Abstract
In the metazoan central nervous system (CNS), serotonergic neurons send projections throughout the synaptic neuropil. Little is known about the rules that govern these widespread neuromodulatory branching patterns. In this study, we utilize Drosophila as a model to examine serotonergic branching. Using single cell GFP labeling we show that within each segment of the Drosophila ventral nerve cord (VNC), each of two serotonergic neurons tiles distinct innervation patterns in the contralateral neuropil. In addition, branches extend only a short distance from the target segment. Through ablation-mediated isolation of serotonergic cells, we demonstrate that the distinct areas of innervation are not maintained through competition between neighboring like-serotonergic neurites. Furthermore, the basic branching pattern of serotonergic neurons within the neuropil remains unchanged despite alterations of initial axonal trajectories.
Keywords: neurite formation, neurotransmitter, CNS development, serotonin, branch morphology, tiling, competition, axon guidance
Introduction
The human brain consists of billions of neurons, which are highly diverse in morphological structure and function. In general, it is important to regulate neurite morphology during development because the geometry of branch patterns can play a central role in the functional properties of a neuron. The serotonergic neuron is one example of a neuron whose branching morphology is likely important for function. Serotonin, a monoamine neurotransmitter, is an important neuromodulator with influences on mood, sleep, appetite, and many other behaviors in humans (Jacobs and Azmitia, 1992). Abnormalities in serotonin levels and potentially innervation have been associated with a variety of neuropsychiatric diseases (Hornung, 2003). This highlights the importance of understanding how the serotonergic system develops and modulates its targets. Serotonergic branches and terminals are generally found distributed throughout the entire synaptic neuropil, presumably to widely release transmitter. How such a dispersed pattern forms is unclear.
The majority of serotonergic synapses, or varicosities are thought to be non-junctional, at which serotonin is released, able to diffuse to distances greater than 5 µm and thereby modulate nearby neuronal circuits (Bunin and Wightman, 1998). Serotonergic varicosities in the insect are also largely non-junctional (Peters and Tyrer, 1987). Humans have approximately 300,000 serotonergic neurons, whose cell bodies are housed within the raphe nuclei in the brainstem. Projections from these serotonergic neurons extend throughout the cortex and spinal cord and are thought to adopt a space-filling pattern. Space-filling patterns are found quite prevalently in nature, and have been extensively studied in the sensory dendrites of the fly and leech peripheral nervous system (PNS) and in mammalian retinal ganglion cells (Gan and Macagno, 1995; Gao et al., 2000; Leventhal et al., 1988). In order to efficiently innervate a sensory field, the dendrites of these neurons organize as tiled systems with minimal overlap. Previous studies have demonstrated that many of these neurons utilize a mechanism of inhibition/competition between dendrites of the same class of neurons, which allows for an efficient, non-overlapping innervation of a receptive field. Although competition between dendrites is found in many of the sensory neurons studied, there are examples of sensory neurons that exhibit little repulsion between dendrites of the same class. For example, duplication of Drosophila class I and II dendritic arborization neurons demonstrated that there is little inhibition between dendrites from these classes of neurons, which contrasts with the strong inhibition seen between dendrites from class IV dendritic arborization neurons (Grueber et al., 2002; Grueber et al., 2003).
To better understand the contributions of these factors on the patterning of the serotonergic system, we have turned to a simple model organism, the Drosophila larva. The entire Drosophila central nervous system (CNS) has less than 100 serotonergic neurons. Serotonergic innervation of the Drosophila larval ventral nerve cord (VNC) is thought to be largely achieved by two serotonergic neurons in each CNS hemiganglion, a medially positioned EW1 and a laterally positioned EW2 serotonergic cell (Novotny et al., 2002). During embryonic development, the EW1 and EW2 serotonergic neurons within each hemiganglion send their axons across the midline to the contralateral side of the VNC. Once reaching the contralateral side, the axons extend dorsally into the neuropil where they form elaborate branch patterns (Prokop, 1999; Sykes and Condron, 2005). The branches have many swellings or varicosities that are thought, on the basis of ultrastructural morphology to be presynaptic terminals responsible for the release of serotonin. The overall branching patterns and varicosity densities have been documented (Sykes and Condron, 2005). However, it is unknown how the EW1 and EW2 serotonergic neurons each contribute to the overall branching patterns found within the neuropil.
In this study, we provide a description of the branching patterns of the serotonergic neurons within the VNC of Drosophila. Using single cell GFP labeling we demonstrate that the medial and lateral cells within each segment have distinct non-overlapping areas of innervation. While the medial cell is most likely EW1 and the lateral cell EW2, we use medial/lateral terminology in this manuscript as we have not unambiguously assigned lineage relationships. We also demonstrate that serotonergic innervation shows clear segmental identity, where serotonergic projections are rarely observed growing beyond a segment away from the point of origin. Genetic ablation of serotonergic neurons early in development reveals that the general branching patterns are unaltered by loss of surrounding serotonergic neurons. This implies that competition between neighboring serotonergic neurons does not play a significant role in creating the clearly demarcated boundary between the serotonergic medial and lateral cells, nor the segmental boundaries of serotonergic innervation. Furthermore, alterations in axonal routing do not alter the stereotypical branching patterns. These data all indicate that the serotonergic growth cones in each segment are intrinsically programmed to target the correct neuropil irrespective of neighboring like-cells and the initial guidance route.
Materials and Methods
Fly Stocks and Genetics
For GFP analysis, we used the hs-FLP;UAS>y+>cd8-GFP approach, kindly provided by Barry Dickson, Institute of Molecular Biotechnology, Vienna. Tph-GAL4 was a gift from by Jaeseob Kim, Korea Advanced Institute of Science and Technology. Cell ablation was achieved by targeted expression of UAS-rpr-hid, kindly provided by Paul Taghert, Washington University School of Medicine. UAS-drl was donated by John Thomas, Salk Institute for Biological Studies. UAS-hb, UAS-mCD8-GFP, UAS-robo2, eg-GAL4, and ddc-GAL4 were obtained from the Bloomington stock center.
GFP analysis
GFP labeled cells were produced through the combination of the FLP/FRT system and the GAL4/UAS system as described in (Theodosiou and Xu, 1998). hs-FLP;UAS>y+>cd8-GFP flies were crossed to ddc-GAL4 flies. Approximately 10 hour old embryos (stage 12) were heat shocked at 37°C for 1–2 hrs to induce FLP expression, and the generation of single GFP-labeled cells.
Immunohistochemistry and imaging
Drosophila ventral nerve cords were dissected in Schneider’s insect media and mounted on #1 glass microscope coverslips (18 mm2). Tissue was fixed for 1 hour in 4% paraformaldehyde. Samples were incubated in PBS containing 0.1% Triton X-100 (PBT) overnight at room temperature (RT) with primary antibodies, rabbit anti-serotonin (ImmunoStar, 1:1000), anti-Hunchback (obtained from Chris Doe, 1:100), chicken anti-GFP (Abcam, 1:1000), anti-FMRFamide (Dr. Paul Taghert, U. Washington, 1:2000), mAb 1D4 anti-fasciclin II (Developmental Hybridoma Bank, 1:100), mAb 7G10 anti-fasciclin III (Developmental Hybridoma Bank, 1:100) and mAb BP102 (Developmental Hybridoma, 1:100). Alexafluor goat anti-mouse, goat anti-rabbit, and goat anti-chicken secondary antibodies (Molecular Probes) were used at 1:1000 in PBT overnight at RT. Samples were mounted to slides in 90% glycerol/2.5% DABCO. For more details, see (Daubert and Condron, 2007)
Analysis
Imaging was done with Nikon eclipse E800 confocal microscope (100x) and recorded with Perkin-Elmer software. Confocal stacks were taken from the top of the neuropil through the cell bodies. Images were auto-leveled in Adobe Photoshop and imported into Volocity 3.7 for 3D reconstruction (Sykes and Condron, 2005). For branch analysis, confocal stacks were viewed in Volocity and each varicosity hand marked according to its branch position. Student t-tests were performed with Instat software.
