Abstract
Purpose
To determine the effect of repeated intermittent apnea and resuscitation with 100% vs. 21% oxygen enriched gas on levels of key regulatory proteins contributing to cell death (Bax, Caspase-3) or protecting neurons from hypoxic/ischemic injury (Bcl-2, p-Akt, p-CREB).
Methods
The anaesthetized, mechanically ventilated newborn piglets underwent 10 episodes of apnea with resuscitation either with 21% or with 100% oxygen. Following 6-hrs recovery the animals were euthanized, the striatum, frontal cortex, midbrain and hippocampus were dissected out and used to determine levels of Bcl-2, Bax, Caspase-3, p-Akt and p-CREB.
Results
In hippocampus and striatum, Bcl-2 expression was higher with 100% vs. 21% group (173±29% vs.121±31%, p<0.05 and 189±10% vs.117±47%, p<0.01, respectively) whereas the Bax expression was lower (88±3% vs.100±9%, p<0.05 and 117±5% vs.133±10%, p<0.05, respectively). Expression of Caspase-3 in striatum, was higher with 21% vs.100% group (263±33% vs.197±35%, p<0.05, respectively) but not different in hippocampus. p-Akt expression was higher with 100% vs. 21% oxygen in hippocampus and striatum (225±44% vs. 108±35%, p<0.01 and 215±12% vs.164±16%, p<0.01, respectively). The p-CREB expression was higher with 100% vs. 21% oxygen resuscitation in hippocampus (217±41% vs.132±30%, p<0.01) with not changes in striatum. Much smaller or not significant differences between 100% vs.21% oxygen groups were observed in frontal cortex and midbrain, respectively.
Conclusion
In neonatal piglet model of intermittent apnea, selectively vulnerable regions of brain (striatum and hippocampus) are better protected from apneic dependent injury when resuscitation was conducted with 100%, rather than 21%, oxygen enriched gas.
Keywords: Asphyxia, Neonatal resuscitation, Oxygen, Brain injury
INTRODUCTION
Brief but repeated, intermittent severe episodes of asphyxia, as may occur with central or obstructive apnea in the human neonate, may result in clinically important deficits in neuropsychological function. Prior studies document that brain injury, caused by repeated hypoxic-ischemic insults, was more pronounced than that caused by the same period of continuous hypoxia-ischemia.1–4 The mechanism(s) by which repetitive apnea causing hypoxia affects the metabolic pathways in the brain, that sustain the normal processes of cell survival and cell death are not well known. Also, the parameters that, alone or in combination, can determine the pathogenic outcome of repetitive apnea in neonate remain to be elucidated. These parameters include the timing, intensity, duration and variability of the asphyxial event and resuscitation interventions. A debate also rages regarding the proper oxygen concentration to use for neonatal resuscitation. In the clinical setting, the oxygen concentration used for resuscitation ranges from 21% to 100%. Saugstad and colleagues have presented data that room air resuscitation is theoretically equivalent, perhaps even superior, to 100% oxygen in a setting of sustained hypoxic injury.5, 6 However, a recent analysis of existing clinical data from the Cochrane Database concluded that there is insufficient evidence to recommend one oxygen concentration over the other.7 In meta-analysis the authors identified a reduction in mortality in the neonates randomized to air but later neurological outcomes showed no difference between groups. Also, failure of resuscitation was not different between the air and 100% oxygen groups. Understanding how the differences in oxygen concentration during resuscitation and recovery affect brain metabolism and cellular neuropathologic processes leading to cell recovery or cell death is critical to improving outcomes of repeated severe apnea.
The present study was designed to evaluate the degree of regional brain injury following experimental repeated intermittent apnea and controlled resuscitation, assessed by markers of neuronal injury (activation of apoptotic pathway-increase in expression of Bax and Caspase-3) and markers for cellular survival (increase in expression of Bcl-2, Bcl-2/Bax ratio, p-Akt, and p-CREB) at 6 hours following brain injury and resuscitation. We hypothesized that neonatal piglets subjected to10 repeated intermittent episodes of severe apnea and resuscitated with 100% oxygen gas for 10 minutes between apneic episodes, would show less evidence of regional brain cell injury at 6 hours following apnea as compared to the piglets where 21% oxygen enriched gas was used.
METHODS
Animal model
Twenty one newborn piglets, 2 to 4 days of age (1.4–2.5 kg) were randomized and assigned to one of 3 groups: 1) repetitive apnea with resuscitation with 21% oxygen (n=7); 2) repetitive apnea with resuscitation with 100% oxygen (n=7); 3) sham operated with no apnea or resuscitation (n=7). Anesthesia was induced with 4% halothane/96% oxygen and 1.5% lidocaine-HCl was used as a local anesthetic and tracheostomy performed. Halothane was withdrawn entirely after the tracheotomy, and pancuronium was used for neuromuscular blockade and mechanical ventilation (1.5 mg/kg). Fentanyl-citrate (30 μg/kg) was intravenously injected, and the animals were mechanically ventilated with a mixture of oxygen (FiO2 21%) and 0.5% isoflurane. The femoral artery and vein were then cannulated and anesthesia was maintained with isoflurane 0.5% and boluses of pancuronium (1mg/kg/hr). Temperature, anesthetics, blood pressure, glucose, fluids, acidosis, and mechanical ventilation were similarly titrated by prospectively designated laboratory protocol for all piglets. Following apnea protocol, the animals recovered for 6 hours while anesthetized and then were euthanized with 4M KCl.
All animal procedures were in strict accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Animal Care and Use Committee.
Repetitive Apnea Model
The animals underwent 10 episodes of repeated, severe hypoxia induced by apnea and followed by assisted ventilation resuscitation. Apnea was initiated by disconnecting the animal from the ventilator during iatrogenic paralysis, and completed by reconnecting it to the ventilator. There was no gasping or spontaneous respiratory effort. The apneic episodes were terminated at 3.5min, or 0.5 minutes after the heart rate reached the bradycardic threshold of 60 beats/min. Resuscitation was with 21% oxygen (room air) or 100% oxygen gas followed by stabilization for 10 minutes, and then the FiO2 was maintained at 21% for and additional 5 minutes. The time interval between apneic episodes and resuscitation was 15 minutes. After resuscitation from the tenth apneic episode, the animals were supported with mechanical ventilation and maintained for 6 hours under anesthesia titrated by prospectively designated protocol, and then euthanized humanely. The brain tissue was rapidly removed and placed in ice cold saline. The striatum, frontal cortex, midbrain and hippocampus were rapidly dissected out and frozen at 80°for later analysis.
Western blot analysis
Samples of frozen tissues were homogenized in a buffer containing 2% SDS, 10 mM Tris-HCl (pH 7.4) freshly supplemented with NaF (10 mM), Na pyrophosphate (10 mM), Na3Vo4 (1 mM), Na2MoO4 (1 mM), phenylarsine oxide (1 μM), and aprotinin (10 μg/ml). The homogenate was boiled for 5 min after addition of SDS-PAGE sample buffer. Protein concentration was determined in a homogenate aliquot with a BCA Protein Assay kit (Pierce, IL). Equal amounts of protein from each sample were separated by 12% SDS- PAGE and transferred onto a nitrocellulose membrane (Hybond C, Amersham Pharmacia Biotech.). The membrane was incubated in a blocking solution (phosphate buffered saline (PBS), pH 7.4, containing 5% non-fat milk powder) for 1 h at room temperature and then with specific antibodies for Bax (N-20) and Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phospho Akt, Akt (Cell Signaling Technology, Beverly, MA, USA); anti-phosphorylated CREB antibodies (a-p-CREB; Upstate Biotechnology, NY, USA). For determination of caspase expression the membranes were incubated with rabbit Cleaved Caspase-3 antibody (Cell Signaling Technology, Beverly, MA, USA) following with incubation in the HRP-conjugated secondary antibody solution.
