Abstract
Advances in the understanding of the cellular and molecular basis of hepatic fibrogenesis over the past 2 decades have allowed the emergence of a field dedicated to anti-fibrotic therapy. The liver responds to injury by wound healing and subsequently, fibrosis. This response is after essentially all kinds of injury (whether virus, alcohol, or other) and ultimately leads to cirrhosis in some patients. The observation that any of several types of liver diseases and their injury result in cirrhosis suggests a common pathogenesis. It is now recognized that a population or populations of effector cells play a critical role in the fibrogenic process. A classic effector cell, the hepatic stellate cell, is one of the most important fibrogenic cells in the liver. This cell undergoes a transformation during injury, termed “activation”. The activation process is complex, but one of its most prominent features is the synthesis of large amounts of extracellular matrix, resulting in deposition of scar or fibrous tissue. Thus, the hepatic stellate cell and/or other fibrogenic cell types have been a therapeutic target. It is further noteworthy that the fibrogenic process is dynamic and that even advanced fibrosis is reversible. The best anti-fibrotic therapy is elimination of the underlying disease process. For example, elimination of hepatitis B or C virus can lead to reversal of fibrosis. In situations in which treating the underlying process is not possible, specific anti-fibrotic therapy would be highly desirable. To date, many specific anti-fibrotic treatments have been tried, but none have succeeded yet. Nonetheless, because of the importance of fibrosis, the field of anti-fibrotic compounds is rapidly growing. This review will emphasize mechanisms underlying fibrogenesis as they relate to putative anti-fibrotic therapy, and will review current and potential future anti-fibrotic therapies.
Keywords: fibrosis, cirrhosis, stellate cell, extracellular matrix, myofibroblast, liver biopsy, complication, portal hypertension
Introduction
Chronic injury results in a wound healing response that eventually leads to fibrosis. The response is a generalized one, with features common to multiple organ systems. In the liver, a variety of different types of injury lead to fibrogenesis - implying a common pathogenesis. Although a number of specific therapies for patients with different liver diseases have been successfully developed, including anti-viral therapies for patients with hepatitis B and hepatitis C virus infection, specific and effective anti-fibrotic therapy remains elusive.
Over the past 2 decades, great advances in the understanding of fibrosis have been made and multiple mechanisms underlying hepatic fibrogenesis have been uncovered. Elucidation of these mechanisms has been of fundamental importance in highlighting novel potential therapies. Indeed, preclinical studies have pointed to a number of putative therapies that might abrogate fibrogenesis. The objective of this review will be to emphasize mechanisms underlying fibrogenesis, and to review the current status of the field with regard to available and future therapeutics.
Fibrogenesis – Pathophysiology
The fibrogenic process
A fundamental concept is that although the wounding process is complicated, it is characterized by common features that include increased production of extracellular matrix, as a result of a “coordinated” response that includes the action of various events on effector cells that in turn lead to extracellular matrix synthesis. In the liver and in most organs, inflammation often drives the response. Excellent examples include hepatitis B and C infection, autoimmune hepatitis, and alcoholic hepatitis to name a few. The chronicity of inflammation is often important in many types of liver disease, as well as the type of inflammation (i.e., Th2 vs. Th1), and the interplay of inflammation with environmental/metabolic/genetic factors.
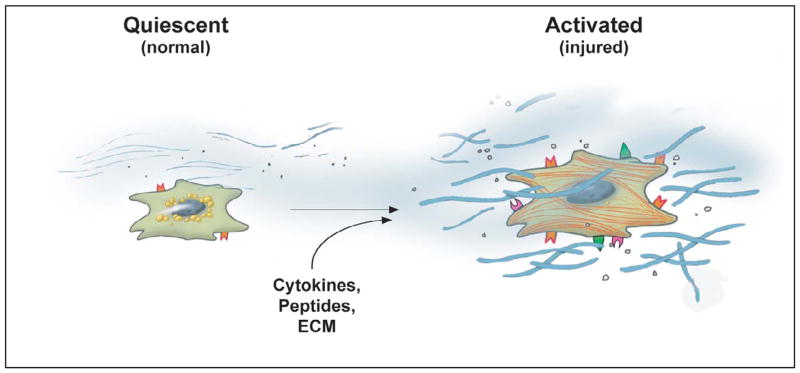
The effectors of the fibrogenic response in the liver are diverse and include different cell types including activated stellate cells, peri-portal and peri-central fibroblasts, myofibroblasts (which may be derived from all 3 of the above cell types), bone marrow derived cells, fibroblasts derived from epithelial cells, and even bile duct epithelial and endothelial cells 29,73,81,82,139,168,169,176. Considerable attention has focused on hepatic stellate cells, which transform from a “quiescent” (normal) to an “activated” (injured liver) state (Figure 1, See the article, ABCD). Although straightforward in concept, the activation process is remarkably complex, and consists of many important cellular changes. Characteristic features of this transition include loss of vitamin A, acquisition of stress fibers, and development of prominent rough endoplasmic reticulum. As intimated above, since the stellate cell has been identified as a key effector of the fibrogenic response, one of the most prominent features of activation is a striking increase in secretion of extracellular matrix (ECM) proteins, including types I, III and IV collagens, fibronectin, laminin and proteoglycans. Some ECM molecules are increased by greater than 50-fold, consistent with the conclusion that stellate cells are the cellular source of the enhanced ECM production at the whole organ level 96. A further critical feature of activation is de novo expression of smooth muscle specific proteins, such as smooth muscle α actin 133. This feature identifies activated stellate cells as liver specific myofibroblasts.
Figure 1. Stellate cell activation.
The current consensus is that the key pathogenic feature underlying liver fibrosis and cirrhosis is activation of hepatic stellate cells. This process is complex, both in terms of the events that induce activation and the effects of activation. Multiple and varied stimuli participate in the induction and maintenance of activation, including, but not limited to cytokines, peptides, and the extracellular matrix itself. Key phenotypic features of activation include production of extracellular matrix, loss of retinoids, proliferation, of upregulation of smooth muscle proteins, secretion of peptides and cytokines (which have autocrine effects), and upregulation of various cytokine and peptide receptors (From reference 129). It is likely that other effector cells (fibroblasts, fibrocytes, bone marrow derived-cells), similarly undergo activation and contribute to the fibrogenic response. With permission, Rockey DC: Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol 3:95, 2005
The field of stellate cell biology has exploded over the past 20 years, and a review of this area can be found in the article, ABCD. Importantly, the science has led to multiple therapeutic approaches based on an understanding of this cell’s biology. For example, many pathways lead specifically to stellate cell fibrogenesis, and these have or can be targeted. Theoretical approaches to anti-fibrotic therapy are highlighted in Box 1. A final important concept is that the complexity of the wounding response allows for multiple different “therapeutic” interventions, including those based on stellate cell biology, but also based on other mechanisms active in the wounding milieu. Therefore, it is possible that more than one anti-fibrotic agent may be prescribed, or that an anti-fibrotic agent may be taken along with another agent having a different (anti-inflammatory, anti-oxidant, etc…) mechanism of action.
Pathophysiology of the fibrogenic process and considerations for therapy
When considering anti-fibrotic therapy, it is important to recognize that fibrosis is a dynamic process. While at one time, it had been believed that the fibrotic lesion was static, abundant evidence indicates that this is not the case. Indeed, a prominent feature of liver fibrosis is that of extracellular matrix turnover, including not only its synthesis, but also its degradation 11. During fibrosis progression, there is increased expression of matrix metalloproteinases (MMPs) as well as their tissue inhibitors (TIMPs). Further, there appears to be an imbalance between MMPs that degrade “good” or normal matrix, and those that degrade “bad” or abnormal matrix. Early in the wounding process, MMPs appear to degrade normal matrix proteins, and this itself may perpetuate the fibrogenic phenotype of effector cells 11,21,49,53,123,157. In advanced fibrosis, overexpression of MMP8 was shown to lead to partial reversal of fibrosis, providing proof of concept for a therapeutic role for overexpressing MMPs 55,149.
An enormous body of literature in animal models 49,71,149,172,177, and a surprisingly robust amount of data in human liver disease emphasizes that fibrosis is reversible. The data come primarily from treatment studies in which the disease has been removed or eliminated (Table 1). For example, eradication or inhibition of hepatitis B virus (HBV) 60,85,97 or hepatitis C virus (HCV)1,122 leads to reversion of fibrosis, even in some patients with histological cirrhosis. Additionally, fibrosis (and cirrhosis) in patients with autoimmune hepatitis who respond to medical treatment (prednisone or equivalent) is reversible 47. Fibrosis may improve in patients with alcoholic liver disease who respond to anti-inflammatory therapy such as corticosteroids 126,151. Fibrosis reverts in patients with hemochromatosis during iron depletion 23,120,173 and after relief of bile duct obstruction 62. Finally, in patients with non-alcoholic steatohepatitis (NASH) treated with the peroxisomal proliferator active receptor (PPAR) gamma agonist, rosiglitazone reduced both steatosis and fibrosis 110.
