Abstract
Previous research has shown that chronic restraint stress exacerbates Theiler’s virus infection, a murine model for CNS inflammation and multiple sclerosis. The current set of experiments was designed to evaluate the potential role of glucocorticoids in the deleterious effects of restraint stress on acute CNS inflammatory disease. Exposure to chronic restraint stress resulted in elevated levels of corticosterone as well as increased clinical scores and weight loss (Experiment 1). In addition, corticosterone administration alone exacerbated behavioral signs of TMEV-induced sickness (i.e. decreased body weight, increased symptoms of encephalitis, and increased mortality) and reduced inflammation in the CNS (Experiment 2). Infected subjects receiving exogenous corticosterone showed exacerbation of acute phase measures of sickness and severe mortality as well as decreased viral clearance from CNS (Experiment 3). These findings indicate that corticosterone exposure alone is sufficient to exacerbate acute CNS inflammatory disease.
1.0 Introduction
Physical and psychological stress can affect immune function, thereby altering the body’s ability to recover from injury, infection, and disease [ 1,2]. Stress results in activation of two parallel pathways, the sympathetic-adrenal-medullary (SAM) axis and the hypothalamic-pituitary-adrenal (HPA) axis. The end result of activating these two pathways is release of catecholamines and glucocorticoids (GCs), respectively. Historically, stress has been described as having a reciprocal relationship with the immune response whereby the stress response suppresses immunity (for review [3,4]). This reciprocal control is primarily attributed to the release of GCs, such as cortisol in humans and corticosterone (CORT) in rodents, which decrease immune/inflammatory responses through a variety of mechanisms [5]. For example, GCs have been shown to modulate peripheral inflammatory responses by inhibiting the release of immune mediators such as cytokines [6]. In fact, preventing the GC response increases sickness severity, inflammation, and mortality following acute bacterial endotoxin (LPS) and cytokine administration [6,7,8,9,10,11,12] suggesting a protective role for GCs against the negative effects of inflammation. While the ability of GC to decrease/impair the immune response is well accepted, evidence suggests that this relationship is more complex particularly when the area of interest is the brain.
In the central nervous system (CNS), GCs have been shown capable of enhancing or inhibiting inflammation. GCs are important modulators of cytokine release and the CNS immune response [13,14]. Nadeau & Rivest (15) showed GCs to be protective against the inflammatory-mediated neurodegenerative effects of lipopolysaccharide (LPS) administration to the pre-frontal cortex. Blocking GC receptors prior to LPS administration into the brain results in inflammatory-mediated neurodegeneration. GCs also decrease cytokine production, proliferation, and antigen presentation in microglia and astrocytes, the immune cells of the CNS [16,17,18,19]. These findings support the role of GCs as potent modulators of the CNS inflammatory response, primarily through negative feedback, however there are circumstances under which GCs are not anti-inflammatory within the CNS. For example, exposure to a variety of stressors has been shown to increase IL-1β expression in the brain [20,21,22,23,24]. Moreover, chronic stress exacerbates LPS-induced degeneration of neurons and glia, an effect that is GC receptor dependent [25] and dexamethasone administration does not decrease inflammation resulting from tuberculosis meningitis [26].
Chronic stress has been shown to impair the peripheral immune response through a variety of mechanisms, including reduction in NK cells and suppression of CD4+/CD8+ and virus-specific T-cell responses [27,28,29,30,31,32,33,34,35]. In addition, chronic stress has been shown to reduce inflammatory cell infiltrate and pro-inflammatory cytokine levels in CNS [36,37,38]. Chronic restraint stress, with its associated increase in circulating CORT, suppresses both innate and adaptive immune responses to Theiler’s murine encephalomyelitis virus (TMEV) through these same mechanisms (36,37,38,34,35]. While an established body of literature supports the immunosuppressive effects of stress, recent evidence suggests the stress-immune response connection is more complex than a simple reciprocal relationship. Specifically, chronic stress-induced elevations in CORT have been shown to enhance the inflammatory response within the CNS rather than suppress it (25,39,40, for review see 41]. Basal (i.e. resting) levels of CORT are responsible for tight regulation of the immune/inflammatory response, which is consistent with the traditional view that GCs are immunosuppressive. However, chronically elevated CORT levels have been shown to exacerbate inflammatory-mediated neuronal damage after insult, suggesting at a minimum that there is a dysregulation of the traditional stress-immune relationship under these circumstances [42,43,44,45].
The present studies examine the impact of CORT on acute TMEV infection, a model of multiple sclerosis (MS) characterized by an acute CNS inflammatory syndrome followed by a chronic demyelinating disease in genetically susceptible strains of mice (include refs for TMEV model). Previous research has shown that both chronic restraint stress and chronic social stress result in elevated circulating levels of CORT, but these manipulations differ greatly in their effects on TMEV infection outcomes. Exposure to chronic restraint stress during acute infection decreases CNS inflammation while exacerbating behavioral signs of infection and mortality during the acute infection [46,36,37,38] and increasing the severity of the demyelinating phase of disease [47]. Restraint stress has been shown to decrease chemokine and cytokine activity during this period and increase CNS viral load [36,37,38], suggesting that pathogen clearance is impaired. In contrast, exposure to repeated social stress during acute TMEV infection results in moderate levels of CNS inflammation, attenuation of behavioral sickness syndrome and motor impairment as well as decreased autoimmunity during acute and chronic disease [48,49]. Thus, it appears that stress-induced increases in GCs are associated with different immune response-related outcomes as well as differential effects on disease course.