Beta-actin antibody (ABCAM, Cambridge, MA, USA) served as a loading control. After being washed in PBS containing 0.05% Tween 20 (PBS-T; Sigma–Aldrich), the membranes were incubated for 1 h with peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Amersham Pharmacia Biotech.). The final reaction was visualized using enhanced chemiluminescence (ECL Western Blotting Detection Reagents, Amersham Pharmacia Biotech.), and the membranes were exposed to x-ray film.
Data analysis
Autoradiographic films were analyzed using Scion Image software (NIH). Each blot contained two sets of samples, one for an experimental group and another for the control group. The data were normalized to the values obtained for the untreated control group (assigned a value of 100). Statistical analysis was performed using one-way analysis of variance followed by Mann–Whitney test. p < 0.05 was considered statistically significant.
RESULTS
Twenty one newborn piglets completed the protocol in one of 3 groups: 1) repetitive apnea with resuscitation with 21% oxygen; 2) repetitive apnea with resuscitation with 100% oxygen; 3) sham operated with no apnea or resuscitation. Four representative regions of brain in each animal were evaluated: frontal cortex, hippocampus, striatum and midbrain. The profile characterizing 100% vs. 21% gas resuscitation reflected in physiological parameters and cortical oxygen pressures was described in detail in our earlier paper.8 Briefly, within each experimental group of animals, resuscitated with 21% of oxygen or 100% of oxygen, there were no significant differences in PaCO2, PaO2 and blood pressure during all ten apneas. The significant difference between apnea with resuscitation with 21% oxygen and apnea with resuscitation with 100% oxygen was in level of PaO2 (about 5 times higher in 100% oxygen resuscitation). The pH values decreased progressively with increasing number of apnea in 21% oxygen and 100% oxygen group of piglets. The control value of pH was 7.47±0.01 and at the end of the last apnea, was 7.19±0.04 (p<0.005) in group resuscitated with 21% oxygen and 7.22±0.03 (p<0.005) in group resuscitated with 100% oxygen.
The control mean cortical oxygen pressure was 40±4 mmHg and when the apnea was initiated, the oxygen pressure in the cortex fell rapidly, attaining a minimal value near 0 mmHg during each apneic episode. During resuscitation with 21% oxygen, following the first and second apneas there was a significant “overshoot” in the oxygen levels. This overshoot reached 78±7 mmHg after the first apnea but was markedly attenuated with increasing numbers of apnea. After six apneic episodes the maximum had decreased to 50±6 mmHg and then remained nearly constant during rest of post-apneic resuscitation periods. Similarly, during resuscitation with 100% oxygen, the maximal cortical oxygen pressure following an apnea also decreased with increasing number of apnea. After the first apnea, the value of oxygen was 173±9 mmHg and this decreased progressively to137±8 mmHg through the first 6 apnea and then remained at the same level through the 10th apnea.
The calculated volume fractions of the cortical microcirculation with oxygen pressures less than 5, 10 or 15 mm Hg shown that after the 10th apnea with 21% oxygen resuscitation, volume fractions of 10±2%, 20±4% and 27±5.5% of the microcirculation were below 5, 10 and 15 mm Hg, respectively. When resuscitation was with 100% oxygen, after the 10th apnea these values were 0.54±0.15%, 2.6±0.49% and 5.9±0.8%, respectively. The pre-apnea (control) values were 0.14±0.1%, 0.51±0.35% and 1.4±0.7%, respectively.
Expressions of Bcl-2 and Bax in four regions of brain following repeated apnea and resuscitation with 21% and 100% oxygen
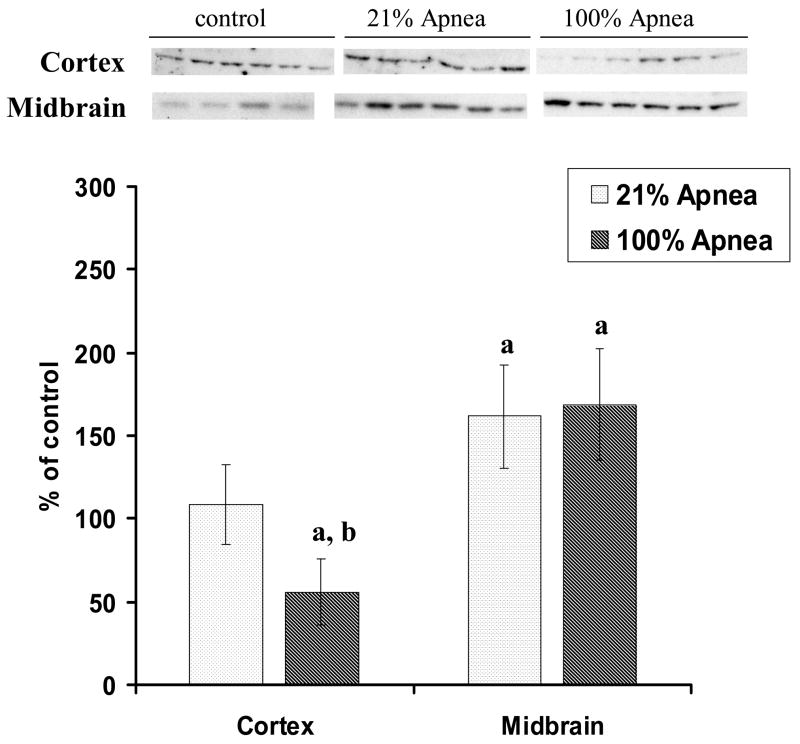
The expression of Bcl-2 and Bax in four regions of piglet brain at 6 hours following 10 repeated apnea and resuscitation events with 21% vs.100% oxygen are shown in Fig 1A–B and Fig 2A–B, with non-apneic piglets serving as controls.
