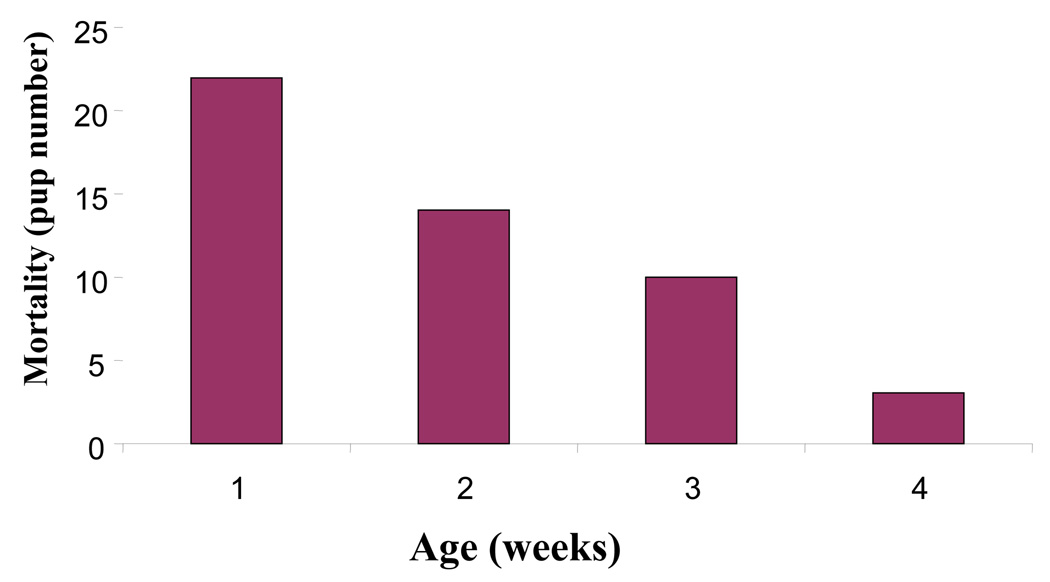Abstract
Developmental exposure of mice to the environmental contaminant and AhR agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), causes persistent postnatal suppression of T cell-mediated immune responses. The extent to which prenatal TCDD may induce or exacerbate postnatal autoimmune disease remains unknown. In the present study, time-pregnant high affinity AhR C57BL/6 mice received a single oral administration of 0, 2.5, or 5 µg/kg TCDD on gestation day (gd) 12. Offspring of these mice (n=5/gender/treatment) were evaluated at 24 weeks-of-age and showed considerable immune dysregulation that was often gender-specific. Decreased thymic weight and percentages of CD4+CD8+ thymocytes, and increased CD4+CD8− thymocytes, were present in the female but not male offspring. Males but not females showed decreased CD4−CD8+ T cells, and increased Vβ3+ and Vβ17a+ T cells, in the spleen. Males but not females also showed increased percentages of bone marrow CD24−B220+ B cell progenitors. Antibody titers to dsDNA, ssDNA and cardiolipin displayed increasing trends in both male and female mice, reaching significance for anti-dsDNA in both genders and for ssDNA in males at 5 µg/kg TCDD. Immunofluorescent staining of IgG and C3 deposition in kidney glomeruli increased in both genders of prenatal TCDD-exposed mice, suggestive of early stages of autoimmune glomerulonephritis. Collectively, these results show that exposure to TCDD during immune system development causes persistent humoral immune dysregulation as well as altered cell mediated responses, and induces an adult profile of changes suggestive of increased risk for autoimmune disease.
Keywords: TCDD, prenatal exposure, autoimmunity, C57BL/6 mouse
Introduction
Several reports suggest TCDD may increase risk of autoimmune responses. Similar patterns of inhibited thymocyte differentiation, as seen after TCDD, occur spontaneously in murine models of autoimmune disease (Kakkanaiah et al., 1990) and in mice treated in vivo with monoclonal antibodies to major histocompatibility complex (MHC) class I and class II molecules (Kruisbeek et al., 1985). These thymic MHC class I and class II antigens are required for normal thymocyte differentiation as well as for deletion of autoreactive cells (Blaylock et al., 2005). DeWall et al. (1992) observed reduced MHC class II antigen expression in the thymic epithelium of TCDD-treated mice; MHC class I antigen expression was not evaluated in these or subsequent studies. However, TCDD was found to down-regulate expression of an MHC class I gene (Q1b) in a mouse hepatoma cell line (Dong et al., 1997). These authors demonstrated that MHC Q1b cDNA encoded for the a3 domain and transmembrane domain of the Q1b class I protein, implying that the MHC gene product could interact with b2-microglobulin. The authors therefore suggested that the MHC Q1b molecule down-regulated by TCDD may function in antigen presentation, similar to MHC class I antigen in the thymus. These effects on MHC class I and II molecules by TCDD raise questions regarding ability of TCDD to impair autoreactive thymocyte deletion.
In addition to potentially increasing thymic release of autoreactive cells, TCDD increased extrathymic production of T cells, i.e., in compartments where negative selection does not occur or is less efficient than in thymus. For instance, Silverstone et al. (1994) found that TCDD induced T cell differentiation in the liver of young adult mice. These extrathymic-derived T cells expressed elevated levels of CD4+ Vβ 17a and Vβ 3+ TcR. Such TcR variable β (Vβ) chains are usually deleted in the thymus by reaction with self-MHC and minor lymphocyte stimulatory antigens (Okuyama et al., 1992; Hanawa et al., 1993) and have been associated with autoimmunity in experimental mouse models (Rocha et al., 1992).
Exposure to immunotoxic compounds during the prenatal period of immune system establishment often results in more dramatic and persistent immunologic damage than does adult exposure (Holladay and Smialowicz, 2000; Dietert and Piepenbrink, 2006; Luebke et al., 2006). Using an autoimmune nephritis mouse model (SWR × NZB) and developmental exposure, Silverstone et al. (1998) observed significantly reduced time to postnatal onset of glomerulonehpritis in the male SNF1 mice offspring, after a single maternal exposure to 10 µg/kg TCDD. T cells, which may be preferentially targeted by developmental TCDD (Faith and Moore, 1977; Luster et al., 1980; Blaylock et al., 1992; Gehrs and Smialowicz, 1999), do not appear to play a direct role in tissue damage in SNF1 lupus nephritis. However cognate interaction between autoreactive Th cells and autoreactive B cells may be involved in the proliferation of autoantibodies (Mohan et al., 1993). Sera of SNF1 mice also contained high titers of IdLNF1 + IgG antibodies, which peaked at 22–24 weeks and coincided with an increase in the CD4 to CD8 ratio of IdLNF1-reactive T cells and IdLNF1 Ig (IgG + IgM) deposition in the kidneys (Uner et al., 1998). The presence of pathogenic autoantibody-inducing Th cells specific for chromatin subparticles or histones has also been described in human patients with lupus as well as lupus-like SNF1 mice (Fournel et al., 2003). These data suggest the possibility that early onset of autoimmune nephritis in SNF1 mice after TCDD could result from a T cell lesion. However, postnatal day 3 thymectomy, which depletes CD4+CD25+ regulatory T cells and leads to multiple independent organ-specific autoimmune diseases, paradoxically protects SNF1 mice from lupus-like glomerulonephritis and may suggest lack of T cell involvement (Bagavant et al., 2002).
In contrast to the unproven contributions by T cells, autoantibodies produced by B cells display strong polyreactive responses to DNA and glomerular substrate dominating the deposits in lupus kidneys (Xie et al., 2003). The possibility that prenatal TCDD may directly alter B cell activity to induce or exacerbate the autoimmune disease remains uninvestigated. In this regard, the lack of studies investigating B cell function following developmental TCDD exposure was recently identified as an important data gap worthy of further examination (Luster et al., 2003; Holsapple et al., 2005). The present studies therefore examined secondary lymphoid organ T cell phenotypes including Vβ TcR expression, and signs of altered B cell lymphopoiesis and autoantibody production, and immune complex deposition in kidneys, in high affinity AhR C57BL/6 mice after prenatal TCDD.