The current set of experiments was designed to investigate the role of stress-induced and exogenous CORT on TMEV infection. We have previously attributed the exacerbating effect(s) of restraint stress on TMEV infection to GC-mediated immunosuppression but given that GCs can act to suppress or enhance the immune response it was necessary to experimentally test this hypothesis.
2.0 Materials and Methods
2.1 Animals
Male CBA mice (21-day-old Harlan Laboratories, Houston, TX) were housed 6 per cage in Experiment 1, and 4 per cage in Experiments 2 and 3 matching on body weight across cages. TMEV infects neurons and glial cells of the CNS [50], and typically causes an acute viral infection of the CNS, followed by a chronic demyelinating disease in susceptible strains of mice [51]. CBA mice have previously been shown to be asymptomatic during acute TMEV and moderately susceptible to TMEV persistence and chronic phase disease [52]. However, following stress-induced immunosuppression, CBA mice develop behavioral signs of encephalitis during acute infection [46,53,35]. Mice were maintained on a 12-h light/dark cycle with ad libitum access to food and water. Upon arrival, mice were allowed to habituate for one week before the initiation of any experimental procedures. All animal care protocols were in accordance with the Texas A&M University Laboratory Animal Care and Use Committee.
2.2. Experimental designs
2.2.1. Experiment 1: Effects of restraint stress on plasma CORT and TMEV infection
Mice (N=48) were divided into groups based on 1) stress condition (restraint or no stress) and 2) infection status (TMEV infected or uninfected). Mice were weighed each day prior to initiation of restraint stress. Mice were infected with TMEV on D0 post-infection (pi). On D -5, 1, 2, 7, 16, 24, and 35 pi blood samples were taken from all mice for determination of plasma CORT levels using RIA. On D-1, 7, and 14 pi mice were evaluated for clinical signs of encephalitis.
2.2.2. Experiment 2: Effects of exogenous CORT on TMEV symptomatology and CNS inflammation
Mice (N= 32) were divided into cages of 4 based on whether they were to receive exogenous CORT (400 mg/mL with 2% ethanol) or vehicle (2% ethanol) [54]. Twenty-four hours later, mice were infected with TMEV or innoculated with the same volume of sterile saline. Mice were weighed, monitored for fluid volume intake, and evaluated for clinical signs of encephalitis twice each week beginning one week prior to CORT administration and infection (D0 pi). Mice in the CORT and/or infection groups were euthanized when they reached a clinical score of 3 along with a randomly selected subject from each of the other groups. Following euthanization, a subset of mice from each group (n = 3) were perfused with saline followed by 10% formalin. Spinal cord tissue was collected and stored for staining with H&E in order to determine the extent of CNS inflammation during acute phase TMEV infection. CNS inflammation was determined as described below and in Johnson et al. [48].
2.2.3 Experiment 3: Effects of exogenous CORT on TMEV viral load
Mice (N=14) were divided into cages of 3 or 4. On pnd 28/D-1 pi, cages were randomly assigned to receive exogenous CORT (400 mg/ml with 2% ethanol) or vehicle (2% ethanol) in their drinking water throughout the remainder of the experiment. Twenty-four hours later, all mice were infected with TMEV. Mice were monitored daily for body weight, food intake, horizontal activity level, and clinical scores. Mice in the CORT group were euthanized when they reached a clinical score of 3, along with a randomly selected subject from the vehicle control group. Following euthanization, mice were perfused with saline, and their brain and spinal cord tissue were collected for evaluation of viral load as described below.
2.3 Restraint Stress Protocol
Beginning at approximately pnd 28/day -1 post-infection (D-1 pi), mice in the restraint condition were exposed to 12 hours of restraint, beginning at 2000 h for 5 consecutive nights each week for 4 weeks as described previously [36,37,38,47,53]. Briefly, during each restraint session, mice were placed in well-ventilated 60 ml plastic tubes placed in their home cage. Although movement was limited, the mice were not physically compressed during the restraint period. These tubes were less confining than those used in our previous study [46]. This schedule of chronic restraint stress has been shown to result in chronically elevated circulating CORT levels during the entire restraint period, though there is evidence of a partial attenuation of the CORT response across days of restraint [53]. Mice in the non-restrained groups remained undisturbed in their home cages.
2.4. Administration of exogenous CORT
Mice were administered exogenous CORT or vehicle in their drinking water beginning on D-1 pi and continuing throughout the experiment. This administration method for exogenous CORT has been shown to result in an elevation in circulating CORT comparable to that seen following repeated restraint stress [54]. The timing of CORT administration was designed to mimic the elevated levels produced by the restraint schedule in Experiment 1. Mice were presented with the CORT solution at 2000 h the day before infection (D-1 pi) and had continuous access to the solution for four weeks. On D0 pi, all mice were infected with TMEV as described below. All mice in the CORT condition continued to receive CORT throughout the duration of the experiment.
2.5. Infection
The BeAn strain of Theiler's virus (obtained from Dr. H.L. Lipton, Dept. of Microbiology-Immunology, Univ. Illinois, Chicago, IL.) was initially propagated in L2 cells [55]. Mice were anesthetized with Isoflurane (Vedco Inc., St. Joseph, MO) and inoculated into the right mid-parietal cortex (1.5 mm depth) with 5 × 104 pfu (plaque forming units) of TMEV or vehicle in a 20 µl volume [46,55]. Inoculation of all mice occurred at 1000 h.