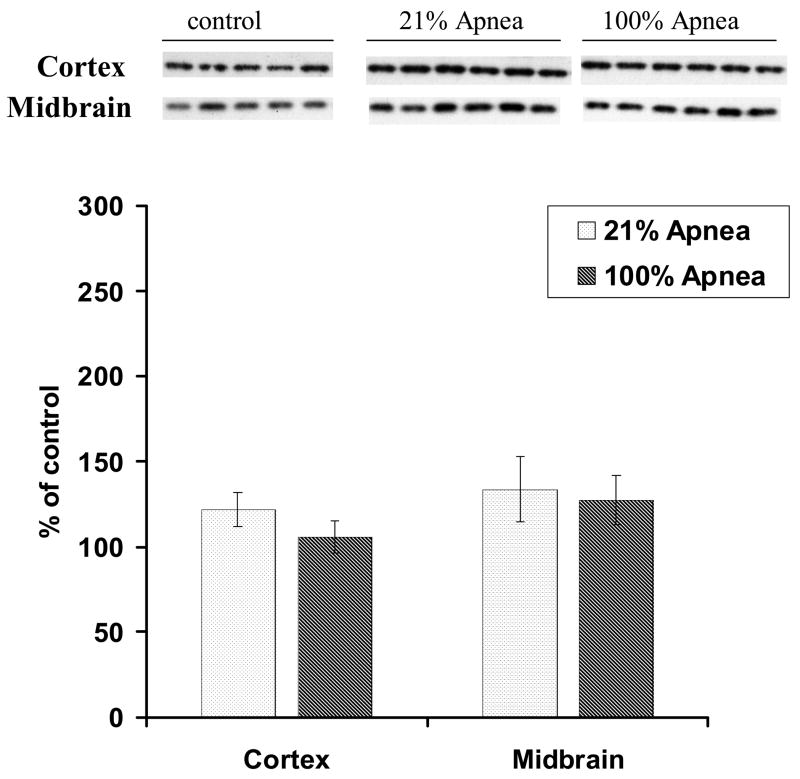
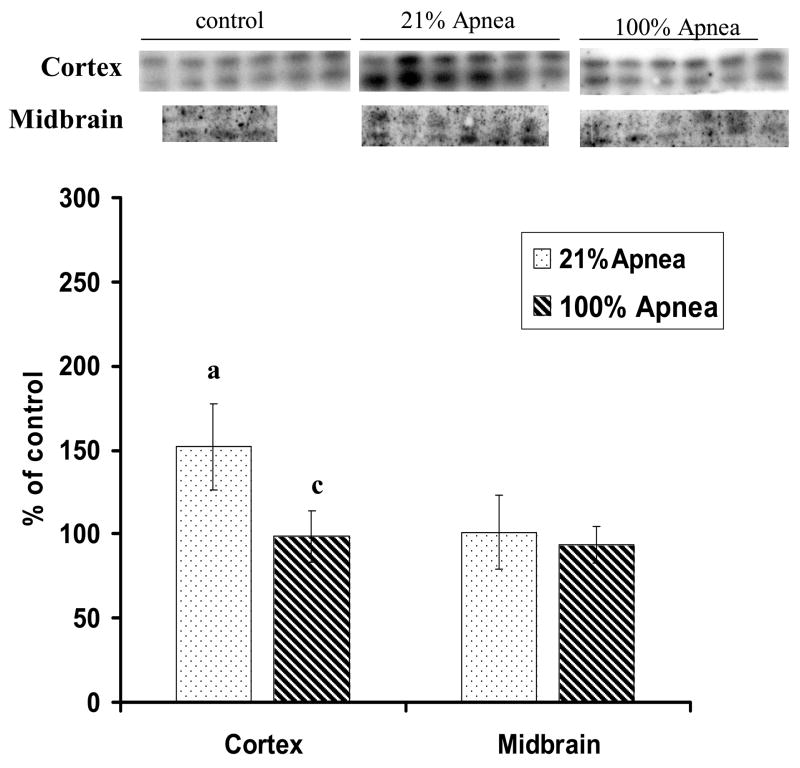
Figure 1. A and B. Level of Bcl-2 in four regions of piglet brain measured after 6-hr recovery following repetitive apnea with resuscitation with 21% and 100% of oxygen.
The results are means from 7 experiments ± SD. The data are expressed in % of the control (sham operated). ap<0.01 for significant difference from control; bp<0.05 and cp<0.01 for significant difference between 21% oxygen and 100% of oxygen groups of animals, as determined by one-way analysis of variance, followed by Mann–Whitney test.
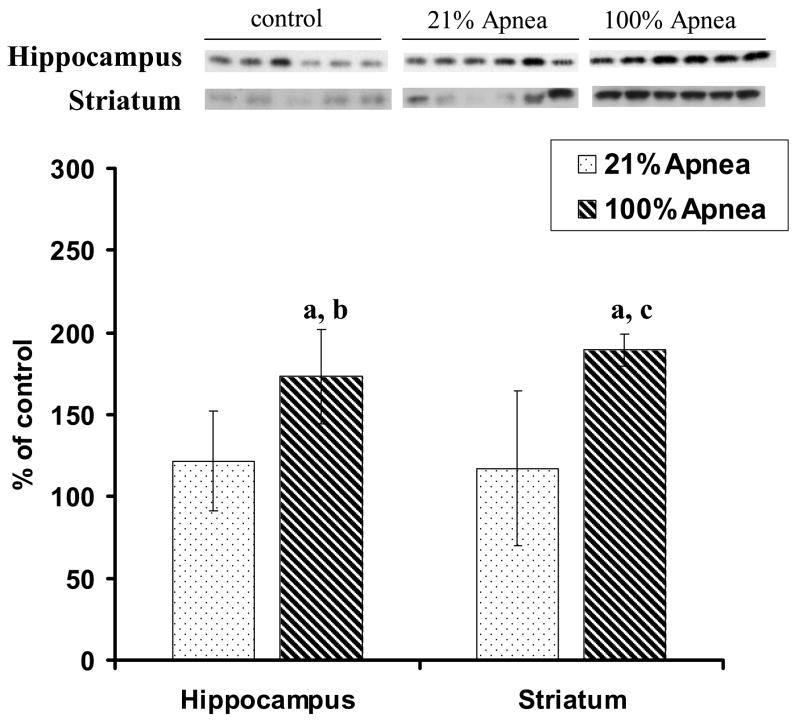
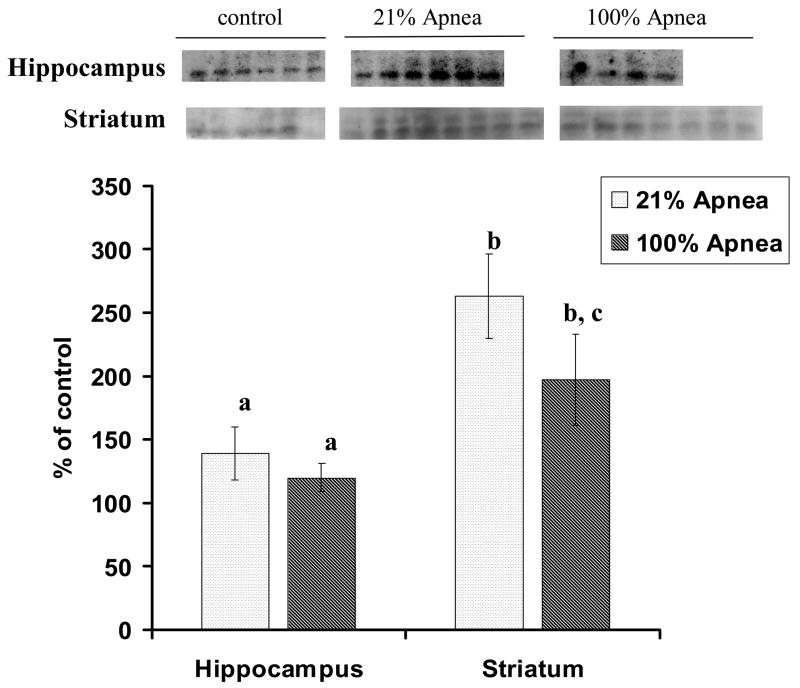
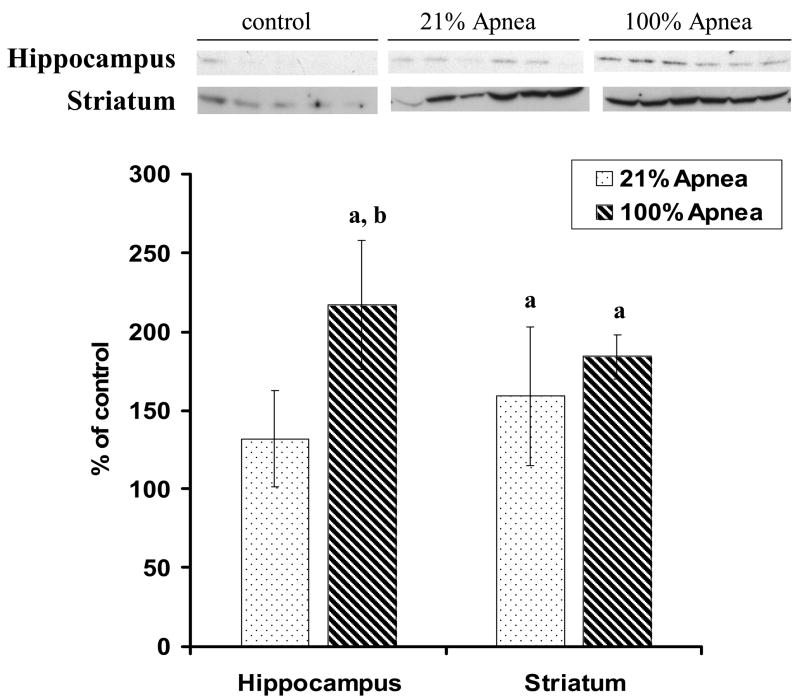
Figure 2. A and B. Level of Bax in four regions of piglet brain measured after 6-hr recovery following repetitive apnea with resuscitation of 21% and 100% of oxygen.
