.45) | 0.07 (.38) |
| K-L score | 0.35 (.0001) | 0.06 (.46) | 0.01 (.89) | −0.19 (.01) |
HA = hyaluronan, AgKS = antigenic keratan sulfate, COMP = cartilage oligomeric protein, TGF-β1 = transforming growth factor- β1, JSW = joint space width. Values shown are correlation coefficients followed by p values in parentheses.
Table 5.
Association between baseline biomarker levels and outcome measures, adjusting for age, gender, BMI (except for weight), baseline value, group and visit.
| Outcomes | HA | AgKS | COMP | TGF-β1 |
|---|---|---|---|---|
| WEIGHT | −.003 (.023) .90 |
.02 (.01) .01 |
.12 (.16) .46 |
.02 (.08) .78 |
| WOMC function | −.0004 (.001) .77 |
−.001 (.0004) .16 |
−.01 (.01) .24 |
.0005 (.005) .92 |
| WOMC pain | −.001 (.001) .38 |
−.003 (.001) .50 |
−.01 (.01) .49 |
−.003 (.005) .46 |
| WOMC stiffness | .00002 (.002) .99 |
−.001 (.001) .03 |
−.01 (.01) .22 |
−.004 (.01) .45 |
| Distance walked | .58 (.63) .36 |
.13 (.20) .53 |
−6.5 (4.24) .13 |
−.71 (2.00) .72 |
| Stair climb time | −.01 (.01) .19 |
.01 (.004) .08 |
−.08 (.08) .30 |
−.03 (.04) .49 |
| JSW lateral | −.001 (.002) .67 |
.0002 (.001) .74 |
.02 (.01) .17 |
−.01 (.01) .52 |
| JSW medial | −.004 (.002) .10 |
.001 (.001) .46 |
.01 (.01) .55 |
.01 (.01) .26 |
| K-L score | .001 (.001) .34 |
−.0003 (.0003) .35 |
.01 (.01) .40 |
−.002 (.004) .54 |
HA = hyaluronan, AgKS = antigenic keratan sulfate, COMP = cartilage oligomeric protein, TGF-β1 = transforming growth factor- β1, JSW = joint space width. Values shown are Beta coefficients (SE) followed by p values.
There were a few weak but significant associations between the change in biomarker levels and change in outcome measures at 18-months (Table 6). These were for change in weight with change in AgKS (p=0.03) and COMP (p=0.02) and for change in WOMAC pain and change in AgKS (p=0.04).
Table 6.
Association between change in biomarker levels at 18-months and change in outcome measures 18-months. Beta coefficient (SE) and p-value
| HA-Chg | AgKs-Chg | COMP-Chg | TGF-Beta1-Chg | |
|---|---|---|---|---|
| Outcomes | ||||
| 1.WEIGHT-Chg | 0.05 (.04) P=.20 |
0.04 (.02) P=.03 |
0.68 (.29) P=.02 |
−0.02 (.15) P=.89 |
| 2.WOMCFUNC-Chg | 0.004 (.003) P=.12 |
−0.002 (.001) P=.15 |
−0.03 (.02) P=.13 |
0.01 (.01) P=.92 |
| 3.WOMCPAIN-Chg | 0.001 (.003) P=.64 |
−0.003 (.001) P=.04 |
−0.03 (.02) P=.15 |
0.02 (.01) P=.79 |
| 4.WOMCSTIF-Chg | 0.001 (.003) P=.69 |
−0.0003 (.001) P=.85 |
−0.03 (.02) P=.25 |
0.003 (.01) =.79 |
| 5.DISTANCE-Chg | 0.55 (1.24) P=.66 |
−0.03 (.60) P=.96 |
−0.076 (9.9) =.94 |
−5.5 (5.1) P=.28 |
| 6.STCLTIME-Chg | −0.01 (.03) P=.56 |
0.006 (.01) P=.60 |
−0.23 (.20) P=.26 |
−0.02 (.11) =.85 |
| 7.JSW_LA-Chg | −0.0001 (.002) p=.98 |
0.0001 (.001) P=.91 |
0.04 (.02) P=.05 |
.003 (.01) =.81 |
| 8.JSW_MED-Chg | −0.004 (.002) P=.08 |
0.001 (.001) P=.65 |
−0.02 (.02) P=.34 |
−0.004 (.01) P=.68 |
| 9.SCORE-Chg | 0.001 (.001) P=.70 |
−0.0003 (.001) =.60 |
−0.001 (.01) P=.92 |
−0.01 (.01) P=.12 |
HA = hyaluronan, AgKS = antigenic keratan sulfate, COMP = cartilage oligomeric protein, TGF- β1 = transforming growth factor- β1, JSW = joint space width. Values shown are Beta coefficients (SE) followed by p values.