Table 1.
Therapeutic Considerations for Hepatic Fibrosis
|
Approach to therapy for fibrosis
Monitoring of Hepatic Fibrosis
One of the major challenges in the field of fibrosis therapy currently is now to monitor fibrosis. Emerging evidence suggests that the presence of fibrosis has important prognostic implications. For example, in patients with hepatitis C virus infection after liver transplantation, adverse clinical events appeared to be increased in those patients with the greatest degree of fibrosis 22. Also, progression of non-alcoholic fatty liver disease, and even liver-related mortality also appeared to be related to initial fibrosis stage 48.
Additionally, in patients with many different types of liver disease, histological grade and stage may be helpful in identifying those who should receive therapy and those who should not. This is particularly true now for patients with hepatitis C virus infection 43,155. Nonetheless, it should be emphasized that use of fibrosis data in treatment algorithms for HCV patients remains controversial. On one hand, patients with advanced fibrosis (e.g. Batts and Ludwig stages 3 and/or 4) may be less likely to respond to antiviral therapy than those with less advanced fibrosis, and moreover it is well appreciated that patients with advanced fibrosis are more likely to experience greater side-effects and often require more aggressive supportive measures (e.g., growth factors) to maintain adequate blood cell counts during treatment. Further, patients with advanced fibrosis may have poorer response rates, and should probably be managed differently (e.g. treated with gradual increments in drug doses and/or for longer periods) than patients with absent or mild fibrosis. Moreover, it has been proposed that patients with absent or minimal fibrosis should simply be observed and perhaps undergo periodic liver biopsy for follow-up staging.
Liver biopsy and histological analysis of the liver has long been considered to be the gold standard for determining the extent of fibrosis and as well to assess fibrosis progression. Qualitative assessment of fibrosis has been made simple by the widespread use of connective tissue stains such as including reticulin, Masson’s trichrome, and picrosirius red (which each readily identify extracellular matrix within tissue). Quantitative measure of collagen content can be performed by colorimetric assay of sirius red in liver tissue or by image analytic quantitation of collagen containing tissue 134. Additionally, scoring systems can quantitate fibrosis 18,113,115 and help standardize the interpretation of biopsies among different centers; such systems are most useful for standardization and comparison of fibrosis in large studies. In single patients, simple inspection of biopsies over time is often the most helpful.
Despite the fact that histological analysis of the liver has been traditionally considered to be the gold standard tool to assess fibrosis, it is not perfect. First, liver biopsy is associated with significant potential morbidity, including a finite risk of death 160. Additionally, it is subject to inter-observer variability, and sampling error may be important, as evidenced by studies examining liver samples from different regions of the liver 127,128. For this reason, noninvasive tools to measure fibrosis would be ideal 132. Noninvasive methods used to assess fibrosis include routine clinical parameters such as physical exam findings, laboratory tests 121,166, radiographic tests 33, combinations of laboratory tests 69,166, and specific serum markers 30,100,132. Serum marker panels, including those that utilize mathematical algorithms 69,166, have recently been emphasized.
Most recently, transient elastography, an ultrasound-based technology, has gained considerable attention 130. This examination involving acquisition of pulse-echo ultrasound signals to measure liver stiffness 141 following the simple placement of an ultrasound transducer probe between two ribs, over the right lobe of the liver. The probe transmits a low amplitude (vibration and frequency) signal to the liver, which induces an elastic shear wave that propagates through the liver. This pulse-echo ultrasound measurement provides a measure of liver stiffness (reported in kilopascals). An advantage of this measurement, beyond its non-invasive nature, is that this technique allows measurement of stiffness across a relatively large area of the liver (1–2 cms), which is at least 100 times greater than for a liver biopsy. Normal liver stiffness is reported to be in the range of 4–6 kilopascals. However, cirrhosis is generally present at levels above 12–14 kilopascals, the higher the level, the more likely that the patient has cirrhosis 31,52,179.
Overview of treatment
Preclinical studies have reported a scientific rationale and experimental evidence supporting the use of many potential therapies for fibrosis. Such therapies have been targeted to any of several different biological targets (e.g., inhibition of collagen synthesis, interruption of matrix deposition, stimulation of matrix degradation, modulation of stellate cell activation, or induction of stellate cell death). In general, these therapies have been highly effective in animal models. A number of these preclinical approaches have been transitioned to clinical trials in humans, which are highlighted below and in Tables 2/3. Therapies have been divided into those that target specifically fibrosis (Table 2) and those that target a more general component of the liver disease process (i.e. oxidative stress) (Table 3). Highlighted below are the major anti-fibrotic agents that have been examined in clinical studies in humans.
Table 2.
Diseases and Therapies in which Fibrosis can be Reduced by Treating the Underlying Disorder
| Disease | Therapy |
|---|---|
| Hepatitis B | Lamivudine, others |
| Hepatitis C | * Interferon alpha |
| Autoimmune hepatitis | Corticosteroids |
| Bile duct obstruction | Surgical decompression |
| Hemochromatosis | Iron depletion |
| ¶ Alcoholic hepatitis | Corticosteroids |
| ¶ Primary biliary cirrhosis | Ursodeoxycholic acid, MTX |
| § Non-alcholic steatohepatitis | PPAR gamma ligands |
or PEG-interferon alpha, with or without ribavirin
The effect is minimal if present
Evidence is preliminary at this point
References are given in the text
Abbreviations: MTX = methotrexate; PPAR = peroxisomal proliferator activated receptor
Table 3.
Potential anti-fibrotic therapies with “specific effects”
| Agent | Disease | Efficacy | Safety | Comments |
|---|---|---|---|---|
| Colchicine | Misc | +/− | ++++ | Inhibits collagen synthesis |
| Interferon gamma | HCV | +/− | ++ | Stellate cell specific effects |
| ARBs | Misc | +/− | ++ | Stellate cell specific effects |
| PPAR ligands | NASH | ++ | ++ | Stellate cell specific effects |
| Pirfenidone | Misc | +/− | +++ | Mechanism via cytokines? |
The scale for efficacy and safety is - to ++++ with - being the lowest rating and ++++ the highest rating. The ratings are empiric based on the aggregate available literature. See text for references and discussion of mechanisms.
Abbreviations: ARBs = angiotensin receptor blockers; PPAR = peroxisomal proliferator activated receptor, HCV = hepatitis C virus, NASH = non alcoholic steatohepatitis, misc = miscellaneous.
Specific anti-fibrotic therapies (studied in human subjects)
Angiotensin II antagonists
The angiotensin II system represents an extremely attractive anti-fibrotic target. Abundant experimental evidence points to overproduction of angiotensin II in the injured liver, and for a role of angiotensin II in stimulation of stellate cell activation and fibrogenesis 16,17. A number of studies have also demonstrated specific anti-fibrotic effects angiotensin II inhibition in a variety of animal models 67,75,107. Angiotensin II may also play a role in the pathogenesis of portal hypertension 131, and thus its inhibition could potentially abrogate not only fibrosis, but also portal hypertension.
Several human studies have examined the effects of angiotensin receptor blockers in humans 37,38,57,144,163, most in the setting of advanced liver disease – and most often in an attempt to reduce portal pressure. A 6 week trial of losartan in 25 patients did not significantly reduce HVPG compared to propanolol in patients with cirrhosis treated after a variceal bleeding episode 57. In a randomized trial of 36 patients with cirrhosis and portal hypertension, irbesartan reduced the hepatic venous pressure gradient by 12.2% +/− 6.6% after 7 days, but it also induced arterial hypotension 144.
In a trial of 39 subjects with cirrhosis who were randomized to losartan (19 patients) or propranolol (20 patients), HVPG was measured at baseline and on day 14 of therapy 37. With losartan, 15 of 19 (79%) patients had a reduction in HVPG≥20%, while with propranolol, nine of 20 (45%) patients had a reduction in HVPG≥20% (p < 0.05). Although the hepatic venous pressure gradient reduction (i.e., percentage from baseline) with losartan (27 +/− 22%) was higher than with propranolol (15 +/− 32%), the difference was not significant. In another study, 47 compensated Child A and Child B (8) cirrhotic patients were randomly assigned to receive candesartan (8 mg/d, n = 24) and no treatment (n = 23) for 1 year. The HVPG was decreased significantly in patients treated (−8.4%+/−2.4mmHg) with candesartan and 25% of patients had a reduction > 20% compared to an increase of +5.6%+/−2.9 mmHg in the untreated group. Plasma hyaluronic acid levels were also significantly reduced in candesartan treated patients in whom HVPG diminished while they rose in untreated patients in whom HVPG increased 38.