2.6. Behavioral measures
2.6.1.Clinical scores
Clinical scoring for encephalitic-like symptoms during the acute phase of Theiler’s virus infection was as previously described [47]. The severity of encephalitis signs of infection was scored on a 0–4 scale that assessed the degree of encephalitis symptoms on several dimensions including hunched posture, piloerection, grooming, lethargy and sunken eyes. The following criteria were used: 0=healthy, grooming complete; 0.5=fur slightly ruffled, grooming incomplete; 1=ruffled fur and/or slightly hunched posture; 2=hunched, slightly lethargic or irritable; 3=very lethargic, unresponsive, very hunched, sunken eyes; 4=dead. This scale was derived by observing the natural progression of behavioral signs of illness and encephalitis in Theiler’s virus infected mice subjected to chronic restraint stress [46,47,35].
2.6.2. Activity monitoring
To examine the effects of restraint and infection on sickness behavior, open field horizontal activity was assessed on D7 pi. The apparatus consisted of six optical beam activity monitors (Model RXYZCM-16), equipped with two banks of eight photocells on each wall. These open field boxes are interfaced with a digital-multiplexor and Versamax software (Model DCM-4, Omnitech Electronics, Columbus, OH). White noise (64 dB) was continually present to mask extraneous disturbances. Mice were habituated in the chambers for 1 h, on pnd 28, prior to baseline measure collection. Baseline assessment occurred on pnd 29. All testing was conducted in the dark beginning at 1500 h, for 30 min. The baseline level of activity for each animal was subtracted from the D7 pi levels for assessment, to control for individual differences.
2.7. Immunological and histological measures
2.7.1. Radioimmunoassay for plasma CORT
Blood was obtained from the saphenous vein within 5 min of cage disturbance to minimize stress artifacts as previously described [47]. The plasma CORT concentration was determined using a 125I-RIA kit (ICN Biomedicals, Inc., Costa Mesa, California) [56]. In experiment 1, mice were bled on D-5, 1, 2, 7, 16, 24, and 35 pi. In experiment 2, mice underwent a one-time saphenous vein bleed on D9 pi. All blood samples for determination of CORT in plasma were collected between 0900 h and 1000 h following restraint (Experiment 1) or the dark cycle (Experiments 2 & 3). This time point was selected due to previous research showing that CORT levels are highest approximately 12 h after the initiation of restraint, corresponding to 0800h in Experiment 1, and the fact that fluid intake/CORT administration would be increased when animals are most active (i.e. dark cycle) in Experiments 2–3 [58,46].
2.7.2. Histology
Subjects were sacrificed by pentobarbital injection, i.p. and perfused via the left ventricle with saline followed by 10% formalin. The spinal cords were removed and stored at −80°C. Acute TMEV infection is characterized by CNS inflammation. To evaluate the impact of exogenous CORT on CNS inflammation, spinal cords were sectioned transversely into 12 pieces [46]. Tissues were processed for hematoxylin and eosin (H&E) staining. Sections were evaluated for perivascular cuffing (PVC; accumulation of lymphocytes and macrophages around blood vessels) and meningitis (MN; accumulation of lymphocytes and macrophages in the meninges). Histological evaluations were made without prior knowledge of experimental condition. Sections were scored according to the degree of lesion expression based on severity (number of cell layers in the meninges or perivascular cuffs) and area (percentage on meninges with inflammation and the number of perivascular cuffs) of inflammation [47,48].
2.7.3 CNS viral load
Subjects were sacrificed by pentobarbital injection, i.p. and perfused via the left ventricle with sterile PBS. The brains and spinal cords were removed and stored at −80°C. All samples were later homogenized and the viral content of the supernatant was determined with a plaque assay on L2 cells [55]. After being incubated at 37°C at 5% CO2 for 72 h, plates were stained with 0.1% cresyl violet to visualize the plaques that formed. Plates were scored based on the number of plaques formed on the L2 cells and calculated per gram of tissue [55].
2.8. Statistical analysis
Data are presented as mean± SEM. Analysis of variance (ANOVA) was used to evaluate differences across conditions; repeated measure ANOVA were used for analysis over time as appropriate (e.g. % survival across days). Where possible, baseline measures were used as a covariate, and analyses of covariance (ANCOVA) were conducted instead. Clinical scores were analyzed using nonparametric statistics, the Kruskal-Wallis and Mann-Whitney U as appropriate, due to the ordinal categorical nature of the clinical scoring scale.
3.0. Results
3.1. Experiment 1
Figure 1 depicts the effects of restraint and infection on body weight, clinical scores, and plasma CORT during acute TMEV infection. Body weight was significantly reduced as a result of infection and restraint. A 2 (restrained vs. non-restrained) × 2 (TMEV infected vs. uninfected) repeated measures ANOVA was used to analyze body weight. This analysis revealed significant main effects of restraint, F (1, 36) = 141.65, p < 0.05, infection, F (1, 36) = 11.46, p < 0.01, and time, F (7, 252) = 71.19, p < 0.001 on body weight (Figure 1A). The ANOVA also revealed significant restraint × time, F (7, 252) = 41.04, p < 0.001, infection × time, F (7, 252) = 3.18, p < 0.01, and restraint × infection × time, F (7, 252) = 5.18, p < 0.001, interactions.
Figure 1.
Effects of restraint stress exposure during acute TMEV infection. A. Subjects exposed to restraint stress have reduced body weights compared to subjects not exposed to restraint. Subjects infected with TMEV also showed significantly reduced body weights compared to non-infected controls. B. Final clinical scores were higher as a result of restraint and infection, indicating more severe encephalitis. C. Serum corticosterone levels were elevated as a result of infection and restraint. Restraint stress elevated circulating corticosterone in both infected and non-infected subjects, an effect that resolves when restraint stress is discontinued (end of week 4).