Discussion
There is very little data in the literature about the effects of therapeutic interventions on biomarker levels in an OA population and how outcome measures commonly used in clinical trials might correlate with these levels. In this study of participants in an exercise and weight loss intervention, we found that the serum levels of HA, COMP, and TGF-β1 remained relatively stable during the 18-month intervention period while there was an overall slight decline in AgKS. Any differences observed between intervention groups with quite minimal.
The most consistent and strongest correlations noted in the present study between biomarker levels and clinical outcome measures were noted with HA and radiographic measures of OA. Serum HA levels at baseline were negatively correlated with medial joint space width and were positively correlated with the K-L score. These findings are consistent with other studies that have shown similar correlations between HA levels and radiographic OA13–15,30. Serum levels of HA in people with knee OA are thought to reflect the level of synovial inflammation because HA is produced by synovial cells and inflammation in the synovium may allow greater amounts to enter the systemic circulation5.
Our study also showed a significant but weak negative correlation between baseline TGF-β1 levels and K-L score. TGF-β has anabolic effects on cartilage that might account for a negative association with systemic levels and K-L score, but it has also been implicated in osteophyte formation in OA31,32. Separate osteophyte scores would have been of interest to correlate with levels of TGF-β but were not available. Our results failed to confirm previous work suggesting that serum TGF-β might serve as a marker for clinical outcomes in OA20.
The effect of weight loss on OA biomarkers has not been previously studied and most reports on the effects of exercise have been on biomarkers in healthy athletes or endurance runners. Sweet et al. found no differences in the serum levels of AgKS in marathon runners before and after the completion of a marathon run33 and showed that serum AgKS levels decreased during periods of immobilization, but rose after return to ambulation34. Serum levels of COMP have been shown to increase just after exercise and return to baseline levels after 30 minutes and with no increase in baseline levels noted after 6 weeks of an exercise program35. In two recent studies using serum samples from the ADAPT subjects, we have shown that the dietary weight loss but not the exercise intervention significantly reduced levels of the inflammation markers, C-reactive protein, interleukin-6, and soluble tumor necrosis factor α receptor 136, and reduced the levels of the adipokine leptin37. In the present study, only weak correlations were noted with change in AgKS and COMP and change in weight over18-months.
Although serum COMP has been widely studied as a potential OA biomarker5, we did not find that COMP levels at baseline, 6- or 18-months correlated with either subjective or objective outcome measures other than the change in weight. In the Johnston County Osteoarthritis Project, levels of COMP varied by ethnicity and sex and were associated with age, BMI and radiographic OA38. Our study did not find any significant differences between the levels of COMP among our subject populations, which were predominantly Caucasian women. Of interest, a recent longitudinal observational study has shown an association of an increase in COMP with increased risk of cartilage loss on MRI in subjects with knee OA39. Our radiographic measure of joint space width on plain films would be unlikely to detect a similar association given the relatively small sample size of the current study and the inherent variability in joint space width measures.
There are several important limitations in the present study. When present, correlations with clinical outcomes, though statistically significant, were relatively weak. Because this was an exploratory study we examined all results with p values <0.05. Because multiple comparisons were examined, some of the apparent significant observations may have occurred by chance alone. Also, recent work has suggested that measurement of markers that reflect cleavage of type II collagen, such as CTX-II, may serve as better OA biomarkers than those measured here22,40,41 although this is still controversial42. However, the most promising of these assays require measurements in urine and only serum samples were collected in the present study. Finally, as with most biomarker studies in OA, a major limitation is that a systemic measure is being correlated with local disease activity. About 55% of the subjects in the ADAPT study reported having arthritis in more than one joint23 but the clinical and radiographic outcome measures focused on knee OA.