Results
Overall development of the fly serotonergic abdominal branch pattern
The CNS neuropil is extensively innervated by serotonergic branches during Drosophila larval development (Landgraf et al., 2003; Sykes and Condron, 2005). This is illustrated in (Fig.1) which shows the dorso-ventral view of the VNC serotonergic abdominal pattern from late embryogenesis to late adulthood. Although the pattern is complex, the overall density of varicosities remains constant during larval stages (Sykes and Condron, 2005). A distinct circular pattern of branches, seen in the dorso-ventral axis, forms during embryogenesis and lasts until early pupation when the branches undergo retraction (Fig. 1). After retraction, remodeled branches re-emerge during late pupal development that give rise to the adult branching pattern. While large branches are also seen in the adult, the circular pattern is difficult to discern.
Figure 1. Developmental profile of abdominal CNS serotonergic innervations.
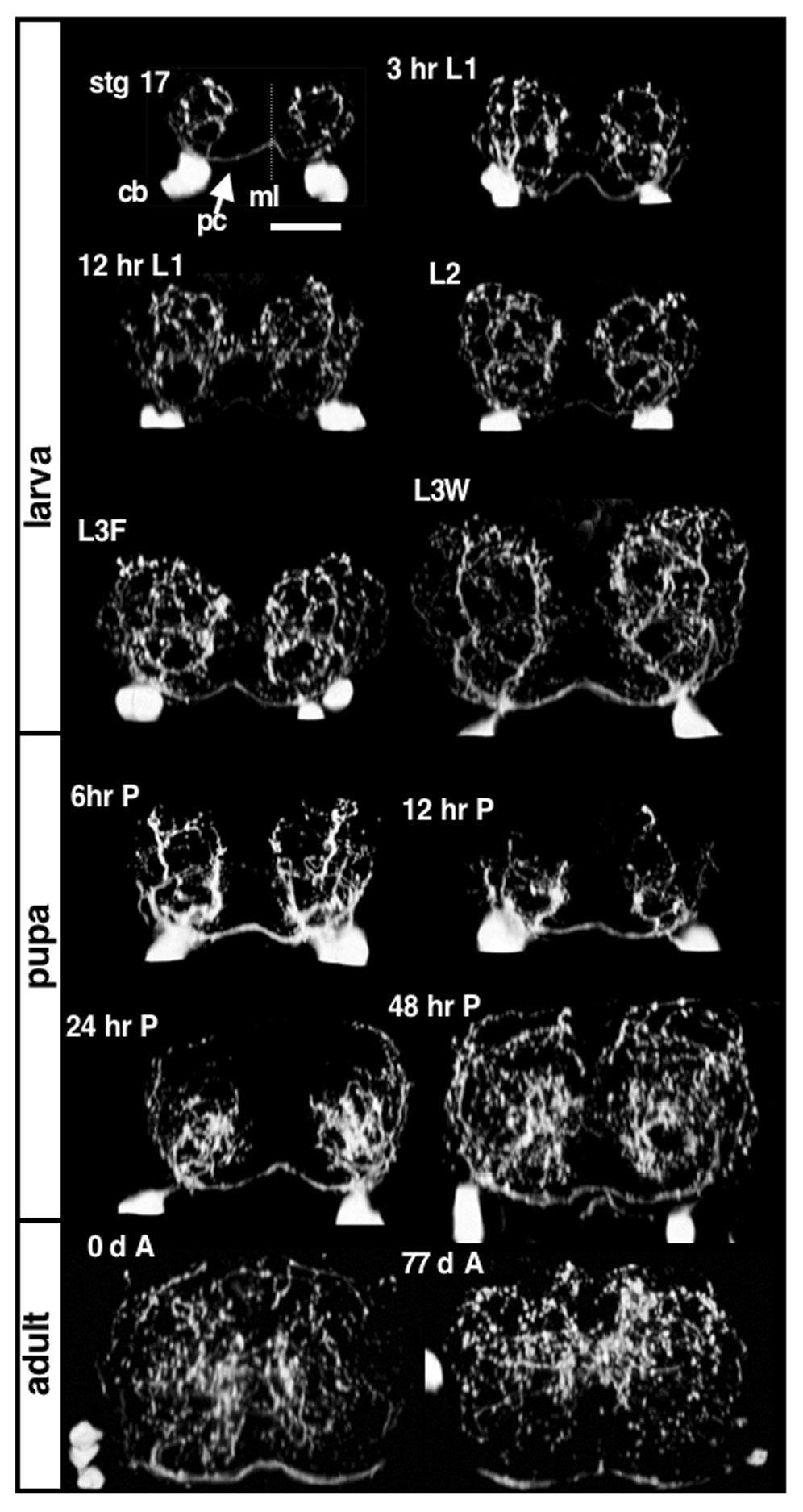
Each panel is a dorso-ventral view of a single serotonin-stained A5 abdominal segment, cropped and rotated 90 degrees to provide the frontal view with dorsal up and ventral down. The top left panel is from the end of embryogenesis, stage 17. The scale bar is 10µm and is the same for all panels. The midline is marked with ml and is indicated with a dashed line; the serotonergic axons crossing the midline along the posterior commissure are marked with a pc and the cell bodies are marked with a cb. The larval stages shown are 3 hours after hatching: 3 hr L1; 12 hours after hatching: 12 hr L1; 24 hours after hatching: L2, 72 hours after hatching: L3F and 5 days after hatching or wandering third instars: L3W. Four pupal stages are shown and two adults stages: immediately after hatching: 0 d A, and 77 days after hatching: 77 d A. During embryogenesis, the serotonergic neurons extend main branches (primary branches) that form a circular pattern that are maintained throughout larval development. The primary branches retract during early pupal development, and reform a new pattern during later pupal development, which is maintained throughout adult development.
During embryonic and larval development two serotonergic neurons within each hemiganglion can be seen extending axons across the midline, but it is not known how each contributes to the final pattern. It is also not known what, if any, contributions are made from each cell to the ipsilateral side. In order to investigate the individual cellular contributions further, we took advantage of a genetic tool to individually label cells
Medial and Lateral Serotonergic Neurons Have Characteristic Branching Patterns
Two serotonergic neurons form in each abdominal hemisegment, with only one in the most posterior portion of the VNC. This latter segment has been previously referred to as either A8 or A9. In order determine the actual segmental designation of this terminal segment, two thoracic markers were used (Supplemental Fig. 1). In each thoracic segment, fasciclin III stains a prominent group of cells and anti-FMRFamide stains a single cell. Comparison of these markers indicates that the terminal serotonin-staining segment is A8 (Supplementary Fig 1). The overall serotonergic innervation pattern is visualized by antibody staining against their neurotransmitter, serotonin (Figs. 2A and 2E). Serotonergic neurons can also be visualized by driving GFP within serotonergic neurons with ddc-GAL4, which drives expression within the dopaminergic and serotonergic neurons, or Tph-GAL4 for serotonergic neurons alone. Serotonin highlights varicosities in a manner very similar to synaptotagmin-GFP (Sykes and Condron, 2005). In order to identify the branching patterns of individual serotonergic neurons, we utilized the previously described method of combining the FLP/FRT system and GAL/UAS system (Ito et al., 1997). Using heat shock control with hs-FLP;UAS-FRT-stop-FRT-mcd8-GFP x ddc-GAL4, we were able to generate single mCD8-GFP labeled serotonergic neurons.