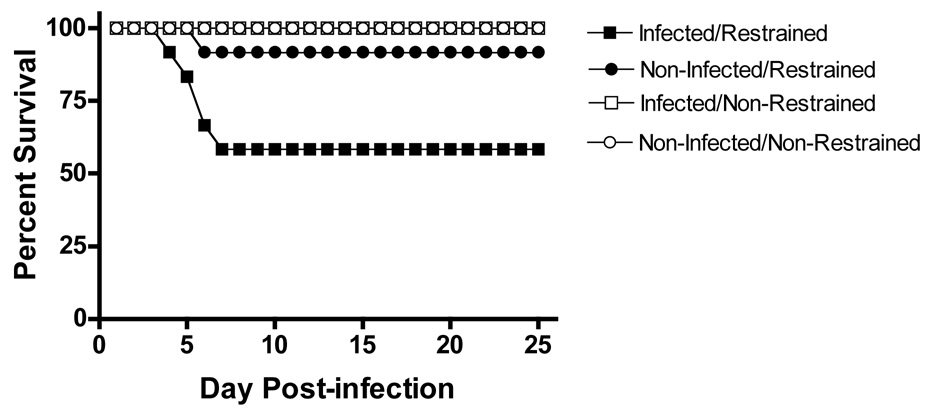
As can be seen in Figure 1B, final clinical score was higher for infected and restraint stressed subjects. A Kruskal-Wallis Test verified that clinical score was significantly different between groups, X2= 40.576, p < 0.001. In addition, a priori planned means comparisons were performed based on previous findings [46] and revealed that the INFECTED/RESTRAINT subjects were significantly more symptomatic than the other groups combined, F (1,44) = 55.18, p < 0.001, and than the NONINFECTED/RESTRAINT group, F (1,44) = 8.36, p < 0.01. As shown in panel C, plasma CORT levels were also elevated as a result of restraint and infection. An ANCOVA using baseline CORT level as a covariate revealed significant main effects of restraint, F (1, 29) = 268.43, p < 0.001, and infection, F (1, 29) = 5.08, p < 0.05, on plasma CORT levels (Figure 1C). No other significant main effects or interactions were present, all F (1, 29) ≤ 0.956, p > 0.05. As can be seen in Figure 2, mortality was increased for infected/restrained subjects during acute TMEV infection. There was 40% mortality in the infected/restrained group within the first week following infection while only 8% of the non-infected/restrained mice died during the same period. A 2 (restrained vs. nonrestrained) × 2 (infected vs. uninfected) repeated measures ANOVA confirmed significant main effects of restraint, F (1, 44) = 10.15, p < 0.01, infection, F (1, 44) = 5.20, p < 0.05, and time, F (24, 1056) = 8.10, p < 0.001, on mortality. The ANOVA also revealed significant interactions of restraint × infection, F (1, 44) = 5.20, p < 0.05, restraint × time, F (24, 1056) = 8.10, p < 0.001, and infection × time, F (24, 1056) = 3.848, p < 0.001. These effects were clarified by a significant restraint × infection × time interaction, F (24, 1056) = 3.848, p < 0.001, indicating that INFECTED/RESTRAINED subjects had greater mortality during the acute phase of infection with TMEV. These findings are similar to those seen previously [46] and establish that the modified restraint stress procedure results in exacerbation of acute TMEV infection while decreasing the mortality rate from 80% reported by Campbell to 40% in the present study.
Figure 2.
Effects of restraint stress exposure and TMEV infection on percent survival during acute infection. During the first week following infection with TMEV, 40% of the Infected/Restrained group died. This rate of mortality was higher than all other groups.
3.2. Experiment 2
As can be seen in Figure 3 (A–C), administration of exogenous CORT treatment decreased body weight, increased clinical scores, and increased mortality during acute TMEV infection. Separate 2 (cort vs vehicle) × 2 (infected vs non-infected) ANOVA were used for analysis of body weight and final clinical scores. Body weight was reduced as a result of infection and cort administration. ANOVA confirmed a significant main effect of infection, F (1, 26) = 8.493, p < 0.01. In addition, there was a significant infection × time interaction, F (1, 26) = 9.272, p < 0.01. Exogenous CORT significantly reduced body weight for both infected and uninfected subjects, and this difference was greater as time post-infection increased. ANOVA confirmed a significant main effect of cort, F (1, 26) = 37.383, p < 0.001, and a significant cort × time (day pi) interaction, F (1, 26) = 117.258, p < 0.001, on body weight. These significant main effects and interactions were further clarified by a significant infection × cort × time interaction, F (1, 26) = 5.353, p < 0.05, indicating that the CORT/INFECTED group showed the greatest reduction in body weight during the post-infection period. Fluid intake was not affected by CORT administration or by time, suggesting that fluid intake and therefore CORT administration was sustained for the duration of the experiment, all F’s < 4.147, p > 0.05 (data not shown). CORT administration resulted in more severe sickness as indicated by higher final clinical scores. A Kruskal-Wallis test comparing final clinical scores showed a significant group difference, X2= 26.754, p < 0.001, with the CORT/INFECTED group showing the highest final clinical scores compared to all groups. The only group to show mortality was the CORT/INFECTED group. An ANOVA verified significant main effects of infection, cort, and time post-infection on percent survival during the acute phase of TMEV infection, all F > 6.83, p < 0.01. Moreover, ANOVA revealed significant cort × infection, cort × time, and infection × time interactions, all F > 6.83, p < 0.01. These effects were further clarified by a significant cort × infection × time interaction, F (5, 135) = 6.83, p < 0.01.