The results are means from 7 experiments ± SD. The data are expressed in % of the control (sham operated). ap<0.05 for significant difference from control; bp<0.05 for significant difference between 21% oxygen and 100% of oxygen groups of animals, as determined by one-way analysis of variance, followed by Mann–Whitney test.
There were no significant differences for Bcl-2 or Bax expression in 100% vs. 21% oxygen resuscitation groups in frontal cortex and midbrain regions (Fig 1A and 2A).
Bcl-2 expression, in hippocampus and striatum, was significantly higher with 100% vs. 21% oxygen resuscitation (173±29% vs.121±31%, p<0.05 and 189±10% vs.117±47%, p<0.01, respectively) (Fig 1B).
Bax expression, in hippocampus, was significantly lower with 100% vs. 21% oxygen resuscitation (88±3% vs.100±9%, p<0.05) (Fig 2B). Similarly, in striatum Bax expression was significantly lower with 100% vs. 21% oxygen resuscitation (117±5% vs.133±10%, p<0.05) (Fig 2B).
The calculated ratios of Bcl-2 to Bax in frontal cortex, midbrain, striatum and hippocampus are presented in Table 1. In frontal cortex and midbrain regions, there were no significant differences for Bcl-2 to Bax ratio in 100% vs. 21% oxygen resuscitation groups. In striatum and hippocampus, this ratio was significantly higher with 100% vs. 21% oxygen resuscitation: (striatum 1.6 vs. 0.9 and hippocampus 2.0 vs.1.2).
Table 1.
Ratio of Bcl-2 to Bax in four regions of piglet brain measured after 6-hr recovery following repetitive apnea with resuscitation with 21% and 100% of oxygen.
| Regions of brain | Experimental Conditions | |
|---|---|---|
| 21% Apnea | 100% Apnea | |
| Frontal Cortex | 1.13 | 0.91 |
| Midbrain | 1.47 | 1.24 |
| Hippocampus | 1.22 | 1.96 |
| Striatum | 0.88 | 1.62 |
The ratios were calculated from results presented on Figures 1A–B and 2A–B.
Expressions of Caspase-3 in four regions of brain following repeated apnea with resuscitation with 21% and 100% oxygen
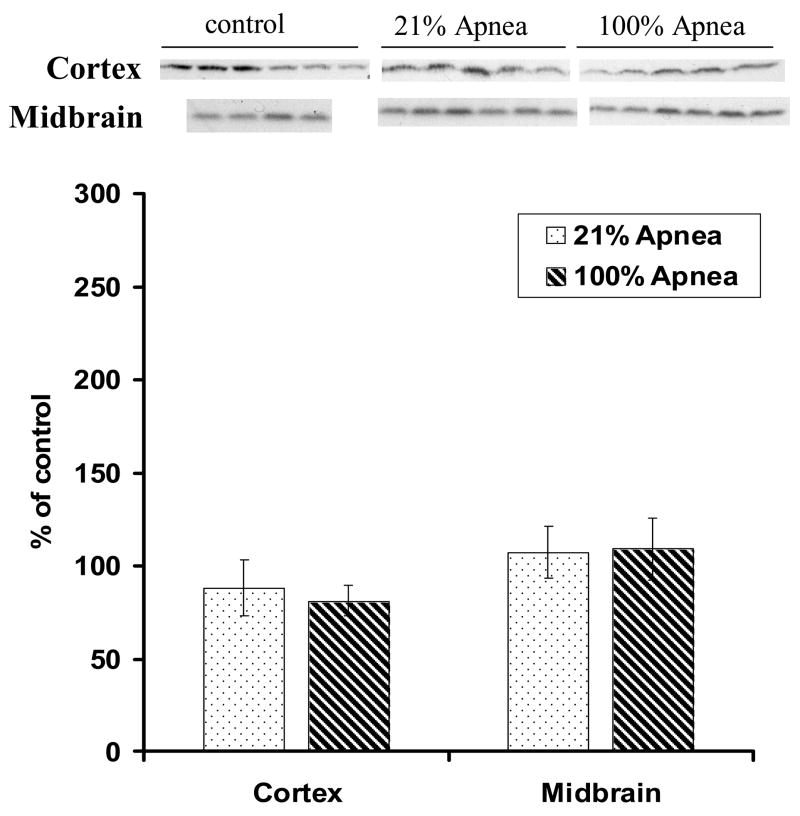
Expression of Caspase-3 in four regions of piglet brain at 6 hours following 10 repeated, severe apnea and resuscitation events with 21% vs. 100% oxygen are shown in Fig 3A–B with non-apneic piglets serving as controls. In midbrain and hippocampus, the differences for Caspase-3 expression were not significant between 100% vs. 21% oxygen resuscitation groups. In frontal cortex and striatum the differences in Caspase-3 expression were statistically significant. In cortex, Caspase-3 expression with 100% vs. 21% oxygen resuscitation was 99±15% vs.152±25% (p<0.05) (Fig 3A) whereas in striatum, was 197±35% vs. 263±33% (p<0.05), respectively (Fig 3B).
Figure 3. A and B. Effect of repetitive apnea with resuscitation of 21% and 100% of oxygen on level of Caspase-3 in four regions of piglet brain tissue measured after 6-hr recovery.
The results are means from 7 experiments ± SD. The data are expressed in % of the control (sham operated). ap<0.05 and bp<0.01 for significant difference from control; cp<0.05 for significant difference between 21% oxygen and 100% of oxygen groups of animals, as determined by one-way analysis of variance, followed by Mann–Whitney test.