The data in humans are thus mixed, and further have been performed in small numbers of patients. Given the particularly supportive preclinical data, the evidence suggests that there is likely to be some element of anti-fibrotic effect in humans for the angiotensin receptor blockers (or perhaps angiotensin converting enzyme inhibitors). Larger and longer studies appear to be warranted, several of which have recently been closed or are currently underway (http://clinicaltrials.gov/; Clinical Trials.gov Identifier: NCT00298714, NCT00265642).
Interferon gamma
The interferons consist of a family of 3 major isoforms including α, β and γ There are many different interferon α subtypes, while there appear to be only single interferon β and interferon γspecies. Interferon α and β bind to the same receptor and therefore share many common properties. Interferon α has much more potent antiviral effects than does interferon γ Interferon γ has been shown to specifically inhibit extracellular matrix synthesis in fibroblasts 36. Preclinical work with interferon γ in hepatic stellate cells demonstrated that this cytokine inhibited multiple aspects of stellate cell activation 135,136. These data led to an initial pilot study demonstrating that interferon γ 1b was safe and well tolerated in humans with HCV infection and advanced fibrosis 106. In addition, it led to reduction in fibrosis in selected patients 106. A subsequent double-blind, placebo-controlled, multi-center study examined interferon-γ1b in 488 patients with an Ishak fibrosis score of 4–6 examined 3 treatment groups; interferon-γ1b 100 micrograms (group 1, n=169), interferon-γ1b 200 micrograms (group 2, n=157), or placebo (group 3, n=162) 3 times a week for 48 weeks 116. The vast majority of patients (83.6%) had cirrhosis at baseline (Ishak score=5 or 6). Among the 420 patients in whom pre- and post treatment liver biopsies were evaluable, there was no improvement in Ishak score among the 3 groups. Analysis of interferon-γinducible biomarkers revealed that interferon-inducible T cell-alpha chemoattractant (ITAC), an interferon-γ-inducible CXCR3 chemokine was an independent predictor of stable or improving Ishak score. Interferon-γ was well tolerated, suggesting that interferon-γ could be effective in certain subgroups of patients.
In a randomized, open-labeled, multicenter trial of interferon-γ in patients with HBV infection and biopsy proven hepatic fibrosis 170, a total of 99 patients who were not receiving anti-HBV antiviral medications were divided into those receiving diammone-glycyrrhizinate and potassium-magnesium aspartate alone (n = 33), and those receiving these medications plus 50 micrograms interferon-γ intramuscularly on a daily basis for 3 months, and on alternate days the subsequent 6 months (n = 66). The majority of patients had follow-up biopsies at 9 months. Hepatic fibrosis scores were significantly reduced in 63% of interferon-γtreated patients compared with 24.1% in the control group. Using a semiquantitiative scoring system combining the previously described Chevallier 32 and Knodell 83 systems, mean total fibrosis scores decreased from 13.8 +/− 5.8 to 10.1 +/− 5.1 in the interferon-γ group (p = 0.0001), whereas they were unchanged in control subjects (13.2 +/− 6.8 vs 12.6 +/− 4.8, p = 0.937). Using the Scheuer histologic grading system, 12 out of 54 patients improved ·1 stage(s) in the interferon-γ group compared with 1 of 29 in the control group. Interestingly, of 35 patients with compensated cirrhosis, 26 receiving interferon-γ and 9 in the control group, 5 patients in the interferon-γ group were found to have histological reversal of cirrhosis while no patient in the control group had an improvement in fibrosis and 9 month follow-up biopsy.
In summary, the biologic rationale for use of interferon-γ is strong. The data suggest that there are likely to be subgroups of patients for whom interferon-γ may be effective, though whether it would be a cost-effective therapy is not clear.
Peroxisomal proliferator activated receptor (PPAR) gamma ligands
The PPAR family of nuclear hormone receptors consists of 3 subgroups, alpha, gamma, and delta (beta). These receptors heterodimerize with the retinoid X receptor (RXR) and bind to specific regions on target gene DNA. PPAR gamma ligands have received great attention because hepatic stellate cell activation during liver injury is associated with reduced PPAR gamma expression 104, and activation of this receptor by exogenous PPAR gamma ligands 104, or re-expression of PPAR gamma itself in stellate cells reversed the activated phenotype 64. Additionally, expression of PPAR gamma during liver injury led to substantial improvements in fibrosis 174.
In a pilot study, 30 subjects with histologic evidence of NASH, received the PPAR gamma ligand, rosiglitazone, for 48 weeks 110. Twenty-six patients had posttreatment biopsies. Overall, there was significant improvement in hepatocellular ballooning and zone 3 perisinusoidal fibrosis. For the 25 patients completing 48 weeks of treatment, insulin sensitivity and mean serum alanine aminotransferase (ALT) levels (104 initially, 42 U/L at the end of treatment) improved significantly. Weight gain occurred in 67% of patients during treatment. Liver tests returned to baseline after stopping treatment, consistent with data from a subsequent study that demonstrated a return of histologic NASH after cessation of a PPAR gamma ligand used for therapy 95.
In another small study, pioglitazone was examined in subjects with impaired glucose tolerance or type 2 diabetes and liver biopsy-confirmed NASH 19. Subjects were randomized to 6 months of a hypocaloric diet (a reduction of 500 kcal per day in relation to the calculated daily intake required to maintain body weight) plus pioglitazone (45 mg daily) or a hypocaloric diet plus placebo 19. Compared to placebo, diet plus pioglitazone, improved glycemic control and glucose tolerance and led to normalization of aminotransferase levels. Pioglitazone also decreased hepatic fat content, histologic evidence of steatosis (p = 0.003), ballooning necrosis (p = 0.02), and inflammation (p = 0.008), but did not reduce fibrosis significantly compared to placebo (p = 0.08).
The findings in these small studies suggest that treatment of underlying NASH may be associated with an improvement in fibrosis, and warrant larger studies. Particularly given the preclinical data suggesting a direct affect of PPAR gamma ligands on stellate cell activation, one study examining features of stellate cell biology is currently underway (see http://clinicaltrials.gov/; Clinical Trials.gov Identifier: NCT00244751). Preliminary results are expected in 2008.
Pirfenidone
Pirfenidone ((5-methyl-1-phenyl-2-(1H)-pyri-) is a small orally bioavailable molecule that appears to inhibit collagen synthesis 40,41, though its molecular mechanism of action is not clearly understood. Pirfenidone has been shown to have anti-fibrotic effects in a variety of fibrogenic animal models, including the lung, kidney and liver 54,90,147,156.
Pirfenidone has been evaluated in small number of patients with fibrosing parenchymal organ diseases. In a double-blind, randomized, placebo-controlled trial of 107 patients with idiopathic pulmonary fibrosis, pirfenidone led to an improvement in vital capacity, and a reduction in the number of episodes of acute exacerbation of IPF compared to placebo (p = 0.0031) 12. Significant adverse events were associated with pirfenidone. In 15 patients with treatment naïve HCV related fibrogenesis, the compound was administered for 12 months (dose 1,200 mg/day) 10 and pre- and post treatment biopsies were compared. Fibrosis was reduced in 5 of 15 patients (30%) by the end of 12 months of treatment.
Colchicine
Colchicine is a plant alkaloid that inhibits polymerization of microtubules, a process that is believed to be required for collagen secretion. Thus, this compound is believed to work as an anti-fibrotic compound by preventing collagen secretion and deposition. Colchicine effectively inhibits collagen synthesis and fibrosis in experimental animal models 117,137,138.