Figure 3.
Behavioral effects of exogenous corticosterone administration during acute TMEV infection. Panel A. Infected subjects receiving exogenous corticosterone (CORT/INFECTED) during acute infection showed the greatest reduction in body weight during the post-infection period. Panel B. Final clinical scores were significantly higher (indicating more severe encephalitis) for CORT/INFECTED subjects. Panel C. CORT/INFECTED subjects showed higher mortality than all other conditions.
Figure 4 is a representative section taken from a subject that received CORT followed by infection with TMEV showing moderate inflammation in the form of meningitis and perivascular cuffing. Figure 5 (A–D) illustrates the effects of infection and exogenous CORT administration on CNS inflammation, a process vital to effective viral clearance during initial infection. Uninfected subjects showed no inflammation regardless of whether they received exogenous CORT or vehicle. For infected subjects only, administration of exogenous CORT during acute TMEV infection reduced meningitis and perivascular cuffing (Figure 5 A–D). Separate ANOVA confirmed significant main effects of infection and CORT administration as well as significant cort × infection interactions on both measures of meningitis (% of perimeter with meningitis and number of layers present in those areas), all F > 9.36, p < 0.05. Separate ANOVA also confirmed significant main effects of infection and CORT administration as well as significant infection × cort interactions on both measures of perivascular cuffing (number of cuffs and average number of cell layers present in those cuffs), all F ≥ 37.23, p < 0.001, (see Figure 5 B & D).
Figure 4.
Representative histological image, stained with H & E, showing CNS inflammation in the form of meningitis and perivascular cuffing. Dark cells indicate infiltrating immune cells. Meningitis can be seen as layers of dark cells in the outer perimeter of the section. Perivascular cuffing can be seen (inset) as a collection of darkly stained cells in very close proximity to a blood vessel, seen here as a clear opening
Figure 5.
Effect of exogenous corticosterone administration during acute TMEV on CNS inflammation. Panel A and B. The administration of exogenous corticosterone (CORT) attenuated the infection-induced increase in percent meningitis (A) and the layers of cells involved in areas of meningitis (B). Panel C–D. The administration of exogenous corticosterone attenuated the infection-induced increase in the number of perivascular cuffs present (C) and the layers of cells involved in those cuffs (D).
3.3. Experiment 3
Figure 6 depicts the effects of exogenous CORT administration on body weight, behavioral sickness syndrome (i.e. horizontal activity and clinical scores), and plasma levels of CORT during acute TMEV infection. Separate ANOVA were used for analysis of body weight, clinical scores, and horizontal activity. Exogenous CORT significantly reduced body weight (F (1, 12) = 9.03, p < 0.05) and horizontal activity, F (1, 12) = 4.09, p < 0.05, on D7 pi (Figure 6A & B). A Mann-Whitney U test confirmed that CORT subjects had significantly higher final clinical scores than vehicle controls, U (7, 7) = 4.500, p < 0.01 (Figure 6C). As can be seen in Figure 6D, exogenous CORT administration significantly increased plasma CORT levels on D9 pi, F (1, 12) = 35.493, p < 0.001, though plasma CORT was lower than seen following restraint stress on D9 pi in Experiment 1 (refer to Figure 1C). As can be seen in Figure 7, exogenous CORT administration also resulted in higher CNS viral titers. An ANOVA (CORT vs. vehicle) revealed a significant main effect of CORT administration on viral load. Viral titers were higher in both brain, F (1, 12) = 54.528, p < 0.001, and spinal cord, F (1, 12) = 8.833, p < 0.01, for mice receiving CORT compared to vehicle controls.
Figure 6.
Behavioral effects of exogenous corticosterone administration during acute TMEV infection. A. Body weight (D7 pi) was significantly reduced as a result of exogenous corticosterone administration. B. Horizontal activity (D7 pi) was reduced as a result of restraint stress exposure, indicating more severe sickness as a result of exogenous corticosterone administration during acute TMEV infection. C. The final clinical scores were more higher for subjects receiving exogenous corticosterone compared to those receiving vehicle (indicating more severe infection-related encephalitis symptoms). D. Administration of exogenous corticosterone in the subjects’ drinking water resulted in elevated levels of serum corticosterone.
Figure 7.
Impact of exogenous corticosterone administration during acute TMEV on viral load in brain and spinal cord. Subjects receiving exogenous corticosterone showed significantly higher viral titers in both brain and spinal cord, indicating impaired viral clearance in the CNS.