Expressions of p-Akt in four regions of brain following repeated apnea with resuscitation with 21% and 100% oxygen
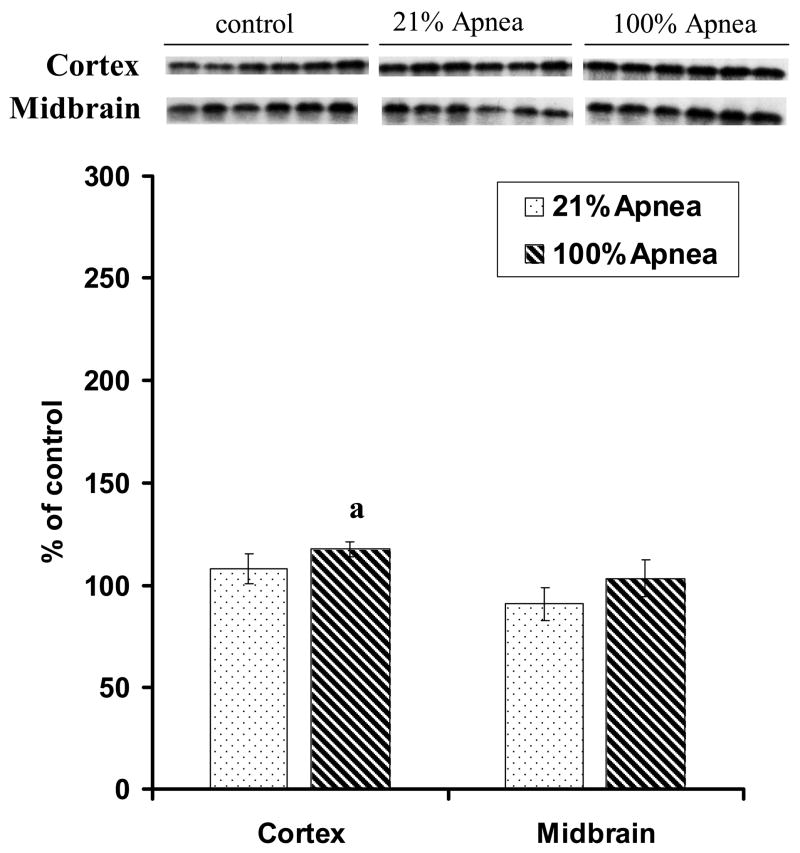
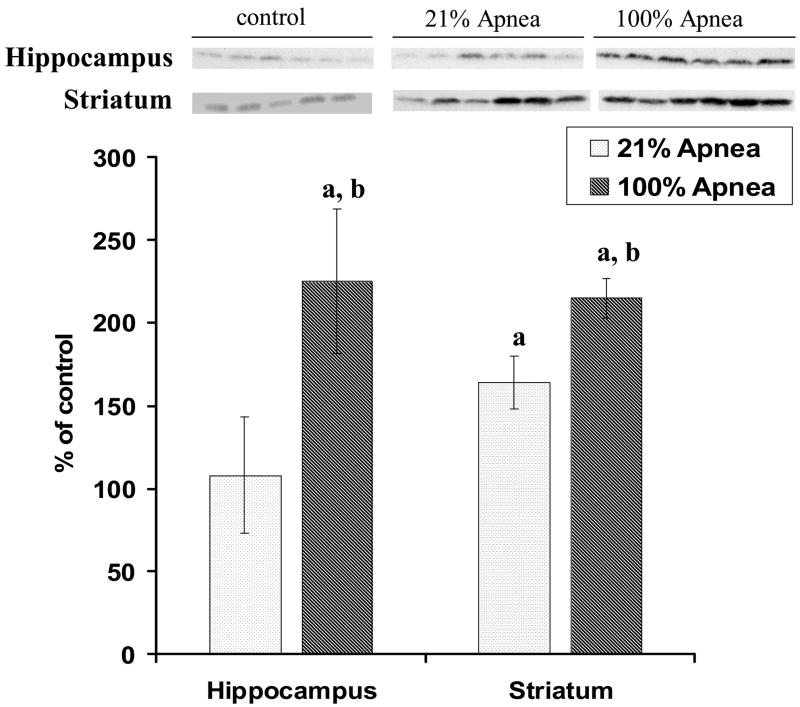
The p-Akt expressions in four regions of piglet brain at 6 hours recovery following 10 repeated, severe apnea and resuscitation with 21% vs. 100% oxygen are shown in Fig 4A–B, with non-apneic piglets serving as controls.
Figure 4. A and B. Effect of repetitive apnea with resuscitation of 21% and 100% of oxygen on level of phosphorylated Akt in four regions of piglet brain tissue measured after 6-hr recovery.
The results are means from 7 experiments ± SD. The data are expressed in % of the control (sham operated). ap<0.01 for significant difference from control; bp<0.01 for significant difference between 21% oxygen and 100% of oxygen groups of animals, as determined by one-way analysis of variance, followed by Mann–Whitney test.
In frontal cortex and midbrain regions, there were no significant differences for p-Akt expression in 21% vs. 100% oxygen resuscitation groups (Fig 4A). In hippocampus, p-Akt expression was significantly higher with 100% vs. 21% oxygen resuscitation (225±44% vs. 108±35%, p<0.01) (Fig 4B). Similarly, in striatum, p-Akt expression was significantly higher with 100% vs. 21% oxygen resuscitation (215± 12% vs. 164± 16%, p<0.01) (Fig 4B).
Expressions of p-CREB in four regions of brain following repeated apnea with resuscitation with 100% and 21% oxygen
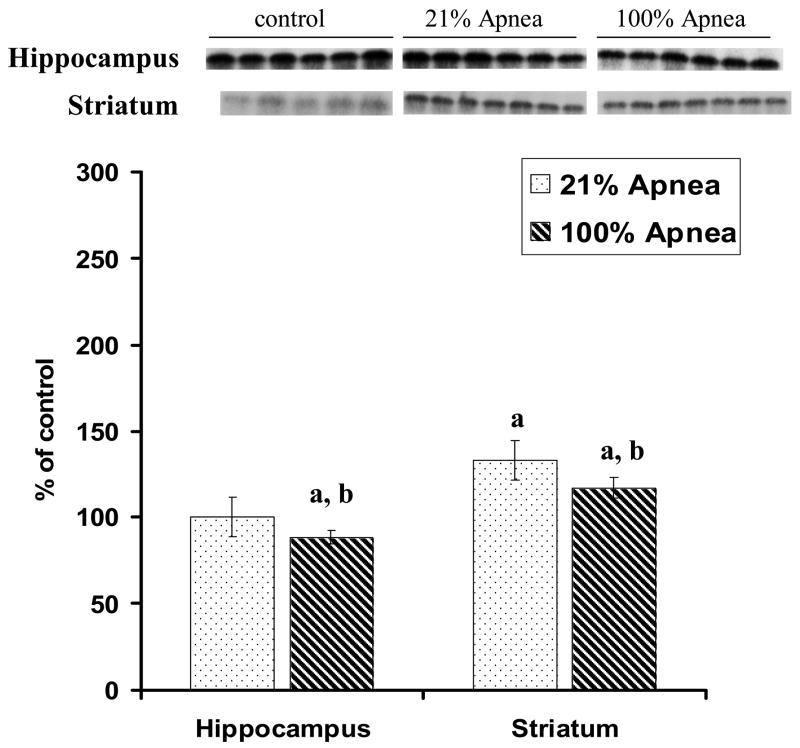
The p-CREB expressions in four regions of piglet brain at 6 hours recovery following 10 repeated, severe apnea and resuscitation events with 100% vs. 21% oxygen are shown in Fig 5A–B, with non-apneic piglets serving as controls.