In conclusion, the present study showed that serum levels of four potential biomarkers were relatively stable during the 18-month intervention. Previous observations that worse radiographic knee OA correlates with higher HA serum levels were confirmed. Based on the recent suggested classification criteria of biomarkers (burden of disease, investigative, prognostic, efficacy of intervention, and diagnostic)43the only marker in the present study that could be classified would be HA as a marker for burden of disease. Although it was not possible to conclude from the biomarker measures that the diet and exercise interventions improved joint structures, the finding of little change in levels of the four markers over the course of the study is consistent with the premise that the interventions did not result in measurable harm to the joints. This was a potential concern with overweight and obese subjects in an exercise program that included weight-bearing exercises which result in periods of increased joint loading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
References
- 1.Mazzuca SA, Brandt KD, Buckwalter KA. Detection of radiographic joint space narrowing in subjects with knee osteoarthritis: longitudinal comparison of the metatarsophalangeal and semiflexed anteroposterior views. Arthritis Rheum. 2003;48:385–390. doi: 10.1002/art.10765. [DOI] [PubMed] [Google Scholar]
- 2.Vignon E, Piperno M, Le Graverand MP, Mazzuca SA, Brandt KD, Mathieu P, et al. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: comparison of standing anteroposterior and Lyon schuss views. Arthritis Rheum. 2003;48:378–384. doi: 10.1002/art.10773. [DOI] [PubMed] [Google Scholar]
- 3.Buckland-Wright JC, Bird CF, Ritter-Hrncirik CA, Cline GA, Tonkin C, Hangartner TN, et al. X-ray technologists' reproducibility from automated measurements of the medial tibiofemoral joint space width in knee osteoarthritis for a multicenter, multinational clinical trial. J Rheumatol. 2003;30:329–338. [PubMed] [Google Scholar]
- 4.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968. doi: 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Poole AR. Biochemical/immunochemical biomarkers of osteoarthritis: utility for prediction of incident or progressive osteoarthritis. Rheum Dis Clin North Am. 2003;29:803–818. doi: 10.1016/s0889-857x(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Thonar EJ, Manicourt DH. In osteoarthritis:diagnosis and medical /surgical management Philadelphia. WB Saunders; 2001. Noninvasive biomarkers in osteoarthritis; pp. 293–313. [Google Scholar]
- 10.Poole AR. National Institutes of Health, NIAMS News and Events; NIH white papers: biomarkers, the osteoarthritis initiative. 2000 Available at: http://www.niams.nih.gov/ne/oi/oabiomarwhipap.htm.
- 11.Georges C, Vigneron H, Ayral X, Listrat V, Ravaud P, Dougados M, et al. Serum biologic markers as predictors of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1997;40:590–591. doi: 10.1002/art.1780400333. [DOI] [PubMed] [Google Scholar]
- 12.Manicourt DH, Lenz ME, Thonar EJ. Levels of serum keratan sulfate rise rapidly and remain elevated following anterior cruciate ligament transection in the dog. J Rheumatol. 1991;18:1872–1876. [PubMed] [Google Scholar]
- 13.Sharif M, George E, Shepstone L, Knudson W, Thonar EJ, Cushnaghan J, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38:760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 14.Pavelka K, Forejtova S, Olejarova M, Gatterova J, Senolt L, Spacek P, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:277–283. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Bruyere O, Collette JH, Ethgen O, Rovati LC, Giacovelli G, Henrotin YE, et al. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. J Rheumatol. 2003;30:1043–1050. [PubMed] [Google Scholar]
- 16.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34:306–310. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 17.Petersson IF, Boegard T, Dahlstrom J, Svensson B, Heinegard D, Saxne T. Bone scan and serum markers of bone and cartilage in patients with knee pain and osteoarthritis. Osteoarthritis Cartilage. 1998;6:33–39. doi: 10.1053/joca.1997.0090. [DOI] [PubMed] [Google Scholar]