Figure 2. The medial and lateral serotonergic cells of Drosophila VNC have different innervation patterns.
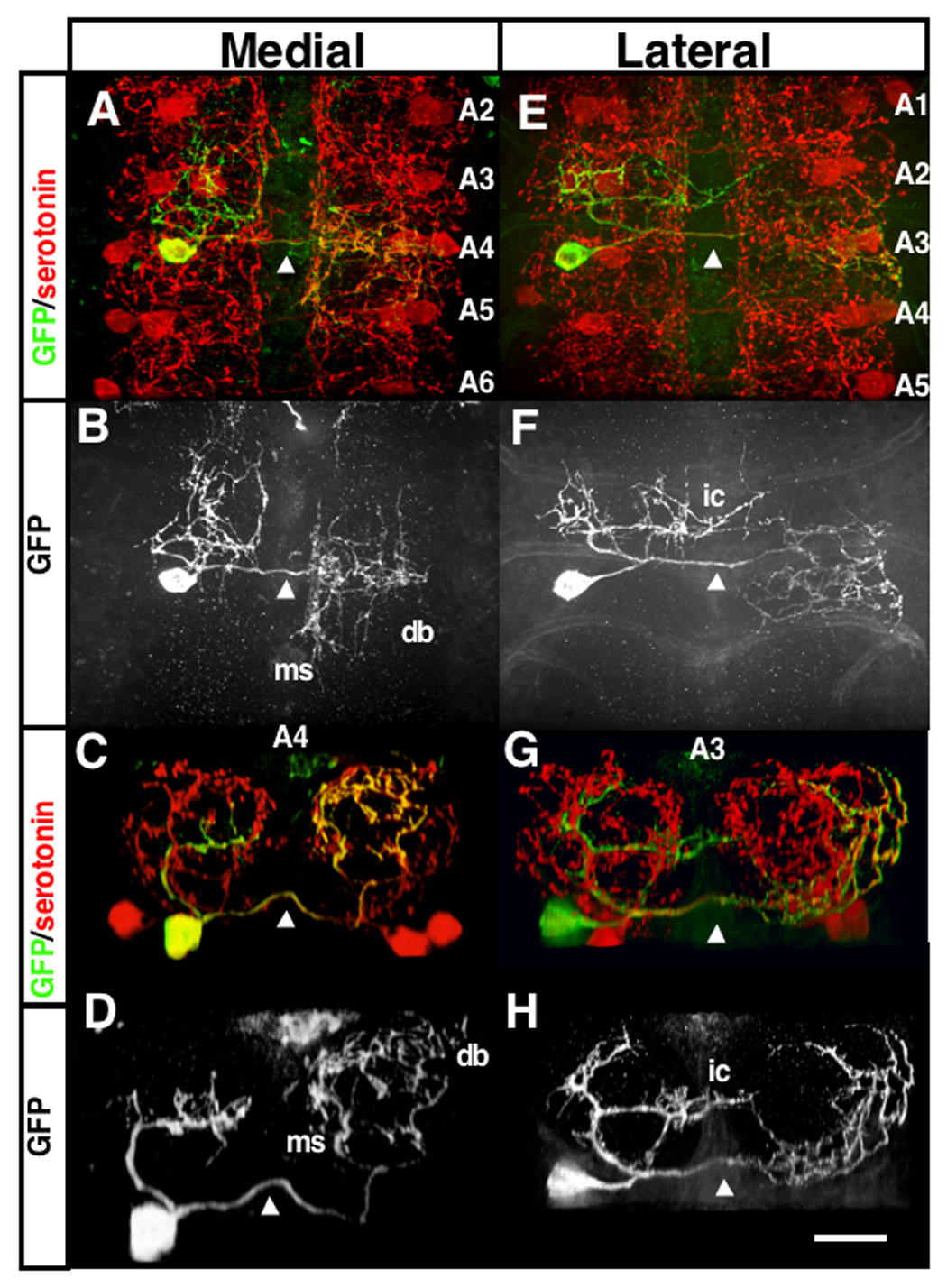
Images of L3F A4 medial (A–D) and A3 lateral (E–H) branch patterns using single cell labeling with GFP (see text). (A,B,E,F) are top views and (C,D,G,H) are a single segment cropped and rotated 90 degrees such that dorsal is at top. The axons crossing the midline in the posterior commissure are indicated at the midline with an arrowhead in each panel. The ipsilateral branches are to the left of each panel and the contralateral branches are to the right. db: dorsal bifurcation, a secondary branch that usually appears from the dorsal lateral point of the medial contralateral primary; ms: secondary branches that run along the edge of the midline; ic: the contralateral projecting secondary branch that originates from the lateral cell ipsilateral branches. The scale bar in H is 10µm and is the same for all panels.
Genotypes hs-FLP;UAS-FRT-mcd8-GFP-FRT x ddc-GAL4
GFP expression within a single medial serotonergic cell reveals that the axon bifurcates into two thick branches as it enters dorsally into the contralateral neuropil (Figs. 2A–D). These two branches, which we will refer to as primary branches, initially diverge in opposite directions and grow along the medial two-thirds of the neuropil forming a circular pattern, with the branches meeting at the dorsal end of the neuropil (Figs. 2C–D) (Supplemental Movie 1). In contrast, lateral cell contralateral branches form a complex pattern at the ventral border of the contralateral neuropil that extends along the lateral margins (Figs. 2E–H) (Supplemental Movie 2). Both serotonergic neurons also extend ipsilateral projections. These ipsilateral projections are slightly different between the medial and lateral cell (Fig. 2, 3, Supplementary Fig. 2). The overall pattern is depicted in (Fig. 3). The ipsilateral connections of both medial and lateral cells seem to fasciculate along a similar pathway except that the lateral cell’s ipsilateral branches extend a contralateral neurite (ic, Fig. 3) about mid level in the dorso-ventral and anterior-posterior axis. This contralateral branch extends a short way anterior along a midline tract commonly innervated by contralateral serotonergic secondary branches (see below). The main ipsilateral branches have a structure that is similar to the contralateral medial primary branches except that they form more centrally. In addition, they project anteriorly and form the majority of their branches in the next anterior segment. A small number of short secondary branches can be seen, mostly from the medial ipsilateral main branches. Thus the neuropil of one hemisegment is tiled into three main regions: branches from the contralateral lateral cell occupy the lateral and ventral margins, branches from both the medial and lateral ipsilateral cells occupy the central region and branches from the contralateral medial cell occupy all other regions (Fig. 3). While this pattern is shared among the majority of abdominal segments of the VNC, the serotonergic neurons within the A7 segment have a different pattern, where the medial cells have a single primary branch that extends centrally and disperses into a dense cloud of varicosities (Supplemental Fig. 2). For simplicity, we have chosen to focus primarily on the non-A7 segment contralateral projections in the following experiments.
Figure 3. Summary of overall branch structure of the abdominal serotonergic neurons.