Methods
Mice and TCDD exposure
C57BL/6 mice were obtained from Charles River Laboratories (Portage, MI) at 4–5 weeks-of-age. Mice were acclimated to the animal care facility for at least 2 weeks prior to breeding. Briefly, 80 female C57BL/6 mice were bred overnight using one C57BL/6 male per two females, and plug positive mice the next morning were designated gd 0. Pregnant C57BL/6 mice were orally gavaged on gestation day 12 with 0, 2.5, 5 or 10 µg/kg TCDD dissolved in corn oil (n= 12 pregnant mice/treatment). The F1 offspring remained with dams until weaning, then were separated by treatment and allowed to mature to 24 weeks-of-age. All animals were fed a commercial pelleted diet and provided water ad libitum, and were housed under controlled conditions of temperature (22 °C), humidity (40–60%), and lighting (12:12 light:dark cycle). Animal maintenance, care and use were at all times in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines at Virginia Tech, and approved prior to initiation of experiments.
Body weights and tissue collection
Mice were euthanized by cervical dislocation and weighed. The thymus, spleen, bone marrow and mesenteric, axillary and inguinal lymph nodes (LN) were immediately collected post-euthanasia under aseptic conditions, using dissection scissors and curved forceps. Messenteric LN were used for histopathology, while axillary and inguinal LN were pooled for flow cytometry. Non-LN organs were weighed and then placed individually into pre-labeled sterile Petri dishes (Corning, Corning, NY), containing 8 mL of RPMI-1640 culture medium (Mediatech, Herndon,VA). Dishes were placed on ice until tissue dissociation.
Cell dissociation and enrichment
Each organ was gently dissociated over a stainless steel sieve screen (Sigma, St. Louis, MO) using curved forceps. Cells were then pipetted through the sieve screen following dissociation to remove debris. Cells were washed in RPMI-1640 (Mediatech) for 10 min, 240 × g, and 23 °C. The supernatant was then discarded and the cell pellet was resuspended in 8 mL of RPMI-1640. Spleen cells were then resuspended in 1 mL incomplete RPMI-1640. To each tube, 2 mL of ACK 0.83% ammonium chloride lysis buffer (pH 7.29) were added, to lyse red blood cells, and tubes were incubated for 5 min at 23°C. After lysis incubation, the cells were resuspended in 5 mL of incomplete RPMI-1640 (Mediatech) and washed twice (7 min, 290 × g and 7 °C). The splenic leukocyte-rich cells were then resuspended in 5 mL complete RPMI-1640 media containing 10% FBS (Sigma), 2 mML-glutamine (ICN, Costa Mesa, CA), 50 IU/mL penicillin (ICN), and 50 mg/mL of streptomycin (ICN), and maintained on ice. For bone marrow isolation, femurs were removed and the bone marrow cavities flushed with 2% FBS-PBS, washed once (7 min, 290 × g and 7 °C), resuspended in 1 mL incomplete RPMI-1640 media (Mediatech) and stored at 4 °C. Cells were adjusted to 5.0×106 cells/mL in complete media.
Cell enumeration
Cells were enumerated and size-analyzed using a Beckman Multisizer 3® Coulter cell counter (Fullerton, CA) according to the manufacturer’s protocol. Briefly, a 10 µL aliquot of enriched cell suspension was transferred to a plastic counting-chamber containing 10 mL of PBS (Mediatech). The plastic chamber was capped, mixed by repeated gentle inversion, and counted.
Flow cytometric evaluation of cell-surface phenotypic markers
Cell suspensions (5 × 105/100 µL) from the thymus, spleen, lymph node and bone marrow were aliquoted into individual wells of a 96-well round-bottom tissue culture plate (Corning, NY). Monoclonal antibodies (mAbs) with phycoerythrin (PE) fluorescent labels were used according to manufacturer (BD Pharmingen; San Diego, CA) recommendation at a concentration of 0.2 µg/µL; mAbs with fluorescein isothiocyanate (FITC) fluorescent labels were similarly used at the recommended concentration of 0.5 µg/µL. Cells were stained as previously described (Gogal et al., 2001; Klein et al., 2006). Briefly, lymphocyte aliquots (5 × 105 cells/ 100 µL) from thymus, spleen, lymph node and bone marrow were incubated with the following primary mAbs: PE-anti CD4, FITC-anti CD8, FITC-anti CD25, PE-anti CD45/B220 (ebioscience, San Diego, CA); FITC-anti Vβ 3 TCR, FITC-anti Vβ17a TCR, FITC-anti CD45/B220, PE-anti CD24 (HSA), FITC-anti CD1, PE-anti CD23 (BD Pharmingen). For double staining protocols, mAbs with different fluorescent labels were simultaneously added to the sample. For bone marrow, aliquots of 5 × 105 cells were pre-blocked with anti-Fc III/IIR (clone 2.4G2, Rat IgG2b). Following staining, cells were washed and evaluated on a Coulter Epics XL flow cytometer (Coulter, Miami, FL). From each sample, 5000 events were collected and analyzed using the Immuno-4 software program. Dead cells, clumps, and debris were excluded electronically by gating on forward (FSC) versus side scatter (SSC).
ELISA for autoantibodies to double-stranded DNA, single-stranded DNA and cardiolipin
To detect anti-dsDNA antibody, 96-well high-binding microtiter plates (Corning, Corning, NY) were coated with heat-denatured calf thymus DNA (100 mg/ml; Sigma). For anti-ssDNA antibody, DNA from calf thymus was used (10 mg/mL; Sigma), and for autoantibodies to cardiolipin a solution from bovine heart was used (10 mg/ml; Sigma). All pre-coated plates were incubated at 4°C overnight. The plates were washed three times with 300 µL PBS/0.05% Tween 20 (Mediatech), blocked with 1% BSA (Sigma) for 2 h at 23 °C, washed and then incubated with diluted serum samples to be tested (1/25). After 3 h at 23 °C, the serum-coated plates were washed three times with 300 µL of PBS-0.05% Tween 20. To each well, 0.2 mL of diluted alkaline phosphatase-conjugated anti- IgG antibody (1/3000) (Sigma) were added and the plates were incubated for 30 min at 23 °C. The plates were then washed three times with 300 µL of PBS-0.05% Tween 20. To each well, 0.2 mL prepared substrate for alkaline phosphatase conjugated secondary antibody (SIGMA FAST™ p-Nitrophenyl phosphate tablets) were added and allowed to develop for 45 min at 23 °C before adding 50 µL of 3 M NaOH stop solution (Sigma, St. Louis, MO). The absorbance (A405) of the initial dilution was measured. Optical density (OD) readings represent the average from sera from each mouse performed in duplicate.
Histology of the kidneys, livers and lymph nodes
Kidneys, livers and mesenteric lymph nodes were collected at the time of euthanasia and immediately fixed in 10% formalin (Fisher Scientific, Pittsburgh, PA) for 48 h, processed and embedded in a paraffin block. After embedding, a 5 µm section was cut from each tissue block, which was subsequently stained with hematoxylin and eosin (H&E, Richard-Allen Scientific, Kalamazoo, MI) using standard histologic methods. The prepared slides were then evaluated, with a light microscope, in a blinded-manner by a boarded veterinary pathologist (co-author PS).
Immunohistochemistry of the kidneys: C3 and IgG deposition
Kidney frozen sections were cut into 5 mm sections and stained with FITC conjugated antibodies. Briefly, tissue sections were thawed at room temperature and dried for 30 min. Slides were fixed in acetone for 10 min and then washed with PBS (Mediatech) three times for 3 min/wash. Goat anti-mouse IgG diluted 1:100 (Pierce, Rockford, IL) or goat anti-mouse C3 diluted 1:100 (Pierce, Rockford, IL) were incubated with tissues sections in humid chamber for 60 min at 23 °C. The sections were then rinsed three times for 5 min/wash with PBS (Mediatech). The slides were mounted using Vectashield™ mounting media (Vector Labs, Burlingame, CA) and then examined on an Olympus BX-60 fluorescence microscope (Center Valley, PA). The severity of glomerulonephritis and immune complex deposition was scored using a range from 0 to 3+, where 0 corresponded to a non-autoimmune healthy mouse and 3+ to the maximal alteration observed in the study. All slides were scored in a blinded manner by an experienced investigator (co-author CR).