Given the rationale for use of colchicine as an anti-fibrotic, as well as its presumed favorable safety profile, colchicine has been studied in a number of clinical trials 77,80,105,124, including in primary biliary cirrhosis, alcoholic cirrhosis, and in various other liver diseases 80. In one primary biliary cirrhosis trial, improvements were noted in a number of biochemical markers, but colchicine failed to reduce fibrosis 77. Interestingly, in a study comparing colchicine and methotrexate for PBC in 42 subjects, there was no change in fibrosis after 24 months of treatment with colchicine (or methotrexate), and in those with stable or improving histologic stage, interleukin-1beta synthesis was elevated in peripheral blood mononuclear cells 103. In a double blind, randomized, controlled trial of colchicine versus placebo in 100 patients with different liver diseases (mostly alcohol or posthepatitic), colchicine led to improved fibrosis as well as a dramatic improvement in survival when followed for up to 14 years 80. However, this study has been questioned because many patients were lost to follow-up, and there there was substantial unexplained excess mortality in the control group from causes unrelated to liver disease. A meta-analysis including 1138 subjects found that colchicine had no effect on fibrosis or mortality 124.
In a multicenter study involving 549 patients comparing colchicine (0.6 mg p.o. Bid) to placebo in patients with alcoholic liver disease, there was no effect of active treatment on survival (histologic data were not obtained) 105. In a more recent small trial of colchicine of 38 subjects with miscellaneous liver diseases randomized to receive either colchicine 1 mg per day (n=21) or no agent (n=17) for at least 12 months 111. Liver biopsy was performed prior to beginning colchicine and after 12 months. Interestingly, mean albumin serum levels increased 12 months post-treatment period only in the colchicine group (p < 0.05). Although Knodell fibrosis scores remained unchanged at 12 months, 7 patients were noted to have a reduction in mean serum PIIINP levels during 24-month post-treatment follow-up period.
In summary, the data indicate that colchicine is generally safe (although one report suggested that it may alter the response to interferon alpha bases anti HCV therapy 8) and may lead to improvement in markers of liver disease and even mortality from liver disease. Overall, however, colchicine does not appear to reduce hepatic fibrosis, and it cannot therefore be recommended as a primary anti-fibrotic treatment. It is also noteworthy that in small randomized studies, colchicine did not appear to be effective for treatment of pulmonary fibrosis 44,145.
Herbal Medicines
A number of herbal medicines have been shown to have anti-fibrotic properties in experimental animal models 98,140,146,177,178. Many of these medications have arisen from China 93. While the mechanism(s) of many of these agents is unknown, these compounds are being used extensively in a wide array of patients with liver diseases 167. Medications containing herbs of the Salvia genus have been popular as anti-fibrotics; salvianolic acid B, a major water-soluble polyphenolic acid appears to be the major active ingredient 93,167. This compound appears to have specific effects on stellate cells. The active ingredients of other agents, including curcumin, glycyrrhizin, celastrol, tetrandrine, berberine, oxymatrine appear to have a wide variety of biologic effects, accounting for their purported activity in human disease.
It is important to emphasize that although some studies have suggested effectiveness of specific herbal medicines 93,167, rigorous evidence is sorely lacking. Since it is well appreciated that such herbal medicines may have significant toxicity, including hepatotoxicity 152, these medications should be used with extreme caution.
Compounds with a potential anti-fibrotic effect occurring due to upstream effects
A number of compounds appear to be capable of affecting fibrogenesis, not through a direct effect on stellate cells or on matrix synthesis per se, but rather by having an effect on other important biologic events such as on lipid peroxidation, on the immune system, or others. These are highlighted briefly below and in Table 3.
Silymarin
The major active component of the milk thistle Silybum marianum, silymarin extract (in turn the major component of which is silybinin), reduces lipid peroxidation and inhibits fibrogenesis in small animals 26,74, as well as in baboons 86. Although fibrosis was not studied as a primary outcome, the compound has been found to be safe. It has been reported to have variable effects 50,114. One study revealed a putative benefit on mortality in patients with alcohol induced liver disease 50. Those with early stages of cirrhosis also appeared to benefit. However, in another study focused solely on alcoholics, no survival benefit could be identified 114. Given the apparent safety of silymarin, and its common use as a complementary and alternative medicine, studies in patients with NASH or in those who have failed conventional antiviral treatment for HCV infection have been initiated (http://clinicaltrials.gov/;Clinical Trials.gov Identifier: NCT00680407 and NCT00680342). Although fibrosis is not a primary outcome measure, histological data are planned, and thus information about the effect of silymarin on liver fibrosis is anticipated.
Polyenylphosphatidylcholine
Polyenylphosphatidylcholine has gained considerable interest in the treatment of patients with liver injury. The therapeutic compound is derived from purified soybean extract, consisting of 95–96% polyunsaturated phosphatidylcholines. Polyenylphosphatidylcholine has both has both antioxidant and anti-fibrotic and properties. It is attractive in alcoholic liver injury because this disease is often associated with oxidative stress. Oxidative stress in turn leads to lipid peroxidation, cellular injury, inflammation and subsequently fibrogenesis. It has thus been proposed that because phosphatidylcholine is a prominent component of cell membranes, that supplementation of it should protect cell membranes and might lead to reduced cellular injury and fibrogenesis. Experimental data support this concept 7.
Several large studies have been undertaken in an attempt to determine whether polyenylphosphatidylcholine is beneficial. A multicenter, prospective, randomized, double-blind placebo-controlled VA cooperative clinical trial examined 789 alcoholics (average alcohol intake of 16 drinks/day) 87. Subjects were randomized to either polyenylphosphatidylcholine or placebo for 2 years. Although the majority of subjects substantially reduced their ethanol consumption during the trial (which was felt to result in improvement in fibrosis in the control group), polyenylphosphatidylcholine failed to lead to a comparative improvement in fibrosis. A subsequent study examining the effect of polyenylphosphatidylcholine is currently underway (http://clinicaltrials.gov/;Clinical Trials.gov Identifier: NCT00211848).
Ursodeoxycholic acid
Ursodeoxycholic acid, a non-toxic bile acid, binds to hepatocyte membranes and is presumably cytoprotective, thereby reducing inflammation and therefore downstream fibrogenesis 108. It is important to emphasize that neither experimental data nor human studies indicate a primary anti-fibrotic effect of ursodeoxycholic acid in the liver. However, an extensive body of literature, in a variety of liver diseases generally has examined ursodeoxycholic 34,39,59,72,88,89,118,119,154. The aggregate data suggest that ursodeoxycholic acid may impede progression of fibrosis in primary biliary cirrhosis via effects on biliarly ductal inflammation, particularly if initiated early in the disease course. Ursodeoxycholic acid is safe, and while it is expensive, in the absence of definitively effective agents, it is this author’s belief that the available data justify its use was an “anti-fibrotic”, primarily in patients with primary biliary cirrhosis.
Interleukin-10
Interleukin-10 is a potent immunomodulatory cytokine which can down regulate production of proinflammatory T cell cytokines, such as tumor necrosis factor-α, interleukin-1, interferon γ, and interleukin-2. Endogenous interleukin-10 appears to attenuate the intrahepatic inflammatory response and reduce fibrosis in several models of liver injury 161. A direct anti-fibrotic effect for interleukin-10 has not been established. Notwithstanding, interleukin-10 was given to 30 subjects with HCV infection and advanced fibrosis who had failed antiviral therapy for 12 months in an effort shift the intrahepatic immunologic balance away from Th1 cytokine predominance (SQ interleukin-10 given daily or thrice weekly) 109. This therapy decreased hepatic inflammatory activity and fibrosis, but led to increased HCV viral levels. Thus, while these results suggest that interleukin-10 might be an attractive anti-fibrotic agent, the adverse effects on HCV viral levels are problematic.
Miscellaneous antioxidants and anti-inflammatory compounds
Oxidative stress has been implicated in a wide variety of biological processes in liver injury (including stellate cell activation and stimulation of extracellular matrix production) 6. Thus, antioxidants have received considerable attention as putative anti-fibrotics 27,63,68,102,153. Compounds, including the vitamin E precursor, d-alpha-tocopherol (1200 IU/day for 8 weeks) 68, vitamin E 102,153, malotilate 9, propylthiouracil 125, penicillamine 24,42,143, and S-adenosylmethionine 92,99 have all been tested in humans; evidence supporting their effectiveness remains lacking. It is noteworthy that for many of these compounds, fibrosis was not typically measured as a specific outcome. Thus, it is not appropriate to consider these agents as primary anti-fibrotics, but rather as compounds that could have secondary effects on fibrogenesis due to other properties.
Metrothrexate is also considered to be an anti-inflammatory compound. However, it has also been believed to be profibrogenic 2,112, although the risk of fibrosis progression when used in patients with skin or rheumatologic disease may be less than commonly believed 2,158. Nonetheless, it has been studied in a substantial number of human trials as a therapeutic agent in patients with primary biliary cirrhosis 13,14,65,103,158. Although improvement in disease and fibrosis have been reported, including reversion of fibrosis 79, the majority of the data on methotrexate are either negative 13,65 or show that its effects are marginal, either alone 65, or in combination with colchicine 78. If methotrexate is used to treat patients (with primary biliary cirrhosis), an experienced Hepatologist must manage its use.