4.0 Discussion
Previous research has shown that restraint stress results in increased signs of sickness and decreased inflammation in male CBA mice following infection with TMEV. The present experiments were designed to evaluate the impact of chronically elevated CORT on acute TMEV-related disease course. The current findings indicate that chronic CORT elevation alone mimics the restraint stress-induced attenuation of responding to TMEV infection in CBA mice. Using a restraint stress procedure that was less intense than that used previously by Campbell et al. [46], Experiment 1 verified that restraint resulted in increased circulating CORT as well as exacerbation of the behavioral signs of infection and mortality associated with acute TMEV infection. These findings are consistent with previous research from our laboratory showing that restraint and infection produce significant elevations in CORT early on [46] and throughout the four-week period of restraint [58]. The findings from Experiment 2 indicated that administration of exogenous CORT alone mimicked the adverse effects of restraint stress, resulting in a similar pattern of exacerbated behavioral sickness syndrome and mortality. CORT administration also decreased CNS inflammation resulting from infection, as measured by meningitis and perivascular cuffing. Experiment 3 was designed to 1) replicate and expand the number of behavioral measures used to evaluate TMEV-induced behavioral syndrome and 2) verify that exogenous CORT administration results in increased circulating CORt and 3) evaluate the impact of exogenous CORT administration on viral clearance following TMEV infection. The results indicate that CORT administration during acute TMEV infection increased the severity of the behavioral syndrome and impaired viral clearance. Interestingly, even though exogenous CORT administration resulted in less circulating CORT compared to the level seen in Experiment 1 following restraint, the pattern of results suggests that exogenous CORT was sufficient to mimic the restraint stress-induced exacerbation of acute TMEV infection. Taken together with previous research on the impact of restraint on acute TMEV pathogenesis, the present findings indicate that exogenous CORT administration induces a similar pattern of behavioral and immunological exacerbation to that seen following restraint.
Chronic restraint stress has previously been shown to alter the progression of both acute and chronic TMEV disease severity, a proposed mechanism of which has been GC-induced immunosuppression [46, 47, 53]. Previous research from our laboratory indicates that chronic restraint-induced elevations in circulating CORT are accompanied by a reduction in various measures of immune function, including NK and T cell function and cytokine/chemokine activity [34,36,37,38]. Specifically, chronic restraint caused a 50% reduction in NK cell lytic activity 24 hours after TMEV infection [35]. Chemokine mRNA expression for Ltn, IP-10 and RANTES were significantly decreased in both the brain and spleen at 7 days post-infection [36]. Restraint stress also attenuated infection-related increases in IFN-γ in the brain. In addition, CNS levels of lymphotoxin-β, TNF–α, and IFN-γ were negatively correlated with virus levels in CNS [37,38]. More recently, four weeks of restraint stress has been shown to decrease virus-specific CD4+ and CD8+ T cell responses [34]. Interestingly, while other evidence suggests an attenuation of the CORT response with repeated restraint exposures [59], the immunosuppressive effects of restraint remain high following repeated periods of restraint. Moreover, subjects receiving exogenous CORT in the present experiments showed a smaller elevation in circulating CORT levels but a similar pattern of disease exacerbation to that seen with restraint. Elevated CORT has been linked with immunosuppression following restraint during acute TMEV infection, but other evidence from our laboratory suggests that elevated CORT is not always associated with inhibition of the immune response [48]. For example, exposure to social stress during early infection with TMEV results in increased levels of CORT but induces protective effects on acute TMEV disease course. Given the divergent effects of social stress and restraint stress-induced elevations of CORT, it was important to investigate the impact of CORT alone on acute TMEV pathogenesis. The present findings suggest that exogenous CORT administration more closely mimics the effects of restraint stress, rather than social stress, on TMEV infection.
The TMEV model of chronic demyelination is characterized by a persistent viral infection of the CNS that leads to demyelination through both direct mechanisms (e.g. virus-induced lysis of oligodendrocytes) and indirect mechanisms (e.g. bystander demyelination, autoimmunity, etc.) [55,60,61,62,63]. As a result, stress- and/or GC-induced suppression of the initial response to viral infection would be expected to increase viral persistence and, potentially, increase subsequent demyelination [47]. However, previous research from our laboratory indicates that this relationship is more complex than this statement suggests. While restraint stress during acute infection has been shown to subsequently exacerbate the chronic demyelinating disease in male and female SJL mice and female CBA mice, restrained male CBA mice exhibit reduced susceptibility to the chronic demyelinating disease [47,53].
Lipton and Dal Canto [64] reported similar contrasting effects on the development of the acute viral infection and chronic demyelinating phase of TMEV infection. Immunosuppressive treatments (cyclophosphamide and rabbit anti-mouse thymocyte serum) during the first 3 weeks of infection resulted in exacerbation of acute viral infection and a reduced susceptibility to the chronic demyelinating phase of TMEV-induced disease. Likewise, we have shown that restraint stress induces significant lymphopenia and thymus atrophy [34,35,46], which may disrupt viral clearance during acute infection while also dysregulating the autoreactive T and B cell responses that contribute to autoimmune demyelination. According to this logic, immunosuppressive manipulations (e.g. CORT administration) during acute TMEV-induced disease should negatively impact acute phase symptomatology while also altering the development of the autoimmune response during the chronic phase disease.
In the current experiments, we have shown that elevation of GCs, either as a result of restraint stress or exogenous CORT administration, alters the CNS inflammatory response to TMEV infection (sufficiency) offering further support of the strong regulatory role of GCs within the CNS. We have also established that CORT is necessary for the negative effects of restraint stress on TMEV disease [34,65]. Specifically, we have shown that both administration of the glucocorticoid synthesis inhibitor metyrapone and the glucocorticoid receptor antagonist RU486 during restraint stress attenuated the adverse effects of restraint stress on TMEV infection.
Although the etiology of MS remains uncertain, several environmental risk factors have been identified, including viral infection and stress. Epidemiological studies have linked exposure to certain viruses (e.g., herpes, rubella, mumps, Epstein-Barr) with an increased likelihood of developing MS [66,67,68,69,70] and several retrospective studies indicate that MS patients report more severe life events prior to disease onset [71,72]. The current findings help shed light on the mechanism(s) by which chronic stress could alter susceptibility to the initial processes involved in chronic autoimmune diseases like MS. In addition, these results suggest that exposure to endogenous (e.g. Cushing’s syndrome, adrenal disease) or exogenous (e.g. immunosuppressive therapy) sources of elevated GCs may alter disease susceptibility and/or progression regardless of exposure to environmental stress.