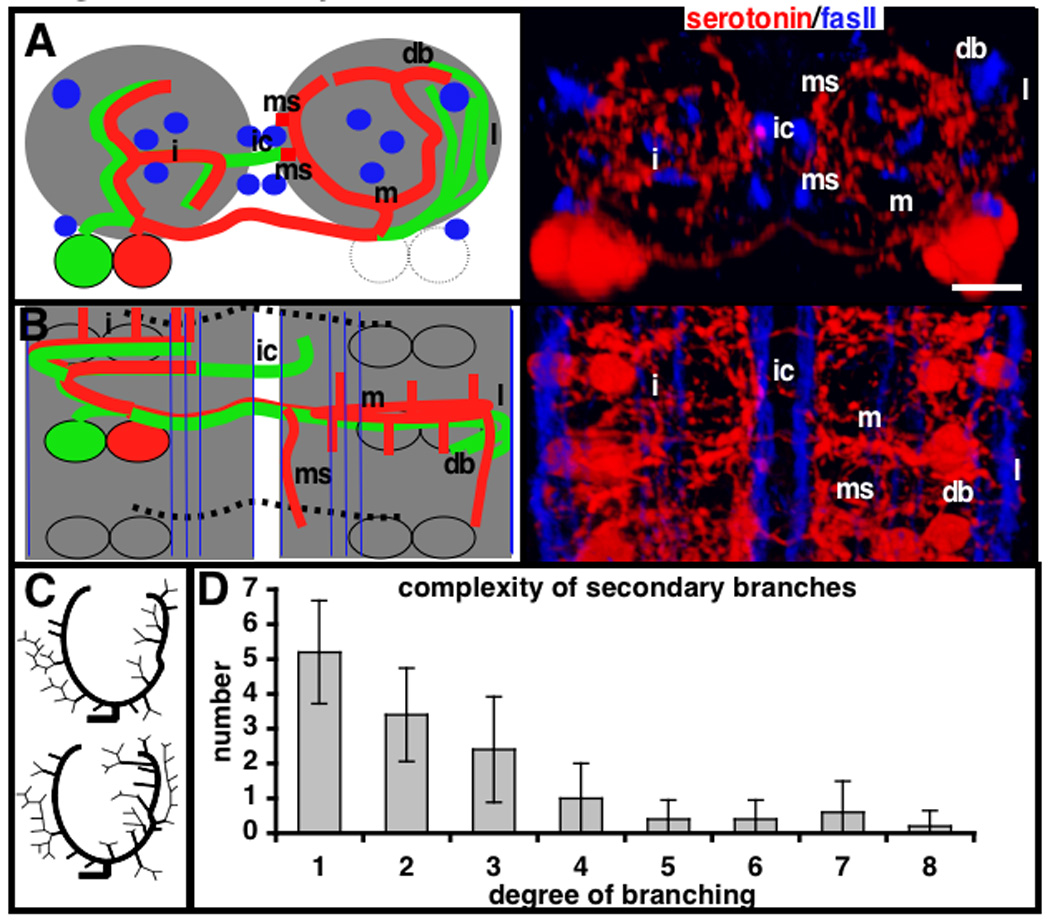
(A) Frontal view with dorsal at top and (B) top view with anterior at top of an A4, A5 or A6 segment. Left columns are cartoon representations and right panels are both stained for serotonin (red) and fasciclin II (blue). Also refer to Fig. 2 and 3 for images of these branches. (A and B) In the cartoon, the medial cell is drawn in red and lateral in green. The fasciclin II bundles are shown in blue and the neuropil in grey. m: medial contralateral branches; i: ipsilateral branches; l: lateral contralateral branches; db: dorsal bifurcation; ms: secondary branches that run along the edge of the midline, just lateral of the dorsal medial fasII bundle; ic: the contralateral projecting secondary branch. (C) Dendrograms of two reconstructed medial cell contralateral branches showing pattern of the secondary branches. The branches are drawn in to show the structure, but are not drawn to scale and do not represent the direction of secondary branch projections. (D) Plot of branch complexity as defined by number of branch points. The x-axis represents the number of segments on a branch and the y-axis the average number of secondary branches of this complexity extending from a medial contralateral primary branch. A secondary branch without any daughter bifurcations is designated 1 by this measure and a branch with one bifurcation a 2.
Secondary branches originate at non-fixed positions along the primary branches and have non-stereotypical projections
In the larval VNC, the two primary branches from the medial cell give rise to numerous smaller branches. We refer to all branches originating off the primary branches, including their sub-branches, as secondary branches. Two prominent secondary branches often form off the contralateral medial cell primary branches (Fig 3). The lateral primary branch of the medial cell extends a prominent secondary branch at the dorsal point, that projects in a posterior direction for variable distances (db, Fig. 3). The medial primary branch of the medial cell has a prominent secondary branch that extends posteriorly along the midline edge of the neuropil at the mid dorso-ventral axis. This secondary branch does not always derive from the same point along the medial primary branch. Besides these two often-occurring secondary branches, no other obvious pattern exists among this class. To analyze these further, 11 wild type patterns were reconstructed for the positions and trajectories of secondary branches and 5 were reconstructed for the positions of all varicosities on branches. Two dendrograms are shown schematically in Figure 3. These secondary branches form the majority of the serotonergic cell’s contralateral projections. There are 406+/−102 (n=6) varicosities in a single hemisegment (counted from the posterior side of one set of serotonergic cell bodies to the posterior of those in the next segment, as done in (Sykes and Condron, 2005)). On the basis of the reconstructions, there are 28+/−4 and 191+/−29 (n=5) varicosities on the medial cell contralateral primary and secondary branches respectively. This accounts for over half of varicosities in a hemisegment. The remaining varicosities are derived from the lateral cell contralateral projections primarily, some from the ipsilateral anterior intersegmental projections and a few from medial cell posterior intersegmental projections.
Any given secondary branch has no obvious pattern in trajectory or position of origin along the primary branches (Fig. 3). There are 14.4+/−2.1 (n=5) secondary branch roots on the medial primary branches. Based on the examination of 111 branch points, all have a varicosity at the bifurcation point. Of 33 assayable branch points, 25 (75%) are asymmetric, in that one daughter branch terminates and the other continues to branch. The distribution of branch complexity, as defined by number of branch points, is shown in (Fig. 3D). This shows a ‘heavy-tail’ distribution, with the majority of branches having few if any bifurcations, but a consistent small number with many bifurcations.