Cytokine ELISA Assays
Splenocytes were plated into each well (1 mL in complete media; 5 × 106 cells per well) of a 24-well tissue-culture plate (Corning Cell Wells™, Corning, Corning, NY). Cells were co-cultured with 1 mL Con A (10 µg/mL, Sigma) and incubated at 37 °C, 5 % CO2 for 48 h. The plates were centrifuged (7 °C, 250 × g, 7 min), and the supernatants were transferred to a sterile 12 × 75 mm cultured tubes (Fisher). Supernatants were stored at − 80 °C until use. The levels of interleukin 2 (IL-2), IL-4, IL-10, IL-12 and INF-γ were determined using ELISA kits (Ready-to-use; ebioscience) according to the manufacturer’s instructions.
Statistical analysis
Data were expressed as arithmetical mean ± SEM. Analysis of variance (ANOVA) was used with Dunnett's t-test to establish significant differences among groups. Group size was 5 for all experiments (n=5/gender/treatment). Results described as different in this report indicate significantly different at p <0.05.
Results
Prenatal 10 µg/kg TCDD exposure resulted in postnatal mortality
The F1 offspring of dams orally dosed with 0.0, 2.5, 5.0 or 10 µg/kg TCDD on gestational day 12 were monitored and allowed to mature to 24 weeks-of-age. By postnatal day 28, ninety–percent of the C57BL/6 pups born to the dams exposed to 10 µg/kg TCDD had succumbed to a wasting-like syndrome (Figure 1). This dose produced no overt toxicity in previous studies that utilized gd 18 data collection (Holladay et al., 1991) or in pups cross-fostered to naïve dams (Blaylock et al., 1992), suggesting sufficient postnatal TCDD exposure occurred by lactation to induce the wasting syndrome. Alternately, the higher dose of TCDD may have diminished lactation due to impaired mammary gland differentiation (Vorderstrasse et al., 2004). The other treatment groups did not show any significant mortality. Therefore, the study was carried on from this point with the vehicle (0.0 µg/kg) and two treatment groups: 2.5 and 5 µg/kg TCDD.
Figure 1.
The incidence of mortality in C57BL/6 pups born from dams prenatally-exposed to 10 µg/kg TCDD. The data represent the number of pup deaths observed up to 30 days post-parturition. No significant mortality was observed in the other treatment groups.
Body and thymus weights
Body weight was reduced relative to controls in both 24-week-old male and female offspring of TCDD-treated dams, at the 2.5 µg/kg dose. Thymic weights showed a numerical trend toward decrease in males and significant decrease in females, in the 2.5 µg/kg and 5.0 µg/kg TCDD exposure groups. Thymic weight/body weight ratios were decreased significantly at 24 weeks, at both doses of TCDD, in females but not males. Similarly, mean thymic cellularity decreased significantly at both doses of TCDD in females but not males. Splenic weight, splenic body weight ratio and cellularity yielded no significant changes across treatment groups (Table 1).
Table 1.
| 0.0ug/kg Mean ± SEM |
2.5ug/kg Mean ± SEM |
5.0ug/kg Mean ± SEM |
||
|---|---|---|---|---|
| Body Weight Grams | Male | 37.71 ± 0.96 | 33.26 ± 1.51 * | 35.05 ± 0.90 |
| Female | 25.43 ± 0.49 | 22.83 ± 0.60 * | 25.27 ± 1.06 | |
| Thymic Weight Grams | Male | 0.061 ± 0.004 | 0.049 ± 0.006 | 0.049 ± 0.003 |
| Female | 0.072 ± 0.003 | 0.055 ± 0.003 * | 0.064 ± 0.004 * | |
| Thymus:Body Weight Ratio | Male | 0.0016 ± 0.0001 | 0.0015 ± 0.0002 | 0.0014 ± 0.0001 |
| Female | 0.0028 ± 0.0001 | 0.0024 ± 0.0001 * | 0.0024 ± 0.0001 * | |
| Thymic Cellularity Cells (Millions) | Male | 87.60 ± 9.82 | 79.40 ± 9.30 | 75.40 ± 7.19 |
| Female | 73.26 ± 7.37 | 49.02 ± 2.91 * | 55.20 ± 6.08 * | |
| Splenic Weight Grams | Male | 0.09 ± 0.01 | 0.08 ± 0.00 | 0.09 ± 0.00 |
| Female | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | |
| Spleen:Body Weight Ratio | Male | 0.0024 ± 0.0002 | 0.0025 ± 0.0001 | 0.0025 ± 0.0002 |
| Female | 0.0058 ± 0.0004 | 0.0072 ± 0.0004 | 0.0061 ± 0.0005 | |
| Splenic Cellularity Cells (Millions) | Male | 65.68 ± 13.09 | 61.41 ± 7.61 | 59.81 ± 7.48 |
| Female | 67.36 ± 8.42 | 62.87 ± 13.41 | 58.19 ± 7.03 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
Thymic T cell differentiation
Female but not male offspring of dams dosed with 5.0 µg/kg TCDD exhibited marginal but significant thymic phenotypic changes at 24 weeks-of-age. The percentage of CD4+CD8+ double positive thymocytes was decreased and of CD4+CD8− was increased by gd 12 TCDD (Table 2). Absolute numbers of cells in these phenotypes was further decreased due to smaller thymuses in the female mice (data not shown).
Table 2.
| THYMUS | 0.0 µg/kg Mean ± SEM |
2.5 µg/kg Mean ± SEM |
5.0 µg/kg Mean ± SEM |
|
|---|---|---|---|---|
| CD4+CD8+ (%) | Male | 76.08 ± 1.41 | 70.18 ± 3.53 | 77.04 ± 1.39 |
| Female | 79.90 ± 0.87 | 75.84 ± 1.49 | 71.35 ± 3.20 * | |
| CD4− CD8− (%) | Male | 2.49 ± 0.11 | 11.58 ± 5.40 | 3.00 ± 0.84 |
| Female | 3.74 ± 0.52 | 4.33 ± 0.87 | 7.12 ± 3.53 | |
| CD4+CD8− (%) | Male | 20.02 ± 1.22 | 16.96 ± 1.95 | 18.56 ± 1.04 |
| Female | 14.70 ± 1.29 | 17.04 ± 0.44 | 19.38 ± 1.05 * | |
| CD4− CD8+ (%) | Male | 1.41 ± 0.15 | 1.27 ± 0.11 | 1.39 ± 0.16 |
| Female | 1.76 ± 0.29 | 2.70 ± 0.35 | 2.10 ± 0.21 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
T cell phenotypes in the spleen and lymph nodes
The 5.0 µg/kg gd 12 dose of TCDD caused a significant decrease in the percentage of CD4−CD8+ cells in the spleens of 24-week-old male, but not female, mice. The percentage of Vβ3+ TCR and Vβ17a+ TCR T cells showed a numeric (non-significant) dose-response trend toward increase in female mice. However, Both of these Vβ phenotypes were significantly increased in the 5.0 µg/kg males (Table 3).
Table 3.
| SPLEEN | 0.0ug/kg Mean ± SEM |
2.5ug/kg Mean ± SEM |
5.0ug/kg Mean ± SEM |
|
|---|---|---|---|---|
| CD4+CD8− (%) | Male | 27.31 ± 0.80 | 27.15 ± 0.54 | 30.58 ± 1.11 |
| Female | 26.91 ± 1.04 | 25.14 ± 0.86 | 29.26 ± 1.80 | |
| CD4−CD8+ (%) | Male | 10.69 ± 0.56 | 10.34 ± 0.26 | 5.67 ± 0.49 * |
| Female | 12.19 ± 0.59 | 11.87 ± 0.27 | 13.42 ± 0.89 | |
| V§17a TCR+ (%) | Male | 2.76 ± 0.54 | 5.44 ± 1.25 | 7.83 ± 1.94 * |
| Female | 3.67 ± 0.58 | 4.82 ± 0.48 | 4.76 ± 0.29 | |
| V§3 TCR+ (%) | Male | 2.02 ± 0.50 | 4.56 ± 0.73 | 10.08 ± 2.49 * |
| Female | 2.92 ± 0.71 | 5.02 ± 0.96 | 5.32 ± 0.54 | |
| CD4+CD25+ (%) | Male | 2.11 ± 0.17 | 2.23 ± 0.07 | 2.00 ± 0.12 |
| Female | 2.19 ± 0.24 | 1.90 ± 0.06 | 2.62 ± 0.29 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
The 5.0 µg/kg gd 12 dose of TCDD caused a significant decrease in the percentage of The CD4+CD8− cells and a significant increase in non-T cells (CD4−CD8−) in lymph nodes of female, but not male, mice. Total activated/regulatory T cells (CD4+CD25+) were not affected by prenatal TCDD (Table 4).