Experimental evidence suggests that inhibition of tumor necrosis factor alpha (TNF-α) signaling during liver injury may ameliorate fibrosis 15,84. TNF-α is upregulated in alcoholic liver disease, and thus an anti-TNF-α compound would be attractive because it should reduce inflammation and thus fibrosis. Several studies have examined the effect of anti-TNF-α compounds in patients in patients alcohol induced liver disease 4,101,150,162. Available evidence suggests suggest an improvement in inflammation, and acute injury (which presumably precede fibrosis in this disease) 162. In a randomized, double blind, placebo-controlled trial of etanercept in patients with moderate to severe alcoholic hepatitis, there was no improvement in mortality at one month, and patients treated with etanercept had a greater mortality after 6 months; of note, however, this study did not evaluate liver histology, 25.
Why have so many potential therapies been effective in animal models, yet so ineffective in humans?
This key question is a major conundrum for the field of anti-fibrotics. A critical consideration is that experimental models and conditions are dramatically different from real life situations. First, in most animal experiments, anti-fibrotic agents have been tested for their ability to prevent development of fibrosis. This almost never happens in the clinical arena (patients present with advanced fibrosis or cirrhosis, and there is little or no opportunity to treat the patient during fibrosis progression). Second, patients in most of the human trials performed to date have had advanced fibrosis, if not cirrhosis, and since the duration of treatment has been relatively short, it seems unlikely that even if a compound actively inhibited fibrosis, a demonstrable benefit may not be apparent within a short (1 or 2 year) time frame.
It is also possible that there are differences in pharmacokinetics of therapeutic agents among animals and humans. For example, drug levels may be pushed to very high levels in animals, but such levels are not realistically attainable in humans. It is also possible that compounds that truly have anti-fibrotic features in animals are simply not anti-fibrotic in humans; this may be because of differences in basic cell or molecular aspects of the fibrogenic platforms.
Finally, the duration of injury differs markedly between rodent models and human disease, which could lead to significant differences in the cross-linking of ECM, and thus its potential for degradation. Whereas human diseases that lead to fibrosis require decades, in rodents this process is condensed into weeks or months, and thus there is less time for the ECM to ‘mature’, meaning that there is less chemical cross linking and instead the scar remains highly cellular and resorbable.
Future specific targets
A comprehensive discussion of the many different putative pathways that could lead to novel anti-fibrotic therapeutics is beyond the scope of this review. However, there are several systems/areas that are particularly attractive; several are highlighted in Table 4 and below. The most central of fibrogenic pathways involves the cytokine, transforming growth factor beta (TGF-β). Several approaches to inhibit the action of TGF-β can interrupt the TGF-β signaling pathway 56,70,175. The concept is clear, although theoretical concerns include the potential (pro-proliferative) effect of inhibiting TGF-β signaling in vivo.
Table 4.
Potential anti-fibrotic therapies with “general” effects
| Agent | Disease | Efficacy | Safety | Comments |
|---|---|---|---|---|
| Interleukin-10 | HCV | ++ | + | Increased viral load |
| Malotilate | ETOH | − | +++ | |
| PPC | ETOH | − | ++++ | |
| Propylthiouracil | ETOH | − | ++ | |
| SAM | ETOH | + | +++ | |
| Anti-TNFα compounds | ETOH | ++ | + | Clearly anti-inflammatory |
| Ursodeoxycholic acid | Multiple | + | ++++ | Most studied in PBC |
| Vitamin E | HCV/NASH | - | ++++ |
The scale for efficacy and safety is - to ++++ with - being the lowest rating and ++++ the highest rating. The ratings are empiric based on the aggregate available literature. See text for references and discussion of mechanisms.
Abbreviations: PPC = Polyenylphosphatidylcholine; SAM = s-adenosylmethionine, TNF = tumor necrosis factor; ETOH = alcohol, HCV = hepatitis C virus, NASH = non alcoholic steatohepatitis, misc = miscellaneous, PBC = primary biliary cirrhosis.
Recent data implicate the cannabinoid system in fibrogenesis. In the injured liver, the endogenous endocannabinoid receptors, CB1 and CB2 are upregulated and thus facilitate endocannabinoid signaling 148. Additionally, in patients with chronic hepatitis C virus infection, daily cannabis use is an independent predictor of fibrosis progression 66. On one hand, upregulation of endogenous hepatic cannabanoid CB2 receptors is associated with progression of experimental liver fibrosis 76. On the other hand, CB1 receptors were induced in human cirrhotic samples and in liver fibrogenic cells 159, and in animals undergoing liver injury, a CB1 receptor antagonist inhibited fibrosis, presumably by inhibiting expression of TGF-β1 and by either inhibiting growth hepatic myofibroblasts and/or stimulating apoptosis 159.
Data is emerging that suggests angiogenesis is important in the fibrogenic response to injury 35,164 and thus, anti-angiogenic compounds are attractive therapeutic targets. Likewise, as biology uncovering stellate cell signaling pathways continues to emerge, therapy targeted at these pathways will become attractive 28, with a caveat being that the signaling pathways are extremely complicated, and moreover may vary among models of injury 5.
An important therapeutic concept is directed or targeted therapy. Since many compounds have adverse affects collateral cells or organs outside the fibrogenic response, it would be most desirable to specifically target fibrogenic cells, particularly hepatic stellate cells 20,45,46,58,61,94,98. The ability to specifically stimulate stellate cell apoptosis and enhance the resolution of fibrosis is especially attractive 172. Additionally, the ability to potentially specifically target siRNAs to the liver also makes this approach appealing 3,142,171. MicroRNAs may also be important in fibrogenesis 165; additional investigation in liver injury models is expected to lead to potential therapies for liver fibrosis. A number of other specific targets are of considerable interest (Table 4).
Farnesoid X receptor (FXR) is a member of the nuclear hormone receptor superfamily or transcription factors that is bile acid-activated. It is not only hepatoprotective in various experimental models of liver injury 51,91, but it may also ameliorate fibrosis. FXR activators may be particularly useful in patients with cholestatic injury.
Summary
Elucidation of the mechanisms responsible for fibrogenesis, with particular emphasis on stellate cell biology, has generated great hope that novel therapies will evolve; indeed, the field of anti-fibrotic compounds is growing rapidly. A central event in fibrogenesis is the activation of effector cells (hepatic stellate cells are the most prominent). The activation process is characterized by a number of important features, including in particular, enhanced matrix synthesis and transition to a myofibroblast-like (and contractile) phenotype. Factors controlling activation are multifactorial and complex, and thus multiple potential therapeutic interventions are possible. A further critical concept is that even advanced fibrosis is dynamic and may be reversible. Currently, the most effective therapy for hepatic fibrogenesis is to attenuate or clear the underlying disease. The most effective specific anti-fibrotic therapies will most likely be directed at fibrogenic effector cells, either in a targeted fashion, or by using generalized approaches that take in to account biologic differences between fibrogenic cells and their non-fibrogenic neighbors. Additionally, approaches that address matrix remodeling (i.e. by enhancing matrix degradation or inhibiting factors that prevent matrix breakdown) will be pursued. Thus, although there are no specific, effective, safe, and inexpensive anti-fibrotic therapies yet, multiple potential targets have been identified, and it is expected that effective therapies will emerge.
Table 5.