Cell boundaries between serotonergic cells are not maintained through competition
The observation that neuropil space is parceled among serotonergic cells, both inter- or intra segmentally, suggests tiling-like competition as seen in peripheral dendrites (Gao et al., 2000). Based on this hypothesis, ablation of a neighboring serotonergic cell should allow the surviving cells to expand into the territory of the ablated cell. In order to determine whether competition between the medial and lateral serotonergic neurons within each hemiganglion is responsible for the demarcated branching pattern, we genetically ablated serotonergic neurons early in development. This ablation was achieved with the expression of reaper-hid (rpr-hid) with eagle-GAL4 (eg-GAL4) early in serotonergic embryonic development, which causes apoptosis of a subset of serotonergic neurons, leaving a variable number of serotonergic neurons intact. Eg is a transcription factor involved in the differentiation of serotonergic neurons, and is transiently expressed in serotonergic cells during embryonic development until late stage 17 (Dittrich et al., 1997). The pattern of ablation was unpredictable, with all serotonergic neurons within the VNC being roughly equally susceptible to this method of genetic ablation. We score ablation as the complete loss of serotonin staining. While it does remain possible that rpr-hid expression is merely removing serotonin staining, we have seen cells with a short degenerating axon using tph-GAL4 expression of rpr-hid (data not shown). Counting hemisegments with at least one surviving cell, we found an average of 7.6+/−6.5 (N=133) out of a possible 22. Of the 133 samples, three VNCs had complete ablation of serotonergic neurons. All VNCs showed some degree of ablation, with the minimum showing 2 hemisegments without staining. All samples have cells remaining in the brain, which is a result of decreased eg expression in the brain (Lundell and Hirsh, 1998, Fig. 1B). In addition, two prominent cells at the VNC/brain boundary are also intact in all VNCs. Larvae with complete ablation of all serotonergic neurons within the VNC have slight developmental delays, but are grossly normal (data not shown). In fact, adult flies can be isolated from this experiment with little or no serotonergic neurons seen in the abdominal VNC (data not shown).
Isolated serotonergic neurons maintained the medial and lateral patterns, even after growing for days in an environment devoid of neighboring serotonergic cells. This is evident by comparing the branch pattern of wild-type GFP labeled cells, whose neighboring serotonergic neurons are intact, with the branch pattern of single isolated serotonergic neurons (Figs. 4 C and D vs G and H). Ablation of a neighboring lateral cell does not affect the remaining medial cell’s branch structure. The medial pattern is maintained and is grossly indistinguishable from the medial pattern seen in the wild-type GFP labeled cells. Ablation of a lateral cell’s medial partner does not affect the remaining lateral cell’s branch structure (Fig. 4). This is found for every segment throughout the VNC (data not shown). These results suggest that there is no competition of growth between the lateral and medial cells. If homotypic competition were responsible for the maintenance of the boundary between medial and lateral projections, removal of one would lead to overgrowth from the remaining cell into the region vacated by the ablation. Because of the timing of ablation, the surviving cells have over 72 hours to potentially fill in the vacated neuropil.
Figure 4. Genetic ablation of serotonergic cells reveals that the cell boundaries are not maintained through competition.
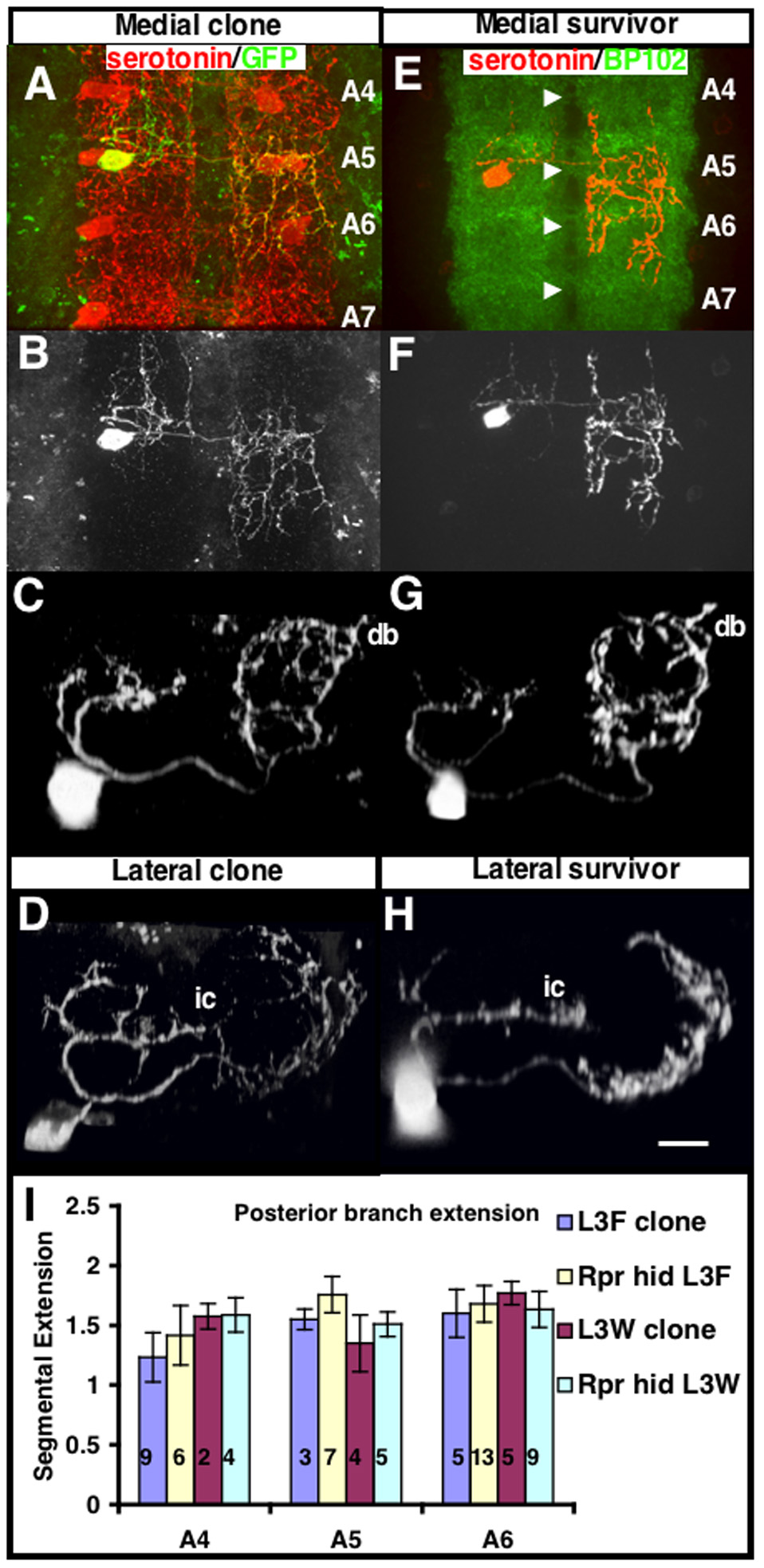
(A–D) Single GFP labeled cells generated in the same way as in figure 1 are shown for comparison. (A) A medial A5 serotonergic neuron is labeled with GFP. The medial A5 serotonergic neuron extends its contralateral axonal projections throughout the 5th abdominal segment and halfway through the 6th abdominal segment (halfway between the A5 and A6 commissure). For quantifying the distance of projections, we define the full innervation of a segment as 1 segment away. Therefore, this cell sends its projections 1.5 segments away. (B) Top view of GFP alone from (A) is shown to highlight the anterior-posterior extensions. (C) Frontal view of a medial A5 serotonergic neuron labeled with GFP. (D) Frontal view of a lateral A3 serotonergic neuron labeled with GFP. The dorsal secondary branch (db) and lateral cell contralateral projection (ic) are also indicated. (E–H) Reaper-hid (rpr-hid) expression is limited to early serotonergic development by driving UAS-rpr hid with the eg-GAL4 promoter. Expression of rpr-hid early in serotonergic development causes apoptosis of a random subset of serotonergic neurons during embryogenesis, leaving a variable number of serotonergic neurons intact. (E) The top view image of a VNC stained with serotonin (red) and BP102 (green) shows a typical outcome of rpr-hid expression. BP102 is a general neuropil marker and is used to demark the segmental boundaries by staining the commissural axons. Serotonergic neurons cross the midline along the posterior commissure of each segment (denoted by the white arrowheads). In this VNC only a single medial A5 remains. The larva before dissection was morphologically and behaviorally grossly normal. The contralateral projection of this serotonergic neuron extends in the posterior direction and spans its segment and 3/4 into the adjacent posterior segment, which is calculated from the overlaid BP102 staining (1.75 segments). (F) Top view of serotonin staining alone from (E) is shown. (G) Frontal view of a single isolated medial A5 stained with serotonin. The medial pattern is maintained and is grossly indistinguishable from the medial pattern seen in medial cells labeled with GFP that have all neighboring cells intact (C). (H) Frontal view of a single isolated lateral A3 stained with serotonin. The lateral pattern is maintained and is grossly indistinguishable from the lateral pattern seen in lateral cells labeled with GFP that have all neighboring cells intact (D). (I) The average farthest posterior intersegmental distance that a medial cell sends its contralateral axons is shown for various segments, with comparisons between GFP labeled and rpr-hid isolated cells during L3F and L3W. Isolated serotonergic cells (rpr-hid) do not send their contralateral axonal extensions any farther than serotonergic cells with all neighboring cells intact (GFP-labeled cell). In L3W rpr-hid larvae, serotonergic cells have grown in isolation for five days with little/no increase in axonal extension in comparison to GFP labeled neurons in L3W. Isolated serotonergic cells (rpr-hid) do not send their contralateral axonal extensions any farther than serotonergic cells with all neighboring cells intact (GFP-labeled cell). Data are mean ±SD. Number of samples for each measure is indicated in the graph.