- 18.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 19.Conrozier T, Saxne T, Fan CS, Mathieu P, Tron AM, Heinegard D, et al. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: a one year prospective study. Ann Rheum Dis. 1998;57:527–532. doi: 10.1136/ard.57.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otterness IG, Weiner E, Swindell AC, Zimmerer RO, Ionescu M, Poole AR. An analysis of 14 molecular markers for monitoring osteoarthritis. Relationship of the markers to clinical end-points. Osteoarthritis Cartilage. 2001;9:224–231. doi: 10.1053/joca.2000.0379. [DOI] [PubMed] [Google Scholar]
- 21.Campion GV, McCrae F, Schnitzer TJ, Lenz ME, Dieppe PA, Thonar EJ. Levels of keratan sulfate in the serum and synovial fluid of patients with osteoarthritis of the knee. Arthritis Rheum. 1991;34:1254–1259. doi: 10.1002/art.1780341008. [DOI] [PubMed] [Google Scholar]
- 22.Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–2478. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 23.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The arthritis, diet, and activity promotion trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 24.Miller GD, Rejeski WJ, Williamson JD, Morgan T, Sevick MA, Loeser RF, et al. The Arthritis, Diet and Activity Promotion Trial (ADAPT): design, rationale, and baseline results. Control Clin Trials. 2003;24:462–480. doi: 10.1016/s0197-2456(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 25.Li XQ, Thonar EJ, Knudson W. Accumulation of hyaluronate in human lung carcinoma as measured by a new hyaluronate ELISA. Connect Tissue Res. 1989;19:243–253. doi: 10.3109/03008208909043899. [DOI] [PubMed] [Google Scholar]
- 26.Thonar EJ, Lenz ME, Klintworth GK, Caterson B, Pachman LM, Glickman P, et al. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 1985;28:1367–1376. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]
- 27.Thonar EJ, Lenz ME, Masuda K, Manicourt DH. Body fluid markers of cartilage metabolism. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of Bone and Cartilage Metabolism. San Diego: Academic Press; 1999. pp. 453–464. [Google Scholar]
- 28.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Wahl SM, Allen JB, Costa GL, Wong HL, Dasch JR. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor beta. J Exp Med. 1993;177:225–230. doi: 10.1084/jem.177.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52:105–111. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 31.van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–290. [PubMed] [Google Scholar]
- 32.Scharstuhl A, Glansbeek HL, Van Beuningen HM, Vitters EL, Van Der Kraan PM, Van Den Berg WB. Inhibition of endogenous tgf-Beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169:507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 33.Sweet MB, Jakim I, Coelho A, Becker PJ, Thonar EJ. Serum keratan sulfate levels in marathon runners. Int J Sports Med. 1992;13:348–350. doi: 10.1055/s-2007-1021279. [DOI] [PubMed] [Google Scholar]
- 34.Sweet MB, Jakim I, Coelho A, Becker PJ, Thonar EJ. Serum keratan sulphate levels during prolonged rest. S Afr Med J. 1990;78:629–630. [PubMed] [Google Scholar]
- 35.Andersson ML, Thorstensson CA, Roos EM, Petersson IF, Heinegard D, Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2006;7:98. doi: 10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 37.Miller GD, Nicklas BJ, Davis CC, Ambrosius WT, Loeser RF, Messier SP. Is serum leptin related to physical function and is it modifiable through weight loss and exercise in older adults with knee osteoarthritis? Int J Obes Relat Metab Disord. 2004;28:1383–1390. doi: 10.1038/sj.ijo.0802737. [DOI] [PubMed] [Google Scholar]
- 38.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48:675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 39.Hunter DJ, Li J, Lavalley M, Bauer DC, Nevitt M, Degroot J, et al. Cartilage markers and their association with cartilage loss on MRI in knee osteoarthritis: The Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9:R108. doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougadosc M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46:2613–2624. doi: 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 41.Garnero P, Mazieres B, Gueguen A, Abbal M, Berdah L, Lequesne M, et al. Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J Rheumatol. 2005;32:697–703. [PubMed] [Google Scholar]
- 42.Mazzuca SA, Brandt KD, Eyre DR, Katz BP, Askew J, Lane KA. Urinary levels of type II collagen C-telopeptide crosslink are unrelated to joint space narrowing in patients with knee osteoarthritis. Ann Rheum Dis. 2006;65:1055–1059. doi: 10.1136/ard.2005.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]