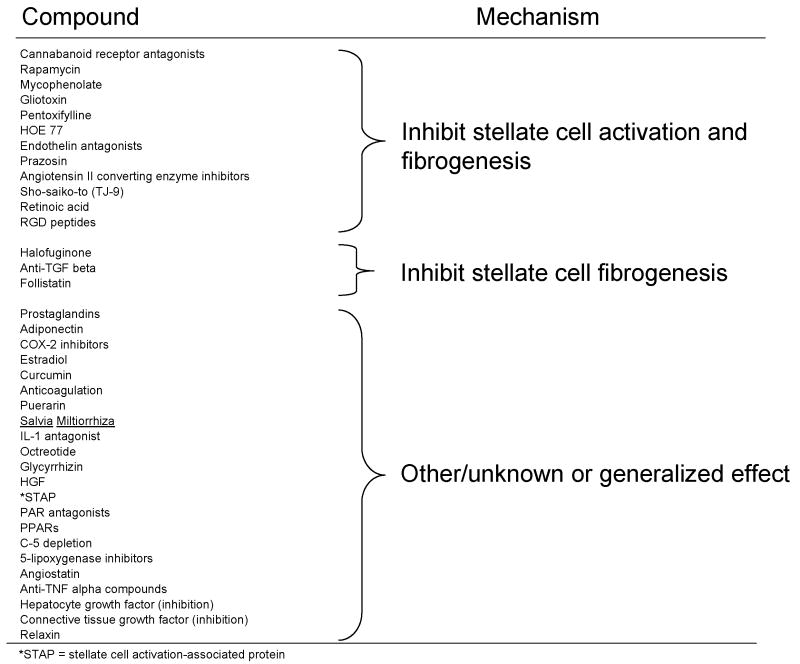
Experimental Anti-Fibrotic Therapies
|
Acknowledgments
This work was supported by the NIH (Grants R01 DK 50574 and R01 DK 60338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abergel A, Darcha C, Chevallier M, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol. 2004;16:1219. doi: 10.1097/00042737-200411000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Aithal GP, Haugk B, Das S, et al. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment Pharmacol Ther. 2004;19:391. doi: 10.1046/j.1365-2036.2004.01819.x. [DOI] [PubMed] [Google Scholar]
- 3.Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 5.Al-karim K, Shao R, Rockey DC. Am J Pathol. 2008 In Press. [Google Scholar]
- 6.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Aleynik SI, Leo MA, Ma X, et al. Polyenylphosphatidylcholine prevents carbon tetrachloride-induced lipid peroxidation while it attenuates liver fibrosis. J Hepatol. 1997;27:554. doi: 10.1016/s0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- 8.Angelico M, Cepparulo M, Barlattani A, et al. Unfavourable effects of colchicine in combination with interferon-alpha in the treatment of chronic hepatitis C. Aliment Pharmacol Ther. 2000;14:1459. doi: 10.1046/j.1365-2036.2000.00857.x. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. The results of a randomized double blind controlled trial evaluating malotilate in primary biliary cirrhosis. A European multicentre study group. J Hepatol. 1993;17:227. doi: 10.1016/s0168-8278(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 10.Armendariz-Borunda J, Islas-Carbajal MC, Meza-Garcia E, et al. A pilot study in patients with established advanced liver fibrosis using pirfenidone. Gut. 2006;55:1663. doi: 10.1136/gut.2006.107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 12.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 13.Bach N, Bodian C, Bodenheimer H, et al. Methotrexate therapy for primary biliary cirrhosis. Am J Gastroenterol. 2003;98:187. doi: 10.1111/j.1572-0241.2003.07173.x. [DOI] [PubMed] [Google Scholar]
- 14.Bach N, Thung SN, Schaffner F. The histologic effects of low-dose methotrexate therapy for primary biliary cirrhosis. Arch Pathol Lab Med. 1998;122:342. [PubMed] [Google Scholar]
- 15.Bahcecioglu IH, Koca SS, Poyrazoglu OK, et al. Hepatoprotective Effect of Infliximab, an Anti-TNF-alpha Agent, on Carbon Tetrachloride-Induced Hepatic Fibrosis. Inflammation. 2008 doi: 10.1007/s10753-008-9067-1. [DOI] [PubMed] [Google Scholar]
- 16.Bataller R, Gabele E, Parsons CJ, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 17.Bataller R, Sancho-Bru P, Gines P, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117. doi: 10.1016/s0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 18.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 20.Beljaars L, Molema G, Schuppan D, et al. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem. 2000;275:12743. doi: 10.1074/jbc.275.17.12743. [DOI] [PubMed] [Google Scholar]
- 21.Benyon RC, Iredale JP, Goddard S, et al. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 22.Blasco A, Forns X, Carrion JA, et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology. 2006;43:492. doi: 10.1002/hep.21090. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg RS, Chopra S, Ibrahim R, et al. Primary hepatocellular carcinoma in idiopathic hemochromatosis after reversal of cirrhosis. Gastroenterology. 1988;95:1399. doi: 10.1016/0016-5085(88)90379-4. [DOI] [PubMed] [Google Scholar]
- 24.Bodenheimer HC, Jr, Schaffner F, Sternlieb I, et al. A prospective clinical trial of D-penicillamine in the treatment of primary biliary cirrhosis. Hepatology. 1985;5:1139. doi: 10.1002/hep.1840050613. [DOI] [PubMed] [Google Scholar]
- 25.Boetticher NC, et al. Gastroenterology. 2008;134:A765. [Google Scholar]
- 26.Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 27.Brown KE, Poulos JE, Li L, et al. Effect of vitamin E supplementation on hepatic fibrogenesis in chronic dietary iron overload. Am J Physiol. 1997;272:G116. doi: 10.1152/ajpgi.1997.272.1.G116. [DOI] [PubMed] [Google Scholar]
- 28.Buck M, Chojkier M. A Ribosomal S-6 Kinase-Mediated Signal to C/EBP-beta Is Critical for the Development of Liver Fibrosis. PLoS ONE. 2007;2:e1372. doi: 10.1371/journal.pone.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caligiuri A, Glaser S, Rodgers RE, et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol. 1998;275:G835. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- 30.Callewaert N, Van Vlierberghe H, Van Hecke A, et al. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 31.Castera L, Foucher J, Bertet J, et al. FibroScan and FibroTest to assess liver fibrosis in HCV with normal aminotransferases. Hepatology. 2006;43:373. doi: 10.1002/hep.21019. [DOI] [PubMed] [Google Scholar]
- 32.Chevallier M, Guerret S, Chossegros P, et al. A histological semiquantitative scoring system for evaluation of hepatic C fibrosis in needle liver biopsy specimens: comparison with morphometric C studies. Hepatology. 1994;20:349. [PubMed] [Google Scholar]
- 33.Colli A, Fraquelli M, Andreoletti M, et al. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 34.Combes B, Carithers RL, Jr, Maddrey WC, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759. [PubMed] [Google Scholar]
- 35.Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 36.Czaja MJ, Weiner FR, Eghbali M, et al. Differential effects of gamma-interferon on collagen and fibronectin gene expression. J Biol Chem. 1987;262:13348. [PubMed] [Google Scholar]
- 37.De BK, Bandyopadhyay K, Das TK, et al. Portal pressure response to losartan compared with propranolol in patients with cirrhosis. Am J Gastroenterol. 2003;98:1371. doi: 10.1111/j.1572-0241.2003.07497.x. [DOI] [PubMed] [Google Scholar]
- 38.Debernardi-Venon W, Martini S, Biasi F, et al. AT1 receptor antagonist Candesartan in selected cirrhotic patients: effect on portal pressure and liver fibrosis markers. J Hepatol. 2007;46:1026. doi: 10.1016/j.jhep.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Degott C, Zafrani ES, Callard P, et al. Histopathological study of primary biliary cirrhosis and the effect of ursodeoxycholic acid treatment on histology progression. Hepatology. 1999;29:1007. doi: 10.1002/hep.510290444. [DOI] [PubMed] [Google Scholar]
- 40.Di Sario A, Bendia E, Macarri G, et al. The anti-fibrotic effect of pirfenidone in rat liver fibrosis is mediated by downregulation of procollagen alpha1(I), TIMP-1 and MMP-2. Dig Liver Dis. 2004;36:744. doi: 10.1016/j.dld.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Di Sario A, Bendia E, Svegliati Baroni G, et al. Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production. J Hepatol. 2002;37:584. doi: 10.1016/s0168-8278(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 42.Dickson ER, Fleming TR, Wiesner RH, et al. Trial of penicillamine in advanced primary biliary cirrhosis. N Engl J Med. 1985;312:1011. doi: 10.1056/NEJM198504183121602. [DOI] [PubMed] [Google Scholar]
- 43.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Douglas WW, Ryu JH, Swensen SJ, et al. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. A randomized prospective study Members of the Lung Study Group. Am J Respir Crit Care Med. 1998;158:220. doi: 10.1164/ajrccm.158.1.9709089. [DOI] [PubMed] [Google Scholar]
- 45.Douglass A, Wallace K, Parr R, et al. Antibody-targeted myofibroblast apoptosis reduces fibrosis during sustained liver injury. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 46.Du SL, Pan H, Lu WY, et al. Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol Exp Ther. 2007;322:560. doi: 10.1124/jpet.107.122481. [DOI] [PubMed] [Google Scholar]
- 47.Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 49.Emonard H, Grimaud JA. Active and latent collagenase activity during reversal of hepatic fibrosis in murine schistosomiasis. Hepatology. 1989;10:77. doi: 10.1002/hep.1840100116. [DOI] [PubMed] [Google Scholar]
- 50.Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 51.Fiorucci S, Antonelli E, Rizzo G, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference. Hepatology. 2007;45:242. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- 54.Garcia L, Hernandez I, Sandoval A, et al. Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol. 2002;37:797. doi: 10.1016/s0168-8278(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Banuelos J, Siller-Lopez F, Miranda A, et al. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002;9:127. doi: 10.1038/sj.gt.3301647. [DOI] [PubMed] [Google Scholar]