Table 4.
| Lymph Nodes | 0.0 µg/kg Mean ± SEM |
2.5 ug/kg Mean ± SEM |
5.0 µg/kg Mean ± SEM |
|
|---|---|---|---|---|
| CD4+ CD8− (%) | Male | 38.20 ± 1.41 | 35.46 ± 1.80 | 38.98 ± 0.66 |
| Female | 42.77 ±0.78 | 40.96 ± 1.90 | 34.33 ± 2.52 * | |
| CD4− CD8+ (%) | Male | 20.76 ± 1.85 | 19.88 ± 1.43 | 18.86 ± 0.74 |
| Female | 22.37 ± 2.15 | 20.32 ± 1.72 | 15.45 ± 1.88 | |
| CD4− CD8− (%) | Male | 39.94 ± 2.93 | 43.46 ± 2.96 | 41.12 ± 0.63 |
| Female | 33.70 ± 1.86 | 37.62 ±3.61 | 49.40 ±4.20 * | |
| CD4+ CD25− (%) | Male | 31.48 ± 0.59 | 31.98 ± 0.86 | 31.20 ± 0.69 |
| Female | 36.52 ± 0.88 | 34.30 ± 0.59 | 30.64 ± 1.80 * | |
| CD4+ CD25+ (%) | Male | 1.46 ±0.13 | 1.30 ± 0.15 | 1.37 ± 0.24 |
| Female | 2.26 ± 0.15 | 2.20 ± 0.19 | 1.98 ± 0.09 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
Phenotype of mature B cells in the spleen
The 5.0 µg/kg gd 12 dose of TCDD resulted in a significant decline in splenic CD24+B220+ B cells at 24 weeks, in both males and females. Staining of these cells with CD21 revealed a significant increase in cells expressing both the CD24-CD21+ and CD23-CD1+ in 5.0 µg/kg TCDD-exposed mice. This phenotype is consistent with the marginal zone (MZ) B cells suggesting an expansion of these cells (Grimaldi etal., 2001, Pillai et al., 2005). Additionally, CD138+ plasma cells increased by dose reaching significance in the 5.0 µg/kg TCDD-exposed male mice (Table 5).
Table 5.
| SPLEEN | 0.0 µg/kg Mean ± SEM |
2.5 µg/kg Mean ± SEM |
5.0 µg/kg Mean ± SEM |
|
|---|---|---|---|---|
| CD24+ B220+ (%) | Male | 44.6 ± 0.9 | 47.5 ± 1.6 | 20.9 ± 8.5 * |
| Female | 40.5 ± 1.3 | 38.8 ± 4.0 | 30.7 ± 2.0* | |
| CD24− B220− (%) | Male | 49.3 ± 0.7 | 45.4 ± 2.2 | 64.5 ± 5.1 * |
| Female | 51.1 ± 1.5 | 49.4 ± 3.7 | 53.5 ± 2.4 | |
| CD24− B220+ (%) | Male | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.1 * |
| Female | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | |
| CD23− CD1+ (%) | Male | 2.8 ± 0.3 | 4.0 ± 0.3 | 9.4 ± 0.5 * |
| Female | 2.7 ± 0.4 | 4.0 ± 0.5 | 8.8 ± 0.4 * | |
| CD24− CD21+ (%) | Male | 6.6 ± 1.0 | 8.8 ± 0.9 | 12.7 ± 0.7 * |
| Female | 5.7 ± 0.6 | 6.7 ± 0.6 | 11.0 ± 0.5 * | |
| CD138+ (%) | Male | 6.0 ± 0.7 | 8.6 ± 0.7 | 14.1 ± 2.5 * |
| Female | 5.9 ± 0.7 | 8.0 ± 1.0 | 8.3 ± 0.5 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
B lymphoid progenitors in bone marrow
The 5.0 µg/kg gd 12 dose of TCDD caused significantly increased bone marrow-derived non B cell committed populations (CD24+B220-) in both genders and, in males, increased percentages of the earliest B cell progenitors (CD24−B220+). Immature and mature B cells (CD24+B220+) were decreased in both genders (Table 6).
Table 6.
| Bone Marrow | 0.0 µg/kg Mean ± SEM |
2.5 µg/kg Mean ± SEM |
5.0 µg/kg Mean ± SEM |
|
|---|---|---|---|---|
| CD24+ B220+ (%) | Male | 32.30 ± 1.15 | 31.20 ± 0.47 | 23.70 ± 1.03 * |
| Female | 28.16 ± 2.47 | 26.98 ± 1.71 | 22.00 ± 1.45 * | |
| CD24+ B220 − (%) | Male | 55.12 ± 1.48 | 56.22 ± 0.55 | 62.56 ± 1.48 * |
| Female | 54.08 ± 3.21 | 56.24 ± 2.07 | 63.98 ± 2.49 * | |
| CD24− B220+ (%) | Male | 0.50 ± 0.12 | 0.50 ± 0.12 | 1.06 ± 0.14 * |
| Female | 0.68 ± 0.20 | 0.68 ± 0.20 | 0.76 ± 0.18 | |
| CD24− B220− (%) | Male | 12.08 ± 0.61 | 11.68 ± 0.39 | 12.68 ± 1.10 |
| Female | 17.06 ± 1.63 | 16.06 ± 1.59 | 13.30 ± 1.37 | |
n = 5 mice /treatment/gender
= p ≤ 0.05, Dunnett’s t-test.
Anti-dsDNA, anti-ssDNA, and anti-cardiolipin titers
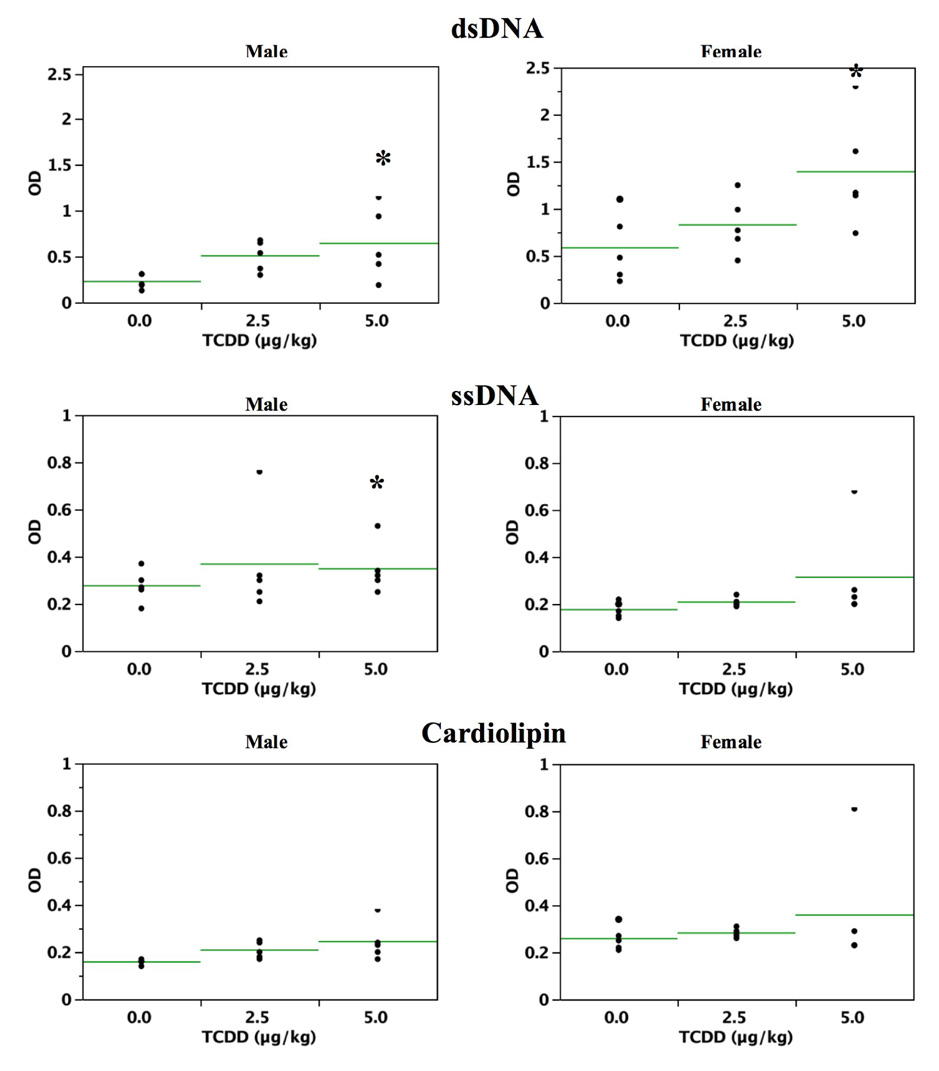
Antibody titers to dsDNA, ssDNA and cardiolipin displayed increasing trends in both male and female mice, reaching significance for anti-dsDNA in both males and females at 5.0 µg/kg TCDD. A significant increase in ssDNA was present in 5.0 µg/kg male mice (Figure 2).