Figure 5. A and B. Effect of repetitive apnea with resuscitation of 21% and 100% of oxygen on level of phosphorylated CREB in four regions of piglet brain tissue measured after 6-hr recovery.
The results are means from 7 experiments ± SD. The data are expressed in % of the control (sham operated). ap<0.01 for significant difference from control; bp<0.01 for significant difference between 21% oxygen and 100% of oxygen groups of animals, as determined by one-way analysis of variance, followed by Mann–Whitney test.
In midbrain and striatum regions, there were no significant differences for p-CREB expressions in 100% vs. 21% oxygen resuscitation groups. In frontal cortex, p-CREB expression was significantly lower with 100% vs. 21% oxygen resuscitation (56±20% vs. 108±24%, p<0.01) (Fig 5A). In hippocampus, p-CREB expression was significantly higher with 100% vs. 21% oxygen resuscitation (217± % vs. 132± %, p<0.01) (Fig 5B).
DISCUSSION
Understanding how the differences in oxygen concentration during resuscitation and recovery affect brain metabolism and critical cellular neuropathologic processes leading to cell recovery or cell death is critical to improving outcomes of repeated, severe asphyxia. Different mechanisms of injury (intermittent vs. sustained hypoxia) and certain selectively vulnerable regions of brain (striatum and hippocampus) may benefit from different resuscitative interventions (100% vs.21% oxygen). This was precisely the reason why we chose to study the effect of 100% vs. 21% oxygen gas resuscitation following repeated, severe hypoxia induced by apnea on selectively vulnerable regions of brain. The tissues of four regions of brain taken at 6 hrs after the last apnea were analyzed for the expression of a selected group of key regulatory proteins believed to participate in pathways contributing to cell death (Bax, Caspase-3) or to protecting neurons from hypoxic/ischemic injury (Bcl-2, p-Akt, p-CREB). The pattern of changes in these proteins can provide significant insight into the extent of neuronal injury likely to result from the apneic insults that occur in neonates.
There is a substantial literature describing the roles of Bcl-2 and Bax in ischemic/hypoxic neuronal cell survival and injury.9–12 The proteins of Bcl-2 family have been shown to be key regulatory factors in apoptotic events and they can either promote cell survival (Bcl-2, Bcl-XL, A1, Mcl-1, and Bcl-W) or promote cell death (Bax, Bak, Bcl-XS, and Bok). Accumulating evidence indicates that over expression of Bcl-2 provides protection against apoptosis and ischemic neuronal death. Several mechanisms have been proposed to explain the anti-apoptotic function of Bcl-2. Bcl-2 might act as a regulator of Ca2+ homeostasis or as an antioxidant.13, 14 Bcl-2 forms heterodimers with the pro-apoptotic protein Bax and might thereby neutralize its death effector property.15 In addition, Bcl-2 prevents the release of potent mitochondrial activators of the cytosolic death effector proteases, the caspase protease family, which mediates the intracellular proteolysis that is characteristic of apoptosis.16 The association of Bcl-2 with the mitochondrial apoptosis-activating factor Apaf1 and the blockade of cytochrome c release may prevent the activation of the two major death proteases, Caspase-9 and Caspase-3.17
In contrast to cytoprotective biomarker Bcl-2, Bax is a pro-apoptotic protein that has been shown to promote cell death by activating caspases.18 Bax is thought to contribute to the vulnerability of neurons to apoptotic cell death induced by exposure to gamma-radiation, glutamate and kainite.19–21 Bax has been shown to form ion-conducting channels or pores in intracellular planar lipid bilayer membranes, and this can lead to nuclear envelope breakdown and allow for increase in intranuclear calcium.22, 23 The active form of Bcl-2 forms heterodimers with Bax, and thus the Bcl-2 to Bax ratio reflects the cellular susceptibility to apoptotic stimuli.15, 18, 19, 21 An increased ratio of Bax to Bcl-2 protein was shown in hypoxic and hypocapnic neonatal piglets, demonstrating an increased susceptibility to apoptosis in the autopsied brains.24, 25
Our current results show significantly elevated protective Bcl-2 and diminished cytotoxic Bax in the vulnerable striatum and hippocampus regions of brain, when severe, repeated apnea was resuscitated with 100% vs. 21% oxygen gas. In these regions, the calculated ratio of Bcl-2 to Bax was significantly higher in the group resuscitated with 100% vs. 21% oxygen, suggesting less apoptotic damage at 6 hours following apnea.
Further support of cytoprotection by resuscitation of 100% oxygen in these vulnerable brain areas is provided by assessment of p-Akt expression. Akt plays an essential role in neuronal survival. Active Akt protein supports the survival of neurons in the absence of trophic factors, whereas a dominant-negative mutant of Akt inhibits neuronal survival even in the presence of survival factors.26 These results establish an essential role for Akt in neuronal survival. The Akt protein kinase has been implicated as a critical transducer of PI3-kinase-dependent survival signals generated by a variety of stimuli and growth factors.27–30 Akt targets several key proteins to keep cells alive, including apoptosis regulators and transcription factors. For example, Bad is a pro-apoptotic member of the Bcl-2 family, which in its unphosphorylated form can bind to Bcl-x L and thus block cell survival.31 But the activation of Akt induces the phosphorylation of Bad and promotes its interaction with the chaperone protein 14-3-3, which sequesters Bad in the cytoplasm and inhibits Bad’s pro- apoptotic activity.32 Akt has been shown to affect, directly or indirectly, three transcription factor families: Forkhead, cAMP-response-element-binding protein (CREB) and NF-kappaB, all of which are involved in regulating cell survival.
The elevated p-Akt expression was detected in the vulnerable striatum and hippocampus regions of brain, when severe, repeated apnea was resuscitated with 100% oxygen gas, suggesting less neuronal damage at 6 hours following apnea in this group of piglets as compared to piglets resuscitated with 21% of oxygen.
Similarly to p-Akt in striatum and hippocampus, repeated apnea was resuscitated with 100% oxygen but not with 21%oxygen caused increase in p-CREB expression. CREB is a transcription factor, constitutively expressed and abundant in brain. Several studies demonstrated that CREB family members are crucial in neuronal survival in various cellular models.33–36 Neuronal survival during post-ischemic recovery was associated with increased CREB phosphorylation, whereas neuronal death was preceded by a decrease in p-CREB levels.37, 38 In striatum, severe ischemic injury caused a transient activation of CREB phosphorylation followed by its rapid disappearance, which preceded ischemia induced morphological changes in the neurons.39 In hippocampal neurons, after 5-min ischemia CREB phosphorylation was decreased and never recovered and in contrast, a shorter (2-min) ischemic episode led to a comparatively steep increase in p-CREB, which was sustained for days.40
Walton et al. (1999) had shown that upregulation of CREB protein inhibited apoptosis in neurons. Substantial evidence indicates that Bcl-2 is positively regulated by CREB via CRE in the 5′ promoter region, and increased phosphorylation of CREB on Ser-133 induces expression of Bcl-2 protein. Cell survival mediated by neurotrophin-induced CREB phosphorylation in sympathetic and cortical neurons was associated with increased Bcl-2 expression.35, 41 Overexpression of CREB decreased apoptosis through upregulation of Bcl-2 expression.42 A study of Sugiura et al. suggested that CRE-mediated expression of Bcl-2 might contribute to neuronal survival in the penumbra after focal cerebral ischemia.43
The exact mechanisms of changes in CREB phosphorylation depending on the severity of ischemic stress are not fully understood. CREB phosphorylation may be activated by several kinases including PKA, PKC, Akt/PKB, CaMK, MAPK-activated protein kinase 2 and the pp90 ribosomal S6 kinase family (Rsks).44, 45
Our data show that the pCREB expression in striatum and hippocampus increased significantly in 100% oxygen group as compared to 21% oxygen. It can therefore be postulated that particularly in striatum and hippocampus, the Akt-pCREB-Bcl-2- mediated survival pathway become activated in 100% group as compared to 21% groups, which probably reflects the less severity of the insults and a substantial less neuronal damage.