Genotypes: (A–D) hs-FLP;UAS-FRT-mcd8-GFP-FRT x ddc-GAL4, (E–H) UAS-rpr-hid x eg-GAL4
Comparison of wild-type GFP labeled cells with isolated serotonergic neurons can also be used to address the role of competition in maintaining intersegmental boundaries of serotonergic innervation. As mentioned previously, wild-type GFP labeled cells demonstrate that serotonergic neurons’ contralateral projections fill their segment and have anterior/posterior projections that extend variable distances into the adjacent segments (Fig. 2). The density and distance of the contralateral extensions were quantified for medial serotonergic neurons in A4–A6 segments (Fig. 4I ). On average, the contralateral projections from the medial cell filled its segment and extended halfway into the adjacent posterior segment. rpr-hid-induced ablation of serotonergic cells within adjacent segments does not cause significant changes in the distance or density of contralateral branches in the remaining isolated medial serotonergic neurons (Figs. 4I ). Furthermore, isolated medial serotonergic neurons in L3W rpr-hid larvae that have grown in isolation for five days do not demonstrate an increase in their contralateral segmental extensions. Therefore, the limited contralateral anterior/posterior extension of branches and varicosities is most likely not due to competition from like-serotonergic neurons within neighboring segments.
The stereotypical target branching patterns are maintained when axon routing is altered
To attempt to elucidate the mechanism behind the stereotypical primary branch patterns, we took advantage of the UAS-GAL4 system to ectopically express genes that cause errors in initial axonal routing. In our first line of experiments, we took advantage of derailed (drl), an atypical receptor tyrosine kinase that plays a role in commissure choice. drl expression driven by eg-GAL4 early in serotonergic development misdirects crossing axons to the anterior commissure of the adjacent posterior segment instead of the posterior commissure of its own segment (Bonkowsky et al., 1999). After crossing in the aberrant segment, the axon grows back to the correct segment (Fig. 5A–E). This can be seen more clearly in single serotonergic cells expressing drl (Fig. 5B and Supplemental Fig. 3), isolated through rpr-hid ablation of the neighboring serotonergic cells. The A6 medial serotonergic neuron sends its commissural axon along the anterior commissure of A7, as indicated by the white arrowhead in Supplemental Fig. 3, instead of the posterior commissure of A6 indicated by the white arrow in Supplemental Fig. 3. Although drl expression puts the serotonergic growth cone in contact with an adjacent serotonergic-vacated neuropil, the axon curves anteriorly after crossing the midline and innervates the correct segment’s neuropil (Supplemental Fig. 3). Isolated serotonergic cells with early drl expression reveal that the medial and lateral patterns are preserved even when the axons enter the contralateral side from an adjacent segment (Figs. 5C–E) and have no like-cells to interact with.
Figure 5. The primary branch pattern is maintained despite altered axon routing or number.
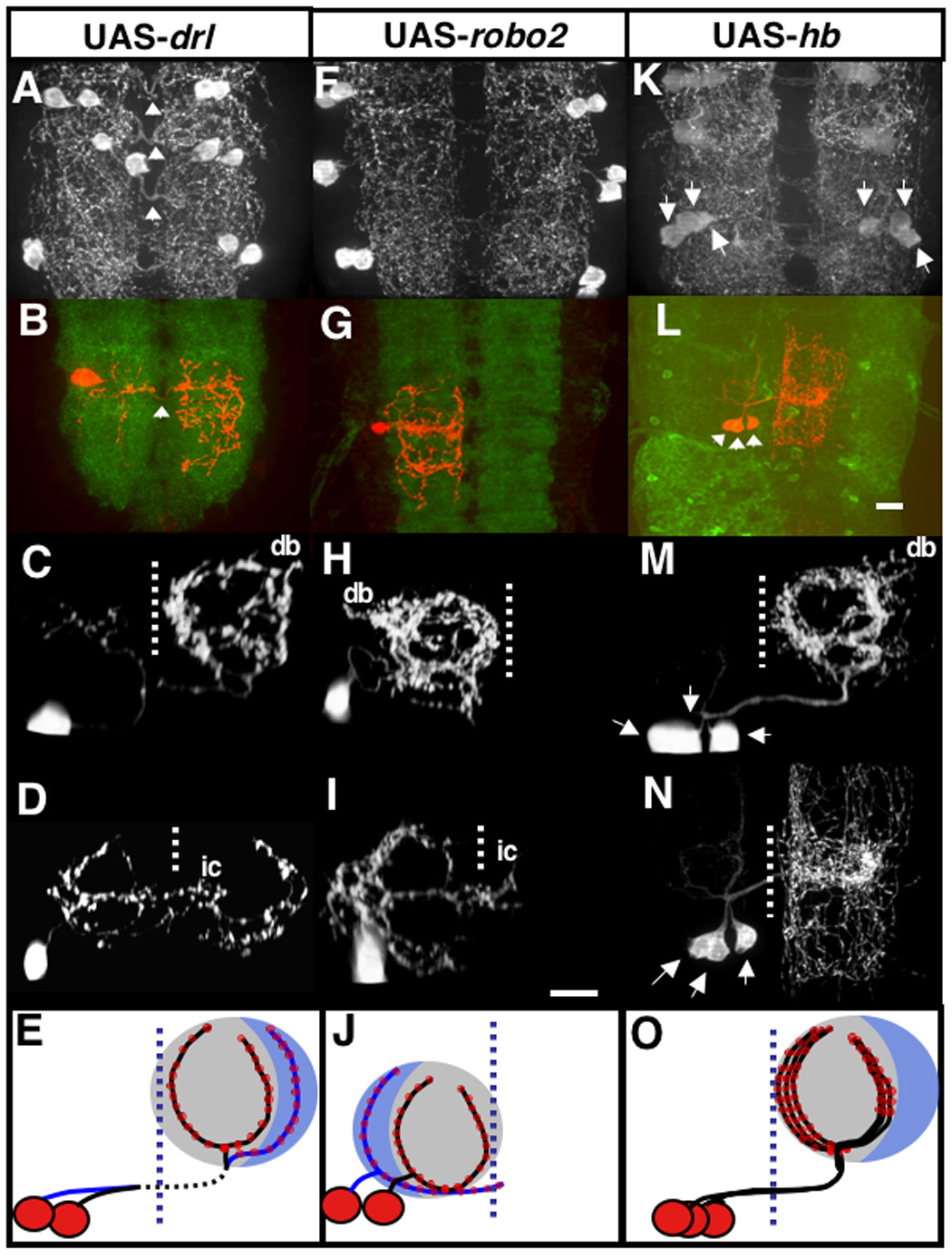
Effects of overexpression of drl (A,B,C,D,E), robo2 (F,G,H,I,J) and hb (K,L,M,N,O). The midline is indicated with a dashed line and the dorsal secondary branch (db) and lateral cell ipsilateral projection (ic) are also indicated. (A,F,K) are top views of serotonin stained segments with eg-GAL4 used as a driver. (B,G,L) shows a top view of segments stained for surviving serotonergic neurons with serotonin (red) and BP102 (B,G) and GFP (L). (C,D,H,I,M,N) are serotonin staining along of surviving neurons are side views from posterior except for (N) which is a top view.