Figure 2.
Sera from 24 week-old C57BL/6 mice that were prenatally exposed to 0.0, 2.5 and 5.0 µg/kg TCDD were analyzed for the presence of autoantibodies to dsDNA (A), ssDNA (B) and cardiolipin (C), (n=5/gender; *=p ≤ 0.05, Dunnett’s t-test). Each horizontal line represents the arithmetic mean for each treatment group.
Deposition of anti-IgG and anti-C3 immune complexes in the kidney
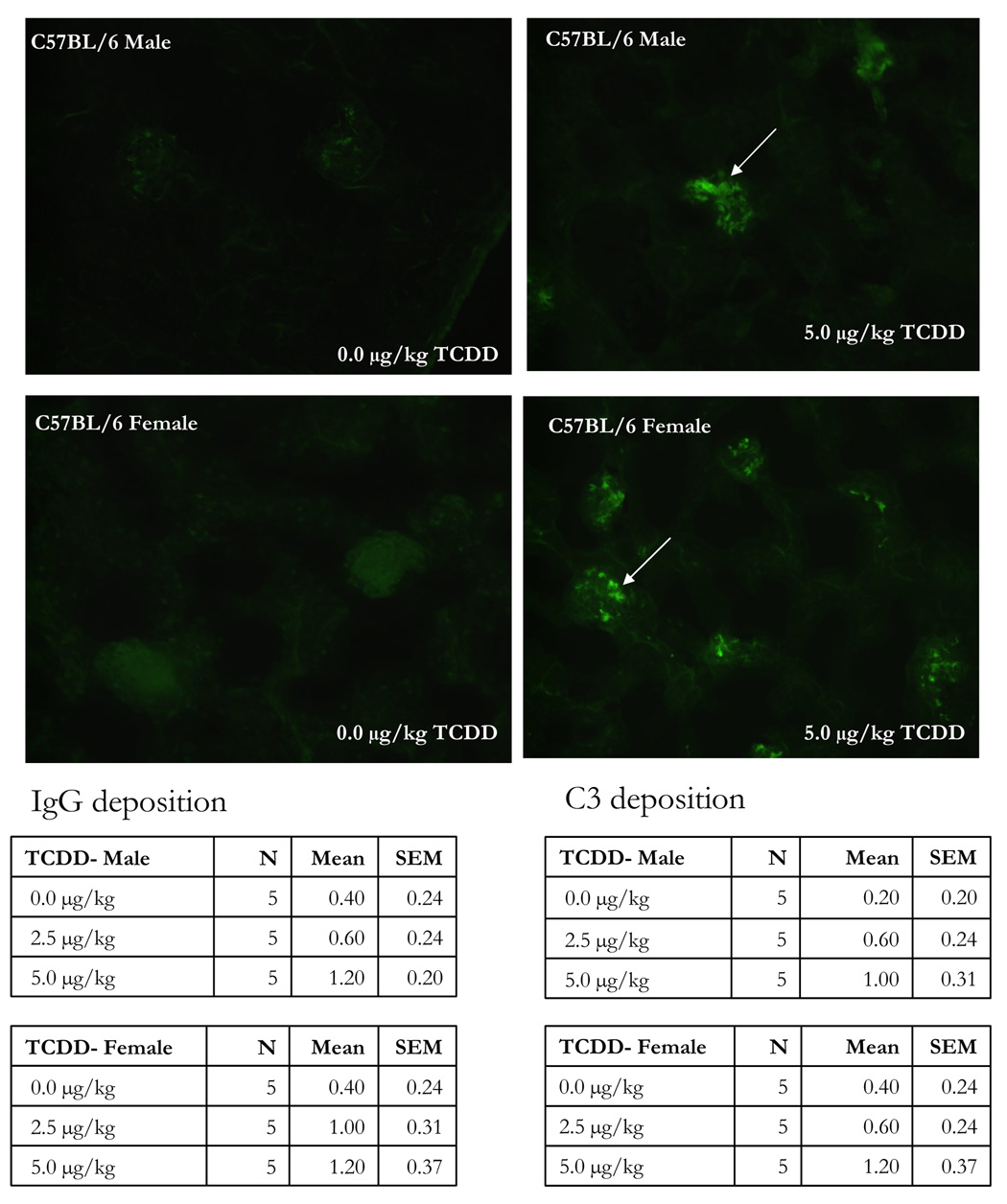
Autoimmune glomerulonephritis was induced in male SNF1 mice by prenatal TCDD exposure (Silverstone et al., 1998). Immunohistochemistry was therefore performed on kidneys from the present 24-week-old mice, and showed dose-related increased deposition of anti-IgG and anti-C3 immune complexes in mice that were exposed to TCDD during development. Visual and numeric increased mean immunofluorescence was clearly present in the 5.0 µg/kg TCDD group, and comparable, for both anti-IgG and anti-C3 probes (representative IgG deposition shown in Figure 3). The 2.5 µg/kg TCDD kidneys were more variable with some samples showing increased mean immunofluorescence and some comparable to control (not shown).
Figure 3.
The kidneys from 24 week-old C57BL/6 mice that were prenatally exposed to 0.0, 2.5 and 5.0 µg/kg TCDD were collected, fixed, section and stained with FITC-labeled anti-IgG. The above figures are representative of kidneys stained with FITC-anti-IgG from control female (A) and male (C) or 5.0 µg/kg TCDD-exposed female (B) and male (D) mice. The table data show the mean± SEM of the IgG and C3 disposition scores of 5 mice/treatment/gender.
Lymph node and liver histopathology
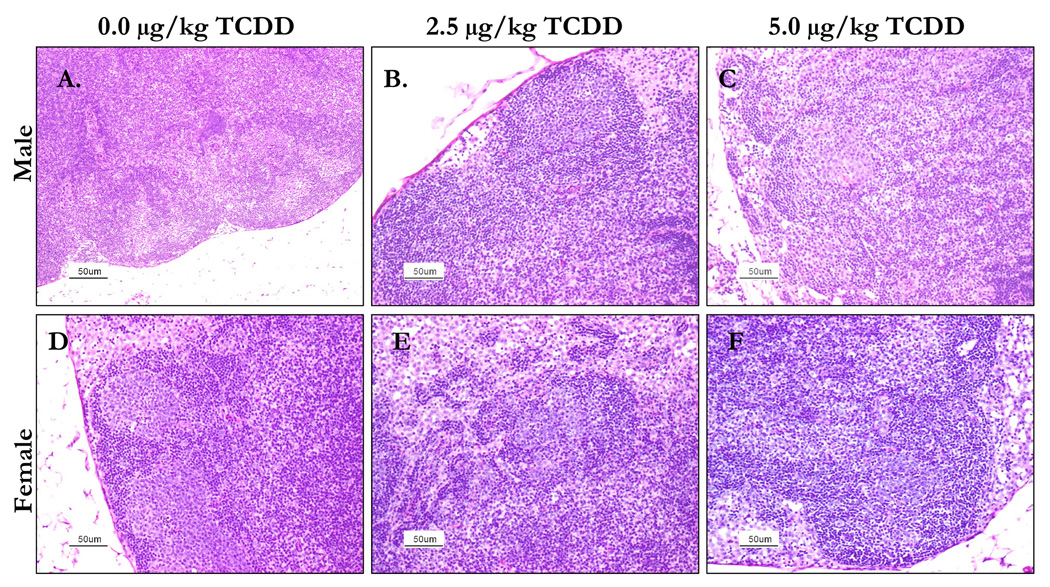
Histological examination of the mesenteric lymph nodes from 2.5 and 5.0 µg/kg TCDD mice showed evidence of disruption of the architecture, including blurring of the usual distinct zones. In some mice the cell populations were regionally monomorphic suggestive of neoplasia. Moderately diminished follicular activity was seen in 5.0 µg/kg female mice and 2.5 µg/kg male mice (Figure 4).