A similar conclusion can be drawn from the response of Caspase-3 to repeated, severe, apnea following resuscitation with 100% vs. 21% oxygen. In all four regions of brain, Caspase-3 expression lower in 100% vs. 21% oxygen resuscitation groups. Activation of the Caspases, cysteine proteases, is an essential component of the process of apoptosis.46 In the brain, Caspase-3 is especially important, where it plays an important role in initiation of the apoptotic pathway and is thought to be responsible for many cytological changes that characterize neuronal apoptosis.47, 48 Caspase-3 exists as an inactive proenzyme of molecular weight 32 kDa. Noxious stimuli precipitate the cleavage of this proenzyme into two active subunits of molecular weight 12 and 17 kDa, which then associate to form the active Caspase-3 enzyme. This active enzyme cleaves a variety of enzymes and substrates including other caspases, initiating the caspase cascade, after entering which the cell is committed to die.49 Thus, Caspase-3 is considered an early marker of the apoptotic pathway activation.
All presented evidences suggests that selectively vulnerable regions of brain (particularly striatum and hippocampus) were consistently protected from apoptotic pathway activation (lower expression of Bax and Caspase-3; higher expression of Bcl-2, p-Akt, p-CREB, and higher Bcl-2/Bax ratios) at 6 hours after apnea when resuscitation was conducted with 100% vs.21% oxygen enriched gas.
One of major concern to use the 100% oxygen during apneic resuscitation is possible increase in production of toxic free radicals. The central nervous system is very sensitive to hyperoxia and has been reported to respond to hyperoxia with increased generation of oxygen-derived free radicals.50–53 Therefore, the question arises whether 100% oxygen gas would be helpful or harmful to selectively vulnerable regions of the brain, following severe, repeated hypoxia induced by apnea. The presented data shows that apoptotic activation is not measurably different in certain regions of brain (frontal cortex and midbrain), but is strikingly different in striatum and hippocampus. These findings are consistent with studies by Agardh et al., showing that generation of free radicals during reoxygenation is more dependent on the extent and duration of ischemia than on the oxygen tension.54 Further, Solas at al, demonstrated higher levels of excitatory amino acids in brain striatum, lower mean arterial blood pressure and a significantly greater degree of cerebral hypoperfusion in piglets resuscitated with room air as opposed to 100% oxygen.55–57 Our earlier study, on striatum of newborn piglets, showed that repeated, intermittent severe apnea with resuscitation of 100% oxygen, as compared to 21% oxygen, suppressed formation of local regions of tissue hypoxia and decreased the hypoxia induced increase in extracellular dopamine, a major source of hydroxyl radicals and possible marker of vulnerable brain striatum injury.8 This finding suggested that the level of free radicals in striatum following apnea with resuscitation of 100% oxygen should be lower than after apnea with resuscitation of 21% oxygen. Although not definitive for mechanism, it is plausible that preconditioning and prevention of suppression of local regional hypoxia, may account for the differences seen in severe, intermittent compared to sustained hypoxic insults.
LIMITATIONS
There are several important limitations to mention for this study. This is an anesthetized (sedated and not spontaneously breathing) neonatal model of intermittent asphyxia, modeling short severe episodes of central apnea without gasping or airway obstruction. The effect of anesthesia, particularly inhaled anesthetic isoflurane, on cytoprotection and apoptosis cannot be ruled out, but would be expected to be equally present in both groups. Variability of blood pressure and cerebral perfusion during the induction of hypoxia with apnea in this model may introduce some variability, which we attempted to minimize with a prospective protocol for management of fluids, sedation, glucose, temperature, ventilation, CO2, paralysis, and acidosis. Finally, the autopsy specimens of brain for biomarker proteins were analyzed at a single time point, 6 hours following brain injury, near the minimum time to see protein changes signaling apoptosis.
CONCLUSIONS
Clinically, the ideal concentration of supplemental oxygen to use for resuscitation remains unknown. Titration of resuscitative intervention to the timing, intensity, duration, and variability of the etiology of hypoxic insult is likely important. In this established neonatal piglet model of severe, repeated intermittent apnea induced brain hypoxia and resuscitation, we found that selectively vulnerable regions of brain (striatum and hippocampus) were consistently protected from apoptotic pathway activation at 6 hours after apnea when resuscitation was conducted with 100%, rather than 21%, oxygen enriched gas. Apoptotic pathway activation was not significantly different in less vulnerable regions of brain (midbrain and cortex). Thus, the specific mechanism (sustained vs. intermittent) of apnea induced hypoxic brain injury may benefit from different resuscitative gas interventions (100% vs. 21% oxygen resuscitation) to achieve optimal support for vulnerable brain regions. We speculate that when the mechanism of severe apnea induced asphyxial brain injury is intermittent with repeated ventilation resuscitation, 100% oxygen (rather than 21% oxygen) may be a preferred resuscitative gas mixture.
Acknowledgments
This study was supported by grants HL-58669, HD041484 and NS 31465 from the National Institutes of Health
Footnotes
Conflict of Interest:
The manuscript has not been previously published and is not under consideration elsewhere. Any authors of this manuscript have not any financial conflict of interest.