- 56.George J, Roulot D, Koteliansky VE, et al. In vivo inhibition of rat stellate cell activation by soluble TGF beta type II receptor: a potential new therapy for hepatic fibrosis. Proceedings of the National Academy of Sciences:USA. 1999;96:12719. doi: 10.1073/pnas.96.22.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Abraldes J, Albillos A, Banares R, et al. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology. 2001;121:382. doi: 10.1053/gast.2001.26288. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalo T, Beljaars L, van de Bovenkamp M, et al. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321:856. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- 59.Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: a meta-analysis. Lancet. 1999;354:1053. doi: 10.1016/S0140-6736(98)11293-X. [DOI] [PubMed] [Google Scholar]
- 60.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 61.Hagens WI, Beljaars L, Mann DA, et al. Cellular targeting of the apoptosis-inducing compound gliotoxin to fibrotic rat livers. J Pharmacol Exp Ther. 2008;324:902. doi: 10.1124/jpet.107.132290. [DOI] [PubMed] [Google Scholar]
- 62.Hammel P, Couvelard A, O’oole D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa T, Yoneda M, Nakamura K, et al. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 64.Hazra S, Xiong S, Wang J, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279:11392. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 65.Hendrickse MT, Rigney E, Giaffer MH, et al. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial [see comments] Gastroenterology. 1999;117:400. doi: 10.1053/gast.1999.0029900400. [DOI] [PubMed] [Google Scholar]
- 66.Hezode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 67.Hirose A, Ono M, Saibara T, et al. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 68.Houglum K, Venkataramani A, Lyche K, et al. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- 69.Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 70.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nature Medicine. 1996;2:418. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 71.Issa R, Williams E, Trim N, et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquemin E, Hermans D, Myara A, et al. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 1997;25:519. doi: 10.1002/hep.510250303. [DOI] [PubMed] [Google Scholar]
- 73.Jarnagin WR, Rockey DC, Koteliansky VE, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia JD, Bauer M, Cho JJ, et al. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392. doi: 10.1016/s0168-8278(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 75.Jin H, Yamamoto N, Uchida K, et al. Telmisartan prevents hepatic fibrosis and enzyme-altered lesions in liver cirrhosis rat induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2007;364:801. doi: 10.1016/j.bbrc.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 76.Julien B, Grenard P, Teixeira-Clerc F, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 77.Kaplan MM, Alling DW, Zimmerman HJ, et al. A prospective trial of colchicine for primary biliary cirrhosis. N Engl J Med. 1986;315:1448. doi: 10.1056/NEJM198612043152304. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan MM, Cheng S, Price LL, et al. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology. 2004;39:915. doi: 10.1002/hep.20103. [DOI] [PubMed] [Google Scholar]
- 79.Kaplan MM, DeLellis RA, Wolfe HJ. Sustained biochemical and histologic remission of primary biliary cirrhosis in response to medical treatment. Ann Intern Med. 1997;126:682. doi: 10.7326/0003-4819-126-9-199705010-00002. [DOI] [PubMed] [Google Scholar]
- 80.Kershenobich D, Vargas F, Garcia-Tsao G, et al. Colchicine in the treatment of cirrhosis of the liver. N Engl J Med. 1988;318:1709. doi: 10.1056/NEJM198806303182602. [DOI] [PubMed] [Google Scholar]
- 81.Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 82.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 84.Koca SS, Bahcecioglu IH, Poyrazoglu OK, et al. The treatment with antibody of TNF-alpha reduces the inflammation, necrosis and fibrosis in the non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Inflammation. 2008;31:91. doi: 10.1007/s10753-007-9053-z. [DOI] [PubMed] [Google Scholar]
- 85.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group [see comments] N Engl J Med. 1998;339:61. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 86.Lieber CS, Leo MA, Cao Q, et al. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 87.Lieber CS, Weiss DG, Groszmann R, et al. II Veterans Affairs Cooperative Study of Polyenylphosphatidylcholine in Alcoholic Liver Disease. Alcohol Clin Exp Res. 2003;27:1765. doi: 10.1097/01.ALC.0000093743.03049.80. [DOI] [PubMed] [Google Scholar]
- 88.Lindblad A, Glaumann H, Strandvik B. A two-year prospective study of the effect of ursodeoxycholic acid on urinary bile acid excretion and liver morphology in cystic fibrosis-associated liver disease. Hepatology. 1998;27:166. doi: 10.1002/hep.510270126. [DOI] [PubMed] [Google Scholar]
- 89.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 90.Liu H, Drew P, Gaugler AC, et al. Pirfenidone inhibits lung allograft fibrosis through L-arginine-arginase pathway. Am J Transplant. 2005;5:1256. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002;27:155. doi: 10.1016/s0741-8329(02)00226-4. [DOI] [PubMed] [Google Scholar]
- 93.Luk JM, Wang X, Liu P, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int. 2007;27:879. doi: 10.1111/j.1478-3231.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 94.Luk JM, Zhang QS, Lee NP, et al. Hepatic stellate cell-targeted delivery of M6P-HSA-glycyrrhetinic acid attenuates hepatic fibrogenesis in a bile duct ligation rat model. Liver Int. 2007;27:548. doi: 10.1111/j.1478-3231.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 95.Lutchman G, Modi A, Kleiner DE, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 96.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mallet VO, Dhalluin-Venier V, Verkarre V, et al. Reversibility of cirrhosis in HIV/HBV coinfection. Antivir Ther. 2007;12:279. [PubMed] [Google Scholar]
- 98.Mandal AK, Das S, Basu MK, et al. Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis. J Pharmacol Exp Ther. 2007;320:994. doi: 10.1124/jpet.106.114215. [DOI] [PubMed] [Google Scholar]
- 99.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 100.McHutchison JG, Blatt LM, de Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 101.Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 102.Mezey E, Potter J, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E in alcoholic hepatitis. Hepatology. 2003;38:264A. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 103.Miller LC, Sharma A, McKusick AF, et al. Synthesis of interleukin-1 beta in primary biliary cirrhosis: relationship to treatment with methotrexate or colchicine and disease progression. Hepatology. 1995;22:518. [PubMed] [Google Scholar]
- 104.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 105.Morgan TR, Nemchausky B, Schiff ER, et al. Colchicine does not prolong life in patients with advanced alcoholic cirrhosis: results of a prospective, randomized, placebo-controlled trial. Gastroenterology. 2002;641A [Google Scholar]
- 106.Muir AJ, Sylvestre PB, Rockey DC. Interferon gamma-1b for the treatment of fibrosis in chronic hepatitis C infection. J Viral Hepat. 2006;13:322. doi: 10.1111/j.1365-2893.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 107.Nabeshima Y, Tazuma S, Kanno K, et al. Anti-fibrogenic function of angiotensin II type 2 receptor in CCl4-induced liver fibrosis. Biochem Biophys Res Commun. 2006;346:658. doi: 10.1016/j.bbrc.2006.05.183. [DOI] [PubMed] [Google Scholar]
- 108.Nava-Ocampo AA, Suster S, Muriel P. Effect of colchiceine and ursodeoxycholic acid on hepatocyte and erythrocyte membranes and liver histology in experimentally induced carbon tetrachloride cirrhosis in rats. Eur J Clin Invest. 1997;27:77. doi: 10.1046/j.1365-2362.1997.910615.x. [DOI] [PubMed] [Google Scholar]
- 109.Nelson DR, Tu Z, Soldevila-Pico C, et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- 110.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, et al. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 111.Nikolaidis N, Kountouras J, Giouleme O, et al. Colchicine treatment of liver fibrosis. Hepatogastroenterology. 2006;53:281. [PubMed] [Google Scholar]
- 112.Nohlgard C, Rubio CA, Kock Y, et al. Liver fibrosis quantified by image analysis in methotrexate-treated patients with psoriasis. J Am Acad Dermatol. 1993;28:40. doi: 10.1016/0190-9622(93)70006-f. [DOI] [PubMed] [Google Scholar]
- 113.O’Brien MJ, Keating NM, Elderiny S, et al. An assessment of digital image analysis to measure fibrosis in liver biopsy specimens of patients with chronic hepatitis C. Am J Clin Pathol. 2000;114:712. doi: 10.1309/D7AU-EYW7-4B6C-K08Y. [DOI] [PubMed] [Google Scholar]
- 114.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial [see comments] J Hepatol. 1998;28:615. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 115.Pilette C, Rousselet MC, Bedossa P, et al. Histopathological evaluation of liver fibrosis: quantitative image analysis vs semi-quantitative scores. Comparison with serum markers. J Hepatol. 1998;28:439. doi: 10.1016/s0168-8278(98)80318-8. [DOI] [PubMed] [Google Scholar]