Figure 4.
The lymph nodes from 24 week-old C57BL/6 mice that were prenatally exposed to 0.0, 2.5 or 5.0 µg/kg TCDD were collected, fixed, section and stained with H&E stain. The above images are representative of control male (A) and female (D), 2.5 µg/kg TCDD male (B) and female (E) and 5.0 µk/kg TCDD male (C) and female (F) lymph nodes. The lymph nodes from male mice prenatally-exposed to either dose of TCDD showed disruption of architecture compared to controls. Lymph nodes from the prenatally-exposed TCDD female mice had regions of diminished follicular activity when compared to controls.
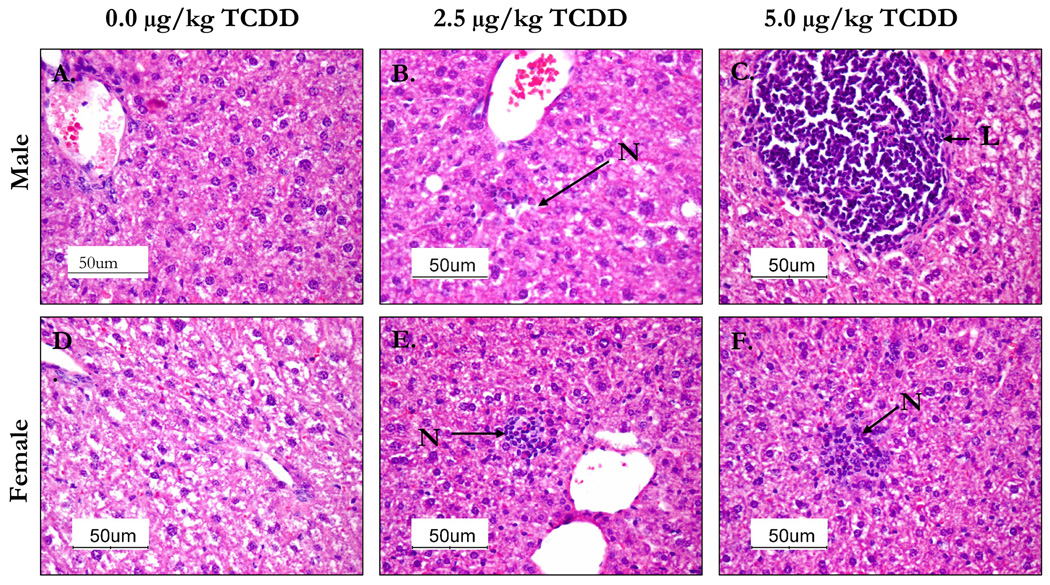
Histological examination of the livers from 5.0 µg/kg TCDD-treated male group mice showed massive infiltration of lymphocytes around some central veins and increased number of small foci of necrosis with infiltration of inflammatory cells. The 2.5 µg/kg male group exhibited scattered lymphoid focus and a few small, scattered foci of necrosis with inflammation. Sections of livers from females in both dosage groups had moderate vacuolation, moderate localized telangiectasis and moderate small foci of necrosis with inflammation. The liver sections from 2.5 µg/kg females exhibited scattered small foci of necrosis with no significant histologic changes compared to controls. In general, livers from females exhibited lesser pathological signs than the similar male dose group (Figure 5).
Figure 5.
The livers from 24 week-old C57BL/6 mice that were prenatally exposed to 0.0, 2.5 and 5.0 µg/kg TCDD were collected, fixed, section and stained with H&E stain. The above images are representative of control male (A) and female (D), 2.5 µg/kg TCDD male (B) and female (E) and 5.0 µk/kg TCDD male (C) and female (F) liver sections. Livers from male mice prenatally-exposed to 5.0 µg/kg TCDD showed infiltration of lymphocytes (L) round central veins compared to control group. Livers from the 2.5 µg/kg prenatally-exposed TCDD males and 5.0 µg/kg TCDD females contained foci of necrosis (N) with inflammatory cells not evident in the controls.
Th1/Th2 cytokine balance
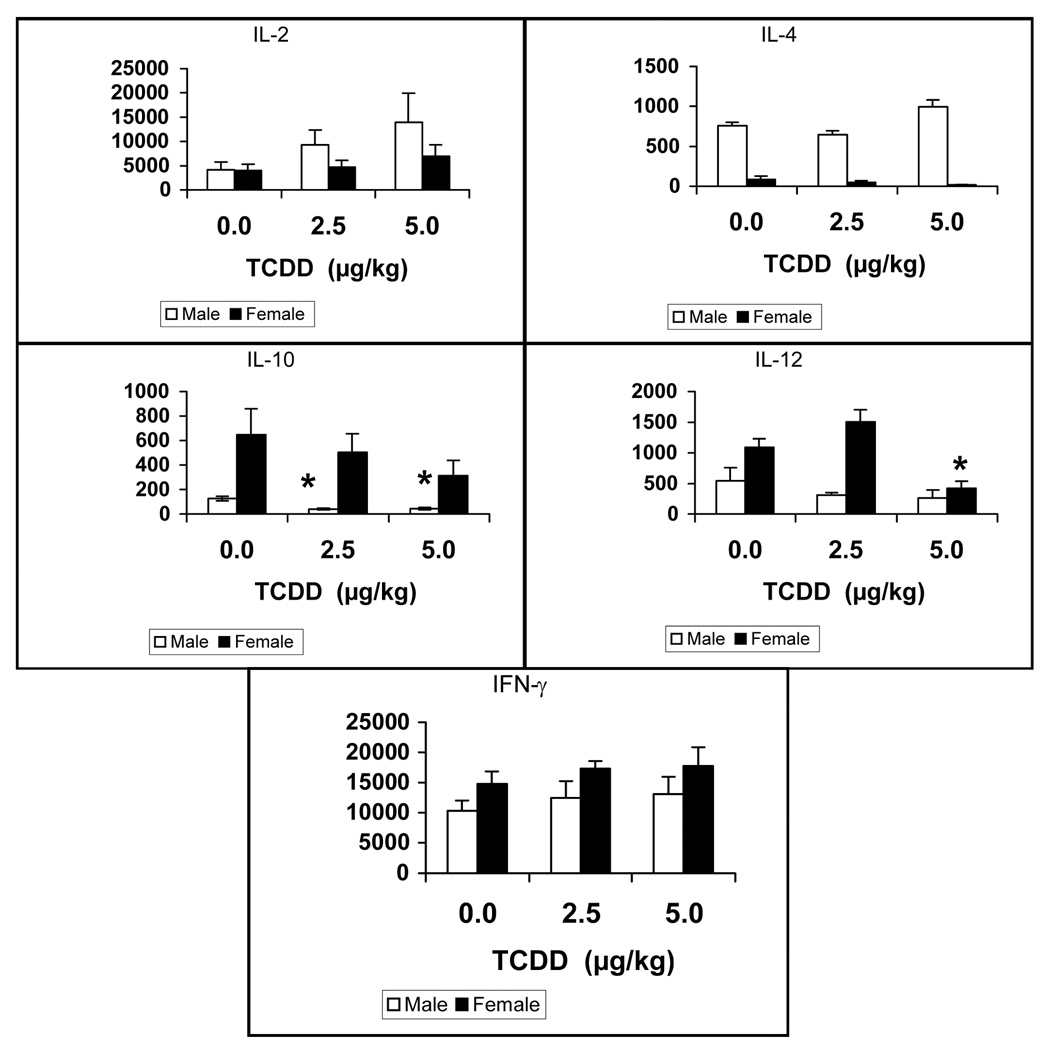
Analysis of cytokine levels from supernatants of Con-A-stimulated splenic lymphocytes showed that the IFNγ and IL-2 levels tended to increase non-significantly across treatment and gender of prenatal TCDD- exposed mice. IL-10 was significantly diminished in males dosed with prenatal TCDD, while females trended in the same direction. IL-12 was depressed in the females but not males at 5.0 mg/kg TCDD. Although statistically not analyzed in this study, IL-4 and IL-10 levels appeared to be markedly different between males and females, however were not affected by TCDD (Figure 6).