Acknowledgments
This research was funded by an NSF Fellowship to R.R.J, and grants to C.J.R.W. and M.W.M. from the National Multiple Sclerosis Society RG 3128 and NIH/NINDS R01 39569.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ader R, Cohen N, Felton D, editors. Psychoneuroimmunology. 2nd ed. Boston: Academic Press; 1991. [Google Scholar]
- 2.Kiecolt-Glaser JK, Glaser R. Psychoneuroimmunology and health consequences: data and shared mechanisms. Psychosom Med. 1995;57(3):269–274. doi: 10.1097/00006842-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Besedovsky HO, Del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17(1):64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, Biron CA, Brunson KW, Bulloch K, chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine, and immune interactions. Brain Res Brain Res Rev. 1997;23(1–2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 5.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids-new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 6.Parant M, Le Contel C, Parant F, Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-a. Lymphotoxin Cytokine Res. 1991;10(4):265–271. [PubMed] [Google Scholar]
- 7.Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60(5):563–572. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Bertini R, Bianchi M, Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goujon E, Parnet P, Laye S, Combe C, Dantzer R. Adrenalectomy enhances pro-inflammatory cytokines gene expression, in the spleen, pituitary and brain of mice in response to lipopolysaccharide. Brain Res Mol Brain Res. 1996;36(1):53–62. doi: 10.1016/0169-328x(95)00242-k. [DOI] [PubMed] [Google Scholar]
- 10.Ozmen L, Pericin M, Hakimi J, Chizzonite RA, Wysocka M, Trinchieri G, Gately M, Garotta F. Interleukin 12, interferon γ and tumor necrosis factor α are the key cytokines of the generalized Schwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol. 1999;162(6):3527–3533. [PubMed] [Google Scholar]
- 12.Zuckerman SH, Shellhaas J, Butler LD. Differential regulation of lipopolysaccharide-induced interleukin-1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]
- 13.Golde S, Coles A, Lindquist JA, Compston A. Decreased iNOS synthesis mediates dexamethasone-induced protection of neurons from inflammatory injury in vitro. Eur J Neurosci. 2003;18(9):2527–2537. doi: 10.1046/j.1460-9568.2003.02917.x. [DOI] [PubMed] [Google Scholar]
- 14.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17(1):13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 15.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23(13):5536–5564. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol. 1990;30(2–3):201–212. doi: 10.1016/0165-5728(90)90104-u. [DOI] [PubMed] [Google Scholar]
- 17.Colton CA, Chernyshev ON. Inhibition of microglial anion production by isoproterenol and dexamethasone. Neurochem Int. 1996;29(1):43–53. doi: 10.1016/0197-0186(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 18.Drew PD, Chavis JA. Inhibition of microglial cell activation by cortisol. Brain Res Bull. 2000;52(5):77–85. doi: 10.1016/s0361-9230(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia. 1997;20(1):23–37. [PubMed] [Google Scholar]
- 20.Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1 beta responses to stress. J Neuroimmunol. 2006;173(1–2):87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigm. Brain Res Bull. 2005;64(6):541–546. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1 beta protein in the rat. J Neurosci. 1998;18(6):2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor KA, Johnson JD, Hansen MK, Wieseler-Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991(1–2):123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106(1–2):109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 25.de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26(21):5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmons CP, Thwaites GE, Quyen NT, Chau TT, Mai PP, Dung NT, Stepniewska K, White NJ, Hien TT, Farrar J. The clinical benefit of adjunctive dexamethasone in tuberculosis meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175(1):579–590. doi: 10.4049/jimmunol.175.1.579. [DOI] [PubMed] [Google Scholar]
- 27.Bonneau RH, Sheridan JF, Feng NG, Glaser R. Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun. 1991;5(2):170–192. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
- 28.Bonneau RH, Sheridan JF, Feng N, Glaser R. Stress-induced modulation of the primary cellular immune response to herpes simplex virus infection is mediated by both adrenal-dependent and independent mechanisms. J Neuroimmunol. 1993;42(2):167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- 29.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48(2):151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- 31.Feng WY, Pagniano R, Tovar CA, Bonneau RH, Glaser R, Sheridan JF. The effect of restraint stress on kinetics, magnitude, and isotype of the humoral immune response to influenza virus infection. Brain Behav Immun. 1991;5(4):370–382. doi: 10.1016/0889-1591(91)90032-6. [DOI] [PubMed] [Google Scholar]
- 32.Padgett DA, Sheridan JF, Dorne J, Bernston GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci. 1998;95(12):7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steelman AJ, Prentice TW, Young CR, Dean DD, Hammons AE, Meagher MW, Welsh CJR. The effects of restraint stress on adaptive immune responses in SJL mice infected with Theiler’s virus. (in prep) [Google Scholar]