(A–E) drl expression driven by eg-GAL4 early in serotonergic development misdirects crossing axons to the anterior commissure of the adjacent posterior segment instead of the posterior commissure of its own segment. After crossing in the adjacent segment, the axon grows back to the correct segment (A), which is denoted by the white arrowheads.
(B–D) drl expression in combination with rpr-hid generates isolated serotonergic cells, which reveals that the medial (C) and lateral patterns (D) are preserved even when the axons enter the contralateral side from an adjacent segment. (B) is a top view showing serotonin in red and BP102 in green. (E) Schematic representation of the medial and lateral pattern seen in serotonergic cells expressing drl. This pattern is identical to GFP labeled cells (Figure 2), except the axon crosses the midline along the adjacent anterior commissure, which is denoted by a dotted line as it crosses the midline (vertical blue dotted line).
(F–J) robo2 expression driven by eg-GAL4 early in serotonergic development blocks axons from crossing the midline (F). (G–I) robo2 expression in combination with rpr-hid creates single isolated serotonergic cells whose branches do not cross the midline, but instead project into the ipsilateral neuropil. (G) Shows a single isolated medial A4 stained with serotonin (red) and BP102 (green). (H) Shows a frontal view of a single medial cell. (I) Shows a frontal view of a single lateral cell. (J) Schematic of the medial and lateral pattern seen in serotonergic cells. This pattern is the mirror image of the normal medial and lateral patterns.
(K–N) Hunchback (hb) expression driven by eg-GAL4 early in serotonergic development causes the creation of multiple cells with circular medial projections. Three to five medially projecting serotonergic neurons can be seen per side of each segment (arrowheads) (K). There is a loss of cells with lateral projections. (L–N) Hb and rpr-hid combined expression can produce isolated multiples of cells with medial circular projections. The axons from these cells fasciculate along the same circular medial branch pattern and cannot be individually resolved under light microscopy. (N) Dorsal view of (M). (O) Schematic representation of serotonergic cells expressing hb. Genotypes: (A) UAS-drl x eg-GAL4 (B–D) UAS-rpr-hid x UAS-drl x eg-gal4; (F) UAS-robo2 x eg-GAL4 (G–I) UAS-rpr-hid x UAS-robo2 x eg-GAL4 (K) UAS-hb;UAS-hb x eg-GAL4 (L–N) UAS-rpr-hid x UAS-hb;UAS-hb x eg-GAL4
Another way of disrupting axonal routing is altering the signaling involved in axon guidance across the midline. Axons are attracted to the midline through signals likely to involve midline-derived Netrin. Once the axon reaches the midline, the roundabout receptors (Robo’s) are expressed, and mediate the repulsion of axons away from the midline (Kidd et al., 1999; Rajagopalan et al., 2000; Simpson et al., 2000). This expression of the Robo receptors, especially robo2, allows axons to cross over to and stay at the contralateral side. Driving early ectopic expression of robo2 with eg-gal4 in serotonergic neurons blocks axons from crossing the midline (Fig. 5F). Instead of sending their projections into the contralateral neuropil, the serotonergic axons branch into the ipsilateral neuropil. robo2 expression in combination with rpr-hid creates single isolated serotonergic cells whose branch patterns can be seen more clearly (Figs. 5G–I). The axon grows ipsilaterally, and enters the neuropil from a lateral point instead of medial but still creates primary branch structures as seen in wild-type serotonergic neurons. Although they are the mirror images of the normal medial and lateral patterns, the medial serotonergic cell expressing robo2 still forms the circular primary branch structure, while the lateral serotonergic cell still grows along the lateral one-third of the neuropil (Figs. 5H–J).
The above experiment indicates that the cues that guide the patterns of the primary branches are maintained in the late larval CNS. In early L1, very low levels of robo2 expression likely remain from the tapering expression driven by eg-GAL4. Therefore serotonergic axons at this developmental time point are still repulsed from the midline and the primary branch patterns cannot be seen (Supplemental Fig. 4). After two days of larval growth (L3F), robo2 expression is no longer being driven by eg-GAL4 and therefore the serotonergic axons are able to fill the neuropil and establish a normal pattern on the ipsilateral side (Supplemental Fig. 4). This can be seen clearly with isolated cells that had expressed robo2 and rpr-hid during early development (Figs. 5H,I). It is unclear why cessation of robo2 overexpression does not result in the axon re-attempting to cross the midline although the contralateral projection from the lateral ipsilateral branches does (marked ‘ic’ in Fig. 5I). After 48–72 hours of larval development, when robo2 expression is still presumably below functional levels, the two primary branches from the medial cell can be seen slowly filling the ipsilateral neuropil resulting in the circular medial pattern seen in wild-type GFP labeled cells (Supplemental Fig. 4). However, continued expression of robo2 in L3F by driving robo2 with eg-GAL4 and ddc-GAL4 prevents axons from ever filling the neuropil (Supplemental Fig. 4B,C). Therefore, the primary branch template, whether intrinsic or extrinsic, is laid out in the embryo but also remains functional in the third instar larva.
Finally, to further examine the role of competition in establishing the primary branch patterns, we looked at the effect of sending multiple medial cell axons into the same segmental neuropil. To do this, we expressed hunchback (hb), encoding a transcription factor that is involved in establishing the medial (EW1) cell fate, the first specified serotonergic neuron from the NB7-3 neuroblast cell line (Novotny et al., 2002). hb expression driven by eg-GAL4 early in serotonergic development induces the birth of multiple cells with characteristics of the medial (EW1) cells at the expense of the lateral (EW2) cell (Novotny et al., 2002). Three to five cells with the circular medial projections are created per side of each segment (Fig. 5K). Only the medial pattern is seen for every segment examined. hb and rpr-hid combined expression can produce isolated multiples of cells, each with circular medial projections. The primary branches from these multiple isolated cells fasciculate along the same circular medial branch pattern and cannot be individually resolved along some sections under confocal microscopy (Figs. 5L–N). This demonstrates that there is little or no competition between primary branches of the same cell type.