Figure 6.
Supernatants were collected from splenocytes, of 24 week-old mice that were prenatally exposed to 0.0, 2.5 and 5.0 µg/kg TCDD, cultured for 48 hr with Con A (10 µg/mL). The levels of IL-2, IL-4, IL-10, IL-12 and INF-γ were determined using commercially-available murine cytokine ELISA kits. The data are presented in a scatterplot analysis with mean bars (n = 5 mice/treatment/gender,*= p ≤ 0.05, Dunnett’s t-test).
Discussion
The incidence of nephritis is low in autoimmune NZB mice, but when crossed with normal SWR mice, almost 100% of the female F1 hybrids (SNF1) develop glomerulonephritis that is fatal by about 8 months-of-age. Autoimmune disease progression in lupus-like SNF1 mice as in the human version of the disease is critically dependent on T cell function, including accelerated antibody production subsequent to inappropriate activity of CD4+ T helper cells. The autoimmune nephritis is then characterized by IgG deposition in kidney glomeruli (Mohan et al., 1993).
Our laboratory and others have suggested that TCDD exposure during the sensitive period of immune system development may increase risk of postnatal autoimmune disease (reviewed by Holladay, 1999). In the single study of its kind, Silverstone et al. (1998) exposed autoimmune-predisposed (SWR × NZB)F1 mice to a mid-gestation dose of 10 µg/kg TCDD. These authors reported significantly reduced time to postnatal onset of glomerulonephritis in the male SNF1 offspring. Mechanisms underlying this induced autoimmunity in the normally resistant males are not known. Both SWR and NZB mice are of the Ahdd phenotype (low affinity AhR/less sensitive to TCDD) whereas C57BL/6 mice are of the Ahbb (sensitive) phenotype (Holsapple et al., 1991). The present experiments therefore used developmental exposure in TCDD- and immunologically-sensitive C57BL/6 mice to detect postnatal changes in T and B cells that may relate to increased risk of autoimmune disease.
The ability of TCDD to alter T cell maturation and differentiation may negatively impact central tolerance. Damage to thymic epithelium by TCDD has been detected, leading to suggestions that thymic epithelium-dependent selective events may be impaired, through which thymocytes expressing autoreactive T cell receptors (TcR) are deleted (Greenlee et al., 1985; De Waal et al., 1992; Schuurman et al., 1992). Thymic epithelial distribution of MHC class II molecules was also altered in TCDD-treated mice, an additional effect hypothesized as having potential to cause defective thymocyte selection (De Heer et al., 1994). These reports may support the present observation of dramatically increased autoreactive T cells expressing Vβ17a+ and Vβ3+ TcR in the spleens of male TCDD-treated mice. The marked difference between Vβ TcR expression by gender in C57BL/6 mice, with females showing no increased expression, suggests possible influence of endogenous hormones. Whether these Vβ T cells are increased in SNF1 males and may contribute to TCDD-induced glomerulonephritis is not yet known.
Previous studies have shown that thymic atrophy, thymocyte hypocellularity and delayed thymocyte maturation rebound shortly after birth in offspring of TCDD-treated pregnant mice (Fine et al., 1990; Holladay et al., 1991). These thymic effects were no longer apparent by postnatal day 14 in mice exposed to 1.5 or 3.0 mg/kg/day TCDD from gd 6–14 (Holladay et al., 1991). Rats exposed to 3 mg/kg TCDD on gd 14 had decreased percentages of CD4−8− thymocytes lasting until three weeks of age and decreased thymic/body weight ratio lasting until 14 weeks of age, in both genders (Gehrs et al., 1997). These rats showed persistent and gender-different T cell function as indicated by significant depression of the delayed-type hypersensitivity (DTH) response lasting to 4 months of age in females and 19 months of age in males (Gehrs and Smialowicz, 1999). The present mice also responded differently by gender to early TCDD exposure, and differently from the above rats, with decreased thymic weight, decreased percentages of CD4+CD8+ thymocytes, and increased CD4+CD8− thymocytes occurring in only the female offspring.
In addition to increased Vβ TcR expression in splenocytes, male but not female offspring of TCDD-treated dams showed decreased CD4−CD8+ T cells in the spleen. Hess and colleagues (1990) demonstrated that the therapeutic immunosuppressive drug cyclosporine A (CsA) interfered with negative selection of autoreactive thymic T cells in Lewis rats following irradiation and syngeneic bone marrow reconstitution, an effect they were able to relate to a TCDD-like down-regulation of thymic MHC antigen presenting molecules. These authors did not examine Vβ TcR expression, however found that autoreactivity, in the form of a syngeneic graft-vs-host response, could be adoptively transferred into naïve hosts by injection of CD4−8+ splenocytes from the CsA-treated rats. The present male mouse spleens contained approximately 6% CD4−8+ splenocytes and approximately 31% CD4+8− splenocytes, suggesting the observed increase in Vβ TcR expression occurred primarily in the Th lymphocyte population. These data suggest the possibility of defective thymic Th lymphocyte selection (MHC class II restricted) in males caused by prenatal TCDD, which may be supported by the observation of De Heer et al. (1994), that distribution of thymic MHC class II molecules is altered by TCDD.
Disruption in normal B cell selection or function can lead to autoimmunity, in which B cells do not merely serve as passive producers of autoantibodies but also have important roles in antigen presentation and cytokine production (Fujimoto and Sato, 2007). The majority of studies assessing developmental immunotoxicity of TCDD have focused on T cells. However, autoimmune glomerulonephritis in SNF1 mice has a major B cell component and was induced by prenatal TCDD (Silverstone et al., 1998). Prenatal TCDD exposure also targets terminal deoxynucleotidyl transferase (TdT)-positive bone marrow cells that include progenitor T and B cells in mice (Fine et al., 1989). Further, the lack of studies investigating altered B cell function after early TCDD exposure is a recognized important data gap in the literature (Luster et al., 2003; Holsapple et al., 2005). For these collective reasons, B cell endpoints were also evaluated in the present mice at 24 weeks-of-age.
Male offspring but not females showed increased percentages of bone marrow CD24−B220+ B cell progenitors at 24 weeks-of-age, while both genders had reduced numbers of B cells in the spleen. These effects suggest long-lasting dysregulation of B lymphopoiesis by developmental TCDD. Autoantibody production against dsDNA was increased in both the male and female TCDD mice, and against ssDNA in males at 5 µg/kg TCDD. These are the first data showing persistent alterations in B-lineage cells, and in humoral immune function, as a consequence of TCDD exposure during immune system development.