- 35.Welsh CJR, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection II: NK cell function and cytokine levels in acute disease. Brain, Behav Immun. 2004;18:166–174. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 36.Mi W, Belyavskyi M, Johnson RR, Sieve AN, Storts R, Meagher MW, Welsh CJR. Alterations in chemokine expression following Theiler’s virus infection and restraint stress. J Neuroimmunol. 2004;151:103–115. doi: 10.1016/j.jneuroim.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJR. Restraint stress decreases virus-induced pro-inflammatory cytokine mRNA expression during acute Theiler's virus infection. Journal of Neuroimmunology. 2006a;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Mi W, Young CR, Storts R, Steelman A, Meagher MW, Welsh CJR. Stress alters pathogenecity and facilitates systemic dissemination of Theiler's virus. Microbial Pathogenesis. 2006b;41:133–143. doi: 10.1016/j.micpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Seavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26(14):3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171(1–2):72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21(3):259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84(4):705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 43.Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115(1–2):36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 44.Meagher MW, Johnson RR, Young EE, Vichaya EG, Lunt S, Harden E, Connor M, Welsh CJR. IL-6 as a mechanism for the adverse effects of social stress on acute Theiler's virus infection. Brain Behavior & Immunity. 2007;21(8):1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229(4720):1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 46.Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJR. The effects of restraint on the neuropathogenesis of Theiler’s virus infection: I. acute disease. Brain Behav Immunity. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- 47.Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity of acute Theiler’s virus infection. J Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RR, Prentice T, Bridegam P, Young CR, Steelman AJ, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity and onset of the chronic phase of Theiler's virus infection. Journal of Neuroimmunology. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Njenga MK, Asakura K, Hunter SF, Wettstein P, Pease LR, Rodriguez M. The immune system preferentially clears Theiler’s virus from the gray matter of the central nervous system. J. Virol. 1997;71:8592–8601. doi: 10.1128/jvi.71.11.8592-8601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theiler M. Spontaneous encephalomyelitis of mice: a new viral disease. J. Exp. Med. 1934;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welsh CJR, Borrow P, Nash AA. Theiler’s virus: An experimental model of virus-induced demyelination. Autoimmunity. 1990;6:105–112. doi: 10.3109/08916939008993375. [DOI] [PubMed] [Google Scholar]
- 53.Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Sex dependent effects of chronic restraint stress during early Theiler’s virus infection on the subsequent demyelinating disease in CBA mice. J Neuroimmunol. 2006;177:46–62. doi: 10.1016/j.jneuroim.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Jellinck PH, Dhabhar FS, Sakai RR, McEwen BS. Long term corticosteroid treatment but not chronic stress affect 11beta-hydroxysteroid dehydrogenase type I activity in rat brain and peripheral tissues. J Steroid Biochem Mol Biol. 1997;60(5–6):319–323. doi: 10.1016/s0960-0760(96)00197-5. [DOI] [PubMed] [Google Scholar]
- 55.Welsh CJR, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68(6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- 56.Keith LD, Winslow JR, Reynolds RW. A general procedure for estimation of corticosteroid response in individual rats. Steroids. 1978;31:523–531. doi: 10.1016/0039-128x(78)90034-x. [DOI] [PubMed] [Google Scholar]
- 57.Hermann G, Tovar CA, Beck FM, Sheridan JF. Kinetics of glucocorticoids response to restraint stress and/or experimental influenza infection in two inbred strains of mice. J Neuroimmunol. 1994;28:219–225. doi: 10.1016/0165-5728(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 58.Satterlee DM, Sieve AN, Welsh TH, Welsh CJR, Meagher MW. Corticosterone mediates the effects of chronice restraint stress on Theiler’s virus infection in mice. Soc Neurosci Abstr. 2001;27:2238. [Google Scholar]
- 59.Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904(2):279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- 60.Clatch RJ, Lipton HL, Miller SD. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb Pathog. 1987;3(5):327–337. doi: 10.1016/0882-4010(87)90003-9. [DOI] [PubMed] [Google Scholar]
- 61.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Neville KL, Yauch RL, Kim BS. Epitope spreading leads to myelin-specific autoimmune responses in SJL mice chronically infected with Theiler’s virus. J Neurovirol. 1997 Suppl 1:S62–S65. [PubMed] [Google Scholar]
- 62.Rodriguez M, Sriram S. Successful therayp of Theiler’s virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988;140(9):2950–2955. [PubMed] [Google Scholar]
- 63.Roos RP, Woolmann R. DA strain of Theiler’s murine encephalomyelitis virus induces demyelination in nude mice. Ann Neurol. 1984;15(5):494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- 64.Lipton HL, Dal Canto MC. Contrasting effects of immunosuppression on Theiler’s virus infection in mice. Infect and Immun. 1977;15:903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faulkner RH, Prentice TW, Johnson RR, McCullough HK, Welsh TH, Storts R, Welsh CJR, Meagher MW. Soc for Neurosci Abstracts. New Orleans, LA: 2003. Corticosterone is both necessary and sufficient to observe the adverse effects of restraint stress on acute Theiler’s virus infection. [Google Scholar]
- 66.Acheson ED. Epidemiology of multiple sclerosis. Brit Med Bull. 1977;33:9–14. doi: 10.1093/oxfordjournals.bmb.a071407. [DOI] [PubMed] [Google Scholar]
- 67.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottlieb S. Epstein-Barr virus may increase risk of multiple sclerosis. BMJ. 2003;326(7392):731. [Google Scholar]
- 69.Kurtzke JF, Hyllested K. MS epidemiology in the Faroe Islands. Rev Neurol. 1987;57:77–87. [PubMed] [Google Scholar]
- 70.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 71.Grant DH, Brown GW, Harris T, McDonald WI, Patteron T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren S, Greehhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case-control evidence of a relationship. J Chronic Dis. 1982;35:821–831. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]