Discussion
Serotonergic neurons form stereotypical primary branching patterns
Our studies demonstrate that the branch architecture of serotonergic neurons within the VNC of Drosophila larvae follows either a medial or a lateral pattern. The medial cells within each pair of serotonergic neurons project their axons to the contralateral side where they enter the neuropil and bifurcate, forming a circular pattern that innervates the medial two-thirds of the contralateral neuropil. The lateral serotonergic cells innervate the lateral one-third of the contralateral neuropil. With the exception of A8 and A7, these patterns are found in all segments within the abdominal VNC. In less than 1% cases studied, patterns are reversed where the medial cell innervates the lateral neuropil and the lateral cell innervates the medial neuropil. This is likely due to the swapping of cell body location, rather than a switch in cell fate. Evidence for this also comes from hb overexpression, where the medial cell and the medial pattern are duplicated at the expense of the lateral cell. This indicates that the medial and lateral cells have different innervation patterns. The medial cell is therefore most likely EW1 and the lateral cell EW2 (Novotny et al., 2002). hb staining was too low in larval serotonergics to be used as an additional EW1-specific marker (data not shown).
The medial and lateral patterns are stereotypical and are maintained even when axonal guidance is altered. Axons can enter from an adjacent segment and still form these patterns as seen when drl is expressed in the serotonergic neurons early in development. When axons are forced to enter the neuropil from the lateral position, as in the case when robo2 is expressed early in development, the medial and lateral patterns are preserved.
The primary branches are formed in the late embryo when the serotonergic axons first begin to branch and are found up to pupal metamorphosis. Even if serotonergic axons do not fill the neuropil during development, it remains amenable to later serotonergic innervation. This is apparent by experiments demonstrating the delayed regrowth of the medial branch pattern seen in isolated serotonergic cells that have early robo2 mis-expression. Early robo2 expression during embryogenesis and early L1 causes serotonergic axons to be clustered in lateral positions away from the midline. However, after robo2 is no longer expressed, serotonergic axons expand into the rest of the neuropil and resume the medial and lateral patterns over a span of two to three days. This argues that the template for serotonergic growth is created during embryogenesis and both grows and persists independently of serotonergic innervation. However, these experiments do not exclusively locate the template to intrinsic or extrinsic factors.
Serotonergic neurons form branch patterns that are independent of their neighboring serotonergic neurons
Ablation studies demonstrate that competition between the medial and lateral serotonergic cells does not play a major role in creating the clearly demarcated medial and lateral patterns. This is supported by the observation that the loss of a medial serotonergic neuron does not lead to an overgrowth into the vacated region from the remaining lateral cell. Conversely when the lateral cell is ablated, the medial cell’s projections do not overstep their normal boundaries and the innervation pattern is still confined to the medial two-thirds of the contralateral neuropil. These findings were surprising because there have been numerous studies demonstrating the importance of tiling/competition in creating non-redundant innervation patterns. Competition also seems to play a very minor role in regulating the intersegmental growth patterns from serotonergic cells. Growth from serotonergic neurons is largely limited to their own segment, even when all neighboring serotonergic neurons have been genetically ablated. GFP wild-type labeled cells and isolated serotonergic neurons both extend their contralateral projections mostly within their segment, with some projections that also extend a half segment away. This limited growth may be influenced by hox-coordinated patterning (Merritt and Whitington, 2002). If the hox rules can be nullified, a role of competition in regulating growth between segments may be unmasked. Hox genes may play a role in providing segment identity within the serotonergic neurons as has been observed for sensory projections. If competition does have a larger more global effect, it remains secondary to segment-specific cell fate regulation. From these studies, it appears that intrinsic determinants may play a larger role than extrinsic cues in regulating the branching patterns of serotonergic neurons with the Drosophila VNC. The reliance on intrinsic determinants for serotonergic branching morphology is also seen in the leech, where it has been shown that a subset of serotonergic neurons almost exclusively utilizes intrinsic factors in developing their axonal morphologies (Acklin and Nicholls, 1990). This may be a common theme among serotonergic neurons that is preserved across species. Studies done in vertebrates suggest that serotonergic innervation of the forebrain by the dorsal and median raphe nuclei involve specific zones of innervation that are independent of projections from the other raphe nuclei (Mamounas, 1991). If serotonergic innervation is predominantly ‘hardwired’, then it follows that the chances of genomically encoded defects are also possible. Given the much ascribed role of the serotonergic system in human psychiatric disease, it will be interesting to see if some aspect of targeting is intrinsically disrupted.
Supplementary Material
Acknowledgements
We would like to thank all members of the Condron lab for helpful discussions. This work was funded by a Keck Scholars Award and NIH-RO1 DA020942 to BGC and by the University of Virginia Medical Scientist Training Program to JJC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acklin SE, Nicholls JG. Intrinsic and extrinsic factors influencing properties and growth patterns of identified leech neurons in culture. J Neurosci. 1990;10:1082–1090. doi: 10.1523/JNEUROSCI.10-04-01082.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky JL, Yoshikawa S, O'Keefe DD, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402:540–544. doi: 10.1038/990122. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. A Solid-Phase Immunostaining Protocol for High-Resolution Imaging of Delicate Structures in the Drosophila Larval Central Nervous System. CSH protocols. 2007 doi: 10.1101/pdb.prot4771. [DOI] [PubMed] [Google Scholar]
- Dittrich R, Bossing T, Gould AP, Technau GM, Urban J. The differentiation of the serotonergic neurons in the Drosophila ventral nerve cord depends on the combined function of the zinc finger proteins Eagle and Huckebein. Development. 1997;124:2515–2525. doi: 10.1242/dev.124.13.2515. [DOI] [PubMed] [Google Scholar]
- Gan WB, Macagno ER. Interactions between segmental homologs and between isoneuronal branches guide the formation of sensory terminal fields. J Neurosci. 1995;15:3243–3253. doi: 10.1523/JNEUROSCI.15-05-03243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. Control of dendritic field formation in Drosophila: the roles of flamingo and competition between homologous neurons. Neuron. 2000;28:91–101. doi: 10.1016/s0896-6273(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Sanchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterization of genetically amenable neurites. Dev Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Schall JD, Ault SJ. Extrinsic determinants of retinal ganglion cell structure in the cat. J Neurosci. 1988;8:2028–2038. doi: 10.1523/JNEUROSCI.08-06-02028.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas L, Mullen C, O'hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: Morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. The Journal of Comparitive Neurology. 1991;314:558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Merritt DJ, Whitington PM. Homeotic genes influence the axonal pathway of a Drosophila embryonic sensory neuron. Int J Dev Biol. 2002;46:633–638. [PubMed] [Google Scholar]
- Novotny T, Eiselt R, Urban J. Hunchback is required for the specification of the early sublineage of neuroblast 7-3 in the Drosophila central nervous system. Development. 2002;129:1027–1036. doi: 10.1242/dev.129.4.1027. [DOI] [PubMed] [Google Scholar]
- Peters BH, Tyrer NM. Electron microscopy of serotonin-immunoreactive neuron branches and terminals in the locust central nervous system. Neuroscience. 1987;23:333–341. doi: 10.1016/0306-4522(87)90294-6. [DOI] [PubMed] [Google Scholar]
- Prokop A. Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Sykes PA, Condron BG. Development and sensitivity to serotonin of Drosophila serotonergic varicosities in the central nervous system. Dev Biol. 2005;286:207–216. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou NA, Xu T. Use of FLP/FRT system to study Drosophila development. Methods. 1998;14:355–365. doi: 10.1006/meth.1998.0591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