In summary, male and female C57BL/6 mice showed persistent changes in both T and B cells as a consequence of gd 12 exposure to TCDD. Among these were numerous gender-specific effects, suggesting possible interactions with endogenous hormones, which may not manifest until puberty when hormone levels significantly shift. In this regard, Karpuzoglu-Sahin et al. (2001) reported an immune system imprinting effect of prenatal diethylstilbestrol (DES), such that T cells in late-adult mice over-produced the cytokine IFNγ, but only following a secondary exposure to this estrogenic compound. The present mice displayed a clear autoimmune profile, including increased Vβ TcR expression, increased autoantibody production, and increased IgG and C3 disposition in kidney glomeruli. The TCDD-related histopathologic changes in kidneys were suggestive of progressing autoimmunity and may be viewed as surprising, in that C57BL/6 mice typically do not express autoimmune disease or glomerulonephritis. Collectively, these data suggest that developmental exposure to TCDD, during ontogenesis of the immune system, may increase risk of autoimmune disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagavant H, Thompson C, Ohno K, Setiady Y, Tung KS. Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int Immunol. 2002;14:1397–1406. doi: 10.1093/intimm/dxf105. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Holladay SD, Comment CE, Heindel JJ, Luster MI. Exposure to tetrachlorodibenzo-p-dioxin (TCDD) alters fetal thymocyte maturation. Toxicol Appl Pharmacol. 1992;112(2):207–213. doi: 10.1016/0041-008x(92)90189-y. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Ahmed SA, Holladay SD. Prenatal immunotoxicant exposure and postnatal autoimmune disease. In: Holladay SD, editor. Developmental Immunotoxicology. New York: CRC Press; 2005. pp. 215–228. [Google Scholar]
- De Heer C, De Waal EJ, Schuurman HJ, Vos JG, Van Loveren H. The intrathymic target cell for the thymotoxic action of 2,3,7,8-tetrachlorodibenzo-p dioxin. Exp Clin Immunogenet. 1994;11(2–3):86–93. doi: 10.1159/000424197. [DOI] [PubMed] [Google Scholar]
- De Heer C, De Waal EJ, Schuurman HJ, Vos JG, Van Loveren H. Alterations in the cortical thymic epithelium of rats after in vivo exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD): an (immuno)histological study. Toxicol Appl Pharmacol. 1992;115(1):80–88. doi: 10.1016/0041-008x(92)90370-8. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Piepenbrink MS. Prenatal immunotoxicity: why adult exposure assessment fails to predict risk. Environ Health Perspect. 2006;11:615–619. doi: 10.1289/ehp.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Ma Q, Whitlock JP., Jr Down-regulation of major histocompatibility complex Q1b gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:29614–29619. doi: 10.1074/jbc.272.47.29614. [DOI] [PubMed] [Google Scholar]
- Faith RE, Moore JA. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Toxicol Environ Health. 1977;3(3):451–464. doi: 10.1080/15287397709529578. [DOI] [PubMed] [Google Scholar]
- Fine JS, Gasiewicz TA, Fiore NC, Silverstone AE. Prothymocyte activity is reduced by prenatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. J Pharmacol Exp Ther. 1990;255(1):128–132. [PubMed] [Google Scholar]
- Fournel S, Neichel S, Dali H, Farci S, Maillere B, Briand JP, Muller S. CD4+ T cells from (New Zealand Black × New Zealand White) F1 lupus mice and normal mice immunized against apoptotic nucleosomes recognize similar Th cell epitopes in the C terminus of histone H3. J Immunol. 2003;171:636–644. doi: 10.4049/jimmunol.171.2.636. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Sato S. B cell signaling and autoimmune diseases: CD19/CD22 loop as a B cell signaling device to regulate the balance of autoimmunity. J Dermatol Sci. 2007;46(1):1–9. doi: 10.1016/j.jdermsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Gehrs BC, Riddle MM, Williams WC, Smialowicz RJ. Alterations in the developing immune system of the F344 rat after prenatal exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin: II. Effects on the pup and the adult. Toxicology. 1997;122(3):229–240. doi: 10.1016/s0300-483x(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Gehrs BC, Smialowicz RJ. Persistent suppression of delayed-type hypersensitivity in adult F344 rats after prenatal exposure to 2,3,7,8-tetrachlorodibenzop- dioxin. Toxicology. 1999;134:79–88. doi: 10.1016/s0300-483x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Gogal RM, Jr, Prater MR, Smith BJ, Johnson MS, Holladay SD. Bilateral dissected spleens and thymuses in rodents exhibit homogeneity in leukocyte markers. Toxicology. 2001;157(3):217–223. doi: 10.1016/s0300-483x(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Greenlee WF, Dold KM, Irons RD, Osborne R. Evidence for direct action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on thymic epithelium. Toxicol Appl Pharmacol. 1985;79(1):112–120. doi: 10.1016/0041-008x(85)90373-4. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Tsuchida M, Matsumoto Y, Watanabe H, Abo T, Sekikawa H, Kodama M, Zhang S, Izumi T, Shibata A. Characterization of T cells infiltrating the heart in rats with experimental autoimmune myocarditis. Their similarity to extrathymic T cells in mice and the site of proliferation. J Immunol. 1993;150(12):5682–5695. [PubMed] [Google Scholar]
- Hess AD, Fischer AC, Beschorner WE. Effector mechanisms in cyclosporine A-induced syngeneic graft-versus-host disease. Role of CD4+ and CD8+ T lymphocyte subsets. J Immunol. 1990;145:526–533. [PubMed] [Google Scholar]
- Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR, Heindell JJ, Luster MI. Prenatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p dioxin (TCDD) Teratology. 1991;44(4):385–393. doi: 10.1002/tera.1420440405. [DOI] [PubMed] [Google Scholar]
- Holladay SD. Prenatal immunotoxicant exposure and postnatal autoimmune disease. Environ Health Perspect. 1999;107 Suppl 5:687–691. doi: 10.1289/ehp.99107s5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect. 2000;108 Suppl 3:463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsapple MP, Snyder NK, Wood SC, Morris DL. A review of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: 1991 update. Toxicology. 1991;69(3):219–255. doi: 10.1016/0300-483x(91)90184-3. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Burns-Naas LA, Hastings KL, Ladics GS, Lavin AL, Makris SL, Yang Y, Luster MI. A proposed testing framework for developmental immunotoxicology (DIT) Toxicol Sci. 2005;83:18–24. doi: 10.1093/toxsci/kfh299. [DOI] [PubMed] [Google Scholar]
- Kakkanaiah VN, Pyle RH, Nagarkatti M, Nagarkatti PS. Evidence for major alterations in the thymocyte subpopulations in murine models of autoimmune diseases. J Autoimmun. 1990;3:271–288. doi: 10.1016/0896-8411(90)90146-j. [DOI] [PubMed] [Google Scholar]
- Karpuzoglu-Sahin E, Hissong BD, Ahmed SA. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- Klein AB, Witonsky SG, Ahmed SA, Holladay SD, Gogal RM, Jr, Link L, Reilly CM. Impact of different cell isolation techniques on lymphocyte viability and function. J Immunoassay Immunochem. 2006;27:61–76. doi: 10.1080/15321810500403755. [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM, Bridges S, Carmen J, Longo DL, Mond JJ. In vivo treatment of neonatal mice with anti-I-A antibodies interferes with the development of the class I, class II and Mls-reactive proliferating T cell subset. J Immunol. 1985;134:3579–3604. [PubMed] [Google Scholar]
- Luebke RW, Holsapple MP, Ladics GS, Luster MI, Selgrade M, Smialowicz RJ, Woolhiser MR, Germolec DR. Immunotoxicogenomics: the potential of genomics technology in the immunotoxicity risk assessment process. Toxicol Sci. 2006;94:22–27. doi: 10.1093/toxsci/kfl074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster MI, Boorman GA, Dean JH, Harris MW, Luebke RW, Padarathsingh ML, Moore JA. Examination of bone marrow, immunologic parameters and host susceptibility following pre- and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Int J Immunopharmacol. 1980;2(4):301–310. doi: 10.1016/0192-0561(80)90030-2. [DOI] [PubMed] [Google Scholar]
- Luster MI, Boorman BA, Dean JH, Harris MW, Luebke RW, Padarathsingh ML, Moore JA. Examination of bone marrow, immunologic parameters and host susceptibility following pre-and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-pdioxin (TCDD) Int J Immunopharmacol. 2003;2:301–310. doi: 10.1016/0192-0561(80)90030-2. [DOI] [PubMed] [Google Scholar]
- Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama R, Abo T, Seki S, Ohteki T, Sugiura K, Kusumi A, Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J Exp Med. 1992;175(3):661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Anni Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- Rocha B, Vassalli P, Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992;13(11):449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Schuurman HJ, Van Loveren H, Rozing J, Vos JG. Chemicals trophic for the thymus: risk for immunodeficiency and autoimmunity. Int J Immunopharmacol. 1992;14(3):369–375. doi: 10.1016/0192-0561(92)90166-i. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Frazier DE, Jr, Gasiewicz TA. Alternate immune system targets for TCDD: lymphocyte stem cells and extrathymic T-cell development. Exp Clin Immunogenet. 1994;11:94–101. doi: 10.1159/000424198. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Gavalchin J, Silvin CA, Staples JE, Ames IH, Shanley P. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on a murine model of lupus-like nephritis. Chemosphere. 1998;18:11–23. [Google Scholar]
- Uner AH, Tatum AH, Knupp CJ, Gavalchin J. Characteristics of auto anti-idiotypic anti-bodies reactive with antibodies expressing the pathogenic idiotype, IdLNF1, in the (NZB × SWR)F1 model for lupus nephritis and its parental strains. J Autoimmun. 1998;11:233–240. doi: 10.1006/jaut.1998.0201. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78:248–257. doi: 10.1093/toxsci/kfh062. [DOI] [PubMed] [Google Scholar]
- Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48:1080–1092. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]