References
- 1.Kreisman NR, Sick TJ, Rosenthal M. Importance of vascular responses in determining cortical oxygenation during recurrent paroxysmal events of varying duration and frequency of repetition. J Cereb Blood Flow Metab. 1983;3:330–8. doi: 10.1038/jcbfm.1983.48. [DOI] [PubMed] [Google Scholar]
- 2.Martin RJ, Miller MJ, Carlo WA. Pathogenesis of apnea in preterm infants. J Pediatr. 1986;109:733–41. doi: 10.1016/s0022-3476(86)80685-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagata N, Saji M, Ito T, Ikeno S, Takahashi H, Terakawa N. Repetitive intermittent hypoxia-ischemia and brain damage in neonatal rats. Brain Dev. 2000;22:315–20. doi: 10.1016/s0387-7604(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 4.Tomida S, Nowak TS, Jr, Vass K, Lohr JM, Klatzo I. Experimental model for repetitive ischemic attacks in the gerbil: the cumulative effect of repeated ischemic insults. J Cereb Blood Flow Metab. 1987;7:773–82. doi: 10.1038/jcbfm.1987.133. [DOI] [PubMed] [Google Scholar]
- 5.Saugstad OD. Room air resuscitation-two decades of neonatal research. Early Hum Dev. 2005;81:111–6. doi: 10.1016/j.earlhumdev.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Saugstad OD, Ramji S, Vento M. Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate. 2005;87:27–34. doi: 10.1159/000080950. [DOI] [PubMed] [Google Scholar]
- 7.Tan A, Schulze A, O’Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev. 2005:CD002273. doi: 10.1002/14651858.CD002273.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schears G, Antoni D, Schultz S, et al. Brain oxygenation and metabolism during repetitive apnea with resuscitation of 21% and 100% oxygen in newborn piglets. Neurochem Res. 2005;30:1453–61. doi: 10.1007/s11064-005-8655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa K, Matsumoto M, Yang G, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MS, Ho DY, Sun GH, Steinberg GK, Sapolsky RM. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16:486–96. doi: 10.1523/JNEUROSCI.16-02-00486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinou JC, Frankowski H, Missotten M, Martinou I, Potier L, Dubois-Dauphin M. Bcl-2 and neuronal selection during development of the nervous system. J Physiol Paris. 1994;88:209–11. doi: 10.1016/0928-4257(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 13.Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–9. [PubMed] [Google Scholar]
- 14.Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–6. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- 15.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 18.Golstein P. Controlling cell death. Science. 1997;275:1081–2. doi: 10.1126/science.275.5303.1081. [DOI] [PubMed] [Google Scholar]
- 19.Gillardon F, Wickert H, Zimmermann M. Up-regulation of bax and down-regulation of bcl-2 is associated with kainate-induced apoptosis in mouse brain. Neurosci Lett. 1995;192:85–8. doi: 10.1016/0304-3940(95)11619-8. [DOI] [PubMed] [Google Scholar]
- 20.Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed JC. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene. 1996;12:187–92. [PubMed] [Google Scholar]
- 21.Krajewski S, Mai JK, Krajewska M, Sikorska M, Mossakowski MJ, Reed JC. Upregulation of bax protein levels in neurons following cerebral ischemia. J Neurosci. 1995;15:6364–76. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady HJ, Gil-Gomez G. Bax. The pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol. 1998;30:647–50. doi: 10.1016/s1357-2725(98)00006-5. [DOI] [PubMed] [Google Scholar]
- 23.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–37. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 24.Fritz KI, Zubrow AB, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. The effect of hypocapnia (PaCO2 27 mmHg) on CaM kinase IV activity, Bax/Bcl-2 protein expression and DNA fragmentation in the cerebral cortex of newborn piglets. Neurosci Lett. 2003;352:211–5. doi: 10.1016/j.neulet.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 25.Ravishankar S, Ashraf QM, Fritz K, Mishra OP, Delivoria-Papadopoulos M. Expression of Bax and Bcl-2 proteins during hypoxia in cerebral cortical neuronal nuclei of newborn piglets: effect of administration of magnesium sulfate. Brain Res. 2001;901:23–9. doi: 10.1016/s0006-8993(01)02109-6. [DOI] [PubMed] [Google Scholar]
- 26.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 27.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 28.Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–36. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 29.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–5. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SX, Gozal D, Sachleben LR, Jr, Rane M, Klein JB, Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-beta receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. Faseb J. 2003;17:1709–11. doi: 10.1096/fj.02-1111fje. [DOI] [PubMed] [Google Scholar]
- 31.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 33.Fienberg AA, Hiroi N, Mermelstein PG, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–42. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg ME, Ziff EB. Signal transduction in the postsynaptic neuron. Activity-dependent regulation of gene expression. In: Cowan WM, Sudhof TC, Stevens CF, editors. Synapses. Baltimore, MD: John Hopkins Univ Press; 2001. [Google Scholar]
- 35.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–61. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 36.Somers JP, DeLoia JA, Zeleznik AJ. Adenovirus-directed expression of a nonphosphorylatable mutant of CREB (cAMP response element-binding protein) adversely affects the survival, but not the differentiation, of rat granulosa cells. Mol Endocrinol. 1999;13:1364–72. doi: 10.1210/mend.13.8.0329. [DOI] [PubMed] [Google Scholar]
- 37.Mabuchi T, Kitagawa K, Kuwabara K, et al. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–13. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka K, Nogawa S, Ito D, et al. Phosphorylation of cyclic adenosine monophosphate response element binding protein in oligodendrocytes in the corpus callosum after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1177–88. doi: 10.1097/00004647-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Nogawa S, Nagata E, et al. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp Neurol. 2000;161:462–71. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- 40.Hara T, Hamada J, Yano S, Morioka M, Kai Y, Ushio Y. CREB is required for acquisition of ischemic tolerance in gerbil hippocampal CA1 region. J Neurochem. 2003;86:805–14. doi: 10.1046/j.1471-4159.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 41.Walton M, Connor B, Lawlor P, et al. Neuronal death and survival in two models of hypoxic-ischemic brain damage. Brain Research - Brain Research Reviews. 1999;29:137–68. doi: 10.1016/s0165-0173(98)00053-8. [DOI] [PubMed] [Google Scholar]
- 42.Pugazhenthi S, Nesterova A, Jambal P, et al. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. 2003;84:982–96. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 43.Sugiura S, Kitagawa K, Omura-Matsuoka E, et al. CRE-mediated gene transcription in the peri-infarct area after focal cerebral ischemia in mice. J Neurosci Res. 2004;75:401–7. doi: 10.1002/jnr.10881. [DOI] [PubMed] [Google Scholar]
- 44.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–4. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 45.Impey S, Obrietan K, Wong ST, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–83. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 46.Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 47.D’Mello SR, Kuan CY, Flavell RA, Rakic P. Caspase-3 is required for apoptosis-associated DNA fragmentation but not for cell death in neurons deprived of potassium. J Neurosci Res. 2000;59:24–31. [PubMed] [Google Scholar]
- 48.Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. Embo J. 1997;16:2271–81. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lincz LF. Deciphering the apoptotic pathway: all roads lead to death. Immunol Cell Biol. 1998;76:1–19. doi: 10.1046/j.1440-1711.1998.00712.x. [DOI] [PubMed] [Google Scholar]
- 50.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 51.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–80. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 53.Yusa T, Beckman JS, Crapo JD, Freeman BA. Hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol. 1987;63:353–8. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- 54.Agardh CD, Zhang H, Smith ML, Siesjo BK. Free radical production and ischemic brain damage: influence of postischemic oxygen tension. Int J Dev Neurosci. 1991;9:127–38. doi: 10.1016/0736-5748(91)90003-5. [DOI] [PubMed] [Google Scholar]
- 55.Solas AB, Kalous P, Saugstad OD. Reoxygenation with 100 or 21% oxygen after cerebral hypoxemia-ischemia-hypercapnia in newborn piglets. Biol Neonate. 2004;85:105–11. doi: 10.1159/000074966. [DOI] [PubMed] [Google Scholar]
- 56.Solas AB, Kutzsche S, Vinje M, Saugstad OD. Cerebral hypoxemia-ischemia and reoxygenation with 21% or 100% oxygen in newborn piglets: effects on extracellular levels of excitatory amino acids and microcirculation. Pediatr Crit Care Med. 2001;2:340–5. doi: 10.1097/00130478-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Solas AB, Munkeby BH, Saugstad OD. Comparison of short- and long-duration oxygen treatment after cerebral asphyxia in newborn piglets. Pediatr Res. 2004;56:125–31. doi: 10.1203/01.PDR.0000128978.90201.1D. [DOI] [PubMed] [Google Scholar]