- 116.Pockros PJ, Jeffers L, Afdhal N, et al. Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-gamma1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology. 2007;45:569. doi: 10.1002/hep.21561. [DOI] [PubMed] [Google Scholar]
- 117.Poo JL, Feldmann G, Moreau A, et al. Early colchicine administration reduces hepatic fibrosis and portal hypertension in rats with bile duct ligation. J Hepatol. 1993;19:90. doi: 10.1016/s0168-8278(05)80181-3. [DOI] [PubMed] [Google Scholar]
- 118.Poupon RE, Bonnand AM, Chretien Y, et al. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology. 1999;29:1668. doi: 10.1002/hep.510290603. [DOI] [PubMed] [Google Scholar]
- 119.Poupon RE, Lindor KD, Pares A, et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 120.Powell LW, Kerr JF. Reversal of “irrhosis”in idiopathic haemochromatosis following long-term intensive venesection therapy. Australas Ann Med. 1970;19:54. doi: 10.1111/imj.1970.19.1.54. [DOI] [PubMed] [Google Scholar]
- 121.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 122.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 123.Preaux AM, Mallat A, Van Nhieu JT, et al. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944. doi: 10.1002/hep.510300432. [DOI] [PubMed] [Google Scholar]
- 124.Rambaldi A, Gluud C. Colchicine for alcoholic and non-alcoholic liver fibrosis or cirrhosis. Liver. 2001;21:129. doi: 10.1034/j.1600-0676.2001.021002129.x. [DOI] [PubMed] [Google Scholar]
- 125.Rambaldi A, Gluud C. Meta-analysis of propylthiouracil for alcoholic liver disease--a Cochrane Hepato-Biliary Group Review. Liver. 2001;21:398. doi: 10.1034/j.1600-0676.2001.210606.x. [DOI] [PubMed] [Google Scholar]
- 126.Ramond MJ, Poynard T, Rueff B, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 127.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic Fatty liver disease. Gastroenterology. 2005;128:1898. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 128.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 129.Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95. doi: 10.1016/s1542-3565(04)00445-8. [DOI] [PubMed] [Google Scholar]
- 130.Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8. doi: 10.1053/j.gastro.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 131.Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4. doi: 10.1053/jhep.2003.50044. [DOI] [PubMed] [Google Scholar]
- 132.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 133.Rockey DC, Boyles JK, Gabbiani G, et al. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193. [PubMed] [Google Scholar]
- 134.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rockey DC, Chung JJ. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994;42:660. [PubMed] [Google Scholar]
- 136.Rockey DC, Maher JJ, Jarnagin WR, et al. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 137.Rodriguez L, Cerbon-Ambriz J, Munoz ML. Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res. 1998;29:109. [PubMed] [Google Scholar]
- 138.Rojkind M, Kershenobich D. Effect of colchicine on collagen, albumin and transferrin synthesis by cirrhotic rat liver slices. Biochim Biophys Acta. 1975;378:415. doi: 10.1016/0005-2787(75)90186-0. [DOI] [PubMed] [Google Scholar]
- 139.Russo FP, Alison MR, Bigger BW, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 140.Sakaida I, Tsuchiya M, Kawaguchi K, et al. Herbal medicine Inchin-ko-to (TJ-135) prevents liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. J Hepatol. 2003;38:762. doi: 10.1016/s0168-8278(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 141.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 142.Sato Y, Murase K, Kato J, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 143.Schaff Z, Lapis K, Szende B, et al. The effect of D-penicillamine on CCl4-induced experimental liver cirrhosis. Exp Pathol. 1991;43:111. doi: 10.1016/s0232-1513(11)80156-8. [DOI] [PubMed] [Google Scholar]
- 144.Schepke M, Werner E, Biecker E, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389. doi: 10.1053/gast.2001.26295. [DOI] [PubMed] [Google Scholar]
- 145.Selman M, Carrillo G, Salas J, et al. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: a controlled clinical trial. Chest. 1998;114:507. doi: 10.1378/chest.114.2.507. [DOI] [PubMed] [Google Scholar]
- 146.Shimizu I, Ma YR, Mizobuchi Y, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats [see comments] Hepatology. 1999;29:149. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- 147.Shimizu T, Kuroda T, Hata S, et al. Pirfenidone improves renal function and fibrosis in the post-obstructed kidney. Kidney Int. 1998;54:99. doi: 10.1046/j.1523-1755.1998.00962.x. [DOI] [PubMed] [Google Scholar]
- 148.Siegmund SV, Schwabe RF. Endocannabinoids and liver disease. II Endocannabinoids in the pathogenesis and treatment of liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G357. doi: 10.1152/ajpgi.00456.2007. [DOI] [PubMed] [Google Scholar]
- 149.Siller-Lopez F, Sandoval A, Salgado S, et al. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122. doi: 10.1053/j.gastro.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 150.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 151.Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582. doi: 10.1016/s0168-8278(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 152.Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195. doi: 10.1055/s-2002-30104. [DOI] [PubMed] [Google Scholar]
- 153.Stewart S, Prince M, Bassendine M, et al. A trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology. 2002;36(S):16. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 154.Stiehl A. Ursodeoxycholic acid in the treatment of primary sclerosing cholangitis. Ann Med. 1994;26:345. doi: 10.3109/07853899409148349. [DOI] [PubMed] [Google Scholar]
- 155.Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 156.Tada S, Nakamuta M, Enjoji M, et al. Pirfenidone inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Clin Exp Pharmacol Physiol. 2001;28:522. doi: 10.1046/j.1440-1681.2001.03481.x. [DOI] [PubMed] [Google Scholar]
- 157.Takahara T, Furui K, Funaki J, et al. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology. 1995;21:787. [PubMed] [Google Scholar]
- 158.Te HS, Schiano TD, Kuan SF, et al. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150. doi: 10.1111/j.1572-0241.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 159.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 160.Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5:301. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thompson K, Maltby J, Fallowfield J, et al. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis [see comments] Hepatology. 1998;28:1597. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 162.Tilg H, Jalan R, Kaser A, et al. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 163.Tripathi D, Therapondos G, Lui HF, et al. Chronic administration of losartan, an angiotensin II receptor antagonist, is not effective in reducing portal pressure in patients with preascitic cirrhosis. Am J Gastroenterol. 2004;99:390. doi: 10.1111/j.1572-0241.2004.04051.x. [DOI] [PubMed] [Google Scholar]
- 164.Tugues S, Fernandez-Varo G, Munoz-Luque J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 165.van Rooij E, Sutherland LB, Qi X, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 166.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 167.Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15(Suppl):E67. doi: 10.1046/j.1440-1746.2000.02100.x. [DOI] [PubMed] [Google Scholar]
- 168.Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology. 2000;31:428. doi: 10.1002/hep.510310224. [DOI] [PubMed] [Google Scholar]
- 169.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 170.Weng HL, Wang BE, Jia JD, et al. Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus infection: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:819. doi: 10.1016/s1542-3565(05)00404-0. [DOI] [PubMed] [Google Scholar]
- 171.Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 172.Wright MC, Issa R, Smart DE, et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 173.Wu SF, Peng CT, Wu KH, et al. Liver fibrosis and iron levels during long-term deferiprone treatment of thalassemia major patients. Hemoglobin. 2006;30:215. doi: 10.1080/03630260600642534. [DOI] [PubMed] [Google Scholar]
- 174.Yang L, Chan CC, Kwon OS, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G902. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- 175.Yata Y, Gotwals P, Koteliansky V, et al. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 176.Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 177.Zhang S, Ji G, Liu J. Reversal of chemical-induced liver fibrosis in Wistar rats by puerarin. J Nutr Biochem. 2006;17:485. doi: 10.1016/j.jnutbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 178.Zhang XL, Liu L, Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induces hepatic stellate cell apoptosis via caspase-3 activation. World J Gastroenterol. 2002;8:515. doi: 10.3748/wjg.v8.i3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]